Abstract
Insect molting and metamorphosis are induced by steroid hormones named ecdysteroids, whose production is regulated by various neuropeptides. We cloned the gene and analyzed the expression of the prothoracicostatic peptide, a unique neuropeptide shown to suppress the production of ecdysteroids in the prothoracic gland of the silkworm, Bombyx mori. We also characterized a Bombyx G protein-coupled receptor, which has previously been identified as an ortholog of the Drosophila sex peptide receptor, as a functional prothoracicostatic peptide receptor. This receptor responded specifically to the prothoracicostatic peptides when examined using a heterologous expression system. The receptor was highly expressed in the prothoracic gland on the day before each larval and pupal ecdysis, when prothoracicostatic peptides are synthesized at a high level in the epiproctodeal glands. These results suggest that the sex peptide receptor functions as a prothoracicostatic peptide receptor in Bombyx and that the peripheral neurosecretory cells as well as the central neuroendocrine system play stage-specific roles in regulating ecdysteroidogenesis.
Keywords: Bombyx mori, G protein-coupled receptor, neuropeptides, steroidogenesis
Ecdysteroids are steroid hormones that play a crucial role in insect development and metamorphosis as molting hormones (1, 2). Their biosynthesis (ecdysteroidogenesis) is regulated by several neuropeptides including the prothoracicotropic hormone (PTTH), which is known to stimulate ecdysteroid production in the prothoracic gland (PG) of the silkworm Bombyx mori as well as of other insects (2 –4). There is also growing evidence that the central neuroendocrine system exerts a prothoracicostatic effect in addition to the tropic effect, thus generating a timely fluctuation of the ecdysteroid titer in the hemolymph during insect development. We previously identified three prothoracicostatic factors: prothoracicostatic peptide (PTSP) (5), bommo-myosuppressin (BMS) (6), and bommo-FMRFamides (BRFas) (7) in Bombyx. PTSP belongs to the W(X)6Wamide peptide family, which shares a -W(X)6Wamide C-terminal sequence motif. These peptides are of widespread occurrence in insects (8 –16) and are also found in crustaceans (17) and in a molluscan species (18). They are also referred to as myoinhibitory peptide (MIP) (8, 9, 13) or allatostatin-B (AST-B) (11, 12), owing to their originally identified biological activities.
To elucidate a specific role of PTSP compared to other prothoracicostatic factors, here we sought the PTSP receptor from among neuropeptide G protein-coupled receptors (GPCRs) expressed in the PG of Bombyx. To our surprise, we found that the GPCR, which was previously reported as the Bombyx sex peptide receptor (SPR) is a functional receptor for PTSP (PTSPR). The expression patterns of PTSPR/SPR suggest a stage-specific role for PTSP in the regulation of ecdysteroidogenesis. This conclusion further supports our working model that various neuropeptides from different neurosecretory cells orchestrate the activity of the PG. In this way, a complex fluctuation of ecdysteroid titer in the hemolymph is achieved during insect development.
Results
Identification and Functional Characterization of PTSP-Related Peptides.
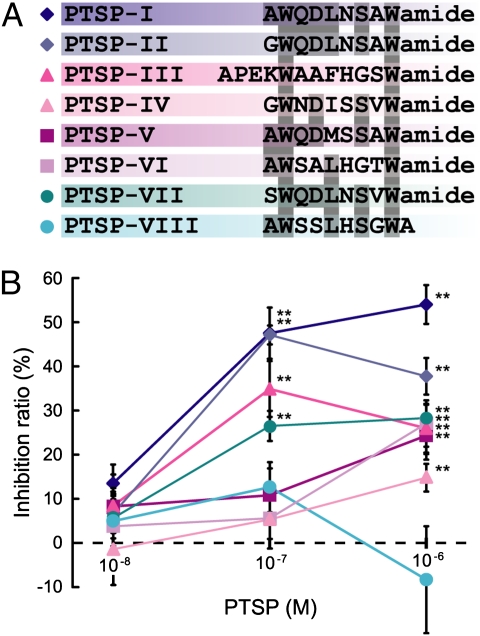
A full-length cDNA clone of PTSP was amplified and sequenced (Fig. S1). Five copies of PTSP and one or two copies of seven PTSP-related peptides were included in the predicted precursor, based on the potential endoproteolytic cleavage sites flanking each peptide and C-terminal amidation sites. The previously identified PTSP was renamed PTSP-I, and the other peptides with identical or similar sequences to Mas-MIPs were named accordingly (Fig. 1A) (10).
Fig. 1.
Structures and prothoracicostatic activities of PTSP-related peptides. (A) Alignment of eight PTSP-related peptides. Amino acid residues conserved in four or more peptides are shaded. Peptides are highlighted in the corresponding colors as in B. (B) Prothoracicostatic activities of PTSPs. The vertical bars represent SE. Statistically significant inhibitions are indicated (**P < 0.01). n = 3–6.
