Abstract
The beneficial effects of probiotics have been described in many diseases, but the mechanism by which they modulate the immune system is poorly understood. In this study, we identified a mixture of probiotics that up-regulates CD4+Foxp3+ regulatory T cells (Tregs). Administration of the probiotics mixture induced both T-cell and B-cell hyporesponsiveness and down-regulated T helper (Th) 1, Th2, and Th17 cytokines without apoptosis induction. It also induced generation of CD4+Foxp3+ Tregs from the CD4+CD25− population and increased the suppressor activity of naturally occurring CD4+CD25+ Tregs. Conversion of T cells into Foxp3+ Tregs is directly mediated by regulatory dendritic cells (rDCs) that express high levels of IL-10, TGF-β, COX-2, and indoleamine 2,3-dioxygenase. Administration of probiotics had therapeutical effects in experimental inflammatory bowel disease, atopic dermatitis, and rheumatoid arthritis. The therapeutical effect of the probiotics is associated with enrichment of CD4+Foxp3+ Tregs in the inflamed regions. Collectively, the administration of probiotics that enhance the generation of rDCs and Tregs represents an applicable treatment of inflammatory immune disorders.
Keywords: regulatory T cell, inflammation, atopic dermatitis, inflammatory bowel disease, rheumatoid arthritis
Gastrointestinal microorganisms affect host physiology through diverse mechanisms, including modulation of the host immune system. Probiotics are nonpathogenic microorganisms that confer a number of beneficial effects on the health of the host (1). Among them, species of lactobacilli and Bifidobacteria are prominent probiotics with antiinflammatory properties (2). For example, administration of Lactobacillus casei suppresses proinflammatory responses by increasing IL-10 levels (3, 4), whereas Lactobacillus acidophilus increases T helper 1 (Th1) type cytokines (5). However, many questions remain unanswered, such as which probiotic strains are the most effective in modulation of specific immune disorders and how orally administrated probiotics affect the systemic immune system (6).
In this study, we sought to identify which probiotics or mixture of probiotics could confer potent antiinflammatory effects by increasing CD4+Foxp3+ regulatory T cells (Tregs). The forkhead family protein Foxp3 is a transcription factor highly expressed in CD4+ Tregs. It is a regulator of T-cell tolerance and is necessary for the development and function of Tregs (7). Following in vitro and in vivo functional studies on Treg generation, we developed a probiotics mixture that exhibits potent antiinflammatory properties and investigated its modulation of diverse immune disorders.
The probiotics mixture, designated IRT5, consists of a combination of five probiotic strains. Oral administration of IRT5 induced T- and B-cell hyporesponsiveness without inducing apoptosis. IRT5 administration increased the level of CD4+Foxp3+ Tregs in mesenteric lymph node (MLN) by augmenting Foxp3+ levels in CD4+CD25− T cells. Conversion of T cells into Foxp3+ Tregs is directly mediated by regulatory dendritic cells (rDCs) that express increased levels of IL-10, TGF-β, COX-2, and indoleamine 2,3-dioxygenase (iDO). Administration of IRT5 suppressed the progression of experimental inflammatory bowel disease (IBD), atopic dermatitis (AD) and rheumatoid arthritis (RA). In addition, migration of Tregs to inflammatory regions in response to chemokines (CCL1 and CCL22) and their receptors (CCR4 and CCR8) mediated disease suppression.
Results
Probiotics Increase CD4+Foxp3+ Tregs in a CD4+CD25− T-Cell Population.
