Abstract
Abscisic acid (ABA) is a ubiquitous phytohormone involved in many developmental processes and stress responses of plants. ABA moves within the plant, and intracellular receptors for ABA have been recently identified; however, no ABA transporter has been described to date. Here, we report the identification of the ATP-binding cassette (ABC) transporter Arabidopsis thaliana Pleiotropic drug resistance transporter PDR12 (AtPDR12)/ABCG40 as a plasma membrane ABA uptake transporter. Uptake of ABA into yeast and BY2 cells expressing AtABCG40 was increased, whereas ABA uptake into protoplasts of atabcg40 plants was decreased compared with control cells. In response to exogenous ABA, the up-regulation of ABA responsive genes was strongly delayed in atabcg40 plants, indicating that ABCG40 is necessary for timely responses to ABA. Stomata of loss-of-function atabcg40 mutants closed more slowly in response to ABA, resulting in reduced drought tolerance. Our results integrate ABA-dependent signaling and transport processes and open another avenue for the engineering of drought-tolerant plants.
Keywords: abscisic acid transporter, drought resistance, guard cell
In both animals and plants, hormones play essential roles in the regulation of growth, development, and environmental response, and they are circulated throughout the organism in part by the extracellular fluid. Two plant hormones known to be transported over long distances, auxin and abscisic acid (ABA), are weak acids, and thus, they exist in either protonated, uncharged forms (e.g., ABAH) or in anionic forms (e.g., ABA-) depending on the prevailing pH relative to their pKa. Because the uncharged forms of these molecules can permeate the cell membrane, it was historically assumed that this diffusive process would obviate a requirement for specific uptake transporters for these hormones (1). However, recent progress in the fields of auxin transport and signaling has established that auxin is in fact transported into plant cells by multiple influx carriers that are intricately regulated. Auxin carriers, including AUX1, LAX3, and several ATP-binding cassette (ABC) transporters (2), have been shown to be integral to auxin regulation of both developmental processes, such as lateral root emergence, and environmental responses, such as gravitropism (3, 4). However, comparable progress has not been made in the field of ABA transport, despite the fact that analyses of the kinetics of ABA uptake suggest that such uptake does not occur solely by a diffusive process (5 –8).
ABA regulates seed germination and seedling growth, and it is required for plant resistance to drought and other abiotic and biotic stresses such as salinity and pathogen infection. During drought, ABA levels increase dramatically in plants (9). ABA is perceived by guard cells, which respond so as to minimize loss of water through transpiration. Each pair of guard cells in the epidermis delineates a stomatal pore through which both carbon-dioxide uptake and transpirational-water loss occur (10, 11). Stomata generally open in response to light, low CO2 concentrations, and high atmospheric humidity and close in response to darkness, high CO2 concentrations, low humidity, and stress-induced ABA. Although secondary messengers and effectors of the ABA signal in guard cells have been studied extensively (11), the initial steps of ABA perception within plant cells are just beginning to be understood.
Recently, soluble intracellular receptors for ABA (12, 13) have been identified. The intracellular localization of these ABA sensors highlights the importance of ABA uptake into the cell for cellular signaling processes to occur, and it suggests the potential importance of an ABA transporter that could deliver ABA in a regulated fashion to initiate rapid and controlled responses to the various stress conditions that are perceived by ABA. We postulated that an ABA transporter would be particularly relevant during stress conditions, because such conditions are known to elevate extracellular pH (8); this pH increase, in turn, causes ABAH to dissociate into its charged form, which cannot passively diffuse across the lipid bilayer, in contradiction to the need to rapidly deliver the stress hormone into the cell to elicit a timely response.
The ABC protein family is one of the largest, and members of this family are found in all phyla (14). Most ABC proteins are integral membrane proteins, and they act as ATP-driven transporters for a very wide range of substrates, including lipids, drugs, heavy metals, and auxin (15). In plants, several reports have shown that mutation of various ABC proteins results in impaired stomatal movement. Deletion of AtMRP5/ABCC5 results in impaired ABA and Ca2+ signaling and reduced anion-channel activity (16). AtABCB14 is highly expressed in guard cells and functions as a malate importer that modulates the stomatal response to CO2 (17). Here, we show that an ABC transporter arabidopsis thaliana pleiotropic drug resistance transporter PDR12 (AtPDR12)/ABCG40 is a plasma-membrane ABA-uptake transporter in guard cells and other types of plant cells. AtPDR12/ABCG40 is necessary for timely closure of stomata in response to drought stress as well as for normal seed germination and lateral root development. From this knowledge, we identified a transporter that mediates the uptake of the phytohormone ABA into plant cells.
Results
Screening of Potential ABA Transporters.
ABA is a sesquiterpene derived from the tetraterpene neoxanthine. The PDR/ABCG subfamily of plant ABC transporters has been reported to transport terpenoids (15, 18). PDR-type ABC transporters have also been reported to be involved in responses to pathogens (19) and a broad range of stresses, including salinity, cold, and heavy metals (15, 20, 21). We, therefore, hypothesized that a member of the PDR family would function as an ABA transporter. To identify the most promising candidate, we tested the seed germination and stomatal movements of 13 of 15 Arabidopsis PDR homozygous knockout mutants (atabcg29–atabcg41). In our screen, atabcg40 exhibited the most pronounced differences from the wild type in seed germination and stomatal movement. Thus, we selected AtABCG40 as a candidate and examined whether or not it indeed transports ABA and if its function as an ABA transporter is critical for plant-stress responses.