The prothoracicostatic activities of all PTSPs were investigated using the PGs from day 8 fifth-instar larvae (Fig. 1B). PTSP-I showed the strongest prothoracicostatic activity at concentrations of 10−7 M and 10−6 M. This result further confirms the previous finding that PTSP-I displays a prothoracicostatic activity, although somewhat lower than that of BMS. PTSP-II, -III, and -VII were also effective at 10−7 M, whereas the others, except PTSP-VIII, were only effective at 10−6 M. We did not detect any significant prothoracicostatic activity of PTSP-VIII (nonamidated PTSP) at up to 10−6 M.
Localization and Developmental Change of PTSP Gene Expression.
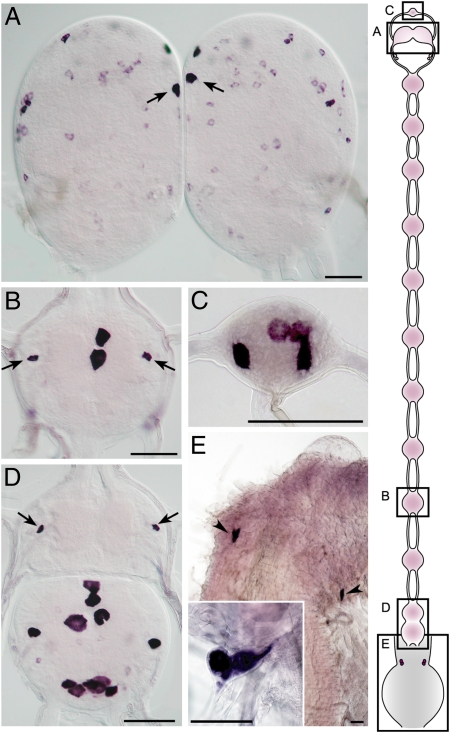
We next performed in situ hybridization to investigate PTSP mRNA localization. PTSP transcripts were detected in numerous cells throughout the central nervous system (Fig. 2 A–D). Among the protocerebral PTSP cells the most prominent signal was observed in a pair of medial neurosecretory cells (Fig. 2A), which have been reported to be immunoreactive to the antiserum against Mas-MIP-I (PTSP-I) and shown to innervate ipsilaterally to the corpora cardiaca (19). Prominent PTSP-expressing cells were also detected in the abdominal ganglia (Fig. 2B), frontal ganglion (Fig. 2C), terminal ganglion (Fig. 2D), and epiproctodeal gland (EPG) (Fig. 2E), the peripheral neurosecretory cells that are located just anterior to the rectum and synthesize and release neuropeptides independent of the central nervous system (19).
Fig. 2.
Expression of PTSP in the nervous system. (A–D) Brain with medial neurosecretory cells (arrows in A), fifth abdominal ganglion with interneuron 704 (IN704; arrows in B), frontal ganglion (C), and terminal ganglion with IN704 (arrows in D) of day 2 fifth-instar larva. (E) Epiproctodeal glands (arrowheads and Inset) of the fourth-instar larva (10 h before ecdysis). (Scale bar, 100 μm.) Schematic illustration of the nervous system is shown on the Right.
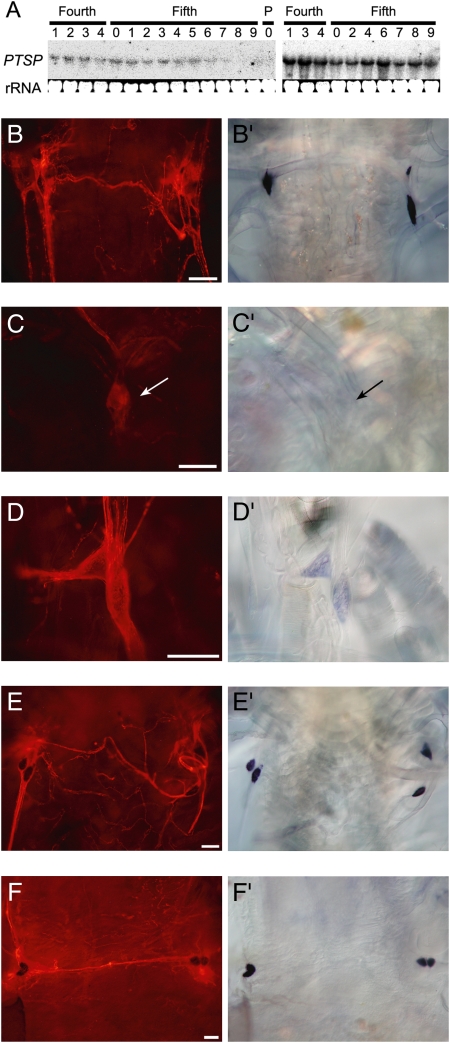
The temporal expression patterns of PTSP in putative endocrine sources were analyzed using Northern and in situ hybridization. An approximate 1.8-kb signal was detected in the brain and the terminal abdominal ganglion, and stronger PTSP expression was observed in the terminal abdominal ganglion rather than in the brain (Fig. 3A). In the brain, PTSP expression was detected during the feeding period but became weaker before each ecdysis (Fig. 3A Left). By contrast, PTSP was constantly expressed in the terminal abdominal ganglion throughout the fourth and fifth instars (Fig. 3A Right).
Fig. 3.