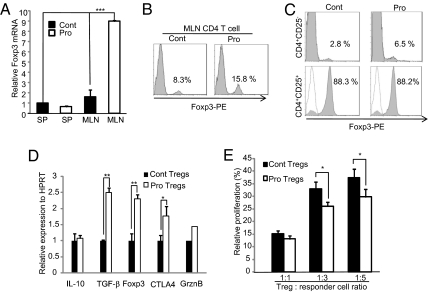
Probiotic bacteria have potent immunomodulatory effects, but their mechanism of action is poorly understood. To identify probiotic strains that could specifically up-regulate CD4+Foxp3+ Tregs, we developed an ex vivo screening system by coculturing freshly isolated MLN with probiotics. Candidate probiotics, or a mixture of them, were selected based on the criteria that showed high levels (>4) of the IL-10/IL-12 production ratio and enhanced Foxp3 expression (>1.5-fold). These include L. acidophilus (LA), L. casei (LC), Lactobacillus reuteri (LR), Bifidobacterium bifidium (BB), and Streptococcus thermophilus (ST). After determination of the optimal dosage (5 × 108 cfu/day; SI Materials and Methods), candidate probiotic strains, individually or in combination, were fed to normal healthy BALB/c mice for 20 days and the population of CD4+Foxp3+ T cells in MLN was measured by fluorescence-activated cell sorting (FACS). A mixture of the five strains (LA/LC/LR/BB/ST) significantly (P < 0.005) increased the CD4+Foxp3+ Treg population compared with control, individual strain, or other mixture groups (Fig. S1 A–C). Thereafter, all experiments were performed with the mixture of five probiotics, designated IRT5. Administration of IRT5 significantly (P < 0.005) increased Foxp3 mRNA expression (Fig. 1A) and protein level (Fig. 1B) compared with the PBS-treated group. Next, we tested whether increased Foxp3 levels originated from naturally occurring CD4+CD25+ Tregs (nTregs) (8) or from induced Tregs in the CD4+CD25− population. Administration of IRT5 [probiotics (Pro)] significantly increased Foxp3 levels (6.5%) compared with the control PBS-fed group (Cont; 2.8%) within the population of CD4+CD25− Tregs (Fig. 1C, Upper). In addition, although IRT5 did not increase the CD4+CD25+ population (Fig. S1D) and no difference in the CD4+CD25+Foxp3+ population was observed between the treatment groups (Fig. 1C, Lower), the CD4+CD25+ Tregs of the Pro group showed increased expression of Treg-associated molecules [TGF-β and cytotoxic T-lymphocyte-associated (CTLA)-4; Fig. 1D], resulting in significantly (P < 0.05) elevated suppressor activity compared with Tregs from the Cont group (Fig. 1E). We also compared the functional difference of CD4+CD25− effector T cells between PBS- and IRT5-fed groups. Compared with the Cont group, CD4+CD25− T cells from the Pro group were hypoproliferative (Fig. S2A) with slightly increased suppression sensitivity by the CD4+CD25+ Tregs (Fig. S2B). These observations indicate that administration of IRT5 induced generation of CD4+Foxp3+ Tregs from the pool of CD4+CD25− cells and potentiated the suppressor function of CD4+CD25+ nTregs.
Fig. 1.
Probiotics increase CD4+Foxp3+ Tregs. Foxp3 levels were analyzed by real-time PCR (A) or FACS (B) in SP and MLN CD4+ T cells from mice fed either PBS (Cont) or IRT5 (Pro) for 20 days. (C) Levels of Foxp3+ cells were analyzed in CD4+CD25+ and CD4+CD25− populations. (D) Expression levels of Treg-associated molecules were compared in MLN CD4+CD25+ Tregs from each group. GrznB, Granzyme B. (E) Suppressor activity of CD4+CD25+ Tregs was measured by T-cell proliferation assay. Tregs from each treatment group (Cont or Pro) were cocultured with responder cells (CD4+CD25−) at various ratios of Treg/responder cells. The proliferation level of responder cells alone was assigned a value of 100%. Data are the average of three independent experiments (10 mice per group); error bars indicate SD. *P < 0.05; **P < 0.005; ***P < 0.001.
Probiotics Induce Hyporesponsiveness in CD4+ T Cells.
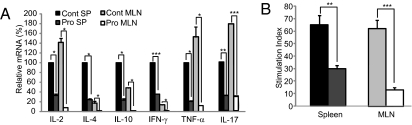
Next, we tested whether increased CD4+Foxp3+ Tregs by IRT5 affects the effector function of T cells and B cells. IRT5 (Pro) or PBS (Cont) was fed to normal BALB/c mice for 20 days, and CD4+ T cells or B220+ B cells isolated from spleen (SP) and MLN were stimulated with phorbol 12-myristate 13-acetone (PMA)/ionomycin. The relative transcript levels of Th1 (IL-2, IFN-γ, and TNF-α), Th2 (IL-4 and IL-10), and Th17 (IL-17) cytokines were measured by real-time PCR. Administration of IRT5 significantly (P < 0.05–0.005) reduced the levels of all tested cytokines in both CD4+ T cells (Fig. 2A) and B cells (Fig. S3A). In addition, administration of IRT5 induced hyporesponsiveness in both CD4+ T cells (Fig. 2B) and B cells (Fig. S3B) without inducing apoptosis (Annexin V+/7-aminoactinomycin D (7-ADD)+) (Fig. S3 C and D).
