Abstract
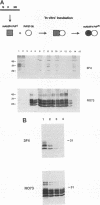
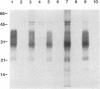
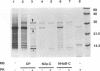
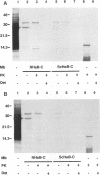
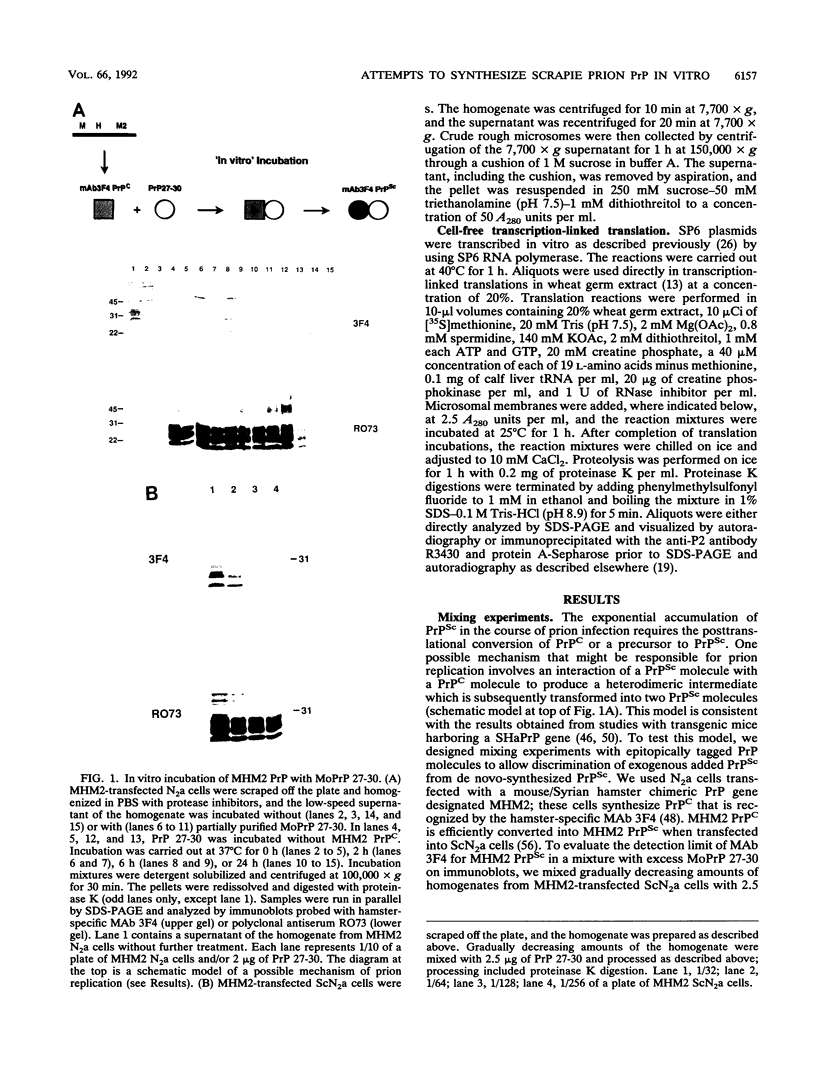
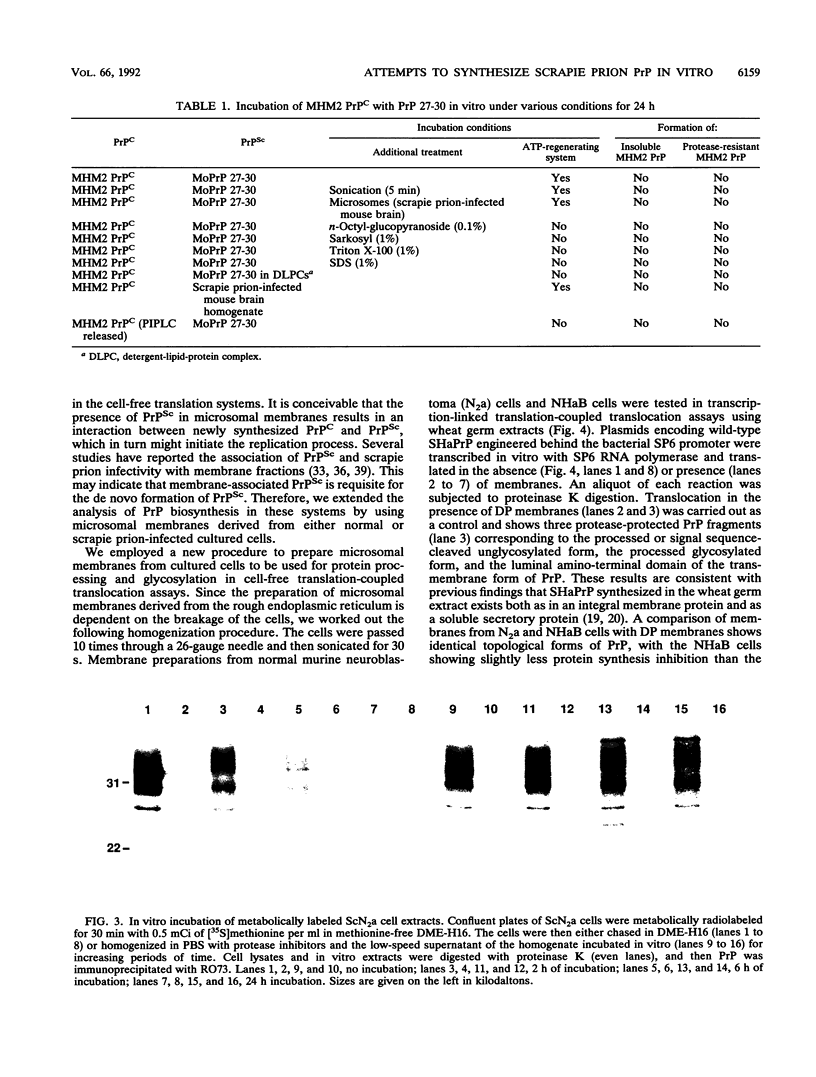
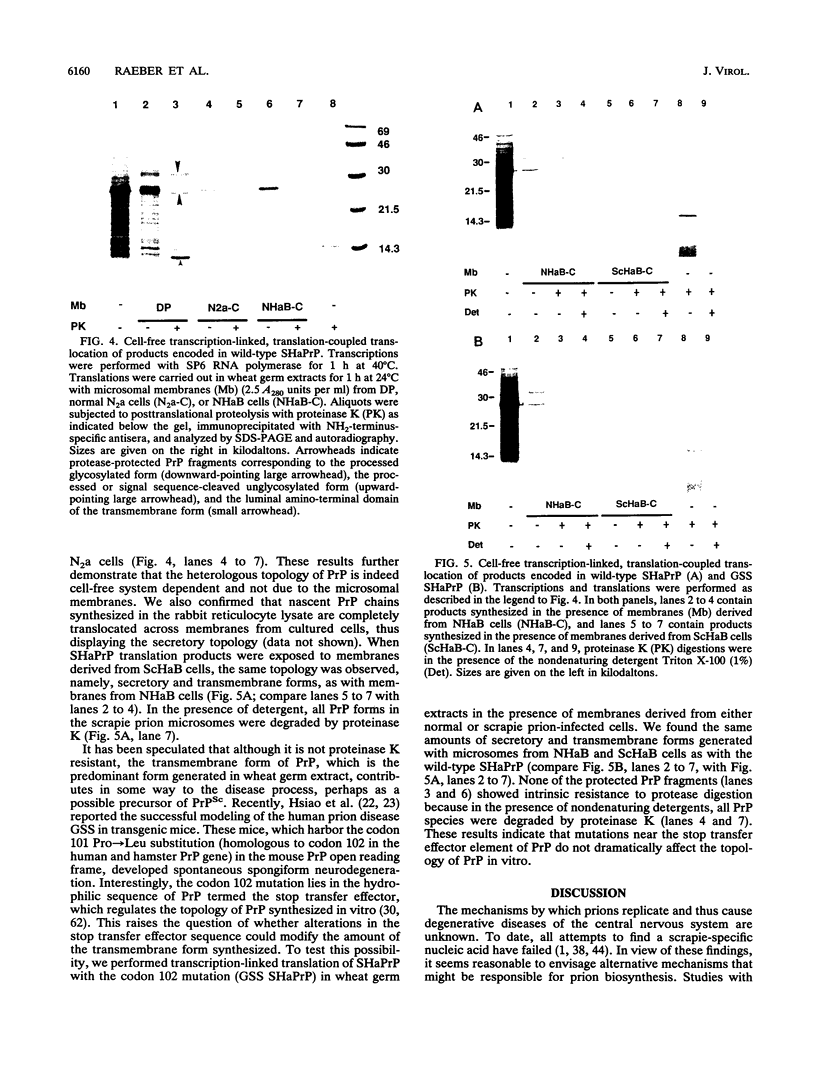
The scrapie prion protein (PrPSc) is derived from a cellular isoform (PrPC) that acquires protease resistance posttranslationally. We have used several different experimental approaches in attempts to reconstitute in vitro the processes leading to protease-resistant PrPSc molecules. In the first study, we performed mixing experiments by adding mouse PrP 27-30 (MoPrP27-30), the protease-resistant core of PrPSc, to PrPC and then incubating the mixture to investigate the possibility of heterodimer formation as a first step in prion replication. We used epitopically tagged PrP molecules, synthesized in murine neuroblastoma (N2a) cells transfected with the chimeric mouse/Syrian hamster MHM2 PrP construct, which are recognized by the Syrian hamster-specific monoclonal antibody 3F4. After as long as 24 h of incubation, the reaction mixture was assayed for heterodimeric intermediates of MHM2 PrPC and MoPrPSc and for protease-resistant 3F4-reactive PrP. We were unable to identify any aggregates of MHM2 PrPC and MoPrPSc on immunoblots; furthermore, we did not observe de novo formation of protease-resistant MHM2 PrP. In a second study, MoPrPC was metabolically radiolabeled in scrapie prion-infected N2a cultured cells, and then the cell extract was homogenized and incubated under various conditions to allow for the formation of protease-resistant MoPrPSc. We observed no radiolabeled MoPrPSc by immunoprecipitation after as long as 24 h of in vitro incubation. In a third approach, Syrian hamster PrP (SHaPrP) was synthesized in a cell-free translation system supplemented with microsomal membranes derived from either normal or scrapie prion-infected cultured cells. We found that all SHaPrP species translocated across microsomal membranes from scrapie prion-infected cells were protease sensitive in the presence of detergents and displayed the same topology as those generated by microsomes from normal cells or from dog pancreas. We also studied PrP molecules that encode the codon 102 mutation that causes the rare human prion disease Gerstmann-Sträussler-Scheinker (GSS) syndrome. On the basis of our data, GSSPrP appears to yield topological forms similar to those of the wild-type PrP when processed by either normal or scrapie prion-derived microsomes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiken J. M., Marsh R. F. The search for scrapie agent nucleic acid. Microbiol Rev. 1990 Sep;54(3):242–246. doi: 10.1128/mr.54.3.242-246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry R. A., Vincent M. T., Kent S. B., Hood L. E., Prusiner S. B. Characterization of prion proteins with monospecific antisera to synthetic peptides. J Immunol. 