Abstract
Long-term depression (LTD) is an important synaptic mechanism for limiting excitatory influence over circuits subserving cognitive and emotional behavior. A major means of LTD induction is through the recruitment of signaling via Gq-linked receptors activated by norepinephrine (NE), acetylcholine, and glutamate. Receptors from these transmitter families have been proposed to converge on a common postsynaptic LTD maintenance mechanism, such that hetero- and homosynaptic induction produce similar alterations in glutamate synapse efficacy. We report that in the dorsolateral and ventrolateral bed nucleus of the stria terminalis (BNST), recruitment of Gq-linked receptors by glutamate or NE initiates mechanistically distinct forms of postsynaptically maintained LTD and these LTDs are differentially regulated by stress exposure. In particular, we show that although both mGluR5- and α1-adrenergic receptor (AR)-dependent LTDs involve postsynaptic endocytosis, the α1-AR-initiated LTD exclusively involves modulation of signaling through calcium-permeable AMPA receptors. Further, α1-AR- but not mGluR5- dependent LTD is disrupted by restraint stress. α1-AR LTD is also impaired in mice chronically exposed to ethanol. These data thus suggest that in the BNST, NE- and glutamate-activated Gq-linked signaling pathways differentially tune glutamate synapse efficacy in response to stress.
Keywords: addiction, norepinephrine, metabotropic glutamate receptor, calcium-permeable AMPA receptor, ethanol
Alterations in key amygdalar and reward circuitries have been proposed as potential mechanisms underlying interrelated anxiety disorders and addiction. The bed nucleus of the stria terminalis (BNST) is a nucleus within a series of structures known as the “extended amygdala,” which receives a mix of glutamatergic inputs from cognitive and systemic brain centers and projects to key nuclei in both the reward and stress circuitries (1, 2). Consistent with this anatomy, a large literature indicates key roles of this region in anxiety-related behaviors, addiction, and other affective disorders (3).
The BNST receives intense noradrenergic innervation through the ventral noradrenergic bundle (VNAB) (4). Disruption of the VNAB or noradrenergic signaling in the BNST alters responses to stressors, preference for opiates, and stress-induced reinstatement to drug seeking (5, 6). α1-Adrenergic receptors (ARs) are Gq-linked G-protein-coupled receptors (GPCRs) that participate in shaping responses to stressors. Signaling through BNST α1-ARs within the BNST potently regulates the hypothalamic-pituitary-adrenal (HPA) stress axis and anxiety responses after stressors (7). The α1-AR antagonist prazosin has been shown to attenuate ethanol self-administration (SA) in ethanol-dependent rats (8) and reduces opiate SA (9). Furthermore, data from clinical trials have demonstrated that prazosin alleviates symptoms of posttraumatic stress disorder (PTSD) (10) and reduces alcohol drinking behavior in alcoholics (11).
Like α1-ARs, group I metabotropic glutamate receptors (mGluR1 and mGluR5) are also coupled to Gq. mGluR5 has been demonstrated to play a critical role in the reinforcing properties of abused drugs (12). Furthermore, multiple drugs of abuse hijack plasticity mechanisms at glutamatergic synapses in the BNST (13–15) and other reward nuclei (16). At hippocampal and cortical synapses, long-term depression (LTD) of excitatory transmission elicited by Gq-coupled GPCRs has been suggested to operate through a common pathway (17, 18). Previously, we described that mGluR5 activation induces LTD of excitatory transmission in the BNST (13). Additionally, norepinephrine (NE) can induce a time-dependent LTD via the α1-AR (19). A particularly interesting feature of α1-AR LTD is that it is heterosynaptic in nature in that it does not occur by augmenting NMDA receptor or mGluR5 signaling, although it can be elicited in the same neurons in BNST as mGluR5-LTD (19). Based on this interaction and the studies in hippocampus (17) and visual cortex (18), we hypothesized that α1-AR LTD and mGluR5 LTD share common mechanisms and are recruited by similar environmental stimuli. Here, we demonstrate that contrary to our hypothesis, α1-AR LTD but not mGluR5 LTD results in the loss of functional calcium-permeable AMPA receptors (CP-AMPARs) from the synapse, suggesting distinct mechanisms. Additionally, we demonstrate that α1-AR LTD and CP-AMPAR function are disrupted in chronic restraint stress-exposed mice, although mGluR5 LTD remains intact. This result is opposite the profile of cocaine-induced modulation of these Gq-GPCR LTDs in BNST (13, 14, 19), suggesting that recruitment of these forms of plasticity is finely tuned to specific stimuli. Finally, potentially related to the ability of prazosin to reduce ethanol dependence-induced SA, α1-AR LTD is attenuated in mice undergoing withdrawal from chronic ethanol exposure.
Results
α1-AR LTD Is Maintained via a Postsynaptic Mechanism.