Fig. 2.
Probiotics induce hyporesponsiveness in CD4 T cells. (A) Levels of cytokine expression in CD4+ T cells were analyzed by real-time PCR. Cytokine expression in spleen CD4+ T cells of Cont group mice was set at 100%. (B) Proliferation of CD4+ T cells from the Cont or Pro group mice was measured after stimulation with anti-CD3/anti-CD28. Data are from 10 mice per group; error bars indicate SD. Data are representative of three independent experiments. **P < 0.005; ***P < 0.001.
Probiotics Administration Induces CD11c+ rDCs, Which, in Turn, Promote Generation of CD4+Foxp3+ T Cells.
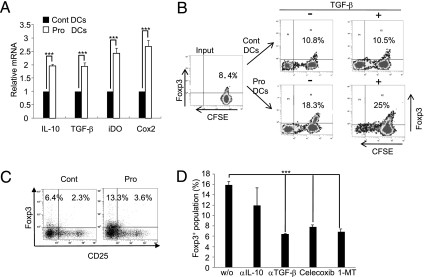
We next investigated the role of CD11c+ dendritic cells (DCs) in the generation of CD4+Foxp3+ Tregs by IRT5. MLN CD11c+ DCs isolated from each treatment group were stimulated with PMA/ionomycin, and the levels of tolerogenic DC markers were measured. Administration of IRT5 significantly up-regulated the expression levels of IL-10, TGF-β, iDO, and COX-2 (Fig. 3A) without increasing CD103+ DCs, which are recognized as a marker of mucosal DCs generating Foxp3+ Tregs (9) (Fig. S4A). We further tested whether rDCs generated by IRT5 have the potential to induce Foxp3+ Tregs. MLN DCs from each group were cocultured with DO11.10 CD4+T cells labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) in the presence of Ova peptide (0.3 μg/mL), with or without exogenous TGF-β, which induces the generation of induced Tregs in vitro. The CD4+Foxp3+ Treg population was then analyzed by FACS. MLN DCs from Pro mice preferentially converted T cells into CD4+Foxp3+ T cells compared with the Cont group in both the absence (18.3% Pro vs. 10.8% Cont) and presence (25% Pro vs.10.5% Cont) of TGF-β (Fig. 3B). In addition, CD4+ T cells cocultured with IRT5-treated MLN DCs showed significantly reduced proliferation compared with control (30% Pro vs. 61% Cont) (Fig. S4B) because of their increased Treg population (Fig. 3B). Like in vivo-generated Tregs by IRT5 administration (Fig. 1C), up-regulation of Foxp3 levels were mainly observed in the CD4+CD25− population (13.3% Pro vs. 6.4% Cont) (Fig. 3C). To characterize the functional property of rDCs generated by IRT5 further, we tested whether addition of inhibitors for rDCs could inhibit rDC-dependent Foxp3+ Treg generation. CD4+ T cells were cocultured with MLN CD11c+ DCs in the presence of various types of inhibitors, and Foxp3+ populations then were analyzed by FACS. Addition of inhibitors such as anti-TGF-β, celecoxib (a COX-2 inhibitor), or 1-methyl-d-tryptophan (1-MT, an iDO inhibitor) significantly down-regulated Foxp3+ generation (Fig. 3D and Fig. S4 C and D). Collectively, these results suggest that IRT5 facilitates the generation of rDCs that have the capacity to convert Foxp3− T cells into Foxp3+ Tregs.
Fig. 3.
Probiotics induce CD11c+ rDCs. (A) CD11c+ DCs isolated from MLN of Cont or Pro group mice were stimulated with PMA/ionomycin. The levels of immunoregulatory molecules were measured by real-time PCR. (B) CD11c+ DCs from each group were cocultured with Do11.10 CD4+ T cells labeled with CFSE and ovalbumin peptide (0.3 μg/mL) in the absence (−) or presence (+) of exogenous TGF-β. (C) Levels of Foxp3+CD25− and Foxp3+CD25+ populations were measured by FACS. (D) MLN CD11c+ DCs from IRT5-fed mice were cocultured with CD4+ T cells in the presence of ovalbumin peptide and inhibitors for rDCs. w/o, without. After coculture, alteration of the Foxp3+ population was measured by FACS. Data are from 10 mice per group and are the average of three independent experiments.
