Abstract
Plant immunity can be induced by two major classes of pathogen-associated molecules. Pathogen- or microbe-associated molecular patterns (PAMPs or MAMPs) are conserved molecular components of microbes that serve as “non-self” features to induce PAMP-triggered immunity (PTI). Pathogen effector proteins used to promote virulence can also be recognized as “non-self” features or induce a “modified-self” state that can induce effector-triggered immunity (ETI). The Arabidopsis protein RIN4 plays an important role in both branches of plant immunity. Three unrelated type III secretion effector (TTSE) proteins from the phytopathogen Pseudomonas syringae, AvrRpm1, AvrRpt2, and AvrB, target RIN4, resulting in ETI that effectively restricts pathogen growth. However, no pathogenic advantage has been demonstrated for RIN4 manipulation by these TTSEs. Here, we show that the TTSE HopF2Pto also targets Arabidopsis RIN4. Transgenic plants conditionally expressing HopF2Pto were compromised for AvrRpt2-induced RIN4 modification and associated ETI. HopF2Pto interfered with AvrRpt2-induced RIN4 modification in vitro but not with AvrRpt2 activation, suggestive of RIN4 targeting by HopF2Pto. In support of this hypothesis, HopF2Pto interacted with RIN4 in vitro and in vivo. Unlike AvrRpm1, AvrRpt2, and AvrB, HopF2Pto did not induce ETI and instead promoted P. syringae growth in Arabidopsis. This virulence activity was not observed in plants genetically lacking RIN4. These data provide evidence that RIN4 is a major virulence target of HopF2Pto and that a pathogenic advantage can be conveyed by TTSEs that target RIN4.
Keywords: bacterial virulence, type III secretion
An important virulence strategy adopted by bacterial phytopathogens is the suppression of plant immunity (1, 2). To accomplish this, Gram-negative bacterial phytopathogens such as Pseudomonas syringae translocate type III secretion effector (TTSE) proteins into the host cytosol via the type III secretion system (TTSS) (3). Several of these TTSEs have demonstrated the ability to suppress plant immunity by targeting important defense-related proteins thereby promoting pathogen growth (reviewed in ref. 1). In response, it is likely that the selective pressures imposed by pathogen effector proteins have contributed to shaping and driving the evolution of effective plant immune responses (4).
Plant effector-triggered immunity (ETI) occurs when specific pathogen effector proteins are detected by plant resistance (R) proteins, which activate an effective resistance response that often culminates in a localized programmed cell death termed the hypersensitive response (HR) (5, 6). The recognition of effectors by R proteins can occur by either direct or indirect interactions (7). In a number of cases described so far, R proteins recognize modifications of host proteins induced by the activity of effector proteins and subsequently induce ETI (6, 8).
The Arabidopsis RPM1-interacting protein (RIN4) is a negative regulator of PAMP-triggered immunity (PTI) that interacts with two R proteins, RPM1 and RPS2 (9 –12). Three unrelated P. syringae type III effectors, AvrRpm1, AvrRpt2, and AvrB, have been demonstrated to target RIN4 (10 –12). The R protein RPM1 can recognize the presence of both AvrB and AvRpm1, whereas RPS2 recognizes AvrRpt2. RIN4 is phosphorylated in response to infection by either AvrB or AvrRpm1, leading to the activation of RPM1 (10). However, kinase activity has not been demonstrated for either AvrB or AvrRpm1 and therefore the phosphorylation may be indirect (13 –15). AvrRpt2 belongs to the CA-clan of cysteine proteases (16). Upon translocation into the plant cell, host cyclophilins, like Arabidopsis ROC1, activate AvrRpt2 protease activity (17, 18). Activated AvrRpt2 undergoes autoprocessing of 71 amino acids from its N terminus to yield the mature protease (17, 19). AvrRpt2 then cleaves RIN4 at two sequences similar to its autoprocessing site (20 –22). Cleavage of RIN4 is believed to activate RPS2 leading to ETI (11, 12). Both recognition events by RPM1 and RPS2 are to the bacteria’s detriment, which raises the question of why these pathogenic TTSEs target RIN4. In fact, AvrRpm1, AvrRpt2, and AvrB all retain virulence functions in the absence of RIN4 and their corresponding resistance proteins (23). Therefore, it remains to be determined whether TTSE manipulation of RIN4 can promote pathogen virulence or whether RIN4 is a decoy for the true virulence targets of TTSEs (24).
The HopF2 (formerly HopPtoF) locus of P. syringae pv. tomato DC3000 (Pto DC3000) encodes two genes, the type III chaperone schFPto and the type III effector hopF2Pto (25). HopF2Pto DC3000 (hereafter referred to as HopF2Pto) uses a rare ATA translation initiation codon that limits protein production in P. syringae (25). Mutation of the native ATA start codon to ATG results in HopF2Pto-mediated virulence in tomato (26). HopF2Pto possesses a predicted myristoylation sequence that is required for localization to the plasma membrane of onion epidermal cells (26). Moreover, HopF2Pto avirulence in tobacco W38 and virulence in tomato require an intact myristoylation sequence, suggesting that HopF2Pto functions at the plasma membrane (26). HopF2Pto can suppress PTI in Nicotiana benthamiana, flagellin-induced NONHOST1 (NHO1) induction in Arabidopsis protoplasts, callose deposition in transgenic Arabidopsis expressing HopF2Pto, and also the HopA1 (formerly HopPsyA)-induced HR in the A. thaliana ecotype Ws-0 (27 –30). However, the biochemical function and host target(s) of HopF2Pto remain to be elucidated.
HopF1Pph7 from P. syringae pv. phaseolicola race 7 (1449B) shares 48% amino acid identity with HopF2Pto and displays virulence or avirulence functions on various bean cultivars (31 –33). The crystal structure of HopF1Pph7 shares limited structural homology to the catalytic domain of bacterial ADP ribosyltransferases (ADP-RT), yet no ADP-RT activity could be demonstrated in vitro (33). Nevertheless, the structural similarity identified a conserved pocket with two amino acids that share structural similarity to catalytic residues of the ADP ribosyltransferase diphtheria toxin, R72 and D174. Mutational analysis of these residues abolished the avirulence and virulence functions of HopF1Pph7 in the bean cultivars Red Mexican and Tendergreen, respectively, demonstrating their importance for HopF function (33).