Previously, we demonstrated that mGluR5 LTD in the BNST is maintained postsynaptically (14); however, the synaptic locus of α1-AR modulation of excitatory transmission has not been thoroughly examined. α1-AR activation by either NE or methoxamine produces LTD of excitatory transmission in the dorsolateral (dl) BNST (18) (Fig. 1A and Fig. S1). This LTD is not associated with alterations in paired-pulse ratios (PPRs) of evoked excitatory responses after the induction of LTD, suggesting a postsynaptic mechanism (18) (Fig. S1 B and C). To explore this possibility further, we assayed the actions of methoxamine on excitatory transmission in the dlBNST with low Ca2+ artificial cerebral spinal fluid (ACSF). Presynaptic modulation has been shown to be more robust in reduced extracellular Ca2+ (20). Previously, we have shown that altering the ACSF divalent cation concentrations to 1 mM Ca2+ and 2.8 mM Mg2+(thus reducing the [Ca2+] from 2.5 mM) significantly increases basal PPR values in the BNST (14). This alteration, however, does not enhance the α1-AR LTD in the BNST (LTD in normal Ca2+: n = 6, 56.7 ± 3.6% of baseline; LTD in low Ca2+: n = 5, 72.3 ± 5.6% of baseline; Fig. S1A), nor does it have any effect on the PPR following induction of the α1-AR LTD (Fig. S1C).
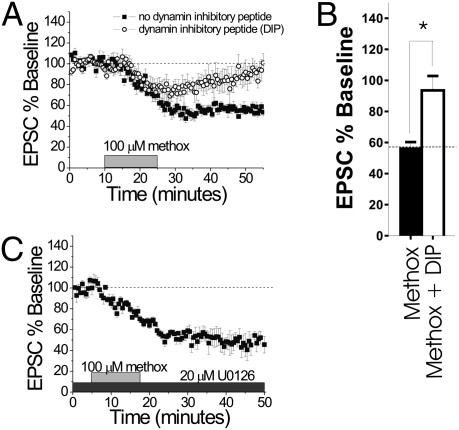
Fig. 1.
α1-AR LTD requires clathrin-dependent endocytosis. (A) Methoxamine (100 μM) produces LTD in control cells (n = 6, ■) but not in cells infused with 2 μM (in patch solution) of the dynamin inhibitory peptide (○, n = 5; P > 0.63). (B) Histogram comparing the EPSCs of the two conditions in A (min 50–55). The black bar is the control, and the white bar is the dynamin inhibitory peptide (*P < 0.03). (C) Coapplication of 20 μM U0126 and 100 μM methoxamine induces LTD (n = 5; P > 0.0001). methox, methoxamine.
To assess the maintenance mechanisms for α1-AR LTD in the dlBNST further, we next analyzed the effect of methoxamine on miniature excitatory postsynaptic currents (mEPSCs) in the presence of 1 μM tetrodotoxin (TTX). Classically, a reduction in mEPSC frequency is indicative of a decrease in glutamate release, whereas a decrease in amplitude is indicative of a decrease in postsynaptic sensitivity. We predicted that the observed LTD of evoked EPSCs produced by methoxamine would be paralleled by a decrease in the amplitude and/or frequency of mEPSCs. Surprisingly, however, methoxamine failed to cause an alteration in either the frequency [n = 4, baseline: 0.70 ± 0.3 Hz, following agonist: 0.68 ± 0.3 Hz, not significant (n.s.)] or the amplitude (n = 4, baseline: 16.3 ± 0.8 pA, following agonist: 15.8 ± 1.0 pA, n.s.) of the mEPSCs 36–40 min after agonist application (Fig. S2 A–C).
The absence of action of methoxamine on mEPSCs suggests that methoxamine-induced LTD is an activity-dependent process. Consistent with this idea, we previously reported that α1-AR LTD requires L-type voltage-gated Ca2+ channel activity (19). We thus tested whether the lack of effect of methoxamine on mEPSCs was attributable to Na+ channel blockade by sampling spontaneous EPSCs (sEPSCs) in the absence of TTX before and after methoxamine application (Fig. S3). Surprisingly, we found that α1-AR activation robustly increased, rather than decreased, the frequency of sEPSCs in the BNST 40 min following methoxamine application (n = 10, baseline: 1.48 ± 0.4 Hz, following agonist application: 4.30 ± 1.3 Hz; P < 0.05; Fig. S3B), with a trend for a decreased sEPSC amplitude (baseline: 18.9 ± 1.9 pA; following agonist application: 17.4 ± 0.9 pA; Fig. S3A). Our group recently showed that dopamine increases sEPSC frequency in the dlBNST in an activity- and corticotropin-releasing factor 1 receptor (CRFR1)-dependent process (21). α1-AR signaling has been shown to increase spontaneous inhibitory postsynaptic currents (IPSCs) in the BNST (22). These IPSCs may originate either from intrinsic neurons or from extrinsic afferents from the central nucleus of the amygdala, both of which can contain corticotropin-releasing factor (CRF) (23). We thus hypothesized that activity-dependent release of CRF by methoxamine may mask a significant decrease of sEPSC amplitude by methoxamine (although we did observe a trend for amplitude decrease). The CRFR1 antagonist 5-chloro-N-(cyclopropylmethyl)-2-methyl-N-propyl-N′-(2, 4,6-trichlorophenyl)-4,6-pyrimidinediamine hydrochloride (NBI 27914; 1 μM) blocked the methoxamine-induced increase in sEPSC frequency (n = 5, baseline: 3.23 ± 1.2, following agonist application: 2.48 ± 1.0 Hz, n.s.; Fig. S3D). Further, the presence of the CRFR1 antagonist revealed that methoxamine produces a significant reduction in sEPSC amplitude (n = 5, baseline: 21.1 ± 1.6 pA, following agonist application: 17.8 ± 1.2 pA; P < 0.01; Fig. S3C) indicative of a postsynaptic mechanism for α1-AR-induced LTD. Additionally, NBI 27914 (1 μM) did not significantly alter the expression of α1-AR LTD in evoked EPSCs (n = 5, 64.9 ± 8.0% of baseline; Fig. S3E), suggesting that the increase in sEPSC frequency we observed in the absence of NBI 27914 (Fig. S3 A and B) is mechanistically independent of the LTD.