Temporal expression patterns of PTSP. (A) Northern blot analysis of PTSP expression during the fourth and fifth instars in the brain (Left) and the terminal abdominal ganglion (Right). (B–F) Immunostaining and (B′–F′) in situ hybridization (of the epiproctodeal glands. (B and B′) Fifth instar day 0 (just after ecdysis). (C and C′) Fifth instar day 1 (first day of feeding). (D and D′) Fifth instar day 6 (1 day before spinning). (E and E′) Fifth instar day 8 (1 day after the initiation of spinning). (F and F′) Fifth instar day 10 (just before pupal ecdysis). (Scale bar, 100 μm.)
In the EPG, PTSP transcript was strongly expressed in the pharate fifth-instar larvae (Fig. 2E) and just after ecdysis (Fig. 3B′), when the immunostaining was also strong (Fig. 3B). But the transcripts disappeared and the immunostaining was reduced in the gland of day 1 fifth-instar larvae (Fig. 3 C and C′) and the expression remained low until day 6 (just before spinning, Fig. 3 D and D′). Then PTSP was strongly expressed again after the larvae started spinning (Fig. 3E′) and continued to be expressed until day 10 (just before pupal ecdysis, Fig. 3F′). PTSP immunostaining in the EPG appeared to be constant during the spinning period (Fig. 3 E and F).
Identification of Bombyx SPR as the Functional PTSP Receptor.
The expression analyses showed that PTSP is expressed in different temporal manners in distinct endocrine sources, making it difficult to predict at which stage these neuropeptides are required in the PG. Therefore we next sought to identify the receptor for PTSP from among orphan neuropeptide GPCRs encoded within the Bombyx genome (20).
We first examined some candidate neuropeptide GPCRs based either on the features of Drosophila orthologs (21, 22, 23) or high expression in the PG, but none of them responded to any PTSPs in our heterologous expression system (Table 1). We then focused on a newly identified Drosophila neuropeptide GPCR, CG16752 (24). This receptor has recently been characterized by Yapici et al. as the receptor for the Drosophila sex peptide (SP) (23). The authors reported that the Bombyx SPR (Bombyx ortholog of SPR) can respond to Drosophila SP, although the Bombyx counterpart of Drosophila SP has not been found in the Bombyx genome (25). Hence, the endogenous ligand for Bombyx SPR remains to be identified.
Table 1.
Bombyx neuropeptide GPCRs analyzed in this study
| GPCR name | Drosophila orthologs (ligands) | PG Expression | Functional features and characteristics |
| BNGR-A15 | CG14484 [MIP/AST-B] (21) | No (20) | No response to PTSPs at up to 10−5 M |
| BNGR-A14 | CG14593 | No (20) | No response to PTSPs at up to 10−5 M; BNGR-A15 paralog |
| BNGR-B4 | No obvious ortholog | Yes (20) | No response to PTSPs, PTTH, BMS, or BRFas at up to 10−5 M |
| BMSR | CG8985, CG13803 [DMS] (21, 22) | Yes (20) | No response to PTSPs; high and moderate response to BMS and BRFas, respectively (6, 7) |
| SPR (PTSPR) | CG16752 [SP] (23) | Yes | High response to PTSPs |
DMS, dromyosuppressin. Expression analysis and functional characterizations were conducted in this study unless specifically referenced.
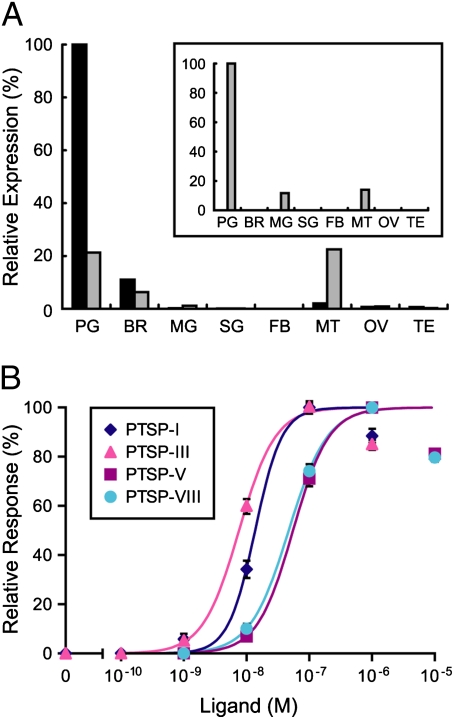
We performed quantitative RT-PCR analysis of SPR in various tissues. Surprisingly, we found that SPR is highly expressed in the PG (Fig. 4A), especially on the last day of the fourth instar. In contrast, BMS receptor (BMSR) was not detected in any tissue at this stage (Fig. 4A Inset).
Fig. 4.
Identification of the PTSP receptor. (A) Tissue specificity of PTSPR/SPR expression analyzed by quantitative RT-PCR. For comparison, BMSR expression pattern is also presented (Inset). Transcript levels of the GPCR genes are presented as relative expression levels, which are normalized for each gene. Expression levels in the fourth instar head capsule slippage period are indicated in black, whereas those in the fifth instar day 2 are indicated in gray. PG, prothoracic gland; BR, brain; FB, fat body; MG, midgut; SG, silk gland; MT, Malpighian tubule; OV, ovary; and TE, testis. (B) Functional characterization of Bombyx PTSPR/SPR. Ca2+ imaging analysis was performed using HEK293 cells expressing PTSPR/SPR. Dose–response curves for PTSP-I, -III, -V, and -VIII are shown. Vertical bars represent SE. Each datum point was calculated from the responses of 150–165 cells in three independent experiments.