To gain insights into HopF function and putative host targets, we examined HopF2Pto transgenic Arabidopsis plants for altered immunity. Arabidopsis plants expressing HopF2Pto are compromised for AvrRpt2-induced ETI. Furthermore, HopF2Pto can interfere with AvrRpt2-induced RIN4 cleavage in vivo and in vitro, suggesting that HopF2Pto may target RIN4. In support of this hypothesis, HopF2Pto interacts with RIN4 both in vivo and in vitro. We also demonstrate that HopF2Pto ATG can enhance the growth of P. syringae in Arabidopsis. The virulence function in Arabidopsis depends on the conserved amino acids important for both avirulence and virulence functions of HopF1Pph7 in bean cultivars. Importantly, RIN4 is required for HopF2Pto-enhanced P. syringae growth, indicating that RIN4 is a major virulence target of the TTSE HopF2Pto.
Results
HopF2Pto-Expressing Arabidopsis Plants Are Compromised for AvrRpt2-Mediated Effector-Triggered Immunity (ETI).
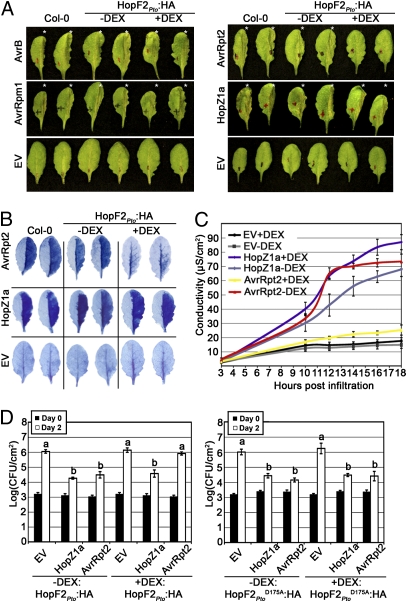
Transgenic Arabidopsis thaliana ecotype Col-0 capable of dexamethasone (DEX)-inducible hopF2Pto or hopF2PtoD175A (corresponding to HopF1Pph7 D174) expression were created with a C-terminal translational fusion to an HA epitope tag (Fig. S1) (34). To assess whether HopF2Pto:HA can delay or suppress the HR associated with ETI, Pto DC3000 expressing various avirulence genes were infiltrated into HopF2Pto:HA transgenic plants. AvrRpt2-mediated HR was suppressed in HopF2Pto-expressing plants, whereas the HR induced by AvrB, AvrRpm1, or HopZ1aPsyA2 was indistinguishable from untreated transgenic plants (Fig. 1A). Loss of AvrRpt2-mediated HR was also observed in two additional independent transgenic lines expressing HopF2Pto (Fig. S1). AvrRpt2-mediated HR suppression was not observed in two independent transgenic lines expressing the mutant HopF2Pto D175A (Fig. S1). The suppression of cell death associated with AvrRpt2-mediated HR was confirmed by Trypan blue staining and electrolyte leakage experiments (Fig. 1 B and C). In addition, loss of AvrRpt2-induced HR was associated with suppression of AvrRpt2-induced ETI as monitored by bacterial growth (Fig. 1D and Fig. S8). The loss of AvrRpt2-induced resistance was not observed in DEX-treated HopF2Pto D175A:HA transgenic plants (Fig. 1D). Therefore, HopF2Pto effectively suppresses AvrRpt2-induced HR and ETI, and this activity depends on the conserved residue D175. Because AvrRpt2 is known to target Arabidopsis RIN4 protein, we examined whether HopF2Pto interferes with AvrRpt2-induced RIN4 modifications.
Fig. 1.
Transgenic expression of HopF2Pto suppresses AvrRpt2-mediated ETI. (A) Half-leaves of untreated HopF2Pto transgenic plants (−DEX) or HopF2Pto transgenic plants treated with DEX for 24 h (+DEX) were infiltrated with Pto DC3000 (5 × 107 cfu/mL) expressing the indicated avirulence gene or empty vector (EV). Photographs of AvrB, AvrRpm1, or corresponding EV control were taken 6 h after inoculation, whereas HopZ1aPsyA2, AvrRpt2, or corresponding EV were taken ≈20 h after inoculation. Asterisks indicate leaves with visible HR collapse. (B) Trypan blue staining of untreated (−DEX) or 24 h DEX-treated (+DEX) HopF2Pto transgenic leaves 14 h postinoculation. (C) Electrolyte leakage of untreated (−DEX) or 24 h DEX-treated (+DEX) HopF2Pto transgenic leaf disks after infiltration with Pto DC3000 expressing the indicated constructs. (D) Growth analysis of Pto DC3000 expressing the indicated avirulence gene or the empty vector (EV) infiltrated into HopF2Pto or HopF2Pto D175A transgenic plants. Plants were treated with 30 μM DEX (+DEX) or water (−DEX) immediately after bacterial inoculation. Bacterial counts were performed 1 h postinoculation (day 0; filled bars) and 2 days postinoculation (day 2; open bars). Although Pto DC3000 empty vector did not grow significantly better in DEX-treated HopF2Pto:HA plants after 2 days of growth, a significant growth enhancement was observed after 3 days of growth (Fig. S7). Results are representative of three independent replicates. Error bars represent the SD from eight samples. “a” or “b” above the bar denotes statistically significant [Fisher’s protected least significant difference post hoc (FLSD) test, P < 0.05] differences between samples. Similar results were obtained with an independent transgenic HopF2Pto line, as well with a lower DEX concentration of 3 μM (Fig. S8).
HopF2Pto Inhibits AvrRpt2-Mediated RIN4 Degradation in Vivo and in Vitro.