Probiotics Suppress Experimental Immune Disorders.
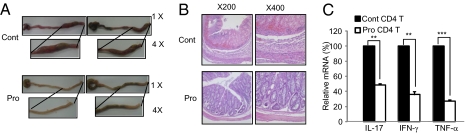
Next, we tested whether IRT5 could ameliorate immune disorders such as IBD, AD, and RA. Trinitrobenzesulfonic acid (TNBS) colitis is a well-characterized animal model of Th1-mediated IBD, and it mimics human Crohn’s disease. Mice were fed with PBS or IRT5 daily for 20 days, and the intestinal colitis was established by an intrarectal injection of TNBS. The prevention effects of IRT5 were monitored by measuring survivalship, weight loss, colitis grade, gross morphology, and histology of the colon (Fig. 4 and Fig. S5). Retardation of IBD progression in the Pro group was associated with reduction of all tested criteria for disease evaluation, including inflammatory cytokines such as IL-17, IFN-γ, and TNF-α (Fig. 4C).
Fig. 4.
Probiotics suppress experimental colitis. (A) Changes in gross intestines were evaluated 3 days after induction of colitis. (B) Histological analysis after H&E staining to detect lymphocyte infiltration. (C) Real-time PCR was performed to analyze changes in the expression of proinflammatory cytokines in response to probiotics administration. Data are from 20 mice per group; error bars indicate SD. Data are representative of three independent experiments. **P < 0.005; ***P < 0.001.
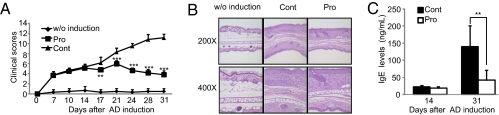
Next, we tested whether IRT5 also has a therapeutical effect on AD, which is known to be a typical Th2-mediated immune disorder. A murine AD model was established by alternate painting of house dust mite extract and dinitrochlorobenzene. Two weeks after AD induction, IRT5 or PBS was administered to mice with AD and the parameters for AD progression were measured. Treatment with IRT5 significantly (P < 0.05) inhibited the clinical symptoms of AD progression (Fig. 5A and Fig. S6A) by reducing IgE levels [total IgE level (Fig. 5C) and mite-specific IgE level (Fig. S6C)], infiltrated lymphocytes and granulocytes (Fig. 5B and Fig. S6B), and levels of AD-associated cytokines, such as IL-4, IL-5, IL-10 and IL-13 (Fig. S6 E and F). In addition, administration of IRT5 significantly slowed the progression of collagen-induced experimental RA by reducing clinical symptoms, lymphocyte infiltration into the joint, and levels of inflammatory cytokines, while increasing Foxp3 levels (Fig. S7).
Fig. 5.
Probiotics suppress experimental AD. The therapeutical efficacy of IRT5 was measured by the following criteria for AD progression: clinical score (A), lymphocyte infiltration by histological analysis (B), and total IgE levels (C). w/o, without. Bars are from 10 mice per group; error bars indicate SD. Data are representative of three independent experiments. **P < 0.005; ***P < 0.001.
Enriched CD4+Foxp3+ Treg Cells at Sites of Inflammation Are Associated with Disease Suppression.
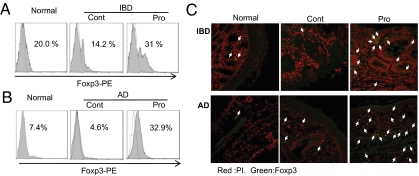
CD4+Foxp3+ Tregs suppress pathogenic effector cells through cell-to-cell contact or focal increment at the inflamed sites (10–12). Thereby, we measured the population of CD4+Foxp3+ Tregs at the sites of disease progression. Mice with AD or IBD were treated with IRT5 or PBS, and the population of CD4+Foxp3+ T cells at the inflammatory sites (colon in experimental IBD and ear in AD) was determined by FACS and immunohistochemistry (IHC). The Pro group showed significantly increased CD4+Foxp3+ T cells compared with the Cont group in both IBD (31% vs. 14.2%; Fig. 6A) and AD (32.9% vs. 4.6%; Fig. 6B) mice. Enrichment of Foxp3+CD4+ Tregs in the IRT5-treated group was also confirmed by IHC in both the inflammatory colon and atopic ear (Fig. 6C and Fig. S8).
