Abstract
Symbioses may be important mechanisms of plant adaptation to their environment. We conducted a reciprocal inoculation experiment to test the hypothesis that soil fertility is a key driver of local adaptation in arbuscular mycorrhizal (AM) symbioses. Ecotypes of Andropogon gerardii from phosphorus-limited and nitrogen-limited grasslands were grown with all possible “home and away” combinations of soils and AM fungal communities. Our results indicate that Andropogon ecotypes adapt to their local soil and indigenous AM fungal communities such that mycorrhizal exchange of the most limiting resource is maximized. Grasses grown in home soil and inoculated with home AM fungi produced more arbuscules (symbiotic exchange structures) in their roots than those grown in away combinations. Also, regardless of the host ecotype, AM fungi produced more extraradical hyphae in their home soil, and locally adapted AM fungi were, therefore, able to sequester more carbon compared with nonlocal fungi. Locally adapted mycorrhizal associations were more mutualistic in the two phosphorus-limited sites and less parasitic at the nitrogen-limited site compared with novel combinations of plants, fungi, and soils. To our knowledge, these findings provide the strongest evidence to date that resource availability generates evolved geographic structure in symbioses among plants and soil organisms. Thus, edaphic origin of AM fungi should be considered when managing for their benefits in agriculture, ecosystem restoration, and soil-carbon sequestration.
Keywords: coevolution, geographic mosaics, mutualism, parasitism
Symbiotic organisms adapt to one another and their abiotic environment. This process varies spatially among populations such that evolutionary mosaics of symbiotic function can arise from geographic differences in the environment (1). The degree to which geographic mosaics of symbioses are caused by variation in the biotic or abiotic environment is not well-understood, but this knowledge is critical for development of a mechanistic understanding of their function within ecosystems (2, 3). Soil fertility mediates the mutualistic function of mycorrhizal symbioses (4, 5); consequently, mycorrhizas from grasslands with varying soil-nutrient environments provide an ideal system to uncouple the importance of biotic (plant and fungal) and abiotic (soil) variation to adaptation by symbionts. Arbuscular mycorrhizal (AM) fungi (Glomeromycota) are obligate biotrophs with vascular plants, and they facilitate plant acquisition of limiting soil resources. Thus, plants and AM fungi may exert reciprocal selective forces on one another. These fungi form arbuscules inside plant roots through which mineral nutrients are exchanged for host photosynthates, and they form extensive networks of extraradical hyphae in the soil through which they forage for mineral nutrients and water. In this way, host plants may benefit from the nutrient-harvesting abilities of their fungal symbionts. The relationship between plant and AM fungus is more likely to be mutualistic when soil-nutrient supply rate is low and fungal access to essential nutrients is beneficial to host growth (6). Parasitic mycorrhizas can develop in fertilized soils if carbon costs of the symbiosis do not outweigh the mineral benefits (4, 5, 7). Glomeromycotan fungi have also been shown to improve soil structure and are important carbon and nutrient sinks in many ecosystems (8 –10). Thus, understanding the factors that control their abundance and symbiotic function can help guide their management so that their desirable community and ecosystem functions can be maximized.
Mutual cooperation is more likely to develop in stable long-term partnerships than in novel combinations of plants and AM fungi (11). Consequently, we looked for evidence of local adaptation and coadaptation in undisturbed or restored grasslands where ecotypes of plants and AM fungi are expected to have a long history of symbiosis in their local soil environment (12). Andropogon gerardii Vitm. is the dominant native grass in many North American tallgrass prairies, and mycorrhizas seem to be critical to the maintenance of this dominance. Whole-community experiments show that removal of AM fungi using fungicides reduces A. gerardii biomass with a concomitant increase in the biomass of less mycotrophic grasses and forbs (13, 14). Soil-phosphorus availability has been linked to differences in the formation of mycorrhizas among regional ecotypes of A. gerardii such that ecotypes from phosphorus-limited sites have coarser roots and benefit more from mycorrhizal symbioses than those from phosphorus-rich sites (15). Enriching soil with chemical fertilizers has been shown to change the species composition and abundance of AM fungi (16) and nearly eliminate populations of A. gerardii (17, 18). Plant communities influence the composition of communities of soil organisms (19), and in turn, these organisms may have beneficial, detrimental, or neutral effects on plant growth and thus, generate positive or negative feedbacks (20). Studies show a preponderance of negative feedbacks in early successional plant species and positive feedbacks in late successional species (21).
For a highly mycotrophic, late successional species like A. gerardii, we hypothesize that adaptation of plants to their biotic and abiotic soil environment may structure AM symbioses such that mutualistic function between plant genotypes and AM fungal communities will be maximized in nutrient-limited sites. This selection is not expected to occur in nutrient-rich sites, because mycorrhizas are not likely to increase plant fitness if mycorrhizal delivery of nutrients provides little benefit to compensate for the carbon cost of the symbiosis (4, 6). Many studies have linked AM function to phosphorus availability (5, 22). Although AM fungi also deliver nitrogen to their host plants (23, 24), the importance of this to plant fitness is not well-established (25, 26). The purpose of our study was to test three hypotheses:
H1: Mycorrhizas improve plant fitness by increasing the uptake of either phosphorus or nitrogen, whichever nutrient is most limiting to plant growth.
H2: The fitness of plants and biomass of associated AM fungi is greater in coadapted symbionts grown in their local soil compared with those grown in novel combinations.
H3: Coadaptation in AM symbioses can maximize mutualism in low-fertility soil or minimize parasitism in high-fertility soil.