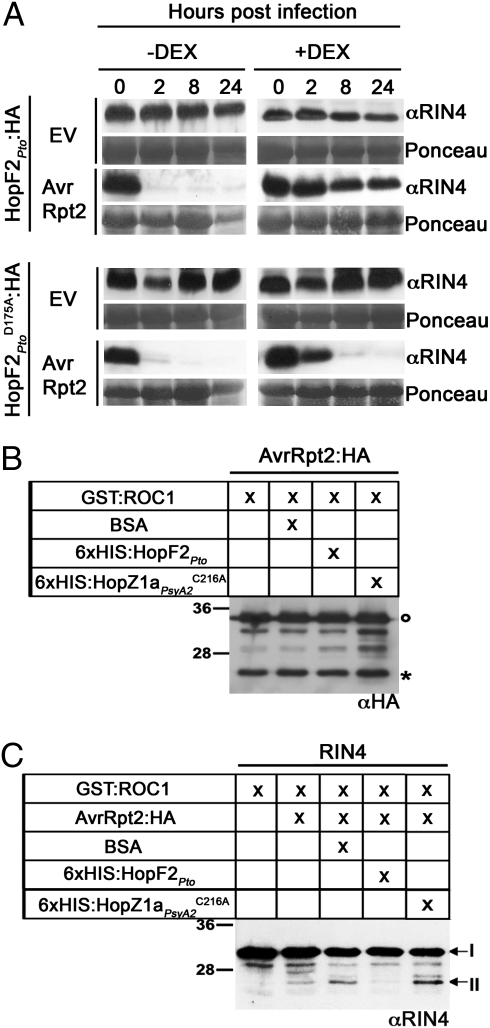
The HR elicited by AvrRpt2 is associated with a rapid depletion of RIN4 within 8 h of inoculation, which is thought to be required for AvrRpt2-mediated ETI (11, 12). To test whether HopF2Pto attenuates AvrRpt2-mediated RIN4 depletion, RIN4 levels were monitored in HopF2Pto:HA and HopF2Pto D175A:HA transgenic leaves inoculated with Pto DC3000 expressing AvrRpt2 (Fig. 2A). In uninduced leaves, RIN4 levels decreased significantly by 2 h postinfection, whereas in DEX-treated leaves RIN4 levels persisted without a detectable decrease up to 24 h after bacterial inoculation. In HopF2Pto D175A:HA-expressing tissues, RIN4 disappearance was still observed (Fig. 2A).
Fig. 2.
HopF2Pto inhibits AvrRpt2-mediated degradation of RIN4. (A) Immunoblot analysis of RIN4 levels in Arabidopsis HopF2Pto and HopF2Pto D175A transgenic leaves infiltrated with Pto DC3000 empty vector (EV) or Pto DC3000 expressing AvrRpt2. Expression of HopF2Pto:HA or HopF2Pto D175A:HA was induced with 30 μM DEX 24 h before infection. Pto DC3000 was infiltrated at 5 × 107 cfu/mL, and samples were collected at the indicated time after infiltration. (B) HopF2Pto does not inhibit AvrRpt2 activation and self-processing in vitro. In vitro cleavage reactions (see Methods) were probed with HA antisera. Cleavage reactions were performed five times with similar results. ○, full-length AvrRpt2; *, processed AvrRpt2. (C) HopF2Pto interferes with AvrRpt2-mediated RIN4 proteolysis in vitro. Reactions were conducted as in B and probed with RIN4 antisera. Arrows indicate full-length RIN4 (I) and proteolytically cleaved RIN4 (II).
Because HopF2Pto interferes with AvrRpt2-mediated cleavage of RIN4 in planta (Fig. 2A), we performed a series of in vitro cleavage reactions using purified recombinant proteins to analyze the effect of HopF2Pto on AvrRpt2 activation and RIN4 cleavage. As previously shown, incubation of AvrRpt2:HA with GST:ROC1 resulted in activation of AvrRpt2:HA and self-processing as indicated by the presence of an ≈21-kDa band corresponding to the C-terminal portion of AvrRpt2:HA (asterisk in Fig. 2B) (17). The addition of recombinant 6xHIS:HopF2Pto had no effect on the ability of GST:ROC1 to activate AvrRpt2:HA, indicating that HopF2Pto does not interfere with the activation of AvrRpt2 (Fig. 2B). When recombinant 6xHIS:HopF2Pto was incubated with RIN4, GST:ROC1, and AvrRpt2:HA, cleaved RIN4 product was greatly diminished relative to the control reactions (Fig. 2C), whereas BSA or the recombinant protein 6xHIS: HopZ1aPsyA2 C216A did not interfere with the proteolytic cleavage of RIN4 (II, Fig. 2C) (20 –22). These results suggest that HopF2Pto hinders the ability of activated AvrRpt2 to cleave RIN4 in vitro.
HopF2Pto Interacts with RIN4 in Vitro and in Vivo.
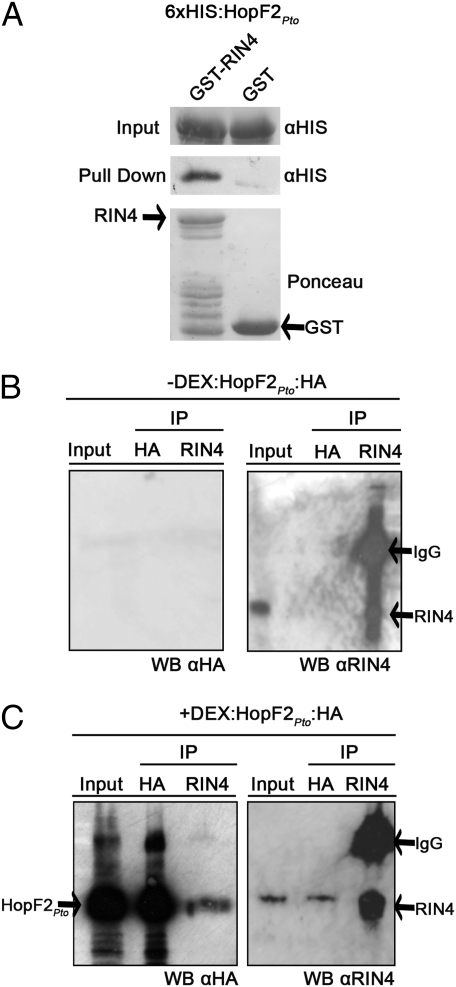
To test whether HopF2Pto directly interacts with RIN4, recombinant GST:RIN4 or GST was incubated with 6xHIS:HopF2Pto and precipitated with glutathione GST-binding resin (Fig. 3A and Fig. S9). Equal amounts of purified 6xHIS:HopF2Pto recombinant protein were added to either GST:RIN4- or GST-bound glutathione beads. Only GST:RIN4 provided an enrichment of 6xHIS:HopF2Pto upon precipitation, indicating that recombinant RIN4 interacts with HopF2Pto in vitro (Fig. 3A). To determine whether HopF2Pto and RIN4 interact in vivo, DEX-induced and uninduced HopF2Pto:HA transgenic leaves were subjected to immunoprecipitation with anti-RIN4 antibodies. HopF2Pto:HA was detectable in the induced fraction immunoprecipitated with anti-RIN4 sera (Fig. 3C). Conversely, RIN4 was detectable in the induced fraction immunoprecipitated with anti-HA antibodies but not in the uninduced fraction or in fractions immunoprecipitated with uncoupled magnetic beads (Fig. 3 B and C and Fig. S2A). Immunoprecipitations carried out using the stringent RIPA wash buffer showed no marked decrease in the amounts of RIN4 immunoprecipitated with HopF2Pto:HA, indicating that the interaction between HopF2Pto and RIN4 or a RIN4 complex is specific (Fig. S2B) (35). Collectively, these results indicate that HopF2Pto interacts with RIN4 or a RIN4-associated complex in vivo.