AtABCG40 Transports ABA.
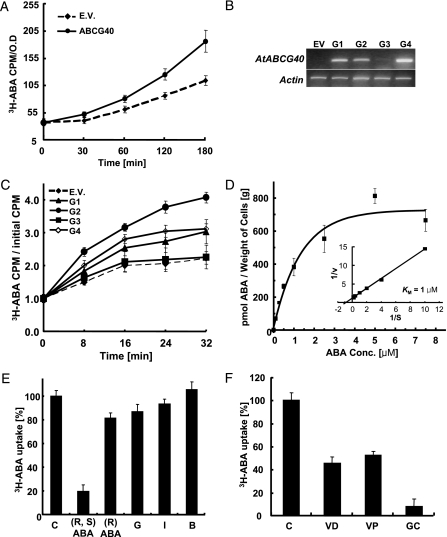
To assess whether or not AtABCG40 is an ABA transporter, we expressed the AtABCG40 cDNA in a heterologous system, namely the YMM12 yeast strain, which carries loss-of-function mutations in eight ABC transporters. Yeast-expressing AtABCG40 took up ABA consistently faster than controls containing the empty vector (Fig. 1A). Further evidence for AtABCG40 as an ABA transporter was obtained by expressing the AtABCG40 cDNA in cultured tobacco BY2 cells. ABA uptake was clearly more efficient in cells expressing AtABCG40 (G1, G2, and G4 in Fig. 1B) than in either control cells or the G3 cell line that expresses AtABAG40 at a very low level (Fig. 1 B and C). AtABCG40 is a high-affinity ABA transporter that displays a K M of 1 μM (Fig. 1D). The transporter also exhibits a high-substrate specificity, because uptake of 3H-ABA was inhibited only by the physiologically active (S)-ABA and not by (R)-ABA, ABA-glucose ester, indole-3-acetic acid, or benzoic acid at 3-fold excess (Fig. 1E). Inhibitors of ABC transporters such as glibenclamide, verapamil, and vanadate inhibited ABA transport (Fig. 1F).
Fig. 1.
AtABCG40 mediates specific uptake of ABA in a heterologous system. (A) Time-dependent uptake of 3H-ABA by YMM12 yeast cells expressing AtABCG40 (ABCG40) or transformed with the empty vector (EV). Yeast was incubated in SG-URA medium containing 4.5 nM 3H-ABA (7.4 kBq, 1.63 Tba/mmol) at pH 7. Radioactivity taken up was normalized to the cell number. Data are mean ± SEM of n = 12 from three independent experiments. (B) AtABCG40 transcripts in independent BY2 cell lines expressing AtABCG40 (G1-G4) or EV. Actin was amplified as a loading control. (C) Time-dependent transport of 4.5 nM 3H-ABA (7.4 kBq, 1.63 Tba/mmol) by BY2 cells expressing AtABCG40 (G1-G4) or EV at pH 5.7. (D) Concentration-dependent uptake of ABA containing 1 nM 3H-ABA (from 1 nM to 5 μM ABA) or 2 nM 3H-ABA (from 10 to 15 μM ABA) in BY2 cells (line G2) for 18 min. Inset shows double reciprocal plot analysis of the data indicating a K M of 1 μM. Mean is compiled from three experiments. K Ms calculated for the single experiments vary between 0.7 and 1.2 μM. Values were corrected for the effective (S)-ABA concentrations. (E) Uptake of 1 μM (R, S)-ABA containing a trace amount (7.4 kBq) of 3H-ABA in BY2 cells (line G2) in the absence (C) or presence of an additional 3-fold (3 μM) unlabeled (R, S)-ABA, (R)-ABA, ABA-glucose ester (G), indole-3-acetic acid (I), or benzoic acid (B). (F) Inhibition of ABCG40-mediated 4.5 nM 3H-ABA (7.4 kBq, 1.63 Tba/mmol) uptake in BY2 cells (line G2) by 3 mM vanadate (VD), 10 μM verapamil (VP), and 25 μM glibenclamide (GC). C, control without inhibitor pretreatment. In D–F, ABA uptake mediated by AtABCG40 was obtained by subtracting the activity of the empty vector control incubated under the same conditions from the total radioactivity to exclude the portion of ABA transported by diffusion.