We tested these hypotheses using a reciprocal-inoculation experiment with local ecotypes of A. gerardii, AM fungal communities, and soils from two native prairie remnants, Cedar Creek in Minnesota and Konza Prairie in Kansas, and one restored prairie at Fermi National Laboratory Environmental Research Park in Illinois. All available evidence suggests that the soils at Cedar Creek and Konza prairies have been relatively undisturbed since the last glaciation 11,000 years ago and probably much longer in the case of Konza. The reconstructed prairie at Fermi was established 28 years ago using locally collected seeds from nearby prairie remnants on similar soil types. The Fermi prairie was previously cultivated and managed with conventional agricultural methods from the late 1840s through 1975. The soils at the three sites are very different. Available soil phosphorus is very low at Fermi and Konza and very high at Cedar Creek; soil nitrogen is very low at Cedar Creek and higher at the other sites (Table 1). Therefore, we predict that mycorrhizal benefits should be related to phosphorus uptake at Fermi and Konza and related to nitrogen uptake at Cedar Creek.
Table 1.
Location, climate, and soil characteristics at the three prairie sites
| Site | Latitude and longitude | Mean annual precipitation (mm) | Mean January/July temperature (°C) | Soil type | Soil texture | pH | Soil organic matter % | Bray-1 PO4, μg g−1 | NH4-N, μg g−1 | NO3-N, μg g−1 | Soil N:P |
| Fermi | 41°50′ N 88°15′ W | 882 | −6.5/22.5 | Mesic udollic endoaqualfs | Silt loam | 7.6 | 3.3 | 11.5 | 7.2 | 5.8 | 1.1 |
| Konza | 39°05′ N 96°18′ W | 835 | −2.7/26.2 | Mesic argiustolls | Silty clay loam | 6.2 | 5.7 | 18.5 | 13.4 | 8.2 | 1.1 |
| Cedar Creek | 45°24′ N 93°12′ W | 660 | −10.0/22.2 | Typic udipsamments | Sandy loam | 5.3 | 1.4 | 46.5 | 5.1 | 4.9 | 0.2 |
Quantifying adaptation within mycorrhizas requires measurements of the influence of the symbiosis on the fitness of the host plant and the fungal biomass as well as some measure of symbiotic exchange. In this study, we used total plant biomass and inflorescence biomass as surrogate measures of plant fitness, the density of extraradical AM hyphae as a measure of fungal biomass, and arbuscular colonization as a measure of symbiotic exchange. We quantified the tissue nitrogen and phosphorus content of plants to test the prediction that AM colonization should be positively associated with phosphorus uptake in Fermi and Konza soils, and it should be positively associated with nitrogen uptake in Cedar Creek soil. Our experimental design allowed us to determine the degree to which plants and AM fungi adapt to each other and their abiotic soil environment. If local adaptation occurs, then the biomass of AM fungi and/or plant fitness should be highest in their home soil. If coadaptation occurs, then plant fitness, fungal biomass, and symbiotic exchange should be greater in “fully home combinations” of plants, fungi, and soils than in “away combinations.”
Results and Discussion
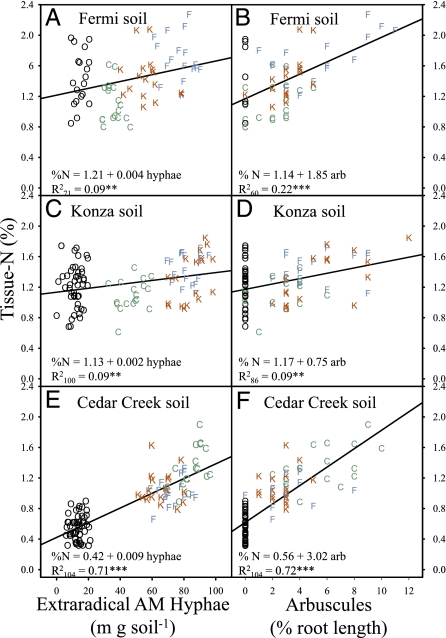
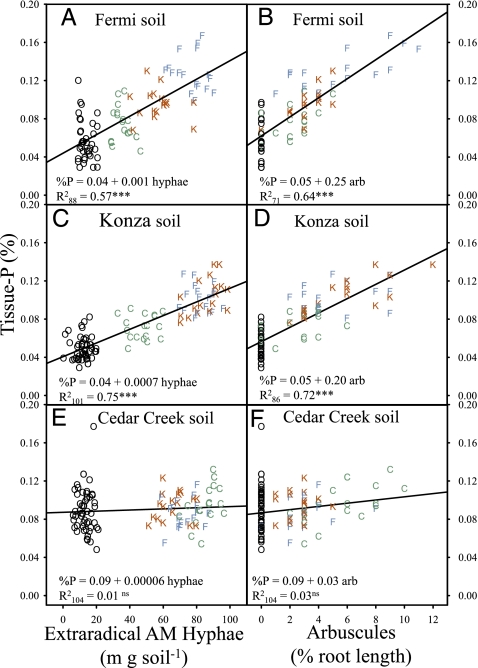
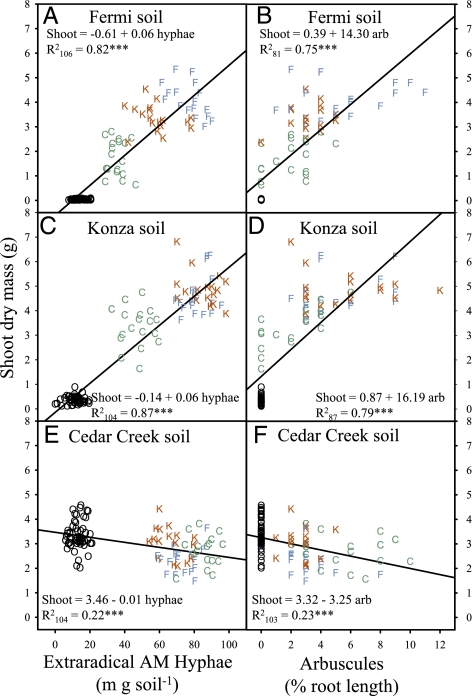
In mesic grasslands, plant tissue N:P < 14 generally indicates nitrogen limitation, N:P > 16 indicates phosphorus limitation, and ratios between 14 and 16 indicate that the two elements are likely to be colimited (27). Liebig's Law of the Minimum suggests that plant productivity can be maximized when nitrogen and phosphorus are equally limited (27). Mycorrhizas had a strong impact on the tissue N:P of A. gerardii in our reciprocal inoculation experiment. Nonmycorrhizal plants grown in Fermi and Konza soils were severely phosphorus-limited (N:P = 26.6 ± 0.91 SE and 24.8 ± 0.58, respectively; n = 6), and nonmycorrhizal plants grown in Cedar Creek soil were severely nitrogen-limited (N:P = 6.3 ± 0.54). In contrast, mycorrhizal plants grown in Fermi and Konza soils had tissue-nutrient concentrations indicative of equal limitation (N:P = 14.5 ± 0.21 and 14.1 ± 0.20, respectively), and those grown in Cedar Creek soil suggest minor nitrogen limitation (N:P = 12.5 ± 0.21). The role of AM fungi in optimizing plant N:P ratios is strongly supported by linear relationships