Fig. 3.
HopF2Pto interacts with RIN4 both in vitro and in vivo. (A) GST:RIN4- or GST-bound glutathione resin was incubated with recombinant 6xHIS:HopF2Pto, washed and probed for the presence of bound 6xHIS:HopF2Pto (see Methods). Equal volumes of 6xHIS:HopF2Pto added to GST:RIN4- or GST-bound glutathione resin were immunoblotted and probed with HIS antisera before washes (Top) or following washes (Middle). (Bottom) Ponceau stain before washes as a loading control. The GST and full-length RIN4 bands are indicated by an arrow. Bands below the full-length RIN4 band are RIN4 degradation products as determined by an anti-RIN4 blot (Fig. S9). (B and C) HopF2Pto associates with RIN4 in vivo. (B) Uninduced HopF2Pto transgenic leaves were harvested 24 h after mock treatment. Ten micrograms of input protein (Input) or entire elution (50 μL) of the immunoprecipitation (IP) by α-HA or α-RIN4 was loaded onto the SDS/PAGE gel and probed with the indicated antisera (see Methods). (C) HopF2Pto transgenic leaves were harvested 24 h after 30 μM DEX application and treated as described in B. α-HA immunoblot detects the presence of HopF2Pto:HA.
HopF2Pto Promotes RIN4-Dependent P. syringae Growth in Arabidopsis.
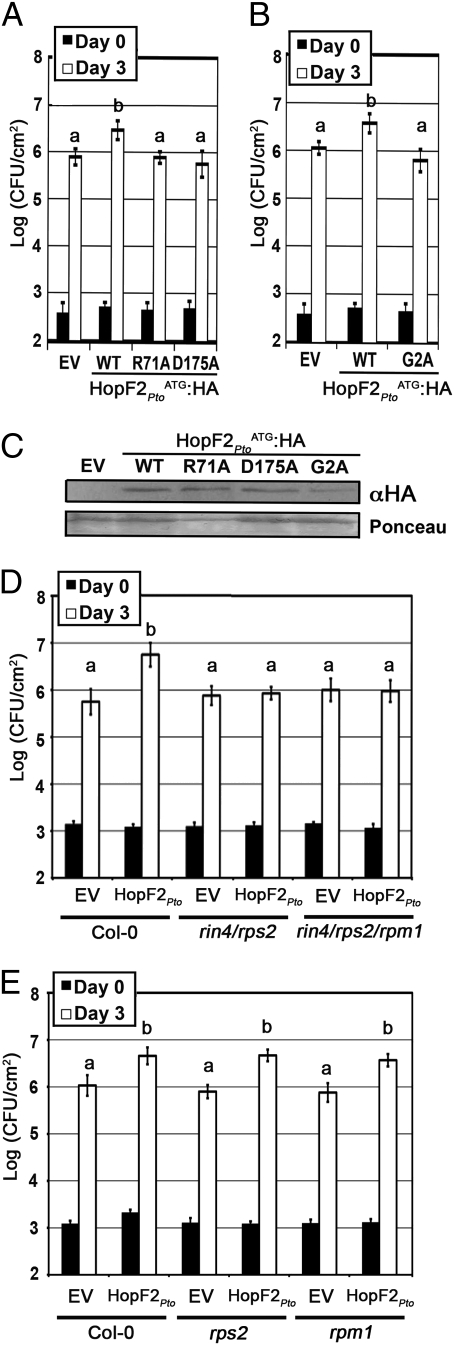
Previous characterization of HopF2Pto virulence function in tomato revealed that growth advantages to hopF2 − mutant of Pto DC3000 complemented with the hopF2Pto gene (schF2 and hopF2) could be enhanced by replacing the native ATA start codon of HopF2Pto with an ATG start codon (26). Similarly, expression of schF2/hopF2ATG:HA in the HopF2 − Pto DC3000 strain resulted in a significant growth advantage of 5-fold in Arabidopsis ecotype Col-0 compared to the empty vector control or schF2/hopF2ATA:HA (Fig. 4A and Fig. S3). The amino acid residues R71 and D175 of HopF2Pto ATG (corresponding to residues R72 and D174 of HopF1Pph7) were required for HopF2Pto virulence function in Arabidopsis (Fig. 4A). Mutation of the predicted myristoylation site (HopF2Pto G2A) also compromised HopF2Pto ATG:HA virulence function (Fig. 4B) (26). Loss of virulence function could not be attributed to lack of expression because HopF2Pto ATG/R71A:HA, HopF2Pto ATG/D175A:HA, and HopF2Pto ATG/G2A:HA were expressed to similar levels as wild-type HopF2Pto ATG:HA in P. syringae (Fig. 4C). Therefore, we conclude that HopF2Pto ATG:HA-enhanced P. syringae growth in Arabidopsis requires intact conserved residues R71 and D175, as well as the myristoylation sequence. This suggests that a membrane localized virulence target may be modified by the action of HopF2Pto.