Fig. 6.
Enriched CD4+Foxp3+ Tregs at inflammatory sites are associated with disease suppression. The Foxp3+ population from the colon of experimental colitis (A) or ear of AD (B) mice was analyzed by FACS or IHC (C) between the Pro and Cont groups. The arrows in C indicate Foxp3+ cells. A control reaction was performed with isotype-matched IgG antibodies (data not shown). Data are from 10 mice per group and represent the average of three independent experiments.
Increased Chemoattractants for Treg Induce Migration of CD4+Foxp3+ Tregs to Inflamed Regions.
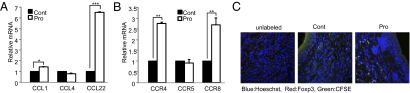
Next, we checked whether increased CD4+Foxp3+ Tregs are directly related to the therapeutical effects of IRT5 in multiple types of immune disorders. As in normal mice (Fig. 1), IRT5 administration in disease conditions also significantly increased the CD4+Foxp3+ Treg populations in MLN (Fig. S9 A and B). We further tested whether enriched levels of CD4+Foxp3+ Tregs in the colon (IBD) or ear (AD) (Fig. 6C) are associated with increased chemoattractants for CD4+Foxp3+ Tregs, resulting in their migration from MLN to the inflammatory sites. We measured the expression levels of chemokines and their receptors involved in the recruitment of CD4+Foxp3+ Tregs in AD mice. Indeed, the group fed with IRT5 (Pro) showed significantly increased expression levels of CCL1 and CCL22 in the ear tissues (Fig. 7A) and their receptors, CCR4 and CCR8 (13 –15), in ear residual CD4+ T cells (Fig. 7B). We further examined the migratory properties of CD4+Foxp3+ Tregs generated in MLN by IRT5 administration to the inflammatory region by means of an adoptive transfer experiment. CD4+ T cells isolated from MLN of the mice fed PBS or IRT5 for 20 days were labeled with CFSE and then adoptively transferred to AD mice by i.p. injection (Fig. S9C). The CFSE+Foxp3+ populations in the ear of AD mice were measured by IHC (Fig. 7C and Fig. S10). As a negative control, the same numbers of unlabeled CD4+ T cells were transferred (Fig. 7C, Left, and Fig. S10). Ear sections from each group of mice were stained by Hoechst (blue) and anti-Foxp3 antibodies (red). CFSE+(CD4+)Foxp3+ cells were detected by bright green staining as a result of overlap of the colors. The Pro group (Fig. 7C, Right, and Fig. S10) showed much higher numbers of CFSE+(CD4+)Foxp3+ cells compared with the Cont group (Fig. 7C, Middle, and Fig. S10) in the ear regions of AD mice. Conclusively, these results suggest that CD4+Foxp3+ Tregs generated by administration of IRT5 in MLN migrated to the inflamed sites to mediate suppression of disease progression.
Fig. 7.
Increased chemoattractant expression leads to enrichment of Tregs in the inflamed region. The expression levels of chemokines in MHC class II-positive antigen-presenting cells (A) and their receptors in CD4+ T cells (B) were analyzed by quantitative real-time PCR. (C) CFSE-labeled T cells obtained from MLN of Cont and Pro groups were adoptively transferred to AD mice. The migration of CFSE+(CD4+)Foxp3+ Tregs to the ear of AD mice was analyzed by IHC. The arrows indicate CFSE+(CD4+)Foxp3+ Tregs. Data are from 10 mice per group; error bars indicate SD. Data are representative of three independent experiments. *P < 0.05; **P < 0.005; ***P < 0.001.
Discussion
In this study, we developed a probiotics mixture (designated IRT5) with potent anti-inflammatory properties and examined its modulation efficacy of experimental immune disorders. Administration of IRT5 induced rDCs, which, in turn, potentiated generation of CD4+Foxp3+ Tregs in MLN. Enrichment of CD4+Foxp3+ Tregs at sites of inflammation is associated with up-regulated levels of chemoattractants (CCL1 and CCL22), which inhibited the progression of experimental immune disorders.