α1-AR LTD but Not mGluR5 LTD Involves Ca2+-Permeable AMPARs.
The lack of PPR change, lack of a more robust LTD in low [Ca2+], and reduction in sEPSC amplitude all support the possibility that a postsynaptic maintenance mechanism underlies α1-AR LTD. Two plausible postsynaptic mechanisms include alteration in function of AMPARs and/or the removal of these receptors from the synapse. To begin to address these possibilities, we infused a dynamin inhibitory peptide (2 mM) into the postsynaptic cell in the dlBNST via the patch pipette 30 min before recording to prevent clathrin-dependent endocytosis, as has been previously shown for mGluR5 LTD (14). In the presence of the dynamin inhibitory peptide, 100 μM methoxamine caused a transient depression that returned to baseline, suggesting a requirement for clathrin-dependent endocytosis (n = 5, 93.6 ± 9.3% of baseline, n.s. from baseline; Fig. 1 A and B). These experiments were also significantly different from experiments lacking the dynamin inhibitory peptide (P < 0.05; Fig. 1 A and B). The observed transient depression has been previously shown to be dependent on α1-AR signaling; however, it appears to be independent of the maintenance of LTD (see ref. 18 and experiments below).
We previously reported that α1-AR LTD is independent of mGluR5 signaling, but its engagement at synapses on BNST neurons blocked subsequent mGluR5 LTD in the same neuron (19), which is consistent with their presence on a common population of neurons. These results suggested that there are similarities between the mechanisms required for expression of both α1-AR and mGluR5 LTD in the BNST. To explore this hypothesis further, we examined the involvement of mitogen-activated protein kinase 1/2 (MEK1/2) in the induction of α1-AR LTD in the dlBNST. Interestingly, and in contrast to mGluR5 LTD (13), preapplication of the MEK inhibitor U0126 (20 μM) did not alter the expression of α1-AR LTD (n = 5, 47.7 ± 6.6% of baseline; Fig. 1C)
Gq-GPCR-induced LTDs in brain regions like the ventral tegmental area (VTA) have been shown to target populations of AMPARs, particularly CP-AMPARs that lack an RNA-edited GluR2 subunit (24, 25). We took a pharmacological approach to investigate if CP-AMPARs are present in the synapses of BNST neurons. Using the selective CP-AMPAR antagonist 1-naphthylacetyl spermine trihydrochloride (Naspm, an analogue of Joro Spider Toxin) (26), we found that application of 100 μM Naspm reduced EPSCs in the dlBNST (n = 6, 71.1 ± 4.8% of baseline; P < 0.01; Fig. 2 A and D). Although Naspm is selective for CP-AMPARs over Ca2+-impermeable AMPARS, NMDARs also contain an external polyamine site that could be potentially sensitive to Naspm application (27). Additionally, NMDARs have been localized to presynaptic afferents in the BNST as well as to postsynaptic neurons (28), suggesting they may play a role in modulating presynaptic cells. To account for an effect on glutamate release, we examined PPR before and following Naspm application and found no significant change, suggesting that the observed decrease was attributable to blockade of postsynaptic CP-AMPARs (Fig. 2A, Insert; n.s.). Further, at −70 mV, we do not see a contribution of postsynaptic NMDARs to the EPSC in the BNST (29) and α1-AR LTD is not dependent on NMDAR activation (19). Following induction of α1-AR LTD, Naspm (100 μM) failed to reduce EPSC amplitude further, suggesting that α1-AR LTD confers a loss of Naspm sensitivity (n = 5, n.s.; Fig. 2 B and D). When 100 μM Naspm was applied following the induction of mGluR5 LTD [via 100 μM (RS)-3,5-dihydroxyphenylglycine (DHPG)], however, there was a similar reduction in EPSC size as compared with the basal condition (n = 8; P < 0.001 compared with pre-Naspm baseline; n.s. for one-way ANOVA vs. Naspm alone; Fig. 2 C and D). Furthermore, this reduction was statistically different from time-matched control experiments (n = 7; P < 0.05; Fig. 2C). These data suggest that α1-AR LTD is maintained, at least in part, by a loss of functional CP-AMPARs and that mGluR5 LTD is maintained by different mechanisms.
Fig. 2.
α1-AR LTD confers a loss of sensitivity to 100 μM Naspm in the BNST. (A) Ten minutes of 100 μM Naspm produced a depression of EPSCs in naive slices (n = 6; P < 0.002). (B) Following induction of LTD by 100 μM methoxamine (methox), 100 μM Naspm failed to reduce EPSCs further (n = 5; P > 0.83). (C) Following induction of mGluR 5 LTD by 100 μM (RS)-3,5-dihydroxyphenylglycine (DHPG), 100 μM Naspm significantly reduced EPSCs (■, n = 8; P < 0.001), and this depression was significantly different from time-matched control experiments without Naspm (○, n = 7; *P < 0.03). (D) Histogram comparing 100 μM Naspm in naive slices (min 30–35), 100 μM Naspm following 100 μM methoxamine (min 65–70 renormalized), and 100 μM Naspm following 100 μM DHPG (min 55–60 renormalized; *P < 0.05 for one-way ANOVA). n.s., not significant.