between plant tissue-nutrient concentrations and extraradical (in the soil) and intraradical (inside roots) fungal structures (Figs. 1 and 2). Extraradical AM hyphae and intraradical arbuscules are strongly positively related to tissue nitrogen in plants grown in nitrogen-limited Cedar Creek soil (Fig. 1 E and F) but weakly related in plants grown in phosphorus-limited Fermi (Fig. 1 A and B) and Konza (Fig. 1 C and D) soils. In contrast, extraradical AM hyphae and arbuscules are strongly related to tissue phosphorus in plants grown in Fermi (Fig. 2 A and B) and Konza (Fig. 2 C and D) soils but not in those grown in Cedar Creek soil (Fig. 2 E and F). These results clearly indicate that AM fungi increase uptake of the nutrient that is most limiting in the soil. However, our results only partially support the hypothesis that AM symbioses improve plant fitness by increasing the uptake of the most limiting nutrient. Extraradical AM hyphae and arbuscular colonization are positively related to plant biomass in Fermi (Fig. 3 A and B) and Konza soils (Fig. 3 C and D) but negatively associated with plant biomass in Cedar Creek soil (Fig. 3 E and F). Similar positive relationships among inflorescence biomass and AM fungal biomass are observed in Fermi (R 2 106 = 0.40; P < 0.0001) and Konza (R 2 104 = 0.29; P < 0.0001) soils, and negative relationships are observed in Cedar Creek soil (R 2 104 = 0.21; P < 0.0001). Thus, although mycorrhizas improve nitrogen uptake in the nitrogen-limited Cedar Creek soil, they do not increase plant fitness.
Fig. 1.
The relationship between tissue nitrogen and extraradical hyphal density and the proportion of root length colonized by arbuscules in Fermi, Konza, and Cedar Creek soils. Symbols represent treatment-inoculum source (blue F, Fermi; red K, Konza; green C, Cedar Creek; black O, nonmycorrhizal controls). Arbuscule-colonization data were arcsine square root transformed before statistical analysis. R 2 subscript indicates the number of samples included in the regression. Significance of the regression equations are indicated as **P < 0.01 > 0.001 and ***P < 0.0001.
Fig. 2.
The relationship between tissue phosphorus and extraradical hyphal density and the proportion of root length colonized by arbuscules in Fermi, Konza, and Cedar Creek soils. Symbols represent treatment-inoculum source (blue F, Fermi; red K, Konza; green C, Cedar Creek; black O, nonmycorrhizal controls). Arbuscule-colonization data were arcsine square root transformed before statistical analysis. R 2 subscript indicates the number of samples included in the regression. Significance of the regression equations are indicated as ***P < 0.0001 (ns, not significant).
Fig. 3.
The relationship between shoot biomass and extraradical hyphal density and the proportion of root length colonized by arbuscules in Fermi, Konza, and Cedar Creek soils. Symbols represent treatment-inoculum source (blue F, Fermi; red K, Konza; green C, Cedar Creek; black O, nonmycorrhizal controls). Arbuscule-colonization data were arcsine square root transformed before statistical analysis. R 2 subscript indicates the number of samples included in the regression. Significance of the regression equations are indicated as ***P < 0.0001.
Why are AM symbioses mutualistic in Fermi and Konza soils but commensal or parasitic in Cedar Creek soil? We propose that the reason is related to the balance of trade between plants and fungi. Plants exchange carbon for fungal phosphorus and nitrogen (22, 26). Tissue N:P ratios in our experiment indicate that AM symbioses completely satisfy the phosphorus requirements of A. gerardii grown in Fermi and Konza soil, but they do not completely satisfy the nitrogen requirements of plants grown in Cedar Creek soil. Consequently, plants grown in this nitrogen-impoverished soil will continue to provision AM fungi with carbon in exchange for nitrogen, and this will increase the biomass of the fungus but not the biomass of the plant. This mechanism accounts for the extremely strong positive correlation between plant nitrogen concentration and extraradical AM hyphae and arbuscules (Fig. 1), despite the negative relationship with plant biomass (Fig. 3) in Cedar Creek soil.
