Abstract
IL-2 is crucial to T cell homeostasis, especially of CD4+ T regulatory cells and memory CD8+ cells, as evidenced by vigorous proliferation of these cells in vivo following injections of superagonist IL-2/anti-IL-2 antibody complexes. The mechanism of IL-2/anti-IL-2 antibody complexes is unknown owing to a lack of understanding of IL-2 homeostasis. We show that IL-2 receptor α (CD25) plays a crucial role in IL-2 homeostasis. Thus, prolongation of IL-2 half-life and blocking of CD25 using antibodies or CD25-deficient mice led in combination, but not alone, to vigorous IL-2–mediated T cell proliferation, similar to IL-2/anti-IL-2 antibody complexes. These data suggest an unpredicted role for CD25 in IL-2 homeostasis.
Keywords: CD25, cytokine, cytokine/mAb complexes, T cell homeostasis, Fc receptor
IL-2 is central to the proper functioning of the immune system by participating in T cell homeostasis and optimizing immune responses (1, 2). IL-2 receptors (IL-2R) consist typically of three receptor subunits termed IL-2Rα (CD25), IL-2Rβ (CD122), and the common gamma chain (γc, CD132) (3 –5). Notably, CD122 and γc are crucial for signal transduction, whereas CD25 is not essential for IL-2 signaling but instead confers high-affinity binding of IL-2 to its receptor, thereby increasing receptor affinities by about 10- to 100-fold (5 –7). High-affinity αβγ IL-2Rs are typically found on CD4+ T regulatory cells (Tregs) as well as recently-activated T cells (1, 2). Low-affinity βγ IL-2Rs are present at a low level on naïve CD8 cells but are prominent on antigen-experienced (memory) and memory-phenotype (MP) CD8+ T cells as well as natural killer (NK) cells (1, 2). Both MP CD8+ T cells and NK cells express very high levels of CD122 and readily respond to IL-2 injections in vivo (8). The vast majority of IL-2 is generated by activated T cells, especially CD4+ cells, with a minor contribution from NK and NK T cells (1, 3, 9). Thus, IL-2 is typically produced by recently-activated T cells and acts in an autocrine or paracrine fashion on these cells, many of which up-regulate CD25 expression upon T cell receptor stimulation (3).
Because of its potent stimulatory properties, IL-2 is subjected to a tight regulation. With regard to IL-2 half-life, renal metabolism has been reported to play an important role in eliminating IL-2 after i.v. injection (10). Thus, for exogenously-administered IL-2, renal elimination results in a very short in vivo half-life of IL-2 in the range of minutes. This has been a major limitation for IL-2-based strategies in the treatment of metastatic cancer and chronic viral infections, thus requiring the use of high doses of IL-2, which, however, can cause severe toxic side effects, termed vascular leak syndrome (11, 12). In an attempt to extend in vivo half-life and increase biological activity, IL-2 has been coupled to larger proteins such as albumin and unrelated antibodies to create IL-2-IgG fusion proteins (IL-2-FP) (12, 13), although with limited success.
Recently, injection of IL-2 bound to particular anti-IL-2 monoclonal antibodies (mAbs) was found to greatly enhance the biological activity of IL-2 in vivo, leading either to vigorous proliferation of MP CD8+ T cells and NK cells or expansion of CD25+ CD4+ Tregs (8). Notably, anti-IL-2 mAbs typified by S4B6 and MAB602, specific to mouse (m) and human (h) IL-2, respectively, formed IL-2/mAb complexes that preferentially bound to MP CD8+ T cells and NK cells expressing high levels of CD122 along with γc (8). We will refer to these CD122-directed complexes as IL-2/mAbCD122 complexes. In contrast, JES6-1 anti-mIL-2 mAb produced IL-2/mAb complexes that interacted almost exclusively with immune cells expressing high levels of CD25 along with βγ IL-2R; these CD25-directed complexes, designated IL-2/mAbCD25 complexes, induced selective expansion of CD25+ CD4+ Tregs (8). In addition to IL-2, cytokine/mAb complexes can also be generated using IL-3, IL-4, IL-6, or IL-7, which have been successfully used in various animal models, including viral infections, antitumor responses, autoimmune disease, and graft tolerance (8, 14–23).