CP-AMPARs lack an edited GluR2 subunit (Q → R, second transmembrane loop) in the AMPAR tetramer. It is thought, however, that nearly all GluR2 receptors are edited in the mammalian brain (30); thus, most CP-AMPARs are composed of GluR1 homomers, GluR3 homomers, and/or GluR1/3 heteromers. We therefore further probed the potential involvement of AMPAR subunit protein–protein interactions in α1-AR LTD and mGluR5 LTD in the dlBNST. By infusing a peptide (via the patch pipette) corresponding to the C terminus of either the GluR1 protein (pep1-TGL; Tocris) or the GluR2 protein (pep2-SKVI; Tocris) into the cell for 30 min before recording, the subunit-specific interactions with intracellular proteins could be selectively disrupted (31). It was necessary to infuse the peptides for this duration because they can alter GluR subunit trafficking and alter basal EPSCs (32). After this initial incubation time, a baseline was collected and 100 μM methoxamine was applied to the cell for 15 min. The cells that were infused with the GluR1 peptide demonstrated significantly attenuated α1-AR LTD (n = 6, 70.5 ± 3.7% of baseline; Fig. 3A) as compared with the cells that were infused with the GluR2 peptide and naive no-peptide recordings (GluR2 peptide: n = 5, 54.6 ± 5.4% of baseline, GluR1 peptide vs. GluR2 peptide: P < 0.05 by one-way ANOVA; naive peptide: n = 7, 52.4 ± 6.4% of baseline, GluR1 vs. naive: P < 0.05 by one-way ANOVA; GluR2 vs. naive: n.s. by one-way ANOVA; Fig. 3A). Furthermore, when the same peptides were used to probe mGluR5 LTD in the dlBNST, neither peptide was able to attenuate LTD (n.s. by one-way ANOVA; GluR1 peptide: n = 7, 65.4 ± 6.8% of baseline; GluR2 peptide: n = 4, 71.1 ± 5.8% of baseline; naive: 70.3 ± 4.0% of baseline; Fig. 3B).
Fig. 3.
GluR1 C-terminal peptide attenuates α1-AR LTD but not mGluR5 LTD. (A) Infusion of a peptide from the C terminus of GluR1 (n = 6) but not GluR2 (n = 5) attenuates the LTD induced by application of 100 μM methoxamine (methox; P < 0.05; GluR1 vs. GluR2). (B) Neither the GluR1 (n = 7) nor GluR2 (n = 4) peptide attenuated the LTD induced by 100 μM (RS)-3,5-dihydroxyphenylglycine (DHPG).
α1-AR LTD but Not mGluR5 LTD Is Disrupted by Stress-Inducing Manipulations.
The BNST is a critical regulator of anxiety-like behaviors (33). Restraint stress is known to increase extracellular NE levels robustly in the BNST and to signal through BNST α1-ARs to increase anxiety-like behaviors and adrenocorticotropic hormone in blood plasma (7, 34). We hypothesized that 10 daily 2-h restraint stress sessions (see Methods) would modulate α1-AR LTD in vivo but not mGluR5 LTD. Recording from the ventrolateral BNST (vlBNST) [which receives the most robust NE projection (4)], we found that a 15-min 100-μM methoxamine application produced a large transient depression but failed to induce LTD in the restrained animals in the vlBNST (stress: n = 6, 95.7 ± 9.0% of baseline, n.s. change from baseline; naive: n = 4, 62.0 ± 9.7% of baseline; Fig. 4A). In the dlBNST, we found that α1-AR LTD was significantly attenuated in restrained mice as compared with naive controls (stress: n = 5, 84.6 ± 6.0% of baseline; naive: n = 5, 54.4% ± 8.1% of baseline; P < 0.05; Fig. 4B). We next examined the expression of mGluR5 LTD following the restraint stress protocol and found that mGluR5 LTD was not diminished in either the dlBNST or the vlBNST (dorsal: n = 5, 74.2 ± 4.4% of baseline; ventral: n = 7, 37.7 ± 6.8% of baseline; Fig. 4 C and D) as compared with naive controls.
Fig. 4.
Chronic restraint stress occludes α1-AR LTD but not mGluR5 LTD. The 10-day restraint stress paradigm blocked α1-AR LTD in the vlBNST (n = 6; P > 0.78) (A) and significantly attenuated α1-AR LTD in the dlBNST (B) (n = 5 in both conditions; P < 0.05). methox, methoxamine. (C and D) Stress exposure does not manipulate mGluR5 LTD induced by 100 μM (RS)-3,5-dihydroxyphenylglycine (DHPG) in either the dlBNST (n = 5 for both conditions) or vlBNST(stress: n = 7, naive: n = 6). (E and F) Stress paradigm significantly attenuated the function of CP-AMPARs in the vlBNST (min 25–30; n = 8 for both conditions; P < 0.05). n.s., not significant.
Because of the disruption of α1-AR LTD in vlBNST in the stressed mice and the data suggesting that α1-AR LTD prevents signaling via CP-AMPARs (Fig. 2), we next probed the signaling through CP-AMPARs in the vlBNST in mice exposed to the same chronic restraint stress. Following application of 100 μM Naspm, naive mice exhibited a similar reduction in EPSCs in the vlBNST as was observed in the dlBNST (n = 8, 71.1 ± 4.4% of baseline; Fig. 4 E and F). Stressed mice, however, had a significantly attenuated Naspm-induced reduction in EPSCs as compared with their naive controls (n = 8, 88.3 ± 4.3% of baseline; P < 0.05; Fig. 4 E and F).