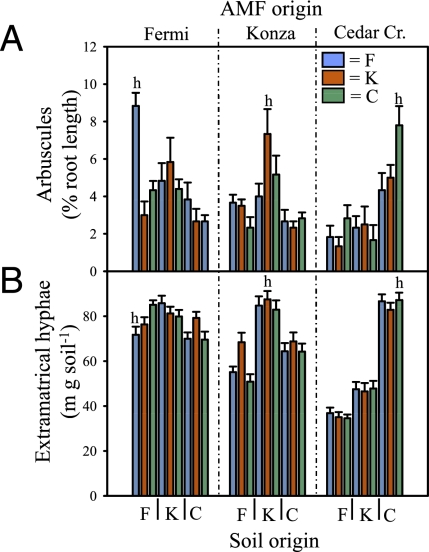
Our findings provide solid evidence that the fitness of plants and their associated AM fungi is greater with coadapted symbionts grown in their local soil compared with those grown in novel combinations. The abundance of home points on the right-hand sides of the scatter plot axes in Figs. 1–3 clearly illustrates a propensity for home-soil advantage in the formation and function of AM symbioses, and the importance of plant genotype origin for arbuscule formation is further illustrated in Fig. 4A. Across all three sites, arbuscule production was highest when fungi were matched with all-home combinations of host plants and soils (letter h in Fig. 4). Recent studies show that AM fungi release signaling molecules, which trigger a series of symbiotic plant genes; this actively prepares the intracellular root environment for colonization and arbuscule formation inside the root cortex (28 –30). Our results indicate that the genetic program of local plant ecotypes responds best to the molecular signals sent by coadapted AM fungal communities. Soils at Fermi and Konza were more similar to each other than to soil at Cedar Creek; nevertheless, the superiority of home-soil inoculum is apparent in soil from Fermi, the most phosphorus-limited site (Figs. 1–3).
Fig. 4.
Mean arbuscular colonization of A. gerardii (A) and extraradical AM hyphae (B) in all possible combinations of soil, plant, and fungal origins. The letter h indicates the all-home combination of soil, plants, and AM fungi. Values are means ± SE (n = 6). Blue, Fermi; red, Konza; green, Cedar Creek.
Production of extraradical hyphae by AM fungi from Cedar Creek and Konza, the two remnant native prairies, also showed strong home-soil fidelity, but hyphal growth was insensitive to host ecotype origin (Fig. 4B). This pattern was not observed for fungi from the reconstructed prairie at Fermi, where the fungi produced equal amounts of extraradical hyphae across all soil types. The absence of home-soil preference of AM fungi at Fermi corresponds to a shorter time for adaptation (28 years versus millennia). Perhaps Fermi AM fungi have lower home-soil fidelity, because the recent agricultural history of this site generated a community of fungi that could forage equally well on a greater diversity of soil conditions.
Another important finding was that tissue N:P was significantly greater in plants inoculated with soil organisms from nitrogen-limited Cedar Creek (N:P = 14.9 ± 0.21) compared with those inoculated with soil organisms from Konza (N:P = 13.0 ± 0.20) or Fermi (N:P = 13.1 ± 0.21). This indicates that the community of AM fungi and other soil organisms in Cedar Creek soil has a superior ability to acquire nitrogen compared with the communities of soil organisms from the phosphorus-limited sites. As with AM hyphal production, this response seems to be entirely driven by soil origin (F 2,156 = 24.6; P < 0.001), inoculum origin (F 2,156 = 24.8; P < 0.001), and an interaction of the two (F 4,156 = 6.9; P < 0.001), whereas plant genotype had no significant effect (F 2,156 = 0.69; P = 0.50).
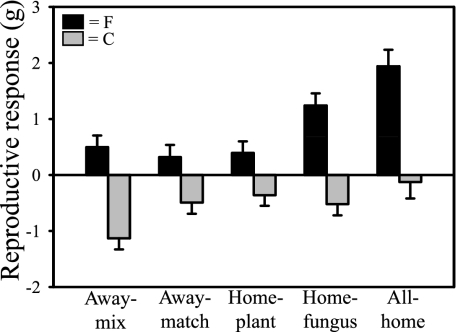
Our results indicate that coadaptation in AM symbioses can maximize mutualism in phosphorus-limited soil and minimize parasitism in phosphorus-rich soil. Response of inflorescence production (i.e., grass fitness) to AM fungi varied among the home and away combinations of soil, fungi, and plant ecotypes in Fermi and Cedar Creek soils, the two sites with the most extreme differences in soil-phosphorus availability. The AM fungi and other soil organisms in all-home combinations were the most beneficial to plants grown in Fermi soil, and they were the least detrimental to plants grown in Cedar Creek soil (Fig. 5). Late successional perennial plants in established grassland swards cannot escape colonization by AM fungi and other soil organisms. Therefore, plant ecotypes that are adapted to phosphorus-rich soils either coadapt with their local community of soil organisms such that parasitism is minimized or receive negative feedbacks between plants and soil organisms that will drive plant community dynamics so that the dominance of these plant ecotypes is reduced (31 –33). The former scenario seems to be occurring in A. gerardii growing in Cedar Creek soil, because their reproductive response to AM colonization with the all-home combination was not significantly different from zero, whereas those grown with the away-mix combination was negative (Fig. 5). This suggests that more parasitic symbioses occurred when novel combinations of plants and AM fungi were grown in Cedar Creek soil.