Little information is available on the mechanism by which administration of probiotics generates regulatory T-cell populations. In the present study, we present evidence that ingestion of a specific probiotics mixture (IRT5) can generate CD4+Foxp3+ Tregs in MLN. The immunomodulatory action of IRT5 was not achieved by increasing the expression of immunosuppressive cytokines such as IL-10 or TGF-β but, instead, by suppressing the expression of inflammatory (IL-17, IFN-γ, and TNF-α) and antiinflammatory (IL-10) cytokines in both T cells (Fig. 2) and B cells (Fig. S3 A and B) without apoptosis induction (Fig. S3 C and D). Administration of IRT5 did not increase the number of nTregs (Fig. 1C) but did increase their suppressor capacity (Fig. 1 D and E). Interestingly, ingestion of IRT5 up-regulated Foxp3 levels more than 2-fold in the CD4+CD25− population (Fig. 1C).
How do probiotics promote the generation of CD4+Foxp3+ cells? They may not directly generate adaptive Tregs but induce rDCs to exhibit immunological tolerance. Indeed, probiotics (IRT5) alone without rDCs failed to induce Foxp3+ Tregs when they were cocultured with CD4+ T cells up to 3 days (Fig. S4E). Recently, two studies reported that oral administration of a certain probiotic strain could increase Foxp3+ Tregs (16, 17) in the SP. However, previous studies did not provide any clue as to how probiotics can enhance the generation of CD4+Foxp3+ Tregs. In this study, we provided evidence and sequential scenarios for how IRT5 can generate CD4+Foxp3+ Tregs in MLN. Administration of IRT5 probiotics induced rDCs, which, in turn, promoted generation of CD4+Foxp3+ T cells in MLN (Fig. 3). DCs can directly present antigens from commensal bacteria to MLN and interact with T and B cells to maintain noninflammatory immune responses (18, 19). Treatment with IRT5 generates CD11c+ DCs that express markers of rDCs such as IL-10, TGF-β, COX-2, and iDO (20). COX-2 is one of several active molecules to generate Foxp3+ Tregs in various types of systems, including cancer (21, 22). iDO DCs promote the development of Treg cells (23). Therefore, we postulated that IRT5 generated tolerogenic DCs, which, in turn, induced generation of CD4+Foxp3+ Tregs. Indeed, we demonstrated that rDCs generated by IRT5 could convert CD4+CD25− T cells into CD4+CD25−Foxp3+ T cells (Fig. 3 B and C), in which, however, addition of inhibitors for TGF-β, COX-2, or iDO strongly inhibited the generation of Foxp3+ Tregs (Fig. 3D and Fig. S4C). Mucosal CD103+ DCs play important roles in the generation of Foxp3+ Tregs through in a retinoic acid TGF-β-dependent manner (24). However, rDCs generated by IRT5 administration were not CD103+CD11c+ but CD103c−CD11c+. IRT5 administration did not up-regulate CD103+ (Fig. S4A), and blockade of retinoic acid (LE540, a retinoic acid inhibitor) did not alter the levels of rDC-mediated Foxp3+ Tregs (Fig. S4D). How does the immune system recognize to the generation of rDCs in MLN? Currently we are trying to identify and elucidate the role of the pattern recognition receptors (e.g., Toll-like receptor-2 and other TLRs) (25, 26) and underlying signal transduction pathways (e.g., Myd88 dependency).
Administration of IRT5 increased the numbers of adaptive Tregs (CD4+CD25−Foxp3+) both in healthy mice (Fig. 1) and in IBD and AD mouse models (Fig. S9 A and B). The protective effect of IRT5 is associated with highly up-regulated levels of CD4+Foxp3+ Tregs in the inflammatory regions (Fig. 6 and Fig. S8). Tregs are able to recirculate through lymphoid tissues and to enter inflamed sites (27) by expressing specific chemokines and their receptors (28). We also found that administration of IRT5 significantly increased the expression of chemokines (CCL1 and CCL22; Fig. 7A) and their receptors (CCR4 and CCR8; Fig. 7B) (13 –15). Furthermore, the results of an adoptive transfer experiment revealed that Tregs generated in MLN by IRT5 can migrate to the inflammatory sites of AD mice (Fig. 7C and Fig. S10). Decreased Tregs are associated with inflammatory diseases, including IBD and AD (29, 30). Interestingly, experimental IBD and AD mice also exhibited reduced numbers of CD4+Foxp3+ Tregs in inflamed sites compared with control mice (Fig. 6 and Fig. S8). Hence, enriched CD4+Foxp3+ Tregs induced by IRT5 may suppress inflammatory effector T cells at the sites of inflammation, resulting in a protective effect in these disease models.