α1-AR LTD Is Disrupted by Chronic Alcohol Exposure.
Stress disorders and alcoholism are highly comorbid (35). Data from human studies suggest that NE is increased in the central nervous system (CNS) of alcoholics (36), where it may play a role in the pathogenesis of alcoholism (35). Additionally, the adrenergic system remains an attractive target for intervention in alcoholism (11). Recently, α1-AR signaling has been linked to drinking behavior in withdrawn-dependent animals (8). Because of these data and our chronic stress results, we examined the persistence of α1-AR LTD in the dlBNST and vlBNST in ethanol-exposed mice. Mice receiving chronic continuous ethanol (CCE) were exposed to 64 h of continuous ethanol vapor, whereas mice receiving chronic intermittent ethanol (CIE) were exposed to 4 days of 16 h of ethanol vapor with 8-h withdrawal periods interspersed. Both the ethanol-exposed and sham animals were administered i.p. pyrazole daily. Animals were killed 4–6 h into the final withdrawal under each condition. Although we examined a shorter time course following methoxamine application, both conditions resulted in significantly attenuated responses to α1-AR signaling from sham mice; however, the LTD was not fully occluded by these treatments (sham mice: n = 6, 55.5 ± 6.5% of baseline; CCE mice: n = 7, 81.1 ± 7.5% of baseline; CIE mice: n = 5, 79.1 ± 6.3% of baseline; one-way ANOVA, P < 0.05; Fig. 5). We did not observe a correlation in the degree of depression between the dlBNST or vlBNST in the ethanol-treated animals.
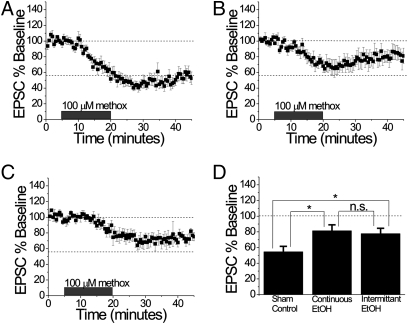
Fig. 5.
Chronic exposure to ethanol attenuates α1-AR LTD in the BNST. (A) Sham mice demonstrated robust LTD when stimulated with 100 μM methoxamine (methox), similar to naive mice (n = 6). (B) Response to 100 μM methoxamine (methox) was attenuated in the BNST in mice that had experienced one withdrawal following CCE (n = 7). (C) Methoxamine (methox; 100 μM) resulted in attenuated LTD following a CIE paradigm (n = 5). (D) Histogram comparing sham, CCE, and CIE conditions: both CCE and CIE conditions are significantly attenuated from control conditions by one-way ANOVA (min 40–45; *P < 0.05 for ANOVA; CIE vs. sham, P < 0.05; CCE vs. sham, P < 0.05).
Discussion
We find that α1-AR activation produces LTD of excitatory transmission in the BNST that involves CP-AMPARs. Despite the fact that both α1-AR and mGluR5 are Gq-linked and can elicit LTD on overlapping neuronal populations in the BNST, we find that these two forms of LTD have distinct maintenance mechanisms. These differences are also apparent when examining the persistence of these LTDs following environmental challenges. We previously found that cocaine disrupts mGluR5- but not α1-AR LTD (13, 14, 19). Here, we report that a chronic stressor disrupts α1-AR LTD and CP-AMPAR transmission but not mGluR5 LTD. Moreover, we report that α1-AR LTD is diminished by chronic ethanol exposure. Finally, our studies uncover an additional acute enhancement of glutamatergic transmission in the BNST by α1-ARs, which, as with dopamine actions in the region, occurs through a CRFR1-dependent process.
α1-AR LTD Is Maintained by a Different Postsynaptic Mechanism Than mGluR5 LTD in the BNST.
Previously, we found that α1-AR LTD induction occluded the further induction of mGluR5 LTD in the BNST (19), leading us to hypothesize that both LTDs involved similar mechanisms (18). Recently, we found that mGluR5 LTD in the BNST is maintained via postsynaptic mechanisms involving endocytosis and rearrangement of the actin cytoskeleton (14). We now show that α1-AR LTD also requires clathrin-dependent endocytosis. Unlike mGluR5 LTD, however, a difference in the required time course of agonist application and a lack of MEK1/2 involvement suggest that different mechanisms underlie both LTDs. Here, we present data suggesting that in contrast to mGluR5 LTD, the AMPARs targeted in α1-AR LTD are CP-AMPARs. Additionally, the mEPSC and sEPSC profiles are different between mGluR5 LTD and α1-AR LTD. In mGluR5 LTD, a decrease in the frequency of events is observed with mEPSCs (14), whereas in α1-AR LTD, a decrease in the amplitude of events is observed with sEPSCs but not with mEPSCs. The lack of an α1-AR agonist effect on the amplitude or frequency of mEPSCs suggests that α1-AR LTD is dependent on the activation of voltage-gated sodium channels. α1-AR signaling can actively desensitize mGluR5 signaling at inositol triphosphate (IP3) receptors (37), providing an explanation for our observed occlusion of mGluR5 LTD following induction of α1-AR LTD in our previous report (19) in the absence of a shared mechanism.
α1-AR LTD but Not mGluR5 LTD Results in the Functional Loss of CP-AMPARs.