Fig. 5.
Reproductive response measured as the difference in total inflorescence biomass of A. gerardii grown with and without AM fungi and associated soil organisms. Biomass differences are shown for five mutually exclusive categories of home and away treatment combinations of plants and inoculum from Fermi (black bars) and Cedar Creek (gray bars). Away-mix, soil, plants, and inoculum originated from three different sites; away-match, plants and fungi originated from the same site but from a different location than the soil in which they were grown; home-plant, plants and soil came from the same location (different from the fungi); home-fungus, fungi and soil came from the same location (different from the plants); all-home, soil, plants, and fungi all originated from the same location. Negative values indicate that the nonmycorrhizal plants have larger inflorescences than those inoculated with AM fungi. Values are means ± SE (n = 6).
Because we used whole soils as our inoculum, an alternative hypothesis for our results could be that soil organisms other than AM fungi are responsible for the observed plant-growth responses (33). In an effort to separate mycorrhizal effects from the effects of other microorganisms in the soil inoculum, a microbial wash composed of non-AM microorganisms from all three sites was uniformly applied to the pots at the beginning of the experiment (34). This should help eliminate differences in the non-AM microbial communities across the three inoculum soils; however, any remaining differences in microbial communities does not change the interpretation of our results, because including these unidentified and uncontrolled soil organisms in our experiment strengthens the evidence for local adaptation. In this regard, AM fungi and their associated microbes may adapt with each other and with their local host and local soil environments in the same way that plants and AM fungi adapt over time.
Use of whole-soil inoculum allowed us to explore symbiotic relationships between naturally coexisting complexes of plant ecotypes and their communities of AM fungi and associated soil organisms. A total of 48 different morphospecies of AM fungal spores were observed in our inoculum soils with more than one-quarter of them occurring across all three grasslands (Table S1). Functional differences in the AM fungal communities in the three inoculum soils are most certainly generating the strong home-soil advantage that we observed in the formation of extramatrical hyphae. Previous studies have also shown that AM fungi generally perform best in their endemic soil (35). Schultz et al. (15) established a mycorrhizal mechanism for the adaptation of A. gerardii to its soil environment by showing that an ecotype of A. gerardii native to low-phosphorus soil benefitted more from mycorrhizas than an ecotype from higher phosphorus soil. Because their study used a single community of AM fungal inoculum that was derived from the low-phosphorous site, Schultz et al. (15) could not assess whether or not there is coadaptation between plant populations and communities of AM fungi, and they encouraged future research to explore this possibility. Our reciprocal-inoculation experiment provides evidence for coadaptation between A. gerardii and AM fungi, because across all three systems, arbuscule formation is consistently highest in the all-home combinations of plants, fungi, and soils. Furthermore, arbuscule formation is highly associated with uptake of the nutrient that is most limiting to plant growth (Figs. 1 and 2). Whether or not arbuscule formation is linked to higher plant biomass production depends on the balance of trade between plants and fungi. The density of extraradical hyphae, our surrogate measure of fungal biomass, was positively related to plant fitness in the two phosphorus-limited soils but not in the nitrogen-limited Cedar Creek soil (Fig. 3). Plant and fungal stoichiometry are key factors in the balance of trade in mycorrhizas (26, 36), and they may help explain the differences in mycorrhizal function among the soil types. Most organisms require ∼10× more nitrogen than phosphorus, and fungi have relatively higher nitrogen requirements than plants (37); therefore, it is possible that the high-phosphorus, low-nitrogen soil at Cedar Creek creates a mycorrhizal marketplace in which host plants can barely break even when exchanging carbon for nitrogen with their AM fungal symbionts.
More research is necessary to explore the importance of selection pressures other than availability of nitrogen and phosphorus, such as the possibility that mycorrhizas at Cedar Creek are selected more for their roles in water relations (38) or protection from pathogens (39) than for their role in nitrogen uptake. Because of the lower water-retention capacity of the Cedar Creek soil (sandy texture and low organic-matter content), it is possible that mycorrhizas at Cedar Creek are an important mechanism for increasing plant drought tolerance through an improved transpiration stream (38). There is evidence that coadapted mycorrhizas may improve plant tolerance to water stress; within the same species of AM fungi, ecotypes isolated from xeric ecosystems have been shown to improve the water relations and drought tolerance of their host plants more than those isolated from mesic environments (40, 41).
This study provides solid evidence that the availability of essential soil resources is an important force in the formation of geographic mosaics of symbiotic function in mycorrhizas. Our reciprocal study design allows us to identify the relative importance of plant genotype, fungal community composition, and soil type in generating a full range of symbiotic function from mutualism to parasitism. Geographic variation among plant genotype was an important factor in the formation of arbuscules, the site for carbon and phosphorus exchange, but it was not important to the formation of extraradical hyphae, the foraging structures of AM fungi. These results combined with recent insights about the evolutionary ecology (42) and population structure (43) of Glomeromycota indicate that coadapted complexes of plants, AM fungi, and other associated soil organisms develop over time such that the fitness of both plants and fungi is maximized under local soil conditions. Selection pressures differ for AM fungi and plants. It behooves AM fungi to be generalist foragers (42), and it behooves plant hosts to be more selective in the fungi with which they establish trading partnerships (28 –30). Nutritional mutualisms are environmentally stable interactions when soil phosphorus is strongly limiting to plant growth. In contrast, when soil-resource availability makes mycorrhizal trading partnerships moot, an evolutionary arms race may occur so that plants minimize mycorrhizal parasitism. These findings may represent general mechanisms of evolutionary processes that occur among communities of plants and soil organisms, and they may help define the phenotypes of communities (44).