Various neuropeptides having stimulatory or inhibitory effects on the PG were tested for their activation of the SPR using our heterologous expression system. Remarkably, HEK293 cells expressing Bombyx SPR responded to all PTSPs at a concentration of 10−7 M, whereas they did not respond to other neuropeptides tested at concentrations up to 10−5 M (PTTH, BMS, and BRFas). The dose–response of SPR to PTSPs was further investigated (Fig. 4B). Of the four PTSPs tested, PTSP-I and -III stimulated SPR at lower concentrations (EC50 values: 13.7 nM and 7.6 nM, respectively), whereas PTSP-V and -VIII showed weaker activities (EC50 values: 55.3 nM and 47.1 nM, respectively). These results are consistent with the relative prothoracicostatic activities of these PTSPs (Fig. 1B), except that PTSP-VIII had no detectable prothoracicostatic activity on the PG in vitro. This may be attributed to the instability of this nonamidated peptide during in vitro PG incubation (3 h). Hereafter, we call this receptor Bombyx PTSPR/SPR.
Temporal Expression Patterns of Neuropeptide GPCR Genes in the PG.
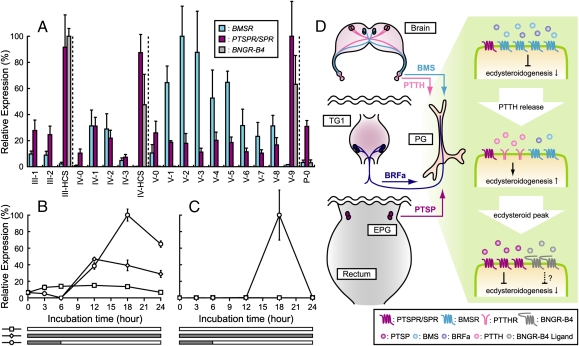
We then examined the expression levels of PTSPR/SPR along with two other neuropeptide GPCR genes in the PG during development. PTSPR/SPR is highly expressed during each head capsule slippage period and on the last day of the spinning stage (i.e., one day before each ecdysis), but only weakly expressed during each intermolt period (Fig. 5A). This expression pattern markedly contrasts with that of BMSR, which is highly expressed in each intermolt feeding stage but not on the day before ecdysis. Our findings clearly suggest that PTSPs are mainly required immediately before or after each ecdysis when there is a dramatic reduction of ecdysteroid titer. We also found that Bombyx neuropeptide GPCR-B4 (BNGR-B4) was expressed in the PG almost exclusively before each ecdysis (Fig. 5A) (20), suggesting the existence of at least one more ligand involved in the regulation of PGs at this period.
Fig. 5.
Expression analyses of neuropeptide GPCR genes in the PG. (A) Developmental expression profile of three neuropeptide GPCRs in the PG from third instar to the first day of the pupal stage. Transcript levels of the GPCR genes are presented as relative expression levels, which are normalized for each gene. Vertical bars represent SE n = 3 batches. HCS, head capsule slippage. (B and C) Effect of 20E on the expression of PTSPR/SPR (B) and BNGR-B4 (C) in the PG in vitro. Lower rectangles in each panel indicate the period of 20E treatment (gray). Vertical bars represent SE n = 3 batches. (D) Current working model on the temporal regulation of the PG activity by various neuropeptides. BMS and BRFa act on BMSR during feeding stage to suppress ecdysteroidogenesis. When enough PTTH is released from the brain, an event probably coupled with the downregulation of BMSR signaling, ecdysteroidogenesis is upregulated to generate an ecdysteroid surge in hemolymph. After the decline of ecdysteroid titer, PTSP and probably BNGR-B4 ligand further suppress ecdysteroidogenesis. TG1, prothoracic ganglion; PTTHR, PTTH receptor. Molecular identity of BNGR-B4 ligand remains unknown.
Regulation of Neuropeptide GPCR Gene Expression in the PG by 20-Hydroxyecdysone.
It is intriguing to hypothesize that the decline of ecdysteroid titer before each ecdysis is mediated by PTSPs. However, such inactivation of the PG has been attributed to the negative feedback regulation by 20-hydroxyecdysone (20E) (26). To examine the putative interaction between these two pathways, we next investigated the regulation of PTSPR/SPR expression by 20E.
When day 2 fourth-instar (i.e., one day before the ecdysteroid peak) larval PGs were incubated for 24 h, the expression level of PTSPR/SPR remained low throughout this incubation period (Fig. 5B). In contrast, when the PGs were incubated with 20E, the expression decreased to an undetectable level after the first 6 h, followed by a sudden increase in the next 6 h. Interestingly, when 20E was removed from the incubation medium after the first 6 h, this increase continued for 12 h, giving the highest expression level after 18 h of total incubation. Taken together, these results show that 20E can induce the expression of PTSPR/SPR in the PG in vitro. Indeed, this effect can be enhanced if 20E is removed from the medium, which mimics the rapid decline of ecdysteroid titer in vivo. Therefore, it is likely that the rapid decline of ecdysteroid titer in hemolymph precedes the full upregulation of PTSPR/SPR expression in the PG, making it improbable that PTSPs are involved in the initial step of this event in vivo. This pattern of expression was much clearer for BNGR-B4, whose expression was transiently upregulated only after the removal of 20E from the medium in the same experiment (Fig. 5C). These results are consistent with their expression patterns in the PG in vivo, where PTSPR/SPR transcript can be detected at a moderate level in the PG during the intermolt period, whereas that of BNGR-B4 is present almost exclusively on the day before each ecdysis (Fig. 5A).