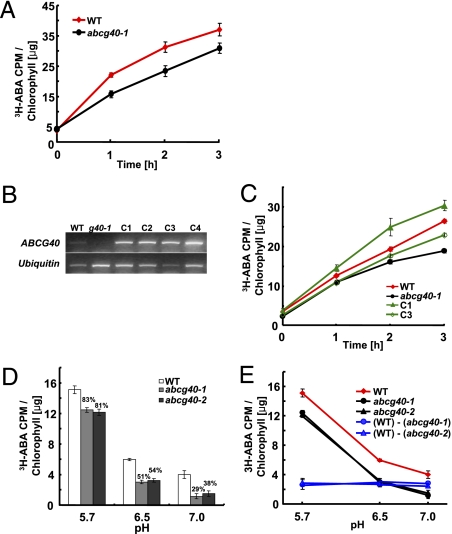
If AtABCG40 is an ABA transporter, then not only should cells transgenically expressing the transporter exhibit enhanced rates of ABA uptake, but cells in which the endogenous AtABCG40 gene is disrupted should show decreased rates of ABA uptake. Accordingly, we assessed ABA uptake in mesophyll protoplasts isolated from two independent T-DNA insertional mutants of AtABCG40, abcg40-1 and abcg40-2 (21). Indeed, ABA was taken up more slowly into protoplasts isolated from atabcg40 leaves compared with those from wild type (Fig. 2A), and in the complemented mutant lines, the uptake rate was restored to wild-type level (Fig. 2 B and C). In both wild-type and atabcg40 plants, ABA uptake was higher at low pH, and it decreased with increasing pH (Fig. 2D), supporting a component of ABA uptake consisting of pH-dependent diffusion of ABAH. However, the rate of ABA uptake was consistently lower in atabcg40 cells compared with wild type, and the proportion of total ABA taken up by the knockout mutants decreased in relation to the corresponding amount taken up by wild type with increasing pH (Fig. 2D). This phenomenon suggests an increasing contribution of AtABCG40 to total ABA uptake with increasing pH. It should, furthermore, be mentioned that, in planta, the ratio of uptake by the transporter versus uptake by diffusion would be higher than that in our experiments where the concentration of (S)-ABA available for transport by AtABCG40 is only one-half of the total concentration indicated. This is because of the high sterospecificity of transport (Fig. 1E) versus the lack of sterospecificity of diffusion. Interestingly, the differences in the absolute amounts of ABA taken up by wild-type and atabcg40 mutant cells, respectively, were independent of pH (Fig. 2E). This pH-independent component of ABA uptake cannot be explained by diffusion, but it is in agreement with the notion of an active transport of ABA by AtABCG40. We propose that AtABCG40 is the major ABA transporter in the leaf-cell protoplasts, because, at pH 7, the residual ABA-transport activity in atabcg40 mutants is only about 30% of that in the wild type (Fig. 2D). However, it cannot be excluded that close homologs of AtABCG40 may also carry out ABA transport functions.
Fig. 2.
AtABCG40 mediates specific uptake of ABA in Arabidopsis. (A) The isolated mesophyll cells were incubated in a bathing solution containing 4.5 nM 3H-ABA at pH 5.7. The chlorophyll content of the protoplasts was used to normalize 3H-ABA uptake. A representative of four independent experiments with similar results is shown (mean ± SEM; n = 4). (B) Presence of the AtABCG40 transcript in atabcg40-1 mutant plants complemented with AtABCG40pro::AtABCG40::sGFP. Ubiquitin was amplified as a loading control. Total RNA was isolated from shoots of wild-type atabcg40-1 mutant (g40-1) and AtABCG40 complemented atabcg40-1 mutant plants (C1, 2, 3, and 4), respectively. (C) Uptake of 3H-ABA into mesophyll protoplasts of wild-type and AtABCG40-complemented atabcg40-1 lines C1 and C3 are shown as a function of time. The chlorophyll content of the protoplasts was used to normalize the 3H-ABA uptake. Data are mean ± SEM of n = 12 from three independent experiments. (D) pH-dependent uptake of 3H-ABA into mesophyll protoplasts from wild-type and atabcg40 lines. The chlorophyll content of the protoplasts was used to normalize 3H-ABA values. Radioactivity of samples incubated for 2 min was subtracted from that of samples incubated for 3 h. Data are mean ± SEM of n = 16 from four independent experiments. (E) To obtain AtABCG40-mediated ABA transport, 3H-ABA taken up by abcg40-1 or abcg40-2 protoplasts was subtracted from that of wild-type protoplasts. The data are from the same experiments as shown in D.
AtABCG40 Is Broadly Expressed and Plasma Membrane Localized.
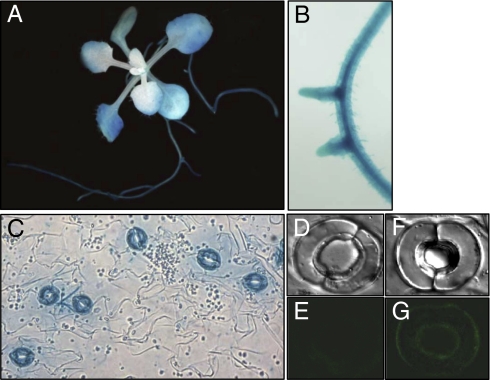
To address the questions of whether or not AtABCG40 functions in a broad range of plant-cell types or if it is specifically associated with a particular cell type, we assessed AtABCG40 expression patterns both in planta and in silico. According to our promoter-β-glucuronidase (GUS) analysis, the AtABCG40 promoter is broadly active, including activity in the leaves of young plantlets and in primary and lateral roots (Fig. 3 A and B), which is in line with published microarray data (www.genevestigator.ethz.ch). In leaves, the expression was by far the highest in guard cells (Fig. 3C), consistent with microarray data that reveal that AtABCG40 is expressed 8-fold more in guard cells than in mesophyll cells (www.bar.utoronto.ca).
Fig. 3.