Our results have direct relevance to sustainable land management and ecosystem-restoration practices, because they stress the importance of the preservation or establishment of edaphic complexes of communities of locally adapted ecotypes of plants and soil microorganisms (45). The current practice of introducing novel genotypes of AM fungi in commercial inoculum may not have the desired outcomes (46) if the soil properties and host genotypes in the inoculated system are very dissimilar to those under which the inoculant fungi were originally selected. Also, our observation that, in the two native prairie sites, AM fungi produced up to 87% more hyphae in their home soil (Fig. 4B) implies that locally adapted fungi have the potential to sequester more carbon and nutrients in their extraradical hyphae than nonadapted fungi (9, 10). In addition to AM-hyphal biomass inputs to the soil-carbon pool, the filamentous hyphae help create a physical framework for stabilizing primary soil particles into larger, relatively stable soil aggregates. By generating these aggregated soil structures, extraradical hyphae increase soil stability and create conditions that favor the protection of detrital inputs (8, 9). Although the contributions of AM hyphae to this mechanism are important for both Fermi and Konza soils, they may play an even more crucial role in the more coarse-textured sandy loam at Cedar Creek (8).
The reduced hyphal densities found in the away-soil combinations emphasize the need for a better understanding of the adaptive processes that contribute to fungal exploitation of the soil environment. A worldwide increase in the cost of chemical fertilizer, as well as a need to decrease chemical-fertilizer runoff, makes it highly desirable to manage AM symbioses to naturally improve or sustain yields of crops and biofuel feedstocks (47). Because of the development of new carbon-negative biofuels, great advancement has been made in identifying and characterizing aboveground traits in prairie grasses relevant to the needs of biofuel feedstocks (48). However, relatively few studies have ventured below ground, primarily because feedstock interests end at the soil surface. This study identifies the importance of local adaption for biomass allocation to mycorrhizal fungal symbionts and suggests that it may be important to identify belowground traits and soil factors that favor symbiotic processes.
Materials and Methods
Seeds of local A. gerardii ecotypes from Cedar Creek, Fermi, and Konza were pregerminated, and 14-day-old seedlings were transplanted into large plastic pots (14 cm tall by 11 cm wide) and filled with 1 kg of sterilized soils from the three prairies. Soils were steam-pasteurized at 80 °C for 2 h and allowed to cool for 72 h to eliminate biotic communities but retain abiotic soil traits. Communities of AM fungi and other soil organisms were added back in a controlled manner by inoculating pots with 20 g of living soil from each of the sites in a complete reciprocal design. The living-soil inoculum was added directly below the seedling roots during transplantation. Differences in fertility among the three types of soil inocula were eliminated by adding 20 g of additional sterilized soil from the two sites that were not used in the AM treatment. Additional pots were established using only sterile soil to create nonmycorrhizal controls for each plant and soil combination. Each of the 54 combinations of treatments and controls were replicated six times for a total of 324 pots. All pots were amended with 60 mL of nonsterile soil sievate, 20 mL from each of the three sites. The sievates were prepared by blending soil and water in a 1:2 ratio and passing the slurry through a 25-μm sieve. The relatively large AM fungal spores were trapped on the sieve, whereas smaller organisms pass through it; this allows for the addition of the majority of soil microbes while excluding AM fungi (34). Plants were grown in a glasshouse at Kansas State University, Manhattan, KS.
Plants were grown during the summer with full sunlight. At the end of 14 weeks, plants were harvested, roots were washed free of soil, and biomass was oven dried at 60 °C for 48 h. Shoot, root, and inflorescence dry mass were measured. Plant reproductive response to mycorrhizas was calculated as the difference between inflorescence biomass in AM colonized plants and the mean of the matching control plants. Extraradical AM hyphae were extracted from 5 g of soil, quantified by the gridline-intercept method, and converted to hyphal lengths (49). Roots were stained with trypan blue and quantified for intraradical arbuscular colonization using the magnified gridline-intersect method (50). Shoot tissue N and P concentrations were determined after wet digestion with sulfuric acid and hydrogen peroxide of dried plant material was ground through a 20-μm mesh. Phosphorus concentrations were determined on the digest using the molybdate-blue method (51), and nitrogen concentrations were determined by a Kjeldahl method with a rapid-flow autoanalyzer.
The relationship between plant-response variables (tissue nitrogen, tissue phosphorus, and shoot dry mass) and fungal-predictor variables (extraradical hyphal density and the proportion of root length colonized by arbuscules) was measured using simple linear regression. Arbuscule colonization was arcsine square root transformed before statistical analysis. Three-factor ANOVA and Tukey HSD tests were applied to the tissue N:P data to assess the amount of variance that can be accounted for by soil origin, AM fungal origin, and host-plant genotype as main effects and all possible interactions. Nonmycorrhizal control plants were analyzed separately from the mycorrhizal plants to assess the importance of mycorrhizas to tissue-nutrient content. Statistical analyses were performed using JMP version 4.0 (52).
Supplementary Material
Acknowledgments
We thank Anita Antoninka for her analysis of the AM fungal-spore communities and Brent Burch and Matthew Lau for their assistance with the statistical analyses. We also thank James Bever, John Klironomos, and Thomas Whitham for their helpful comments on an earlier version of this manuscript. We thank the Cedar Creek and Konza Long-Term Ecological Research (LTER) sites and the Fermi National Laboratory Environmental Research Park for permitting us to collect soil and seed at their research sites. This work was funded by the National Science Foundation Grants DEB-03116136, DEB-0842327, and IBN-9632851 (Konza LTER). R.M.M.’s participation was in part funded by the US Department of Energy, Office of Science, Office of Biological and Environmental Research, Climate Change Research Division under contract DE-AC02-06CH11357 at Argonne, IL.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906710107/DCSupplemental.