Discussion
The importance of the negative regulation of ecdysteroidogenesis has been recognized (27 –30). However, the molecular features of such prothoracicostatic factors were unknown until the identification of PTSP in Bombyx (5). Thereafter, we further identified two prothoracicostatic factors in Bombyx: BMS and BRFas (6, 7). These findings led to the working model that different neurosecretory cells in the central nervous system exert prothoracicostatic effects in response to different cues, thus creating the finely tuned fluctuation of ecdysteroid titer in hemolymph during insect development (Fig. 5D). However, the identification of these physiologically more relevant prothoracicostatic factors raised questions concerning the role of PTSP in the regulation of ecdysteroidogenesis in Bombyx.
To answer this question, we identified a unique functional PTSPR in Bombyx, and elucidated its expression profile. The expression analysis of the receptor in the PG suggested that PTSP acts during or after the rapid decline of ecdysteroid titer before each ecdysis. Because our in vitro PG incubation assay suggested that the decline of the ecdysteroid titer causes upregulation of PTSP receptor expression, PTSP is unlikely to be involved in the initial step of this event. PTSP is probably involved either in full inactivation or in the suppression of reactivation of the PG to finely regulate the ecdysteroid titer in hemolymph.
Interestingly, massive neurohemal release of MIP/PTSP from the EPG has been suggested to occur at the same stage (i.e., before ecdysis) in both Bombyx and another lepidopteran insect, Manduca sexta (19). We also investigated the temporal expression profiles of PTSP in various neurosecretory systems in Bombyx and detected the upregulated expression of PTSP in the EPG during this period, consistent with the pioneering work (19). On the other hand, expression of PTSP in the central nervous system did not correlate with receptor expression in the PG. Taken together, our findings suggest a stage-specific role of PTSP in the suppression of ecdysteroidogenesis during each ecdysis and further expand our current model in that not only central but peripheral neuroendocrine systems have distinct roles in the regulation of ecdysteroid titer in hemolymph (see Fig. 5D for our current working model). It is also important to note here that PTSP is a multifunctional neuropeptide and thus likely to have other physiological roles during ecdysis (31).
In this study, a neuropeptide GPCR previously reported as the ortholog of Drosophila SPR (23) was identified as the PTSP receptor using a heterologous expression system. This result is also supported by the absence of SP in the Bombyx genome, which indicates the existence of different neuropeptide ligand(s) for SPR. Evolutionary conservation of PTSP and SPR also favors their ligand-receptor relationship, because both PTSP and SPR have been identified in almost all sequenced insect genomes (except two hymenopteran species Apis mellifera and Nasonia vitripennis) as well as in a molluscan species (15, 16, 23, 32). Such paired conservation of ligand and receptor genes in diverse species can be observed in a number of cases (e.g., kinin, corazonin, and allatostatin-A) (24).
Combined with the previous characterization of this receptor as a functional SP receptor (23), we speculate that PTSP/MIP/AST-B neuropeptides are the ancestral ligands for SPR, whereas SP has evolved as an additional ligand for PTSPR/SPR in certain orders of insect species, where a developed behavioral control is required. The unexpected molecular identity of the PTSPR/SPR will broaden our knowledge concerning the physiological role of PTSP/MIP/AST-B neuropeptides, and may serve as an ideal model to study ligand–receptor coevolution theory among different organisms (33).
Materials and Methods
Insects.
A Bombyx racial hybrid, Kinsyu × Showa, was used for the expression analysis of PTSPR/SPR. All of the other experiments in this study used the racial hybrid, C145 × N140. Larvae were reared at 25 °C under a photoperiodic regime of 12L/12D on a standard artificial diet (Nihon Nosan Kogyo). Under these conditions Kinsyu × Showa started spinning on day 6 of the fifth instar (one day earlier than C145 × N140).
Peptide Synthesis.
PTSPs were synthesized on a 9050 Plus PepSynthesizer System (PerSeptive Biosystems) using Fmoc [N-(9-fluorenyl)methoxycarbonyl] chemistry according to the manufacturer’s instructions. The crude synthetic peptides were purified by HPLC to a purity exceeding 95%.
In Vitro Bioassay for Prothoracicostatic Activity.
The in vitro PG bioassay was conducted as described (5). Amounts of ecdysteroids secreted by the PG into the medium were determined by RIA. The inhibition ratio, expressed as a percentage, was calculated using the following equation: inhibition ratio = {1 − (amount of ecdysteroids in experimental/amount of ecdysteroids in control)} × 100. Student’s t test was used to evaluate the significant inhibition of ecdysteroidogenesis.
Northern Hybridization.