CP-AMPARs are less abundant in the CNS than the more conventional Ca2+-impermeable AMPARs (30). Although our understanding of their roles is still developing (30, 38), it is apparent that CP-AMPARs are another means for Ca2+ signaling in cells (30). Another Gq-linked LTD, mGluR1-mediated LTD in the VTA, can induce the removal of CP-AMPARs from the membrane (24). Additionally, it has been shown that group I mGluRs and CP-AMPAR activation facilitate a functional switch of AMPAR subunits in cerebellar stellate cells (39). We found that the polyamine Naspm, a selective external pharmacological blocker of CP-AMPARs, produced a reduction of ∼30% of the EPSC in the BNST. This sensitivity was lost following induction of α1-AR LTD, and a peptide inhibitor that mimics the C terminus of the GluR1 subunit attenuated α1-AR LTD. These converging lines of evidence suggest that the loss of CP-AMPAR function comprises a portion of the mechanism underlying α1-AR LTD. Although it is not known if signaling via CP-AMPARs alters plasticity within the BNST, a loss of CP-AMPARs following α1-AR LTD may result in a metaplastic shift within the BNST that could alter circuit activity.
Additionally, we demonstrated that mGluR5 LTD in the BNST is neither maintained by the functional loss of CP-AMPARs from the postsynaptic density nor sensitive to the inclusion of the GluR1 C-terminal peptide in the intracellular solution. (We did not observe an affect of either C-terminal peptide on mGluR5 LTD; however, this LTD may alter the function of postsynaptic AMPARs via other means than C-terminal interactions.) These results, in conjunction with the differing mEPSC and sEPSC profiles, strongly suggest that although the same cell contains the required elements for the induction of either α1-AR or mGluR5 LTD, they occur by distinctly different mechanisms. Furthermore, the induction of one form of Gq-coupled LTD manipulates the plasticity of the other LTD (19), which differs from observations in other brain regions, where dual Gq-coupled LTDs have been studied (17, 18). It is possible to envision that the occlusion of mGluR LTD in the BNST by α1-AR LTD could influence subsequent behavior, and a lack of proper α1-AR LTD induction/expression (see below) may result in pathological mGluR LTD expression.
Expression of α1-AR LTD Is Manipulated by Chronic Stressors in Vivo.
Previously, we demonstrated that α1-AR LTD cannot be induced in two models of affective disorders: the α2A-AR KO mouse and the NE transporter KO mouse (19). We thus wanted to examine if stressful manipulations, which would increase adrenergic tone in vivo, could alter the expression of α1-AR LTD ex vivo. Withdrawal from alcohol intoxication has been shown to increase anxiety (40), and patients experiencing withdrawal have elevated levels of NE and its metabolites (36, 41). Furthermore, CIE can increase anxiety-like behavior in animal models (44). We used a CCE protocol with a single withdrawal and a CIE protocol with four withdrawals. We originally expected to see differences only after the CIE repeated-withdrawal paradigm; however, both protocols significantly reduced the expression of LTD and were not significantly different from each other. This result, however, is perhaps not surprising, because animals are experiencing acute withdrawal in both conditions. Alternatively, other aspects of the exposure regimen, such as pharmacological effects of alcohol, may drive these alterations.
We also examined the expression of α1-AR LTD following a prolonged chronic stressor. Restraint stress has been shown to increase NE levels within the BNST, and α1-AR signaling therein increases anxiety-like behavior and activates the HPA axis (7). Mice that received chronic restraint stress failed to express α1-AR LTD in the vlBNST and demonstrated attenuated α1-AR LTD in the dlBNST. These stress experiments suggest that either the LTD had already been expressed in vivo or that the mechanisms for inducing α1-AR LTD were not functioning following the stressor. Thus, we next probed CP-AMPAR function in the vlBNST in animals exposed to the chronic stress paradigm. Alterations in the function of CP-AMPARs could serve as a physiological “marker” for the induction of LTD. Following chronic stress, the CP-AMPAR inhibitor Naspm had a significantly reduced effect on evoked EPSCs as compared with naive controls (Fig. 4 E and F). These results suggest that the α1-AR LTD is induced during the chronic restraint stress, which thus reduces transmission via CP-AMPARs. There is however, a slight but significant effect of Naspm on EPSCs remaining in the stressed animals, suggesting complex poststressor effects on physiology. One possibility for the remaining CP-AMPAR function in the stressed animals is that although our bath application of α1-AR agonist appears to target all synapses with CP-AMPARs, NE signaling in vivo may only target a subset of synapses.
Understanding the modulation of α1-AR LTD is critical, because the BNST is thought to be part of the braking mechanism that inhibits activation of the paraventicular nucleus of the hypothalamus (PVN) (1). This LTD could thus serve to disengage the inhibitory influence of the BNST on the PVN under stressful conditions. Additionally, the HPA axis has altered function in both human disease states (43) and rodent models of affective disorders (44, 45), which may suggest a pathological role for this plasticity or the lack of α1-AR LTD and CP-AMPAR function. In future studies, it will be important to delineate the pattern and duration of alcohol/stress exposures necessary to modulate α1-AR LTD as well as the persistence of this modulation.
Although α1-AR LTD was disrupted by alcohol exposure and chronic stress, mGluR5 LTD expression was not altered. Previously, we have observed that a single cocaine i.p. injection can prevent the expression of mGluR5 LTD but not α1-AR LTD in the BNST (14, 19), which may suggest that different behavioral saliencies can manipulate the induction of Gq-coupled LTD on neurons within the BNST. Because we have demonstrated that prior induction of α1-AR LTD can manipulate further induction of mGluR5 LTD (19), there may be cross-talk between the induction mechanisms that could contribute to stress/substance abuse pathological findings.