Fig. 4.
HopF2Pto virulence function requires the RIN4 Arabidopsis protein. (A and B) Growth of Pto DC3000 hopF2 − mutant expressing the indicated HopF2Pto ATG construct or the empty vector (EV) in Arabidopsis Col-0 leaves. Bacterial counts were performed 1 h postinoculation (day 0; filled bars) and 3 days postinoculation (day 3; open bars). Error bars represent the SD of eight samples. Experiments are representative of three independent trials. “a” or “b” above a bar denotes statistically significant [Fisher’s protected least significant difference post hoc (FLSD) test, P < 0.05] differences between samples. (C) Immunoblot analysis of indicated HopF2Pto ATG:HA protein expression in the Pto hopF2 − mutant. Bacteria were grown in minimal media to induce the type III secretion system, and equal amounts of protein were immunoblotted with HA antisera (33). Ponceau red staining is shown as a loading control. (D and E) Growth of Pto DC3000 hopF2 − mutant expressing HopF2Pto ATG:HA (HopF2Pto) or the empty vector (EV) in Arabidopsis Col-0 wild-type, rin4/rps2, rin4/rps2/rpm1, rps2, or rpm1 leaves. Bacterial counts were performed as in A and B. This experiment is representative of three independent trials.
To determine whether RIN4 is required for HopF2Pto-mediated virulence, we examined the growth of P. syringae-expressing HopF2Pto ATG:HA in Arabidopsis plants that genetically lack RIN4. Because RIN4 is a negative regulator of RPS2, both RIN4 and RPS2 must be lacking to prevent the lethality associated with activation of RPS2 (11). We observed that HopF2Pto ATG:HA conferred no growth advantage to the HopF2 − mutant of Pto DC3000 in rin4/rps2 or rpm1/rin4/rps2 (Fig. 4D), indicating that RIN4 is required for HopF2Pto virulence function in Arabidopsis. HopF2Pto ATG:HA virulence function did not require RPM1 or RPS2 because the growth advantage conferred to Pto DC3000 expressing HopF2Pto ATG:HA, was retained in rps2 and rpm1 Arabidopsis plants (Fig. 4E). Together, these results demonstrate that HopF2Pto ATG:HA virulence activity in Arabidopsis depends on RIN4 and not the RIN4-associated R proteins RPM1 and RPS2.
Discussion
In this study, we sought to identify potential host targets of the HopF family of phytopathogenic TTSE proteins. Transgenic HopF2Pto-expressing Arabidopsis plants were compromised for AvrRpt2-induced ETI and HR (Fig. 1). HopF2Pto interfered with AvrRpt2-mediated cleavage of RIN4 in planta and in vitro (Fig. 2), and interacted with RIN4 in planta and in vitro (Fig. 3). The marked difference between the immunoprecipitation and the coimmunoprecipitation suggests that only a subset of Arabidopsis RIN4 is targeted by HopF (Fig. 3 B and C). The hopF − mutant of Pto DC3000 did not show decreased virulence in Arabidopsis relative to wild-type Pto DC3000, possibly because of the functional redundancy of TTSE functions (Fig. S4) (1). However, overexpression of HopF2Pto ATG in this strain by changing the native ATA start codon to ATG conferred a growth advantage in wild-type Arabidopsis Col-0 plants but not in rin4/rps2 or rin4/rps2/rpm1 plants genetically lacking RIN4 (Fig. 4). Overall, these results support a model where overexpressed HopF2Pto targets Arabidopsis RIN4 protein to promote pathogen growth.
Transgenic expression of HopF2Pto effectively suppressed AvrRpt2-mediated HR (Fig. 1), In contrast, Pto DC3000 naturally expresses HopF2Pto and has long been used to elicit AvrRpt2-mediated HR in Arabidopsis. Furthermore, we also observed that Pto DC3000 expressing HopF2Pto ATG could not inhibit AvrRpt2-mediated HR and associated RIN4 degradation in bacterial mixing experiments or when expressed in the Pto JL1065, which natively induces an AvrRpt2-mediated HR (Fig. S5) (36, 37). Together, these results indicate an important difference between what HopF2Pto can do when expressed in planta transgenically versus what it does when delivered from P. syringae. One possible explanation for this difference is that AvrRpt2 HR suppression requires an in planta threshold level of HopF2Pto that is attained by transgenic expression but not bacterial delivery (even with the ATG start codon). Another possibility is that bacterially delivered AvrRpt2 and HopF2Pto ATG activities are temporally distinct, similar to the Salmonella type III effectors SopE and SptP; however, transgenic expression of HopF2Pto ATG negates this difference (38). In addition, bacterially delivered HopF2Pto ATG may target only a subset of Arabidopsis RIN4 that is sufficient to promote P. syringae virulence but insufficient to interfere with AvrRpt2-mediated HR. Despite these differences, our data demonstrate that transgenic phenotypes can provide valuable insights into type III effector functions.
Despite the limited structural similarity of HopF2Pto to the ADP ribosyltransferase diphtheria toxin (33), no ADP ribosyltransferase activity could be detected in radiolabeling experiments using purified recombinant RIN4 as a substrate or extracts from HopF2Pto-expressing transgenic plants (Fig. S6) (33, 39). These and previously published results suggest that HopF2Pto is not a bona fide ADP ribosyltransferase (33). Nevertheless, amino acid D175 was required for the ability of HopF2Pto to compromise AvrRpt2-induced ETI as well as RIN4 disappearance, indicating that HopF2Pto may catalytically modify RIN4 to promote pathogen virulence. The observation that HopF2Pto can effectively inhibit AvrRpt2-cleavage of RIN4 suggests that the domain of HopF2Pto action may overlap with the AvrRpt2-cleavage sites, RCS1 and/or RCS2. It remains to be determined whether HopF2Pto requires RIN4 to suppress PTI in Arabidopsis (Fig. S7) (29, 30). Understanding how HopF2Pto manipulates RIN4 promises to uncover the biochemical function of HopF2Pto as well as functional motifs within the RIN4 protein.