1988 Feb 15;140(4):1188–1193. [PubMed] [Google Scholar]
- Basler K., Oesch B., Scott M., Westaway D., Wälchli M., Groth D. F., McKinley M. P., Prusiner S. B., Weissmann C. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell. 1986 Aug 1;46(3):417–428. doi: 10.1016/0092-8674(86)90662-8. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton D. C., McKinley M. P., Prusiner S. B. Identification of a protein that purifies with the scrapie prion. Science. 1982 Dec 24;218(4579):1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- Bolton D. C., Meyer R. K., Prusiner S. B. Scrapie PrP 27-30 is a sialoglycoprotein. J Virol. 1985 Feb;53(2):596–606. doi: 10.1128/jvi.53.2.596-606.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt D. R., Scott M., Taraboulos A., Stahl N., Prusiner S. B. Scrapie and cellular prion proteins differ in their kinetics of synthesis and topology in cultured cells. J Cell Biol. 1990 Mar;110(3):743–752. doi: 10.1083/jcb.110.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D. A., Scott M. R., Bockman J. M., Borchelt D. R., Taraboulos A., Hsiao K. K., Kingsbury D. T., Prusiner S. B. Scrapie-infected murine neuroblastoma cells produce protease-resistant prion proteins. J Virol. 1988 May;62(5):1558–1564. doi: 10.1128/jvi.62.5.1558-1564.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B., Raymond G. J., Ernst D., Race R. E. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): implications regarding the site of conversion of PrP to the protease-resistant state. J Virol. 1991 Dec;65(12):6597–6603. doi: 10.1128/jvi.65.12.6597-6603.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B., Raymond G. J. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J Biol Chem. 1991 Sep 25;266(27):18217–18223. [PubMed] [Google Scholar]
- Endo T., Groth D., Prusiner S. B., Kobata A. Diversity of oligosaccharide structures linked to asparagines of the scrapie prion protein. Biochemistry. 1989 Oct 17;28(21):8380–8388. doi: 10.1021/bi00447a017. [DOI] [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Cell-free translation of messenger RNA in a wheat germ system. Methods Enzymol. 1983;96:38–50. doi: 10.1016/s0076-6879(83)96007-x. [DOI] [PubMed] [Google Scholar]