An intriguing result of this investigation is that α1-AR activation leads to lasting increases in sEPSC frequency, presumably via the release of CRF acting on the CRFR1, akin to dopamine signaling within the BNST (21). Patients with PTSD have been shown to have overengaged central CRF systems (43). In addition, CRF administration within the BNST can potentiate anxiety (44). Moreover, animals experiencing protracted withdrawal from alcohol have altered CRF tone in a component of the dlBNST, which leads to decreased plasticity of the intrinsic excitability of these neurons (46). Heightened adrenergic tone may be one mechanism by which the CRF system is dysregulated in these dependent animals.
In total, the current data, together with our previous results in models of affective disorders, strongly correlate the loss of α1-AR LTD with a number of psychiatric ailments and provide a potential mechanism for the therapeutical effects observed in clinical trials that have demonstrated α1-AR antagonists as potential therapeutical agents for the alleviation of PTSD and alcoholism (14–17).
Methods
Animal Care.
All mice used in experiments were male C57BL/6j mice 5–8 weeks old (The Jackson Laboratories). All animals were provided with food and water ad libitum, with the exception of the 2-h stress (see below) experiments, and were housed in groups within the Vanderbilt Animal Care Facilities. Approved guidelines from the Vanderbilt University and University of North Carolina at Chapel Hill Animal Care and Use Committees were used for all experiments.
Brain Slices and Whole-Cell Recordings.
Brain slices were prepared, and whole-cell recordings were made as previously described (21, 29) (see SI Methods). dlBNST recordings focused heavily on the undifferentiated anterolateral regions and ventral aspects of the oval nucleus.
Stress Procedures.
Mice were stressed for 2 h for 10 consecutive days and recorded from on the 11th day. Restraint devices were 50-mL conical tubes with several (≈15) holes in the front and rear (cap) to maintain airflow. While in restraint devices, animals were placed inside separate sound- and light-attenuating boxes, and they were returned to their home cage immediately following restraint.
Ethanol Procedures.
Ethanol chamber experiments were performed in accordance with the Integrative Neuroscience Initiative on Alcoholism (INIA-Stress) standard operating procedure and as previously reported by Healey et al. (47). For a full description, please refer to SI Methods.
Supplementary Material
Acknowledgments
We thank Heather Gosnell, Drs. Randy Blakely, Roger Colbran and AJ Baucum for comments on an earlier version of the manuscript. This work was supported by NIAAA grant AA015635 and NIDA grant DA019112 (DGW).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905568107/DCSupplemental.
References
- 1.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drolet G, editor. Progress in Neuro-Psychopharmacology and Biological Psychiatry . Vol. 33. New York: Elsevier; 2009. [DOI] [PubMed] [Google Scholar]
- 4.Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Brain Res Rev. 2004;47:145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: An update and clinical implications. Eur J Pharmacol. 2005;526(1-3):36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Olson VG, et al. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science. 2006;311:1017–1020. doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- 7.Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112(1):13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 8.Walker BM, Rasmussen DD, Raskind MA, Koob GF. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42(2):91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenwell TN, Walker BM, Cottone P, Zorrilla EP, Koob GF. The alpha1 adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacol Biochem Behav. 2009;91:295–302. doi: 10.1016/j.pbb.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raskind MA, et al. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: A report of 4 cases. J Clin Psychiatry. 2000;61:129–133. doi: 10.4088/jcp.v61n0208. [DOI] [PubMed] [Google Scholar]
- 11.Simpson TL, et al. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2008;33:255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- 12.Chiamulera C, et al. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 13.Grueter BA, et al. Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J Neurosci. 2006;26:3210–3219. doi: 10.1523/JNEUROSCI.0170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grueter BA, McElligott ZA, Robison AJ, Mathews GC, Winder DG. In vivo metabotropic glutamate receptor 5 (mGluR5) antagonism prevents cocaine-induced disruption of postsynaptically maintained mGluR5-dependent long-term depression. J Neurosci. 2008;28:9261–9270. doi: 10.1523/JNEUROSCI.2886-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumont EC, Mark GP, Mader S, Williams JT. Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nat Neurosci. 2005;8:413–414. doi: 10.1038/nn1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 17.Scheiderer CL, et al. Coactivation of M(1) muscarinic and alpha1 adrenergic receptors stimulates extracellular signal-regulated protein kinase and induces long-term depression at CA3-CA1 synapses in rat hippocampus. J Neurosci. 2008;28:5350–5358. doi: 10.1523/JNEUROSCI.5058-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi SY, et al. Multiple receptors coupled to phospholipase C gate long-term depression in visual cortex. J Neurosci. 2005;25:11433–11443. doi: 10.1523/JNEUROSCI.4084-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McElligott ZA, Winder DG. Alpha1-adrenergic receptor-induced heterosynaptic long-term depression in the bed nucleus of the stria terminalis is disrupted in mouse models of affective disorders. Neuropsychopharmacology. 2008;33:2313–2323. doi: 10.1038/sj.npp.1301635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watabe AM, Carlisle HJ, O’Dell TJ. Postsynaptic induction and presynaptic expression of group 1 mGluR-dependent LTD in the hippocampal CA1 region. J Neurophysiol. 2002;87:1395–1403. doi: 10.1152/jn.00723.2001. [DOI] [PubMed] [Google Scholar]