One of the challenging issues in the probiotic field is how to identify potent immunoregulatory probiotics among the hundreds of probiotic strains. Recently, we developed an ex vivo screening system to identify immune-regulatory probiotics inducing IL-10 and Foxp3+ Tregs but reducing proinflammatory cytokines. After coculturing MLN total cells with each probiotic strain for 3 days, probiotic strains that showed a high level (>4) of the IL-10/IL-12 ratio and more than a 1.5-fold increase in Foxp3+ levels were selected. A mixture of selected strains (IRT5) showed more potent disease suppression efficacy than single or three- and four-strain combinations (Fig. S11). Interestingly, heat-inactivated IRT5 did not have any significant therapeutical effects. Why does a combination of probiotics show more potent therapeutical effects than a single probiotic strain (Fig. 1 and Fig. S11)? The combination of three (Fig. S11; multistrain combination) and four or five (Fig. S11; multispecies combinations) strains may enhance colonization and adhesion activity (31, 32) compared with a single strain, resulting in increased immune-modulatory activities by which Foxp3+ Tregs were generated. How does IRT5 modulate disease progression, regardless of the site of inflammation, in inflammatory diseases? We suggest the following sequence of events. First, DCs recognize and process the administered IRT5 probiotics. During this process, CD11c+ DCs are converted into rDCs that are able to convert CD4+Foxp3− T cells into CD4+Foxp3+ Tregs. Next, the generated Tregs in MLN migrate to the inflammatory sites, directed by chemoattractant produced at the inflammatory sites, resulting in an enrichment of Tregs. Finally, Tregs abolish the inflammatory effector function of pathogenic T cells, which leads to suppression of disease progression.
In summary, the combined probiotics in IRT5 exerted potent immunomodulatory effects by up-regulating or potentiating the generation of Tregs by tolerogenic DCs in MLN. In addition, migration of CD4+Foxp3+ Tregs to sites of inflammation effectively suppressed disease progression. Our results present evidence of the generation of CD4+CD25−Foxp3+ Tregs in response to probiotics, an effect that may be therapeutically useful for the modulation of inflammatory immune disorders.
Materials and Methods
Probiotic Strains.
The probiotics mixtures of live bacteria contained 1 × 109 cfus of each of the following strains: BB, LA, ST, LC, and Lactobacillus leuteri. To test the effect of single strains, each strain was administered to mice at a concentration of 5 × 108 cfus per 100 μL. Probiotic strains used in this study were purchased from Cell Biotech Co., Ltd.
DC-Dependent Treg Cell Differentiation Assay.
MLN CD11c+ DCs were isolated from each group and cultured with splenic CD4+ T cells isolated from Do11.10 transgenic mice or WT BALB/c mice labeled with 10 mM CFSE (Invitrogen) in the presence of ovalbumin peptide (0.2 μg/mL) and soluble anti-CD3 (1 μg/mL) with or without mouseTGF-β (2 ng/mL, PeproTech; catalog no. 100-21c) for 4 days. To test the roles of rDC-related molecules in rDC-mediated Treg generation, inhibitors were added such as anti-IL-10 (10 μg/mL; BD PharMingen), anti-TGF-β (10 μg/mL; R&D Systems), LE540 (0.1 or 1 μM; Wako), celecoxib (50 μM; Biovision) and 1-MT (20 μM; Sigma). The population of Foxp3+ Treg cells was analyzed by FACS.
Adoptive Transfer Experiment.
After 20 days of oral administration of PBS or probiotics (IRT5), mice were killed and MLN CD4+ T cells were isolated from each group. A total of 5 × 106 CD4+ T cells were labeled with 5 μM CFSE for 15 min at 37°C and adoptively transferred into AD-induced mice by i.p. injection. Twenty-four hours after adoptive transfer, mice were killed and ears were removed to examine the migration of Tregs by IHC (33).
Statistical Analysis.
The Student’s t test was used to determine significance, and P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
This research was supported by grants from the 21C Frontier Functional Human Genome Project (FG08-21-11); National Research Foundation of Korea (2009-0079438) funded by the Korean government; Regional Technology Innovation Program of the Ministry of Knowledge Economy (RT105-01-01); and Korea Healthcare Technology R&D Project, Ministry for Health, Welfare, and Family Affairs (A080588-5) and by a Systems Biology Infrastructure Establishment grant provided by the Gwangju Institute of Science and Technology in 2008.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904055107/DCSupplemental.