Tissue-specific expression and subcellular localization of AtABCG40. (A–C) AtABCG40 promoter-GUS (AtABCG40pro::uidA) reporter gene expression in 2-week-old plants (A) and in primary and lateral roots (B). C shows a close-up image of a leaf showing expression in guard cells. (D–G) Plasma-membrane localization of ABCG40::sGFP expression that is driven by the native promoter in Arabidopsis guard cells (F and G). Wild-type cells are shown in D and E. Images show the same two cells under bright field illumination (D and F) and with GFP fluorescence (E and G).
If AtABCG40 functions as a carrier for the initial entry of ABA into plant cells, it would be expected to localize to the plasma membrane. AtABCG40 was previously shown to localize to the plasma membrane of mesophyll protoplasts when transiently expressed under control of the 35S promoter (21). As shown in Fig. 3 F and G, when AtABCG40-sGFP expression is driven by the AtABCG40 native promoter in stably transformed plants, GFP fluorescence is also seen at the cell membrane, indicating that AtABCG40 operates at that locale.
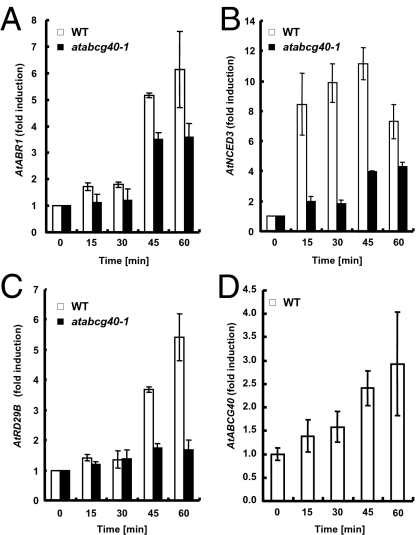
atabcg40 Plants Are Strongly Delayed in Expression of ABA-Responsive Genes on Treatment with ABA.
Given that there is a passive component of ABA uptake into cells, the question arises as to the importance of AtABCG40 in ABA-regulated cellular functions. Accordingly, we investigated the expression of ABA responsive genes in 4-week-old whole-rosette tissue of wild-type and atabcg40 plants. Up-regulation of transcription factors AtABR1 and AtRD29B and an ABA biosynthesis enzyme AtNCED3 in response to exogenous ABA application was considerably delayed and reduced in the mutant plants compared with wild type (Fig. 4 A–C). Evidently, AtABCG40 is required for a fast response to ABA by allowing its efficient uptake into the ABA-responsive cell. In contrast to the altered kinetics of induction of ABA-responsive genes, the kinetics of induction of auxin-responsive genes of the Small Auxin-Up RNAs (SAUR) family did not differ in the mutant from the wild-type plants (Fig. S1). Transcript levels of AtABCG40 itself increased in response to ABA treatment (Fig. 4D), indicating a positive feedback loop.
Fig. 4.
AtABCG40 modulates the expression of ABA-responsive genes. (A–D) Q-PCR analyses of transcripts of ABA-responsive genes in total RNA isolated from shoots of wild-type and atabcg40-1 plants, respectively, that are treated with ABA. Data were normalized using Tubulin 8 and are presented as “fold induction” relative to time = 0. Data are mean ± SEM from four independent experiments. For AtABR1 and AtRD29B, the initial values were similar in the wild type (WT) and atabcg40-1, whereas for AtNCED3, the initial value in atabcg40-1 was 2.4 -fold higher than that of the WT.
atabcg40 Plants Are Impaired in Stress Tolerance.
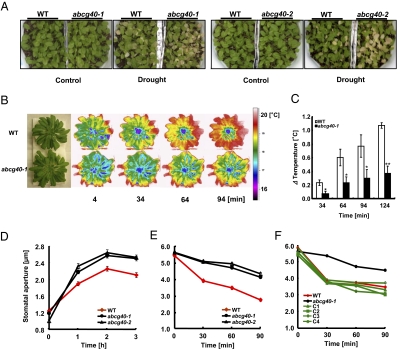
Drought-stress experiments provided further evidence that AtABCG40 is integral to stress tolerance. Plants were grown for 2 weeks under standard conditions, and water is subsequently withheld. Leaves of the two mutant lines wilted faster than those of the wild-type plants (Fig. 5A). This result suggests that effective guard cell response to ABA is impaired in plants lacking expression of the AtABCG40 ABA transporter, but this reaction could also be caused in part by alterations in ABA responsiveness of other physiological aspects. This is because AtABCG40 is widely expressed and is required for rapid transcriptional responses in whole leaves. Additionally, atabcg40 mutants also show impairment in ABA regulation of seed germination and root development (Fig. S2). Because of the high levels of AtABCG40 expression that we observed in guard cells and because of the known importance of ABA in stomatal regulation, we particularly wanted to assess the impact of AtABCG40 knockout on guard-cell function. We pursued two approaches to address this issue. Because transpiration lowers leaf temperature through evaporative cooling, we employed thermal-imaging methods (22) to assess transpirational water loss, which correlates with stomatal apertures. When hydroponically grown plants were treated with 1 μM ABA or 0.5 M mannitol to induce stomatal closure and thus, reduce transpiration, leaf temperature increased more slowly in atabcg40 plants compared with wild-type plants (Fig. 5 B and C and Fig. S3); this indicates that stomatal apertures were not as efficiently reduced in response to ABA or osmotic stress as in the wild type.