- Gabizon R., McKinley M. P., Groth D. F., Kenaga L., Prusiner S. B. Properties of scrapie prion protein liposomes. J Biol Chem. 1988 Apr 5;263(10):4950–4955. [PubMed] [Google Scholar]
- Gabizon R., McKinley M. P., Prusiner S. B. Purified prion proteins and scrapie infectivity copartition into liposomes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4017–4021. doi: 10.1073/pnas.84.12.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek D. C. Unconventional viruses and the origin and disappearance of kuru. Science. 1977 Sep 2;197(4307):943–960. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- Gibbs C. J., Jr, Gajdusek D. C., Asher D. M., Alpers M. P., Beck E., Daniel P. M., Matthews W. B. Creutzfeldt-Jakob disease (spongiform encephalopathy): transmission to the chimpanzee. Science. 1968 Jul 26;161(3839):388–389. doi: 10.1126/science.161.3839.388. [DOI] [PubMed] [Google Scholar]
- Haraguchi T., Fisher S., Olofsson S., Endo T., Groth D., Tarentino A., Borchelt D. R., Teplow D., Hood L., Burlingame A. Asparagine-linked glycosylation of the scrapie and cellular prion proteins. Arch Biochem Biophys. 1989 Oct;274(1):1–13. doi: 10.1016/0003-9861(89)90409-8. [DOI] [PubMed] [Google Scholar]
- Hay B., Barry R. A., Lieberburg I., Prusiner S. B., Lingappa V. R. Biogenesis and transmembrane orientation of the cellular isoform of the scrapie prion protein [published errratum appears in Mol Cell Biol 1987 May;7(5):2035]. Mol Cell Biol. 1987 Feb;7(2):914–920. doi: 10.1128/mcb.7.2.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay B., Prusiner S. B., Lingappa V. R. Evidence for a secretory form of the cellular prion protein. Biochemistry. 1987 Dec 15;26(25):8110–8115. doi: 10.1021/bi00399a014. [DOI] [PubMed] [Google Scholar]
- Hope J., Multhaup G., Reekie L. J., Kimberlin R. H., Beyreuther K. Molecular pathology of scrapie-associated fibril protein (PrP) in mouse brain affected by the ME7 strain of scrapie. Eur J Biochem. 1988 Mar 1;172(2):271–277. doi: 10.1111/j.1432-1033.1988.tb13883.x. [DOI] [PubMed] [Google Scholar]
- Hsiao K. K., Scott M., Foster D., Groth D. F., DeArmond S. J., Prusiner S. B. Spontaneous neurodegeneration in transgenic mice with mutant prion protein. Science. 1990 Dec 14;250(4987):1587–1590. doi: 10.1126/science.1980379. [DOI] [PubMed] [Google Scholar]
- Hsiao K., Baker H. F., Crow T. J., Poulter M., Owen F., Terwilliger J. D., Westaway D., Ott J., Prusiner S. B. Linkage of a prion protein missense variant to Gerstmann-Sträussler syndrome. Nature. 1989 Mar 23;338(6213):342–345. doi: 10.1038/338342a0. [DOI] [PubMed] [Google Scholar]