Despite the considerable interest in IL-2/mAb complexes arising from its broad applications, the exact nature of the underlying mechanism of IL-2/mAb complexes remains elusive. Several mechanisms have been proposed, including protection from enzymatic alteration of the active site, cytokine presentation by Fc receptor (FcR)-bearing cells, and prolongation of in vivo IL-2 half-life, with some evidence supporting the latter (12, 15, 16).
Here, we show that the in vivo activity of IL-2/mAbCD25 complexes crucially depended on the presence of neonatal FcR (FcRn), whereas for IL-2/mAbCD122 complexes, it was a result of prolonged IL-2 half-life and protection from interaction with CD25. These results also suggest an unanticipated role for CD25 in the regulation of IL-2 homeostasis.
Results
Role of FcR on in Vivo Activity of IL-2/mAb Complexes.
As mentioned above, two types of IL-2/mAb complexes with distinct biological activities have been described, namely IL-2/mAbCD122 complexes, generated using S4B6 anti-mIL-2 or MAB602 anti-hIL-2 mAbs, and IL-2/mAbCD25 complexes generated using JES6-1 anti-mIL-2 mAb (8). IL-2/mAb complexes using either mIL-2 or hIL-2 yielded similar results when compared and hence will be described here without specifying the species of IL-2 (this information can be found in the legends for Figs. 1–6).
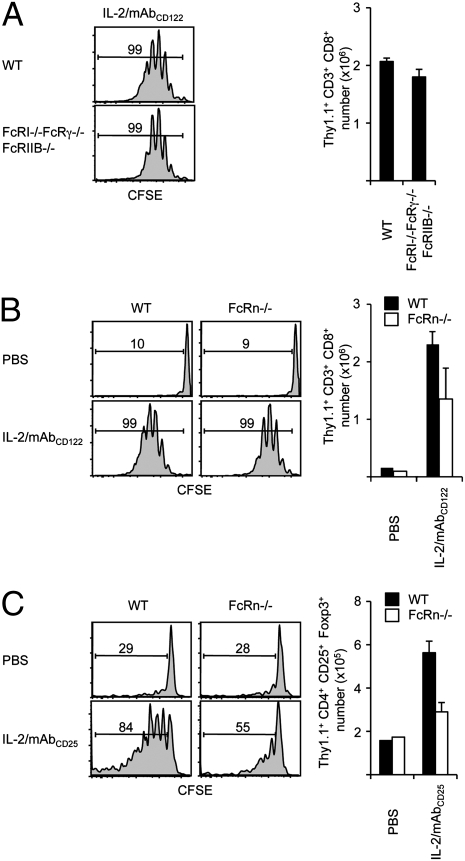
Fig. 1.
In vivo activity of IL-2/mAbCD122 complexes is largely independent of Fc receptors. CFSE-labeled Thy1.1+ MP CD8+ T cells were adoptively transferred to (A) WT mice (Upper) and FcRI−/−FcRγ−/−FcRIIB−/− mice (Lower), or to (B) WT mice (Left) and FcRn−/− mice (Right). (C) CFSE-labeled Thy1.1+ CD4+ T cells were adoptively transferred to WT (Left) or FcRn−/− mice (Right). Host mice subsequently received three injections of rmIL-2 plus S4B6 anti-mIL-2 mAbCD122 (A and B) or rmIL-2 plus JES6-1 anti-mIL-2 mAbCD25 (C) every other day. CFSE dilution and cell recovery from lymph nodes (LN) was analyzed by flow cytometry 7 days after adoptive transfer. Histograms shown are gated on Thy1.1+ CD8+ (A and B) or Thy1.1+ CD25+ FoxP3+ CD4+ donor T cells (C). Numbers indicate percentage of divided donor cells. The data are representative of two (A) or three (B and C) different experiments with each profile representing one of at least two mice.
Fig. 6.