- 21.Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 2008;28:13856–13865. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- 24.Bellone C, Lüscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci. 2005;21:1280–1288. doi: 10.1111/j.1460-9568.2005.03979.x. [DOI] [PubMed] [Google Scholar]
- 25.Grueter BA, McElligott ZA, Winder DG. Group I mGluRs and long-term depression: Potential roles in addiction? Mol Neurobiol. 2007;36:232–244. doi: 10.1007/s12035-007-0037-7. [DOI] [PubMed] [Google Scholar]
- 26.Koike M, Iino M, Ozawa S. Blocking effect of 1-naphthyl acetyl spermine on Ca(2+)-permeable AMPA receptors in cultured rat hippocampal neurons. Neurosci Res. 1997;29(1):27–36. doi: 10.1016/s0168-0102(97)00067-9. [DOI] [PubMed] [Google Scholar]
- 27.Mueller AL, Albensi BC, Ganong AH, Reynolds LS, Jackson H. Arylamine spider toxins antagonize NMDA receptor-mediated synaptic transmission in rat hippocampal slices. Synapse. 1991;9:244–250. doi: 10.1002/syn.890090403. [DOI] [PubMed] [Google Scholar]
- 28.Gracy KN, Pickel VM. Comparative ultrastructural localization of the NMDAR1 glutamate receptor in the rat basolateral amygdala and bed nucleus of the stria terminalis. J Comp Neurol. 1995;362(1):71–85. doi: 10.1002/cne.903620105. [DOI] [PubMed] [Google Scholar]
- 29.Grueter BA, Winder DG. Group II and III metabotropic glutamate receptors suppress excitatory synaptic transmission in the dorsolateral bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:1302–1311. doi: 10.1038/sj.npp.1300672. [DOI] [PubMed] [Google Scholar]
- 30.Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: Synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi Y, et al. Driving AMPA receptors into synapses by LTP and CaMKII: Requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 32.Collingridge GL, Isaac JT. Functional roles of protein interactions with AMPA and kainate receptors. Neurosci Res. 2003;47(1):3–15. doi: 10.1016/s0168-0102(03)00160-3. [DOI] [PubMed] [Google Scholar]
- 33.Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pacak K, McCarty R, Palkovits M, Kopin IJ, Goldstein DS. Effects of immobilization on in vivo release of norepinephrine in the bed nucleus of the stria terminalis in conscious rats. Brain Res. 1995;688:242–246. doi: 10.1016/0006-8993(95)00566-9. [DOI] [PubMed] [Google Scholar]
- 35.Breese GR, et al. Stress enhancement of craving during sobriety: A risk for relapse. Alcohol Clin Exp Res. 2005;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borg S, Kvande H, Sedvall G. Central norepinephrine metabolism during alcohol intoxication in addicts and healthy volunteers. Science. 1981;213:1135–1137. doi: 10.1126/science.7268421. [DOI] [PubMed] [Google Scholar]
- 37.Paladini CA, Fiorillo CD, Morikawa H, Williams JT. Amphetamine selectively blocks inhibitory glutamate transmission in dopamine neurons. Nat Neurosci. 2001;4:275–281. doi: 10.1038/85124. [DOI] [PubMed] [Google Scholar]
- 38.Conrad KL, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly L, Farrant M, Cull-Candy SG. Synaptic mGluR activation drives plasticity of calcium-permeable AMPA receptors. Nat Neurosci. 2009;12:593–601. doi: 10.1038/nn.2309. [DOI] [PubMed] [Google Scholar]
- 40.George DT, Nutt DJ, Dwyer BA, Linnoila M. Alcoholism and panic disorder: Is the comorbidity more than coincidence? Acta Psychiatr Scand. 1990;81(2):97–107. doi: 10.1111/j.1600-0447.1990.tb06460.x. [DOI] [PubMed] [Google Scholar]
- 41.Hawley RJ, Major LF, Schulman EA, Linnoila M. Cerebrospinal fluid 3-methoxy-4-hydroxyphenylglycol and norepinephrine levels in alcohol withdrawal. Correlations with clinical signs. Arch Gen Psychiatry. 1985;42:1056–1062. doi: 10.1001/archpsyc.1985.01790340034005. [DOI] [PubMed] [Google Scholar]
- 42.Kliethermes CL, Cronise K, Crabbe JC. Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcohol Clin Exp Res. 2004;28:1012–1019. doi: 10.1097/01.alc.0000131976.40428.8f. [DOI] [PubMed] [Google Scholar]
- 43.Bremner JD, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker D, et al. Differential effects of the CRF-R1 antagonist GSK876008 on fear-potentiated, light- and CRF-enhanced startle suggest preferential involvement in sustained vs phasic threat responses. Neuropsychopharmacology. 2009;34:1533–1542. doi: 10.1038/npp.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rasmussen DD, et al. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol Clin Exp Res. 2000;24:1836–1849. [PubMed] [Google Scholar]
- 46.Francesconi W, et al. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J Neurosci. 2009;29:5389–5401. doi: 10.1523/JNEUROSCI.5129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Healey JC, Winder DG, Kash TL. Chronic ethanol exposure leads to divergent control of dopaminergic synapses in distinct target regions. Alcohol. 2008;42:179–190. doi: 10.1016/j.alcohol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.