- Kascsak R. J., Rubenstein R., Merz P. A., Tonna-DeMasi M., Fersko R., Carp R. I., Wisniewski H. M., Diringer H. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987 Dec;61(12):3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J. G., Schweizer A., Berger E. G., Hauri H. P., Yuan L. C., Klausner R. D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990 Mar 9;60(5):821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L., Tipper C., Amherdt M., Orci L., Klausner R. D. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991 Nov 1;67(3):601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Lopez C. D., Yost C. S., Prusiner S. B., Myers R. M., Lingappa V. R. Unusual topogenic sequence directs prion protein biogenesis. Science. 1990 Apr 13;248(4952):226–229. doi: 10.1126/science.1970195. [DOI] [PubMed] [Google Scholar]
- Low M. G., Stiernberg J., Waneck G. L., Flavell R. A., Kincade P. W. Cell-specific heterogeneity in sensitivity of phosphatidylinositol-anchored membrane antigens to release by phospholipase C. J Immunol Methods. 1988 Oct 4;113(1):101–111. doi: 10.1016/0022-1759(88)90386-9. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Valley S., Manuelidis E. E. Specific proteins associated with Creutzfeldt-Jakob disease and scrapie share antigenic and carbohydrate determinants. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4263–4267. doi: 10.1073/pnas.82.12.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R. F., Dees C., Castle B. E., Wade W. F., German T. L. Purification of the scrapie agent by density gradient centrifugation. J Gen Virol. 1984 Feb;65(Pt 2):415–421. doi: 10.1099/0022-1317-65-2-415. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Gajdusek D. C., Gibbs C. J., Jr Creutzfeldt-Jakob disease virus isolations from the Gerstmann-Sträussler syndrome with an analysis of the various forms of amyloid plaque deposition in the virus-induced spongiform encephalopathies. Brain. 1981 Sep;104(3):559–588. doi: 10.1093/brain/104.3.559. [DOI] [PubMed] [Google Scholar]
- McKinley M. P., Bolton D. C., Prusiner S. B. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983 Nov;35(1):57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- McKinley M. P., Meyer R. K., Kenaga L., Rahbar F., Cotter R., Serban A., Prusiner S. B. Scrapie prion rod formation in vitro requires both detergent extraction and limited proteolysis. J Virol. 1991 Mar;65(3):1340–1351. doi: 10.1128/jvi.65.3.1340-1351.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley M. P., Taraboulos A., Kenaga L., Serban D., Stieber A., DeArmond S. J., Prusiner S. B., Gonatas N. Ultrastructural localization of scrapie prion proteins in cytoplasmic vesicles of infected cultured cells. Lab Invest. 1991 Dec;65(6):622–630. [PubMed] [Google Scholar]