Fig. 5.
Stomata of atabcg40 plants are less sensitive to ABA. (A) Two-week-old soil-grown plants (24 °C; 16-h light and 8-h dark conditions) were exposed to drought stress by withholding water for 8 days. (B) Delayed elevation of leaf temperature after ABA treatment of atabcg40 plants compared with wild type. Leaf temperature was monitored using an Infrared Thermal Imaging Camera (FLIR systems; P25) after the addition of ABA into the hydroponic culture medium to a final concentration of 1 μM. Results representative of three experiments with similar results are shown. (C) Increase in leaf temperature (Δ Temperature) after ABA treatment of plants is shown. The leaf temperature was quantified from three independent experiments, including the one shown in B. Data represent change in temperatures of the whole-leaf area of the plants. ΔTemperature = (Temperature at indicated time point) − (Temperature at 4 min). The initial temperatures were similar in WT and atabcg40-1. Data are mean ± SEM (*P < 0.05; ** P < 0.01 compared with WT under the same treatment conditions by Student's t test). (D) Opening of stomata in the presence of 1 μM ABA. Epidermal strips were peeled from leaves that had been floated on 10 mM KCl buffer in the presence of 1 μM ABA under 170 μmol m−2sec−1 white light. (E and F) ABA-induced stomatal closure in WT and atabcg40 plants (E) and in four independent lines complemented with AtABCG40 (F). Data are mean ± SEM of n = 83∼108 (D), n = 80∼112 (E), and n = 75∼95 (F) stomata (obtained from three independent experiments).
To confirm the results from thermal-imaging analysis implicating ABCG40 in guard-cell function, we also directly examined the effect of ABA on stomatal movements in wild-type and atabcg40 mutant plants. Wild-type and atabcg40 plants indeed differed in their responses to ABA during light-induced stomatal opening. Stomata of the two mutant lines atabcg40-1 and atabcg40-2 plants opened faster, attaining apertures of 2.64 ± 0.07 μm and 2.57 ± 0.05 μm, respectively, whereas wild-type plants attained 2.25 ± 0.06 μm under the same conditions (Fig. 5D). Furthermore, in comparison with the wild type, the two atabcg40 lines exhibited delayed and reduced stomatal closure in response to ABA (Fig. 5E). Complementation of the atabcg40-1 mutant line with the AtABCG40 cDNA driven by its own promoter confirmed that the observed mutant phenotype was caused by AtABCG40 loss of function (Fig. 5F). In contrast to ABA, no difference was observed for stomatal closure in response to Ca2+ (Fig. S4), suggesting that the mutation affected the level of ABA or its perception rather than the downstream ABA signaling. The results obtained using epidermal strips and thermal imaging of intact plants collectively show that the guard cells of atabcg40 plants exhibit a strongly delayed response to ABA. We propose that AtABCG40 is an ABA importer required for efficient response to ABA.
Discussion
Rapid adjustment to a stress, such as drought stress, is a prerequisite for plant survival. Despite the diffusion of ABAH through the lipid bilayer, our data indicate that a transporter is required for optimal ABA uptake during stress. Neither ABA uptake nor the response to ABA is totally abolished when AtABCG40 is nonfunctional, indicating that a contribution of diffusion to ABA uptake at an apoplastic pH of 5.5–6.0, typical of the nonstressed plant, does occur. However, for rapid and efficient signaling under stress conditions, AtABCG40 activity is required. As mentioned above, the pH of the xylem and apoplast increases during drought stress (8), and the advantage is that uncontrolled diffusion of ABAH into nontarget cells and hence, loss of ABA on its way to its target cells (i.e., the guard cells) is avoided. Passive diffusion of ABA into the target cells would, however, be reduced as well, and thus, a transporter-mediated uptake process is necessary to overcome this disadvantage (Fig. S5). After it is inside the cell, ABA binds to ABA receptors localized in the cytosol (12, 13) or at the plasma membrane (23), thereby initiating ABA signal transduction. The very recent discovery of a class of soluble ABA receptors is in perfect agreement with our results (12, 13). However, ABA receptors localized at the plasma membrane (23) may also recognize ABA by domains exposed to the cytosol. An ABA transporter would allow fast and controlled delivery of ABA to the receptor and thus, ensure a rapid response to the stress hormone, which is not achievable by diffusive influx of ABA alone. The previous observation that stomatal apertures and activity of inward K+ channels that mediate K+ uptake during stomatal opening are both responsive to intracellular application of ABA is also consistent with the importance of an ABA uptake carrier (24). The observation that ABA-responsive transcription factors, present not only in guard cells but in many other cell types, respond much faster in the presence of AtABCG40 (Fig. 4) indicates that transporter-catalyzed ABA transport is important for ABA signaling in many cell types. Supporting this, lateral root formation was delayed in atabcg40 as well (Fig. S2C). Decreased sensitivity of atabcg40 seeds to exogenously added ABA (Fig. S2B) suggests that this transporter is active in seeds as well, although it is expressed at a very low level in this organ (www.bar.utoronto.ca). An intriguing possibility is that the seeds may take up ABA through AtABCG40 during development and store the hormone to prevent precocious germination. Supporting this possibility, atabcg40 seeds exhibited slightly accelerated germination on normal growth medium without added ABA (Fig. S2A).