Total RNA was isolated from each tissue using ISOGEN (Nippongene). Five micrograms of total RNA was denatured, separated on a guanidine thiocyanate 1% agarose gel (34), and transferred to Hybond-NX nylon membrane (GE Healthcare). The blots were hybridized with the PTSP-specific probe (positions 64–720; Fig. S1). The PTSP-specific DNA probe was prepared by the random labeling reaction with [α-32P]dATP using a Strip-EZ DNA Kit (Ambion). Hybridization, washing, and stripping of blots were performed according to the manufacturer’s instructions. Signals were detected by an image analyzer, BAS2500 (Fujifilm).
Production of Monoclonal Antibody Against PTSP.
Synthetic PTSP-I was conjugated to BSA or ovalbumin through carbodiimide coupling. Mice were immunized with the PTSP-I–BSA conjugate and their splenocytes collected and fused with NS-I cells. The hybridomas were then cultured following a previously published method (35). Hybridoma culture supernatants were primarily screened by ELISA using the PTSP-I–ovalbumin conjugate as an antigen, and the positive wells were next screened by immunohistochemistry. Antibodies that specifically labeled a pair of medial neurosecretory cells (based on Fig. 2A and ref. 19) were selected, and six hybridomas producing these antibodies were subsequently cloned and mass cultured. Monoclonal antibodies in the culture supernatant were purified using a protein A column and stored at a concentration of 1 mg/mL. One of the monoclonal antibodies, 1A4, was used for immunohistochemical detection of PTSPs.
In Situ Hybridization and Immunohistochemistry.
Based on the cDNA sequence (Fig. S1) primers for the digoxigenin-labeled probe synthesis were designed as follows: sense primer, 5′-GGTGTTTATTCGCGCTGTG-3′; antisense primer, 5′-TAGGAGCCTGCTGGTAAGGA-3′. The 696-bp amplicon was reamplified using PCR Dig Probe synthesis kit (Roche Applied Science) to synthesize the digoxigenin-labeled antisense DNA probe. The dissected tissues were fixed in 4% paraformaldehyde at 4 °C overnight and subjected to in situ hybridization procedure as described (36).
PTSP was detected using a monoclonal antibody (1A4) applied at a dilution of 1:1,000. The bound antigen-primary antibody complex was visualized with 1:1,000 diluted Alexa Fluor 555-labeled goat anti-mouse IgG (Molecular Probes). A compound microscope, Leica MZ16 and Leica DMLB microscopes with Leica DC300 digital camera (Leica Microsystems), and fluorescent microscope Nikon Eclipse 600 with Nomarski DIC optics and attached Nikon Coolpix 990 digital camera (Nikon) were used for detection.
Quantitative RT-PCR Analysis.
For quantitative RT-PCR analysis, cDNAs were prepared from various tissues of larvae at distinct stages. Quantitative RT-PCR was performed on a Smart Cycler System (Cepheid) as described previously (36). After 1 min at 95 °C, 40 cycles (95 °C for 10 s and 68 °C for 20 s) were carried out for the amplification of neuropeptide GPCRs. PTSPR/SPR, BMSR, and BNGR-B4 specific primers used are as follows: PTSPR/SPR sense primer, 5′-GAAACCACTACAAGCCGCTAAGTCC-3′; PTSPR/SPR antisense primer, 5′-TATCTCTGGACGGCTAAGGCTAACG-3′; BMSR sense primer, 5′-GCAGGCGTTATTTGGCTTACTGAC-3′; BMSR antisense primer, 5′-TTGTGGAAGTGGCAGGACCTTTC-3′; BNGR-B4 sense primer, 5′-GGAACAGAACGATGCTGGATGG-3′; BNGR-B4 antisense primer, 5′-CGGCTTCATTCGGCTGATTTG-3′. Serial dilutions of plasmids containing cDNAs of PTSPR/SPR, BMSR, BNGR-B4, and ribosomal protein L3 were used for standards, and the transcript levels of PTSPR/SPR, BMSR, and BNGR-B4 were normalized with ribosomal protein L3 levels in the same samples.
HEK293 Cell Expression and Ca2+ Imaging Analysis of PTSPR/SPR.
The ORF of PTSPR/SPR was amplified by PCR using PG cDNA. The specific primers had the following sequences: the sense primer 5′-AATCTCGAGGCCACCATGGCGGTCACC-3′ and the antisense primer 5′-CCGACTAGTTTAGAGCACAGTTTCG-3′. The sense primer incorporates a XhoI restriction site and the Kozak sequence (GCCACC) (37), whereas the antisense primer contains an SpeI restriction site. The amplified product was cloned into the pME18S mammalian expression vector and transfected into HEK293 cells with the promiscuous G protein Gα15. Ca2+ imaging analysis was performed as reported previously (20).
Administration of 20E to the PG in Vitro.
PGs were dissected from day 2 fourth-instar larvae in sterile saline and preincubated in 100 μL of Grace’s Insect Medium for 15 min. The glands were subsequently transferred into 100 μL of medium with or without 2 μg/mL of 20E and further incubated for the indicated period. When the glands were transferred from the medium containing 20E to the one without 20E, they were washed thoroughly in 100 μL of Grace’s Insect Medium before transfer. PGs were incubated independently and a total RNA was prepared from six glands for each treatment. The whole experiment was repeated three times, resulting in three total RNAs for each treatment. These total RNAs were converted into cDNA independently. Quantitative RT-PCR was performed as described above.