In some bean cultivars, HopF1Pph7 is recognized by the R1 resistance protein and therefore acts as an avirulence factor (31, 32). Interestingly, the effector AvrB2Pph 7 (formerly AvrPphC) from P. syringae pv. phaseolicola race 7 (1449B) is able to mask the avirulence function of HopF1Pph 7 in the bean cultivar Canadian Wonder (32). RIN4-like proteins are conserved across the plant kingdom in monocots and dicots. Because AvrB2Pph7 is an allele of the AvrB family of TTSEs that can target Arabidopsis RIN4, it is intriguing to speculate that both HopF1Pph7 and AvrB2Pph7 could differentially modify a bean RIN4 ortholog.
The HopF family of proteins has previously been demonstrated to possess HR suppressing activity. HopF1Pph7 can suppress the avirulence function of an as yet unidentified Pph effector, avrβ1 in the bean cultivar Tendergreen (31, 32). HopF2Pto has also been demonstrated to suppress the HR-inducing activity of HopA1 (formerly HopPsyA) in Arabidopsis WS-0 and N. tabacum cv. Xanthi and also BAX-induced PCD in yeast and N. tabacum cv. Xanthi as well as the HR induced by P. fluorescens expressing AvrRpm1 in Arabidopsis (27, 30). Suppression of BAX-induced PCD in yeast and plants would suggest that an additional HopF2Pto target might be broadly conserved across kingdoms. HopF2Pto HR-suppression in transgenic Arabidopsis appears to be specific to AvrRpt2, suggesting that the mechanism of action examined in these experiments differs from the aforementioned assays.
Overall, we have identified Arabidopsis RIN4 as a major virulence target of the P. syringae TTSE HopF2Pto. HopF2Pto joins the P. syringae TTSEs AvrRpm1, AvrRpt2, and AvrB in targeting Arabidopsis RIN4 protein. However, unlike the latter three, HopF2Pto avoids R gene-mediated recognition in the Arabidopsis Col-0 accession and can target RIN4 when overexpressed to promote pathogen virulence, demonstrating that RIN4 can serve as a virulence target of TTSE proteins.
Methods
Bacterial Strains, Plasmids, and Plant Material.
Bacterial cultures and Arabidopsis plants were grown as described in ref. 40. HopF2Pto constructs expressed in P. syringae were cloned into the multicopy plasmid pBBR1 MCS-2 (41). HopF2Pto was cloned with 100 bp upstream of the SchF ATG start codon and contains an in-frame hemagglutinin (HA) tag at the C terminus. Point mutations were generated by PCR, and the coding sequence of all constructs was sequenced. The HopZ1aPsyA2 P. syringae- and HopZ1aPsyA2 C216A E. coli-expressiwon constructs are described refs. 40 –42. HopZ1aPsyA2 C216A possesses a mutation in the catalytic cysteine (C216) required for in vitro protease activity and avirulence functions (40, 42). HopF2Pto E. coli protein expression constructs were cloned into a modified pET-15b vector (Novagen) (43). RIN4 cloned in pGEX6P-3 (GE Healthcare) was provided by Jeff Dangl.
To make transgenic plants, HopF2Pto and HopF2Pto D175A were cloned into pDB vector (40) with an ATG start codon and in-frame with the C-terminal hemagglutinin (HA) tag. The constructs were sequenced, transformed into Agrobacterium tumefaciens GV3-101, and transformed into Arabidopsis Col-0 plants by floral dip (44). Dexamethasone treatment was as described in ref. 40. Bacterial enumeration, in planta growth assays, HR, and ion leakage were conducted as described in ref. 40.
Protein Expression, Purification, and GST Pull Downs.
Recombinant 6xHIS:HopF2Pto and 6xHIS:HopZ1aPsyA2 C216A (33, 42) were purified by size-exclusion chromatography using a HiLoad 16/60 Superdex 200 prep grade column (Amersham Biosciences) in PBS buffer (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4) at a flow-rate of 1 mL/min. Recombinant GST:RIN4 was affinity-purified in batch format by glutathione sepharose affinity chromatography using Glutathione Sepharose 4B (GE Healthcare) with two 10-mL washes of both 20 mM Tris (pH 8.0)/50 mM NaCl and 20 mM Tris (pH 8.0)/350 mM NaCl. For GST pull-down assays, 200 μl of binding buffer-equilibrated [20 mM Tris (pH 8.0), 50 mM NaCl] Glutathione Sepharose 4B slurry was incubated with 50 μg of GST:RIN4 or GST alone for 1 h at 4°C. Fifty micrograms of purified recombinant 6xHIS:HopF2Pto was then added and incubated for 1 h at 25°C. Two subsequent 5-min washes were conducted with RIPA buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% Nonidet P-40, 0.25% Na deoxycholate] (35). The washed beads were then resuspended in 200 μL of the PBS binding buffer. Twenty microliters of the washed beads was then mixed with SDS/PAGE loading buffer, boiled, resolved on 12% SDS/PAGE gels, and immunoblotted.
In Vitro Cleavage Reactions.
Recombinant GST:ROC1, AvrRpt2:HA, and 6xHIS:RIN4 proteins were cloned, expressed, and purified as described in ref. 17. Cleavage reactions were performed in a 50-μL total volume using 1 μg of AvrRpt2:HA, 3 μg of 6xHIS:RIN4, 5 μg of GST:ROC1, and BSA, 6xHIS:HopF2Pto, or 6xHIS:HopZ1aPsyA2 C216A in PBS buffer (140 mM NaCl, 2.7 mM KCl, Na2HPO4, 1.8 mM KH2PO4). Reactions were incubated at 25°C for 16 h, terminated with SDS/PAGE loading buffer, resolved on 12% SDS/PAGE gels, and immunoblotted.
Protein Extraction, Immunoprecipitation, and Immunoblotting.
Arabidopsis leaves were ground in liquid nitrogen and resuspended in the immunoprecipitation (IP) buffer [50 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 1% dodecyl maltoside, protease inhibitor mixture (Roche)]. The slurry was incubated on ice for 1 h and centrifuged at 10,000 × g for 15 min to clear the debris. Seven hundred fifty micrograms of protein was used in all immunoprecipitation experiments. Protein extracts were incubated in either 50 μL of HA-conjugated magnetic beads (Miltenyi Biotech) or 1 μL of RIN4 polyclonal antibodies, followed by 100 μL of Protein A magnetic beads (Miltenyi Biotech), and incubated for 1 h on ice. Following incubation, magnetic beads were washed with IP buffer according to the manufacturer’s instructions. HopF2Pto and RIN4 were detected as described below.