References
- 1.Marteau P. Probiotics, prebiotics, synbiotics: Ecological treatment for inflammatory bowel disease? Gut. 2006;55:1692–1693. doi: 10.1136/gut.2004.051458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braat H, et al. Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4+ T cells via modulation of dendritic cell function. Am J Clin Nutr. 2004;80:1618–1625. doi: 10.1093/ajcn/80.6.1618. [DOI] [PubMed] [Google Scholar]
- 3.So J-S, et al. Lactobacillus casei potentiates induction of oral tolerance in experimental arthritis. Mol Immunol. 2008;46:172–180. doi: 10.1016/j.molimm.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 4.So J-S, et al. Lactobacillus casei suppresses experimental arthritis by down-regulating T helper 1 effector functions. Mol Immunol. 2008;45:2690–2699. doi: 10.1016/j.molimm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Sudo N, et al. An oral introduction of intestinal bacteria prevents the development of a long-term Th2-skewed immunological memory induced by neonatal antibiotic treatment in mice. Clin Exp Allergy. 2002;32:1112–1116. doi: 10.1046/j.1365-2222.2002.01430.x. [DOI] [PubMed] [Google Scholar]
- 6.Shanahan F. Probiotics: A perspective on problems and pitfalls. Scand J Gastroenterol. 2003;(38(suppl 237):34–36. doi: 10.1080/00855910310001476. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler SF. FOXP3: Of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 8.Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 9.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim CH. Migration and function of FoxP3+ regulatory T cells in the hematolymphoid system. Exp Hematol (Charlottesville, Va) 2006;34:1033–1040. doi: 10.1016/j.exphem.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Huehn J, Hamann A. Homing to suppress: Address codes for Treg migration. Trends Immunol. 2005;26:632–636. doi: 10.1016/j.it.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Gobert M, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Bromberg JS. T regulatory cells and migration. Am J Transplant. 2006;6:1518–1523. doi: 10.1111/j.1600-6143.2006.01372.x. [DOI] [PubMed] [Google Scholar]
- 15.Sebastiani S, et al. Chemokine receptor expression and function in CD4+ T lymphocytes with regulatory activity. J Immunol. 2001;166:996–1002. doi: 10.4049/jimmunol.166.2.996. [DOI] [PubMed] [Google Scholar]
- 16.Feleszko W, et al. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007;37:498–505. doi: 10.1111/j.1365-2222.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- 17.Karimi K, Inman MD, Bienenstock J, Forsythe P. Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am J Respir Crit Care Med. 2009;179:186–193. doi: 10.1164/rccm.200806-951OC. [DOI] [PubMed] [Google Scholar]
- 18.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 19.Huang F-P, et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 21. Sharma S et al. (2005) Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res 65:5211–5220. [DOI] [PubMed]
- 22.Sharma S, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 23.Mellor AL, Munn DH. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 24.von Boehmer H. Oral tolerance: Is it all retinoic acid? J Exp Med. 2007;204:1737–1739. doi: 10.1084/jem.20071251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai J, Liu B, Li Z. Regulatory T cells and Toll-like receptors: What is the missing link? Int Immunopharmacol. 2009;9:528–533. doi: 10.1016/j.intimp.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutmuller RPM, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maul J, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 28.D’Ambrosio D, Sinigaglia F, Adorini L. Special attractions for suppressor T cells. Trends Immunol. 2003;24:122–126. doi: 10.1016/s1471-4906(03)00027-9. [DOI] [PubMed] [Google Scholar]
- 29.Leung DYM, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 31.Timmerman HM, Koning CJM, Mulder L, Rombouts FM, Beynen AC. Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int J Food Microbiol. 2004;96:219–233. doi: 10.1016/j.ijfoodmicro.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Collado MC, Meriluoto J, Salminen S. Development of new probiotics by strain combinations: Is it possible to improve the adhesion to intestinal mucus? J Dairy Sci. 2007;90:2710–2716. doi: 10.3168/jds.2006-456. [DOI] [PubMed] [Google Scholar]
- 33.Kwon H-K, et al. Cinnamon extract suppresses tumor progression by modulating angiogenesis and the effector function of CD8+ T cells. Cancer Lett (Shannon, Irel) 2009;278:174–182. doi: 10.1016/j.canlet.2009.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.