Supplementary Material
Acknowledgments
We thank Drs. Erik C. Johnson, Young-Joon Kim, and Barry J. Dickson for sharing unpublished results; Honoo Satake for peptide synthesis; and Michael B. O’Connor for generous support. N.Y. was a research fellow of the Japan Society for the Promotion of Science (JSPS). This work was supported by the Enhancement of Center of Excellence (Y.T. and Y-J.H); Grants-in-Aid for Scientific Research from JSPS (to Y.T. and A.M., Grant 21380040; and to H.K., Grant 19380034); Japan-France bilateral Cooperating Program by JSPS and the Program for Promotion of Basic Research Activities for Innovative Biosciences (H.K.); Slovak grant agencies, Agentúra na podporu výskumu a vývoja (APVV-51-039105) and Vedecká grantová agentúra (VEGA 2-6090-26) (to L.R.); and a grant from the Natural Science Foundation of China (to Y.-J.H.). Y.-J.H. was supported by a grant of guest professorship from the University of Tokyo, Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AB073553).
This article contains supporting information online at www.pnas.org/cgi/content/full/0907471107/DCSupplemental.
References
- 1.Redfern CPF. In: Ecdysone. Koolman J, editor. New York: Thieme; 1989. pp. 182–187. [Google Scholar]
- 2.Gilbert LI, Rybczynski R, Warren JT. Control and biochemical nature of the ecdysteroidogenic pathway. Annu Rev Entomol. 2002;47:883–916. doi: 10.1146/annurev.ento.47.091201.145302. [DOI] [PubMed] [Google Scholar]
- 3.Ishizaki H, Suzuki A. The brain secretory peptides that control moulting and metamorphosis of the silkmoth, Bombyx mori . Int J Dev Biol. 1994;38:301–310. [PubMed] [Google Scholar]
- 4.McBrayer Z, et al. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila . Dev Cell. 2007;13:857–871. doi: 10.1016/j.devcel.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hua YJ, et al. Identification of a prothoracicostatic peptide in the larval brain of the silkworm, Bombyx mori . J Biol Chem. 1999;274:31169–31173. doi: 10.1074/jbc.274.44.31169. [DOI] [PubMed] [Google Scholar]
- 6.Yamanaka N, et al. Identification of a novel prothoracicostatic hormone and its receptor in the silkworm Bombyx mori . J Biol Chem. 2005;280:14684–14690. doi: 10.1074/jbc.M500308200. [DOI] [PubMed] [Google Scholar]
- 7.Yamanaka N, et al. Regulation of insect steroid hormone biosynthesis by innervating peptidergic neurons. Proc Natl Acad Sci USA. 2006;103:8622–8627. doi: 10.1073/pnas.0511196103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoofs L, Holman GM, Hayes TK, Nachman RJ, De Loof A. Isolation, identification and synthesis of locustamyoinhibiting peptide (LOM-MIP), a novel biologically active neuropeptide from Locusta migratoria . Regul Pept. 1991;36:111–119. doi: 10.1016/0167-0115(91)90199-q. [DOI] [PubMed] [Google Scholar]
- 9.Blackburn MB, et al. The identification of two myoinhibitory peptides, with sequence similarities to the galanins, isolated from the ventral nerve cord of Manduca sexta . Regul Pept. 1995;57:213–219. doi: 10.1016/0167-0115(95)00034-9. [DOI] [PubMed] [Google Scholar]
- 10.Blackburn MB, Jaffe H, Kochansky J, Raina AK. Identification of four additional myoinhibitory peptides (MIPs) from the ventral nerve cord of Manduca sexta . Arch Insect Biochem Physiol. 2001;48:121–128. doi: 10.1002/arch.1064. [DOI] [PubMed] [Google Scholar]
- 11.Lorenz MW, Kellner R, Hoffmann KH. A family of neuropeptides that inhibit juvenile hormone biosynthesis in the cricket, Gryllus bimaculatus . J Biol Chem. 1995;270:21103–21108. doi: 10.1074/jbc.270.36.21103. [DOI] [PubMed] [Google Scholar]
- 12.Lorenz MW, Kellner R, Hoffmann KH, Gäde G. Identification of multiple peptides homologous to cockroach and cricket allatostatins in the stick insect Carausius morosus . Insect Biochem Mol Biol. 2000;30:711–718. doi: 10.1016/s0965-1748(00)00042-4. [DOI] [PubMed] [Google Scholar]
- 13.Predel R, Rapus J, Eckert M. Myoinhibitory neuropeptides in the American cockroach. Peptides. 2001;22:199–208. doi: 10.1016/s0196-9781(00)00383-1. [DOI] [PubMed] [Google Scholar]
- 14.Williamson M, et al. Molecular cloning, genomic organization, and expression of a B-type (cricket-type) allatostatin preprohormone from Drosophila melanogaster . Biochem Biophys Res Commun. 2001;281:544–550. doi: 10.1006/bbrc.2001.4402. [DOI] [PubMed] [Google Scholar]
- 15.Riehle MA, Garczynski SF, Crim JW, Hill CA, Brown MR. Neuropeptides and peptide hormones in Anopheles gambiae . Science. 2002;298:172–175. doi: 10.1126/science.1076827. [DOI] [PubMed] [Google Scholar]