References
- 1.Thompson JN. The Geographic Mosaic of Coevolution. Chicago, IL: University of Chicago Press; 2005. [Google Scholar]
- 2.Hoeksema JD, Thompson JN. Geographic structure in a widespread plant-mycorrhizal interaction: Pines and false truffles. J Evol Biol. 2007;20:1148–1163. doi: 10.1111/j.1420-9101.2006.01287.x. [DOI] [PubMed] [Google Scholar]
- 3.Johnson MTJ, Stinchcombe JR. An emerging synthesis between community ecology and evolutionary biology. Trends Ecol Evol. 2007;22:250–257. doi: 10.1016/j.tree.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Johnson NC, Graham JH, Smith FA. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997;135:575–585. [Google Scholar]
- 5.Koide RT. Nutrient supply, nutrient demand and plant response to mycorrhizal infection. New Phytol. 1991;117:365–386. doi: 10.1111/j.1469-8137.1991.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 6.Thrall PH, Hochbert ME, Burdon JJ, Bever JD. Coevolution of symbiotic mutualists and parasites in a community context. Trends Ecol Evol. 2006;22:120–126. doi: 10.1016/j.tree.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Bethlenfalvay GJ, Bayne HG, Pacovsky RS. Parasitic and mutualistic associations between a mycorrhizal fungus and soybean: The effect of phosphorus on host plant-endopyte interactions. Physiol Plant. 1983;57:543–548. [Google Scholar]
- 8.Miller RM, Jastrow JD. Mycorrhizal fungi influence soil structure. In: Kapulnik Y, Douds DD, editors. Arbuscular Mycorrhizas: Physiology and Function. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2000. pp. 3–18. [Google Scholar]
- 9.Zhu Y-G, Miller RM. Carbon cycling by arbuscular mycorrhizal fungi in soil-plant systems. Trends Plant Sci. 2003;8:407–409. doi: 10.1016/s1360-1385(03)00184-5. [DOI] [PubMed] [Google Scholar]
- 10.Wilson GWT, Rice CW, Rillig MC, Springer A, Hartnett DC. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: Results from long-term field experiments. Ecol Lett. 2009;12:452–461. doi: 10.1111/j.1461-0248.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- 11.Klironomos JN. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology. 2003;84:2292–2301. [Google Scholar]
- 12.Hallett SG. Dislocation from coevolved relationships: A unifying theory for plant invasion and naturalization? Weed Sci. 2006;54:282–290. [Google Scholar]
- 13.Wilson GWT, Hartnett DC. Effects of mycorrhizas on plant growth and dynamics in experimental tallgrass prairie microcosms. Am J Bot. 1997;84:478–482. [PubMed] [Google Scholar]
- 14.Hartnett DC, Wilson GWT. The role of mycorrhizas in plant community structure and dynamics: Lessons from the grasslands. Plant Soil. 2002;244:319–331. [Google Scholar]
- 15.Schultz PA, Miller RM, Jastrow JD, Rivetta CV, Bever JD. Evidence of a mycorrhizal mechanism for the adaptation of Andropogon gerardii (Poaceae) to high- and low-nutrient prairies. Am J Bot. 2001;88:1650–1656. [PubMed] [Google Scholar]
- 16.Egerton-Warburton L, Johnson NC, Allen EB. Mycorrhizal community dynamics following nitrogen fertilization: A cross-site test in five grasslands. Ecol Monogr. 2007;77:527–577. [Google Scholar]
- 17.Tilman D. Plant Strategies and the Dynamics and Structure of Plant Communities. Princeton: Princeton University Press; 1988. [Google Scholar]
- 18.Gibson DJ, Seastedt TR, Briggs JH. Management practices in tallgrass prairie: Large- and small-scale experimental effects on species composition. J Appl Ecol. 1993;30:247–255. [Google Scholar]
- 19.Antoninka A, Wolf J, Bowker MA, Classen AT, Johnson NC. Linking above- and belowground responses to global change at community and ecosystem scales. Glob Change Biol. 2009;15:914–929. [Google Scholar]
- 20.Kulmatiski A, Beard KH, Stevens JR, Cobbold SM. Plant-soil feedbacks: A meta-analytical review. Ecol Lett. 2008;11:980–992. doi: 10.1111/j.1461-0248.2008.01209.x. [DOI] [PubMed] [Google Scholar]
- 21.Kardol P, Bezemer TM, Van der Putten WH. Temporal variation in plant-soil feedback controls succession. Ecol Lett. 2006;9:1080–1088. doi: 10.1111/j.1461-0248.2006.00953.x. [DOI] [PubMed] [Google Scholar]
- 22.Smith FA, Grace EJ, Smith SE. More than a carbon economy: Nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytol. 2009;182:347–358. doi: 10.1111/j.1469-8137.2008.02753.x. [DOI] [PubMed] [Google Scholar]
- 23.George E, Marschner H, Jakobsen I. Role of arbuscular mycorrhizal fungi in uptake of phosphorus and nitrogen from soil. Crit Rev Biotechnol. 1995;15:257–270. [Google Scholar]
- 24.Govindarajulu M, et al. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature. 2005;435:819–823. doi: 10.1038/nature03610. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds HL, Hartley AE, Vogelsang KM, Bever JD, Schultz PA. Arbuscular mycorrhizal fungi do not enhance nitrogen acquisition and growth of old-field perennials under low nitrogen supply in glasshouse culture. New Phytol. 2005;167:869–880. doi: 10.1111/j.1469-8137.2005.01455.x. [DOI] [PubMed] [Google Scholar]