We previously reported that the atabcg40 plants are compromised in lead resistance (21). Because ABC transporters are well-known as transporters of structurally unrelated compounds (15), we tested whether or not AtABCG40 transports both lead and ABA using a competition assay; however, lead did not compete with ABA for uptake into plant cells (Fig. S6). Thus, we suggest that the reduced lead tolerance of the mutant is likely caused by compromised ABA transport, which indirectly affects heavy metal tolerance of the plant. Several reports postulate a role of ABA in heavy metal tolerance. For example, ABA content in leaves increases when plants are exposed to the heavy metals lead, cadmium, or aluminum (25 –27). ABA regulates the expression level of Type 4 metallothionein (28), a member of an important metal chelator family. Application of exogenous ABA reduced the root-to-shoot translocation of Cd (29) by inducing stomatal closure. In short, ABA, which is increased in heavy metal-treated plants, plays an important role in plant tolerance to heavy metals through at least two different pathways. First, it promotes stomatal closure, which reduces heavy metal translocation to the shoot through the transpirational stream. Second, it modifies expression of genes that can contribute to heavy metal tolerance (28 –30).
ABCG40 has been suggested to transport diterpenoids associated with plant defense based on three findings (18): (i) it is up-regulated by pathogen infection, (ii) its amino acid sequence is similar to those of other diterpenoid transporters such as NpABC1 and SpTUR2, and (iii) the atabcg40 mutant plant is altered in tolerance to the toxicity of diterpenoid sclareol. ABA is a sesquiterpenoid, and there is increasing evidence that ABA plays an important role in plant-pathogen responses (31 –33). Therefore, either ABCG40 transports various terpenoids or many of the previous findings on pathogen-related phenomena are indirect effects of the ABA-transport function of ABCG40. We favor the latter possibility, because ABCG40 showed very narrow substrate specificity (Fig. 1E). In line with this possibility, we could not observe any difference in growth of the atabcg40 mutants versus the wild type in medium containing sclareol (21). Moreover, although sclareol is structurally similar to ABA, it is not likely to be a physiological substrate of ABCG40, because this compound is not produced in Arabidopsis. Finally, low micromolar concentrations of sclareol did not inhibit (S)-ABA uptake into AtABCG40-expressing BY2 cells, indicating that at least the uptake activity of AtABCG40 is specific for ABA (Fig. S6). In one publication, the authors suggested that AtABCG40 can export sclareol (18); however, they did not perform transport studies. Whether or not AtABCG40 can transport in two directions must be addressed in future work.
In conclusion, we have presented evidence for ABC transporter-mediated ABA uptake and its importance for rapid responses to environmental stress. This work underlines the importance of transporters for hormones that also diffuse into the cell across the lipid bilayer. Our results may promote the production of plants with increased drought tolerance (e.g., through guard cell-specific expression of AtABCG40), which may lead to faster stomatal closure. Manipulation of a very specific pathway for uptake of ABA, a step localized at the apex of the ABA-signaling pathway, may allow development of plants permanently primed to respond quickly to stress. Transporters related to AtABCG40 also may play an important role in other organisms, because ABA has recently been reported to induce Ca2+ release during Toxoplasma infection and in human granulocytes (34, 35).
Materials and Methods
Plant Material and Growth Conditions.
Wild-type (ecotype Columbia-0) and AtABCG40 transgenic plants (21) were grown on soil (22/18 °C; 16/8 h day/night; 40 μmol m−2s−1 light).
DNA Constructs.
The AtABCG40 promoter::uidA reporter gene construct was made by PCR amplification of a 1.8-kb promoter region of AtABCG40 from genomic DNA using primers containing HindIII and BamHI restriction sites (5′-AAGCTTACGCCGGCCGCCGCCGCGGCAG-3′ and 5′-GGATCCTTTGTATCCAAGAAATCAAAGT-3′) and ligation of the PCR product into pBI101.2. The ORF of AtABCG40 was amplified by PCR from cDNA generated from total RNA extracted from wild-type seedlings using primers containing BamHI restriction sites (5′-CCCGGGGGGGATCCATGGAGGGAACTAGTTTTCACCAAGCGAGTA-3′ and 5′-GGATCCGCGGCCGCCTATCGTTTTTGGAAATTGAAACTCTTGATTC-3′). To generate complemented and tagged lines of atabcg40-1 mutants, the AtABCG40 promoter was inserted into pBI101.2 at HindIII and BamHI restriction sites; sGFP was amplified from the 326-sGFP vector and fused to the 3′-end of the AtABCG40 promoter. Finally, the full-length cDNA of AtABCG40 was ligated into the BamHI site of the construct. All constructs were verified by sequencing.
Verification of AtABCG40-Complemented Arabidopsis Plants.
The abundance of AtABCG40 transcript in the homozygous complemented lines C1, C2, C3, and C4 and wild-type plants was assayed by RT-PCR. Total RNA was extracted from whole seedlings, and RT-PCR was performed after DNaseI (Roche) treatment using primers specific for AtABCG40 (5′-CTGCTTTTGGGTCCTCCAAGTTCT-3′, and 5′-GAGATTGAATGTCTCTGGCGCAG-3′). As a loading control, the Tubulin8 transcript was amplified using the primers (5′-CTCACAGTCCCGGAGCTGACAC-3′ and 5′-GCTTC AGTGAACTCCATCTCGT-3′).