For detection of transgenic HopF2Pto:HA, one fully expanded leaf was ground in 500 μL of protein extraction buffer [20 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 5 mM DTT, plant protease inhibitor (Sigma Aldrich)], briefly vortexed, and then centrifuged at 20,000 × g for 10 min at 4 °C. SDS/PAGE loading dye (10×) was added to the supernatants, and samples were resolved on 12% SDS/PAGE gels and blotted onto nitrocellulose. Affinity-purified polyclonal rabbit anti-RIN4 sera (10) was used at a 1:10,000 dilution, anti-HIS (Cell Signaling Technology) was used at 1:4,000, and anti-HA (Roche) was used at 1:15,000.
Supplementary Material
Acknowledgments
We thank Dr. David Mackey (Ohio State University) for RIN4 antisera; Dr. Ai-Jiuan Wu and Dr. Jeffrey Dangl (University of North Carolina) for GST:RIN4 protein expression construct, the modified pDB vector, and rin4/rps2, rin4/rps2/rpm1, rps2, and rpm1 Arabidopsis seeds; Dr. Brian Staskawicz for Pto JL1065 strain; Dr. James Alfano (University of Nebraska, Lincoln) for Pto DC3000 HopF2 − strain; and Dr. Alex Singer for comments on the manuscript. This work was supported by Natural Sciences and Engineering Research Council of Canada and Ontario Graduate Scholarship postgraduate awards (to M.W.), a Natural Sciences and Engineering Research Council of Canada Discovery grant (to D.D.), the Canadian Foundation for Innovation (D.D.), a Canada Research Chair in Plant-Microbe Systems Biology (D.D.), and the Centre for the Analysis of Genome Evolution and Function (D.D.). G.C. and J.E. were supported by U.S. Department of Agriculture–National Research Initiative Grant 2008-00712 (awarded to G.C.). J.E. was also supported by National Science Foundation–Integrative Graduate Education and Research Traineeship Fellowship DGE-0653984.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904739107/DCSupplemental.
References
- 1.Grant SR, Fisher EJ, Chang JH, Mole BM, Dangl JL. Subterfuge and manipulation: type III effector proteins of phytopathogenic bacteria. Annu Rev Microbiol. 2006;60:425–429. doi: 10.1146/annurev.micro.60.080805.142251. [DOI] [PubMed] [Google Scholar]
- 2.Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 3.Nomura K, Melotto M, He SY. Suppression of host defense in compatible plant-Pseudomonas syringae interactions. Curr Opin Plant Biol. 2005;8:361–368. doi: 10.1016/j.pbi.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Ma W, Guttman DS. Evolution of prokaryotic and eukaryotic virulence effectors. Curr Opin Plant Biol. 2008;11:412–419. doi: 10.1016/j.pbi.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Dangl JL, Jones JDG. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 7.Bent AF, Mackey D. Elicitors, effectors, and R genes: the new paradigm and a lifetime supply of questions. Annu Rev Phytopathol. 2007;45:399–436. doi: 10.1146/annurev.phyto.45.062806.094427. [DOI] [PubMed] [Google Scholar]
- 8.van der Biezen EA, Jones JDG. Plant disease-resistance proteins and the gene-for-gene concept. Trends Biochem Sci. 1998;23:454–456. doi: 10.1016/s0968-0004(98)01311-5. [DOI] [PubMed] [Google Scholar]
- 9.Kim MG, et al. Two Pseudomonas syringae type III effectors inhibit RIN4-regulated basal defense in Arabidopsis . Cell. 2005;121:749–759. doi: 10.1016/j.cell.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Mackey D, Holt BF, Wiig A, Dangl JL. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis . Cell. 2002;108:743–758. doi: 10.1016/s0092-8674(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 11.Mackey D, Belkhadir Y, Alonso JM, Ecker JR, Dangl JL. Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell. 2003;112:379–389. doi: 10.1016/s0092-8674(03)00040-0. [DOI] [PubMed] [Google Scholar]
- 12.Axtell MJ, Staskawicz BJ. Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell. 2003;112:369–377. doi: 10.1016/s0092-8674(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 13.Lee CC, et al. Crystal structure of the type III effector AvrB from Pseudomonas syringae . Structure. 2004;12:487–494. doi: 10.1016/j.str.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Ong LE, Innes RW. AvrB mutants lose both virulence and avirulence activities on soybean and Arabidopsis. Mol Microbiol. 2006;60:951–962. doi: 10.1111/j.1365-2958.2006.05162.x. [DOI] [PubMed] [Google Scholar]
- 15.Desveaux D, et al. Type III effector activation via nucleotide binding, phosphorylation, and host target interaction. PLoS Pathog. 2007;3:456–469. doi: 10.1371/journal.ppat.0030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Axtell MJ, Chisholm ST, Dahlbeck D, Staskawicz BJ. Genetic and molecular evidence that the Pseudomonas syringae type III effector protein AvrRpt2 is a cysteine protease. Mol Microbiol. 2003;49:1537–1546. doi: 10.1046/j.1365-2958.2003.03666.x. [DOI] [PubMed] [Google Scholar]
- 17.Coaker G, Falick A, Staskawicz B. Activation of a phytopathogenic bacterial effector protein by a eukaryotic cyclophilin. Science. 2005;308:548–550. doi: 10.1126/science.1108633. [DOI] [PubMed] [Google Scholar]
- 18.Coaker G, Zhu G, Ding Z, Van Doren SR, Staskawicz B. Eukaryotic cyclophilin acts as a molecular switch for effector activation. Mol Microbiol. 2006;61:1485–1496. doi: 10.1111/j.1365-2958.2006.05335.x. [DOI] [PubMed] [Google Scholar]