- Meyer N., Rosenbaum V., Schmidt B., Gilles K., Mirenda C., Groth D., Prusiner S. B., Riesner D. Search for a putative scrapie genome in purified prion fractions reveals a paucity of nucleic acids. J Gen Virol. 1991 Jan;72(Pt 1):37–49. doi: 10.1099/0022-1317-72-1-37. [DOI] [PubMed] [Google Scholar]
- Meyer R. K., McKinley M. P., Bowman K. A., Braunfeld M. B., Barry R. A., Prusiner S. B. Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2310–2314. doi: 10.1073/pnas.83.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Palmer M. S., Dryden A. J., Hughes J. T., Collinge J. Homozygous prion protein genotype predisposes to sporadic Creutzfeldt-Jakob disease. Nature. 1991 Jul 25;352(6333):340–342. doi: 10.1038/352340a0. [DOI] [PubMed] [Google Scholar]
- Pattison I. H. Fifty years with scrapie: a personal reminiscence. Vet Rec. 1988 Dec 24;123(26-27):661–666. [PubMed] [Google Scholar]
- Prusiner S. B., Bolton D. C., Groth D. F., Bowman K. A., Cochran S. P., McKinley M. P. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. doi: 10.1021/bi00269a050. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Molecular biology of prion diseases. Science. 1991 Jun 14;252(5012):1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Scott M., Foster D., Pan K. M., Groth D., Mirenda C., Torchia M., Yang S. L., Serban D., Carlson G. A. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell. 1990 Nov 16;63(4):673–686. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- Race R. E., Fadness L. H., Chesebro B. Characterization of scrapie infection in mouse neuroblastoma cells. J Gen Virol. 1987 May;68(Pt 5):1391–1399. doi: 10.1099/0022-1317-68-5-1391. [DOI] [PubMed] [Google Scholar]
- Rogers M., Serban D., Gyuris T., Scott M., Torchia T., Prusiner S. B. Epitope mapping of the Syrian hamster prion protein utilizing chimeric and mutant genes in a vaccinia virus expression system. J Immunol. 1991 Nov 15;147(10):3568–3574. [PubMed] [Google Scholar]
- Safar J., Wang W., Padgett M. P., Ceroni M., Piccardo P., Zopf D., Gajdusek D. C., Gibbs C. J., Jr Molecular mass, biochemical composition, and physicochemical behavior of the infectious form of the scrapie precursor protein monomer. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6373–6377. doi: 10.1073/pnas.87.16.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M., Foster D., Mirenda C., Serban D., Coufal F., Wälchli M., Torchia M., Groth D., Carlson G., DeArmond S. J. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell. 1989 Dec 1;59(5):847–857. doi: 10.1016/0092-8674(89)90608-9. [DOI] [PubMed] [Google Scholar]