Assays of Stomatal Movements.
Stomatal apertures were determined in 5-week-old leaves. For assays of stomatal opening, detached whole leaves were floated on 10 mM KCl buffer, with or without 1 μM ABA, under 170 μmol m−2sec−1 white light at 23 °C. For assays of stomatal closure, leaves were preincubated on 10 mM KCl buffer for 3 h under 170 μmol m−2sec−1 white light at 25 °C to open stomata, and then, they were transferred to 10 mM KCl buffer containing 1 μM ABA (time = 0). Stomatal apertures were measured as described previously (36).
Infrared Thermography.
Arabidopsis plants were grown under hydroponic conditions (22 °C; 8 h of light photoperiod; 40 μmol m−2s−1 light) for 6 weeks (37). After adding ABA or mannitol to the hydroponic medium to a final concentration of 1 μM or 0.5 M, respectively, the leaf temperature of intact Arabidopsis plants was measured as described previously (22, 36).
3H-ABA Uptake into Arabidopsis Protoplasts.
Transport activity was determined as described previously (38). Protoplasts were incubated in 1 mL of a bathing solution containing 4.5 nM (R, S)-3H-ABA (Amersham Biosciences; 7.4 kBq and 1.63 TBq/mmol).
Heterologous Expression of AtABCG40 in Yeast and Transport Assays.
The yeast YMM12 strain was a kind gift from Karl Kuchler (Vienna, Austria). AtABCG40 was cloned into the BamHI and NotI sites of pYES2NT/C. 3H-ABA uptake was monitored as described previously with minor modifications (17). Cells were cultured in minimum salt-galactose minus uracil (SG-URA) medium, supplemented with 0.5% raffinose, at pH 7.0, and harvested by centrifugation at midlog phase. They were washed two times using SG-URA medium, and they were resuspended in the same medium at an OD600 = 6. 3H-ABA (4.5 nM, 7.4 kBq, 1.63 TBq/mmol) was then added to the cell suspension and gently mixed. At the times indicated, the cell suspension was filtered through nitrocellulose membranes, and the cells remaining on the filter were washed with 500 μL of ice-cold SG-URA medium. The radioactivity on the filter was determined by liquid-scintillation counting.
Transport Assay Using BY2 Cells.
Quantitative Real-Time RT-PCR.
Supplementary Material
Acknowledgments
We thank N. Amrhein and Shaun Peters for reading the manuscript. This work was supported by the Global Research Laboratory program of the Ministry of Science and Technology of Korea and the Crop Functional Genomics Center of Korea Grant CG1-1-23 (to Y.L.). The work in the laboratory of E.M. was partially supported by the Swiss National Foundation. S.M.A. was partially supported by the U.S. National Science Foundation.
Footnotes
Conflict of interest statement: Provisional application of USA (Abscisic Acid Transporters and Transformants Expressing the Genes; application date 2009-01-23 3; application number 61/146705.
This article is a PNAS Direct Submission. Z.-M.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909222107/DCSupplemental.
References
- 1.Salisbury FB, Ross CW. Plant Physiology. 3rd Ed. California: Wadsworth Publishing Company, Belmont; 1985. p. 312. [Google Scholar]
- 2.Vieten A, Sauer M, Brewer PB, Friml J. Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 2007;12:160–168. doi: 10.1016/j.tplants.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Marchant A, et al. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 1999;18:2066–2073. doi: 10.1093/emboj/18.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swarup K, et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol. 2008;10:946–954. doi: 10.1038/ncb1754. [DOI] [PubMed] [Google Scholar]
- 5.Jiang F, Hartung W. Long-distance signaling of abscisic acid (ABA): The factors regulating the intensity of the ABA signal. J Exp Bot. 2008;59:37–43. doi: 10.1093/jxb/erm127. [DOI] [PubMed] [Google Scholar]
- 6.Daeter W, Hartung W. The permeability of the epidermal cell plasma membrane of barley leaves to abscisic acid. Planta. 1993;191:41–47. [Google Scholar]
- 7.Windsor ML, Milborrow BV, McFarlane IJ. The uptake of (+)-S- and (-)-R-abscisic acid by suspension culture cells of Hopbush (Dodonaea viscosa) Plant Physiol. 1992;100:54–62. doi: 10.1104/pp.100.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkinson S, Davies WJ. Xylem sap pH Increase: A drought signal received at the apoplastic face of the guard cell that involves the suppression of saturable abscisic acid uptake by the epidermal symplast. Plant Physiol. 1997;113:559–573. doi: 10.1104/pp.113.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeevaart JAD. Changes in the levels of abscisic acid and its metabolites in excised leaf blades of Xanthium strumarium during and after water stress. Plant Physiol. 1980;66:672–678. doi: 10.1104/pp.66.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hetherington AM. Guard cell signaling. Cell. 2001;107:711–714. doi: 10.1016/s0092-8674(01)00606-7. [DOI] [PubMed] [Google Scholar]
- 11.Israelsson M, et al. Guard cell ABA and CO2 signaling network updates and Ca2+ sensor priming hypothesis. Curr Opin Plant Biol. 2006;9:654–663. doi: 10.1016/j.pbi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Park SY, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Y, et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- 14.Verrier PJ, et al. Plant ABC proteins—a unified nomenclature and updated inventory. Trends Plant Sci. 2008;13:151–159. doi: 10.1016/j.tplants.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Rea PA. Plant ATP-binding cassette transporters. Annu Rev Plant Biol. 2007;58:347–375. doi: 10.1146/annurev.arplant.57.032905.105406. [DOI] [PubMed] [Google Scholar]