- 19.Jin P, Wood MD, Wu Y, Xie Z, Katagiri F. Cleavage of the Pseudomonas syringae type III effector AvrRpt2 requires a host factor(s) common among eukaryotes and is important for AvrRpt2 localization in the host cell. Plant Physiol. 2003;133:1072–1082. doi: 10.1104/pp.103.025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chisholm ST, et al. Molecular characterization of proteolytic cleavage sites of the Pseudomonas syringae effector AvrRpt2. Proc Natl Acad Sci USA. 2005;102:2087–2092. doi: 10.1073/pnas.0409468102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day B, et al. Molecular basis for the RIN4 negative regulation of RPS2 disease resistance. Plant Cell. 2005;17:292–1305. doi: 10.1105/tpc.104.030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HS, et al. The Pseudomonas syringae effector AvrRpt2 cleaves its C-terminally acylated target, RIN4, from Arabidopsis membranes to block RPM1 activation. Proc Natl Acad Sci USA. 2005;102:6496–6501. doi: 10.1073/pnas.0500792102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belkhadir Y, Nimchuk Z, Hubert DA, Mackey D, Dangl JL. Arabidopsis RIN4 negatively regulates disease resistance mediated by RPS2 and RPM1 downstream or independent of the NDR1 signal modulator and is not required for the virulence functions of bacterial type III effectors AvrRpt2 or AvrRpm1. Plant Cell. 2004;16:2822–2835. doi: 10.1105/tpc.104.024117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Hoorn RA, Kamoun S. From guard to decoy: a new model for perception of plant pathogen effectors. Plant Cell. 2008;20:2009–2017. doi: 10.1105/tpc.108.060194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shan L, et al. The hopPtoF locus of Pseudomonas syringae pv. tomato DC3000 encodes a type III chaperone and a cognate effector. Mol Plant Microbe Interact. 2004;17:447–455. doi: 10.1094/MPMI.2004.17.5.447. [DOI] [PubMed] [Google Scholar]
- 26.Robert-Seilaniantz A, Shan L, Zhou J-M, Tang X. The Pseudomonas syringae pv. tomato DC3000 type III effector HopF2 has a putative myristoylation site required for its avirulence and virulence functions. Mol Plant Microbe Interact. 2006;19:130–138. doi: 10.1094/MPMI-19-0130. [DOI] [PubMed] [Google Scholar]
- 27.Jamir Y, et al. Identification of Pseudomonas syringae type III effectors that can suppress programmed cell death in plants and yeast. Plant J. 2004;37:554–565. doi: 10.1046/j.1365-313x.2003.01982.x. [DOI] [PubMed] [Google Scholar]
- 28.Oh HS, Collmer A. Basal resistance against bacteria in Nicotiana benthamiana leaves is accompanied by reduced vascular staining and suppressed by multiple Pseudomonas syringae type III secretion system effector proteins. Plant J. 2005;44:348–359. doi: 10.1111/j.1365-313X.2005.02529.x. [DOI] [PubMed] [Google Scholar]
- 29.Li X, et al. Flagellin induces innate immunity in nonhost interactions that is suppressed by Pseudomonas syringae effectors. Proc Natl Acad Sci USA. 2005;102:12990–12995. doi: 10.1073/pnas.0502425102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gou M, Tiang F, Wamboldt Y, Alfano JR. The majority of the type III effector inventory of Pseudomonas syringae pv. tomato DC3000 can suppress plant immunity. Mol Plant Microbe Interact. 2009;22:1069–1080. doi: 10.1094/MPMI-22-9-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson RW, et al. Identification of a pathogenicity island, which contains genes for virulence and avirulence, on a large native plasmid in the bean pathogen Pseudomonas syringae pathovar phaseolicola . Proc Natl Acad Sci USA. 1999;96:10875–10880. doi: 10.1073/pnas.96.19.10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsiamis G, et al. Cultivar-specific avirulence and virulence functions assigned to avrPphF in Pseudomonas syringae pv. phaseolicola, the cause of bean halo-blight disease. EMBO J. 2000;19:3204–3214. doi: 10.1093/emboj/19.13.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singer AU, et al. Crystal structures of the type III effector protein AvrPphF and its chaperone reveal residues required for plant pathogenesis. Structure. 2004;12:1669–1681. doi: 10.1016/j.str.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 34.Aoyama T, Chua N-H. A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 1997;11:605–612. doi: 10.1046/j.1365-313x.1997.11030605.x. [DOI] [PubMed] [Google Scholar]
- 35.Elion EA, Wang Y. Making protein immunoprecipitates. Methods Mol Biol. 2004;284:1–14. doi: 10.1385/1-59259-816-1:001. [DOI] [PubMed] [Google Scholar]
- 36.Ritter C, Dangl JL. Interference between two specific pathogen recognition events mediated by distinct plant disease resistance genes. Plant Cell. 1996;8:251–257. doi: 10.1105/tpc.8.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim MT, Kunkel BN. The Pseudomonas syringae avrRpt2 gene contributes to virulence on tomato. Mol Plant Microbe Interact. 2005;18:626–633. doi: 10.1094/MPMI-18-0626. [DOI] [PubMed] [Google Scholar]
- 38.Kubori T, Galan JE. Temporal regulation of Salmonella virulence effector function by proteasome-dependent protein degradation. Cell. 2003;115:333–342. doi: 10.1016/s0092-8674(03)00849-3. [DOI] [PubMed] [Google Scholar]
- 39.Coburn J, Wyatt RT, Iglewski BH, Gill DM. Several GTP-binding proteins, including p21c-H-ras, are preferred substrates of Pseudomonas aeruginosa exoenzyme S. J Biol Chem. 1989;264:9004–9008. [PubMed] [Google Scholar]
- 40.Lewis JD, Abada W, Ma W, Guttman DS, Desveaux D. The HopZ family of Pseudomonas syringae type III effectors require myristoylation for virulence and avirulence functions in Arabidopsis thaliana. J Bacteriol. 2008;190:2880–2891. doi: 10.1128/JB.01702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovach ME, et al. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 42.Ma W, Dong FF, Stavrinides J, Guttman DS. Type III effector diversification via both pathoadaptation and horizontal transfer in response to a coevolutionary arms race. PLoS Genet. 2006;2:2131–2142. doi: 10.1371/journal.pgen.0020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuo Y, et al. Crystal structure of RNase T, an exoribonuclease involved in tRNA maturation and end turnover. Structure. 2007;15:417–428. doi: 10.1016/j.str.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.