- 16.Li B, et al. Genomics, transcriptomics, and peptidomics of neuropeptides and protein hormones in the red flour beetle Tribolium castaneum . Genome Res. 2008;18:113–122. doi: 10.1101/gr.6714008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu Q, et al. Hormone complement of the Cancer productus sinus gland and pericardial organ: an anatomical and mass spectrometric investigation. J Comp Neurol. 2005;493:607–626. doi: 10.1002/cne.20773. [DOI] [PubMed] [Google Scholar]
- 18.Moroz LL, et al. Neuronal transcriptome of aplysia: Neuronal compartments and circuitry. Cell. 2006;127:1453–1467. doi: 10.1016/j.cell.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis NT, Blackburn MB, Golubeva EG, Hildebrand JG. Localization of myoinhibitory peptide immunoreactivity in Manduca sexta and Bombyx mori, with indications that the peptide has a role in molting and ecdysis. J Exp Biol. 2003;206:1449–1460. doi: 10.1242/jeb.00234. [DOI] [PubMed] [Google Scholar]
- 20.Yamanaka N, et al. Neuropeptide receptor transcriptome reveals unidentified neuroendocrine pathways. PLoS One. 2008;3:e3048. doi: 10.1371/journal.pone.0003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson EC, et al. Identification of Drosophila neuropeptide receptors by G protein-coupled receptors-beta-arrestin2 interactions. J Biol Chem. 2003;278:52172–52178. doi: 10.1074/jbc.M306756200. [DOI] [PubMed] [Google Scholar]
- 22.Egerod K, et al. Molecular cloning and functional expression of the first two specific insect myosuppressin receptors. Proc Natl Acad Sci USA. 2003;100:9808–9813. doi: 10.1073/pnas.1632197100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451:33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- 24.Hauser F, et al. A genome-wide inventory of neurohormone GPCRs in the red flour beetle Tribolium castaneum . Front Neuroendocrinol. 2008;29:142–165. doi: 10.1016/j.yfrne.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Roller L, et al. The unique evolution of neuropeptide genes in the silkworm Bombyx mori . Insect Biochem Mol Biol. 2008;38:1147–1157. doi: 10.1016/j.ibmb.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Takaki K, Sakurai S. Regulation of prothoracic gland ecdysteroidogenic activity leading to pupal metamorphosis. Insect Biochem Mol Biol. 2003;33:1189–1199. doi: 10.1016/j.ibmb.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Carlisle DB, Ellis PE. Hormonal inhibition of the prothoracic gland by the brain in locusts. Nature. 1968;220:706–707. doi: 10.1038/220706a0. [DOI] [PubMed] [Google Scholar]
- 28.Mala J, Granger NA, Sehnal F. Control of prothoracic gland activity in larvae of Galleria mellonella . J Insect Physiol. 1977;23:309–316. [Google Scholar]
- 29.Budd E, Käuser G, Koolman J. On the control of ecdysone biosynthesis by the central nervous system of blowfly larvae. Arch Insect Biochem Physiol. 1993;23:181–197. doi: 10.1002/arch.940230405. [DOI] [PubMed] [Google Scholar]
- 30.Hua YJ, Jiang RJ, Koolman J. Multiple control of ecdysone biosynthesis in blowfly larvae: Interaction of ecdysiotropins and ecdysiostatins. Arch Insect Biochem Physiol. 1997;35:125–134. [Google Scholar]
- 31.Kim YJ, et al. Central peptidergic ensembles associated with organization of an innate behavior. Proc Natl Acad Sci USA. 2006;103:14211–14216. doi: 10.1073/pnas.0603459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hummon AB, et al. From the genome to the proteome: Uncovering peptides in the Apis brain. Science. 2006;314:647–649. doi: 10.1126/science.1124128. [DOI] [PubMed] [Google Scholar]
- 33.Park Y, Kim YJ, Adams ME. Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin, and AKH supports a theory of ligand-receptor coevolution. Proc Natl Acad Sci USA. 2002;99:11423–11428. doi: 10.1073/pnas.162276199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goda SK, Minton NP. A simple procedure for gel electrophoresis and northern blotting of RNA. Nucleic Acids Res. 1995;23:3357–3358. doi: 10.1093/nar/23.16.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizoguchi A, et al. A monoclonal antibody against a synthetic fragment of bombyxin (4K-prothoracicotropic hormone) from the silkmoth, Bombyx mori: Characterization and immunohistochemistry. Mol Cell Endocrinol. 1987;51:227–235. doi: 10.1016/0303-7207(87)90032-3. [DOI] [PubMed] [Google Scholar]
- 36.Shinoda T, Itoyama K. Juvenile hormone acid methyltransferase: A key regulatory enzyme for insect metamorphosis. Proc Natl Acad Sci USA. 2003;100:11986–11991. doi: 10.1073/pnas.2134232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozak M. The scanning model for translation: An update. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.