- 26.Johnson NC. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 2010 doi: 10.1111/j.1469-8137.2009.03110.x. in press. [DOI] [PubMed] [Google Scholar]
- 27.Koerselman W, Meuleman AFM. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J Appl Ecol. 1996;33:1441–1450. [Google Scholar]
- 28.Bonfante P, Genre A. Plants and arbuscular mycorrhizal fungi: An evolutionary-developmental perspective. Trends Plant Sci. 2008;13:492–498. doi: 10.1016/j.tplants.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Parniske M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat Rev Microbiol. 2008;6:763–775. doi: 10.1038/nrmicro1987. [DOI] [PubMed] [Google Scholar]
- 30.Reinhardt D. Programming good relations—development of the arbuscular mycorrhizal symbiosis. Curr Opin Plant Biol. 2007;10:98–105. doi: 10.1016/j.pbi.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Francis R, Read DJ. The contributions of mycorrhizal fungi to the determination of plant community structure. Plant Soil. 1994;159:11–25. [Google Scholar]
- 32.Bever JD. Soil community feedback and the coexistence of competitors: Conceptual frameworks and empirical tests. New Phytol. 2003;157:465–473. doi: 10.1046/j.1469-8137.2003.00714.x. [DOI] [PubMed] [Google Scholar]
- 33.Klironomos JN. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature. 2002;417:67–70. doi: 10.1038/417067a. [DOI] [PubMed] [Google Scholar]
- 34.Koide RT, Li M. Appropriate controls for vesicular-arbuscular mycorrhizal research. New Phytol. 1989;111:35–44. [Google Scholar]
- 35.Lambert DH, Cole H, Baker DE. Adaptation of vesicular-arbuscular mycorrhizae to edaphic factors. New Phytol. 1980;85:513–540. [Google Scholar]
- 36.Hoeksema JD, Schwartz MW. Expanding comparative-advantage biological market models: Contingency of mutualism on partners’ resource requirements and acquisition tradeoffs. Proc Royal Soc Lond B Bio Sci. 2003;270:913–919. doi: 10.1098/rspb.2002.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sterner RW, Elser JJ. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton: Princeton University Press; 2002. [Google Scholar]
- 38.Auge RM. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza. 2001;11:3–42. [Google Scholar]
- 39.Newsham K, Fitter A, Watkinson AR. Multi-functionality and biodiversity in arbuscular mycorrhizas. Trends Ecol Evol. 1995;10:407–411. doi: 10.1016/s0169-5347(00)89157-0. [DOI] [PubMed] [Google Scholar]
- 40.Bethlenfalvay GJ, Franson RL, Brown MS, Mihara KL. The Glycine-Glomus-Bradyrhizobium symbiosis. IX. Nutritional, morphological and physiological responses of nodulated soybean to geographic isolates of the mycorrhizal fungus Glomus mosseae . Physiol Plant. 1989;76:226–232. [Google Scholar]
- 41.Stahl PD, Smith WK. Effects of different geographic isolates of Glomus on the water relations of Agropyron smithii . Mycologia. 1984;76:261–267. [Google Scholar]
- 42.Helgason T, Fitter AH. Natural selection and the evolutionary ecology of the arbuscular mycorrhizal fungi (phylum Glomeromycota) J Exp Bot. 2009;60:2465–2480. doi: 10.1093/jxb/erp144. [DOI] [PubMed] [Google Scholar]
- 43.Rosendahl S. Communities, populations and individuals of arbuscular mycorrhizal fungi. New Phytol. 2008;178:253–266. doi: 10.1111/j.1469-8137.2008.02378.x. [DOI] [PubMed] [Google Scholar]
- 44.Whitham TG, et al. A framework for community and ecosystem genetics: From genes to ecosystems. Nat Rev Genet. 2006;7:511–523. doi: 10.1038/nrg1877. [DOI] [PubMed] [Google Scholar]
- 45.Harris J. Soil microbial communities and restoration ecology: Facilitators or followers? Science. 2009;325:573–574. doi: 10.1126/science.1172975. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz MW, et al. The promise and the potential consequences of global transport of mycorrhizal fungal inoculum. Ecol Lett. 2006;9:501–515. doi: 10.1111/j.1461-0248.2006.00910.x. [DOI] [PubMed] [Google Scholar]
- 47.Rooney DC, et al. Mycorrhizas and biomass crops: Opportunities for future sustainable development. Trends Plant Sci. 2009;14:542–549. doi: 10.1016/j.tplants.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Tilman D, Hill J, Lehman C. Carbon-negative biofuels from low-input high-diversity grassland biomass. Science. 2006;314:1598–1600. doi: 10.1126/science.1133306. [DOI] [PubMed] [Google Scholar]
- 49.Miller RM, Reinhardt DR, Jastrow JD. External hyphal production of vesicular-arbuscular mycorrhizal fungi in pasture and tallgrass prairie communities. Oecologia. 1995;103:17–23. doi: 10.1007/BF00328420. [DOI] [PubMed] [Google Scholar]
- 50.McGonigle TP, Mille MH, Evans DG, Fairchild GL, Swan JA. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990;115:495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 51.Murphy J, Riley JP. A modified single solution method of the determination of phosphate in natural waters. Anal Chim Acta. 1962;27:31–36. [Google Scholar]
- 52.SAS. JMP Statistical Discovery Software. Cary, NC: SAS Inst.; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.