- 16.Suh SJ, et al. The ATP binding cassette transporter AtMRP5 modulates anion and calcium channel activities in Arabidopsis guard cells. J Biol Chem. 2007;282:1916–1924. doi: 10.1074/jbc.M607926200. [DOI] [PubMed] [Google Scholar]
- 17.Lee M, et al. The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2 . Nat Cell Biol. 2008;10:1217–1223. doi: 10.1038/ncb1782. [DOI] [PubMed] [Google Scholar]
- 18.Campbell EJ, et al. Pathogen-responsive expression of a putative ATP-binding cassette transporter gene conferring resistance to the diterpenoid sclareol is regulated by multiple defense signaling pathways in Arabidopsis . Plant Physiol. 2003;133:1272–1284. doi: 10.1104/pp.103.024182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein M, et al. Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell. 2006;18:731–746. doi: 10.1105/tpc.105.038372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moons A. Transcriptional profiling of the PDR gene family in rice roots in response to plant growth regulators, redox perturbations and weak organic acid stresses. Planta. 2008;229:53–71. doi: 10.1007/s00425-008-0810-5. [DOI] [PubMed] [Google Scholar]
- 21.Lee M, et al. AtPDR12 contributes to lead resistance in Arabidopsis. . Plant Physiol. 2005;138:827–836. doi: 10.1104/pp.104.058107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merlot S, et al. Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J. 2002;30:601–609. doi: 10.1046/j.1365-313x.2002.01322.x. [DOI] [PubMed] [Google Scholar]
- 23.Pandey S, Nelson DC, Assmann SM. Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis . Cell. 2009;136:136–148. doi: 10.1016/j.cell.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz A, Wu WH, Tucker EB, Assmann SM. Inhibition of inward K+ channels and stomatal response by abscisic acid: An intracellular locus of phytohormone action. Proc Natl Acad Sci USA. 1994;91:4019–4023. doi: 10.1073/pnas.91.9.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barcelo J, Cabot C, Poschenrieder CH. Cadmium-induced decrease of water stress resistance in bush bean plants (Phaseolus vulgaris L. cv. Contender). II. Effects of Cd on endogenous abscisic acid levels. J Plant Physiol. 1986;125:27–34. [Google Scholar]
- 26.Atici O, Agar G, Battal P. Changes in phytohormone contents in chickpea seeds germinating under lead or zinc stress. Biol Plant. 2005;49:215–222. [Google Scholar]
- 27.Kasai M, et al. Possible involvement of abscisic acid in increases in activities of two vacuolar H+-pumps in barley roots under aluminum stress. Plant Cell Physiol. 1993;34:1335–1338. [Google Scholar]
- 28.Chatthai M, et al. The isolation of a novel metallothionein-related cDNA expressed in somatic and zygotic embryos of Douglas-fir: Regulation by ABA, osmoticum, and metal ions. Plant Mol Biol. 1997;34:243–254. doi: 10.1023/a:1005839832096. [DOI] [PubMed] [Google Scholar]
- 29.Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C. Heavy metal toxicity: Cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 2002;32:539–548. doi: 10.1046/j.1365-313x.2002.01442.x. [DOI] [PubMed] [Google Scholar]
- 30.Talanova VV, Titov AF, Boeva NP. Effect of increasing concentrations of lead and cadmium on cucumber seedlings. Biol Plant. 2000;43:441–444. [Google Scholar]
- 31.Zhao Z, Zhang W, Stanley BA, Assmann SM. Functional proteomics of Arabidopsis thaliana guard cells uncovers new stomatal signaling pathways. Plant Cell. 2008;20:3210–3226. doi: 10.1105/tpc.108.063263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ton J, Flors V, Mauch-Mani B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009;14:310–317. doi: 10.1016/j.tplants.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, He SY, Assmann SM. The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J. 2008;56:984–996. doi: 10.1111/j.1365-313X.2008.03657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagamune K, et al. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii . Nature. 2008;451:207–210. doi: 10.1038/nature06478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruzzone S, et al. Abscisic acid is an endogenous cytokine in human granulocytes with cyclic ADP-ribose as second messenger. Proc Natl Acad Sci USA. 2007;104:5759–5764. doi: 10.1073/pnas.0609379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeon BW, et al. The Arabidopsis small G protein ROP2 is activated by light in guard cells and inhibits light-induced stomatal opening. Plant Cell. 2008;20:75–87. doi: 10.1105/tpc.107.054544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen A, Komives EA, Schroeder JI. An improved grafting technique for mature Arabidopsis plants demonstrates long-distance shoot-to-root transport of phytochelatins in Arabidopsis . Plant Physiol. 2006;141:108–120. doi: 10.1104/pp.105.072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim D-Y, et al. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. 2007;50:207–218. doi: 10.1111/j.1365-313X.2007.03044.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.