- Serban D., Taraboulos A., DeArmond S. J., Prusiner S. B. Rapid detection of Creutzfeldt-Jakob disease and scrapie prion proteins. Neurology. 1990 Jan;40(1):110–117. doi: 10.1212/wnl.40.1.110. [DOI] [PubMed] [Google Scholar]
- Stahl N., Borchelt D. R., Hsiao K., Prusiner S. B. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987 Oct 23;51(2):229–240. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- Stahl N., Borchelt D. R., Prusiner S. B. Differential release of cellular and scrapie prion proteins from cellular membranes by phosphatidylinositol-specific phospholipase C. Biochemistry. 1990 Jun 5;29(22):5405–5412. doi: 10.1021/bi00474a028. [DOI] [PubMed] [Google Scholar]
- Taraboulos A., Rogers M., Borchelt D. R., McKinley M. P., Scott M., Serban D., Prusiner S. B. Acquisition of protease resistance by prion proteins in scrapie-infected cells does not require asparagine-linked glycosylation. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8262–8266. doi: 10.1073/pnas.87.21.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboulos A., Serban D., Prusiner S. B. Scrapie prion proteins accumulate in the cytoplasm of persistently infected cultured cells. J Cell Biol. 1990 Jun;110(6):2117–2132. doi: 10.1083/jcb.110.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi J., Ohta M., Koga M., Sato Y., Kuroiwa Y. Transmission of chronic spongiform encephalopathy with kuru plaques from humans to small rodents. Ann Neurol. 1979 Jun;5(6):581–584. doi: 10.1002/ana.410050616. [DOI] [PubMed] [Google Scholar]
- Turk E., Teplow D. B., Hood L. E., Prusiner S. B. Purification and properties of the cellular and scrapie hamster prion proteins. Eur J Biochem. 1988 Sep 1;176(1):21–30. doi: 10.1111/j.1432-1033.1988.tb14246.x. [DOI] [PubMed] [Google Scholar]
- Weissmann C. A 'unified theory' of prion propagation. Nature. 1991 Aug 22;352(6337):679–683. doi: 10.1038/352679a0. [DOI] [PubMed] [Google Scholar]
- Wilesmith J. W., Wells G. A. Bovine spongiform encephalopathy. Curr Top Microbiol Immunol. 1991;172:21–38. doi: 10.1007/978-3-642-76540-7_2. [DOI] [PubMed] [Google Scholar]
- Yost C. S., Lopez C. D., Prusiner S. B., Myers R. M., Lingappa V. R. Non-hydrophobic extracytoplasmic determinant of stop transfer in the prion protein. Nature. 1990 Feb 15;343(6259):669–672. doi: 10.1038/343669a0. [DOI] [PubMed] [Google Scholar]