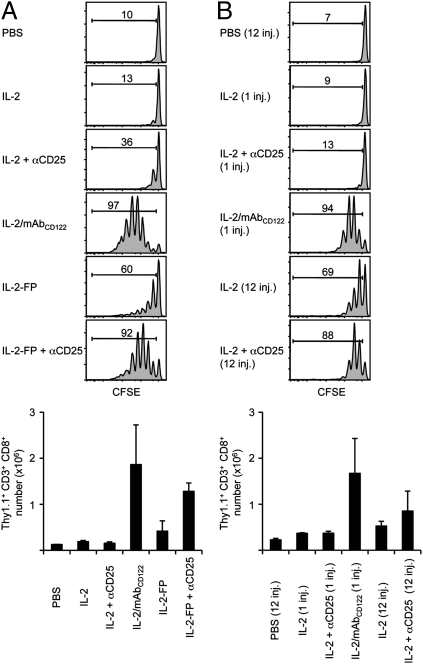
Long-acting IL-2 is equal to IL-2/mAbCD122 complexes in the absence of CD25. CFSE-labeled Thy1.1+ MP CD8+ T cells were adoptively transferred to CD25−/− mice, which then received on the same day a single injection of rhIL-2, rhIL-2 plus MAB602 anti-hIL-2 mAbCD122, chTNT-3/IL-2 IL-2-FP or 12 injections of PBS or rhIL-2 every 2 h for 24 h. CFSE dilution and cell recovery of Thy1.1+ CD8+ donor cells from the host spleen was determined 3 days after adoptive transfer. Numbers indicate percentage of divided cells. The data are representative of three different experiments, with each profile representing one of at least two mice.
The role of the two classes of FcR, namely FcR for IgG (FcRγ), and FcRn, in mediating the potent in vivo activity of IL-2/mAb complexes was investigated by using mice deficient in FcR. For FcRγ, mice deficient in FcRI, FcRγ, and FcRIIB were used to ensure complete absence of FcRγ. Notably, FcRγ−/− and FcRγ−/−FcRIIB−/− mice, previously used by others for the same purpose (24, 25), still express residual FcRγ, including the high affinity FcRI (26). The role of FcRn was investigated using FcRn−/− mice (27). CD44high CD122high MP CD8+ T cells were used as IL-2-responsive reporter cells to measure the presence of IL-2 signals in vivo. These MP CD8+ T cells were obtained from IL-7 transgenic (tg) mice, as these mice contained considerably higher numbers of MP CD8+ T cells, which were phenotypically and functionally indistinguishable from MP CD8+ T cells in WT mice (Fig. S1; refs. 28, 29).
As previously shown, injection of IL-2/mAbCD122 complexes induced strong expansion of purified donor MP CD8+ T cells in WT hosts as evidenced by cell division and recovery (Fig. 1A). Also, strong expansion of donor cells was observed in FcRI−/−FcRγ−/−FcRIIB−/− hosts (Fig. 1A). The lack of reduction in expansion of donor cells in FcRI−/−FcRγ−/−FcRIIB−/− hosts was unlikely to be due to expression of residual FcRγ on donor cells, as similarly strong expansion was observed when MP CD8+ T cells from FcRI−/−FcRγ−/−FcRIIB−/− mice were used as donor cells; moreover, massive expansion of endogenous MP CD8+ T cells occurred in FcRI−/−FcRγ−/−FcRIIB−/− mice injected with IL-2/mAbCD122 complexes. Likewise, endogenous CD25+ CD4+ Tregs underwent comparable expansion in FcRI−/−FcRγ−/−FcRIIB−/− mice and WT mice following administration of IL-2/mAbCD25 complexes. These findings confirm and extend previous reports showing that FcRγ play only minimal role in mediating the in vivo activity of IL-2/mAb complexes (24, 25).
However, different results were obtained for FcRn. For IL-2/mAbCD122 complexes, purified donor MP CD8+ T cells typically underwent one less cell division with a slight reduction in cell recovery in FcRn−/− compared to WT hosts (Fig. 1B). Residual FcRn expression on donor T cells was unlikely to play a role, as similar findings were observed with FcRn−/− donor T cells. These findings indicate that for IL-2/mAbCD122 complexes, FcRn play a partial role in mediating their in vivo activity. This conclusion is consistent with a previous report using β2-microglobulin-deficient mice (24). Moreover, animals deficient in both types of FcR, namely FcRI−/−FcRγ−/−FcRIIB−/−FcRn−/− mice, were able to mediate proliferation of MP CD8+ T cells upon injection of IL-2/mAbCD122 complexes, thus indicating the absence of compensatory mechanisms between the two types of FcR.
In contrast to IL-2/mAbCD122 complexes, IL-2/mAbCD25 complexes were found to be acutely dependent on FcRn for their in vivo activity. Thus, in response to IL-2/mAbCD25 complexes, expansion of donor WT CD25+ CD4+ Tregs was severely reduced in FcRn−/− compared to WT hosts and only slightly above the background level of expansion (Fig. 1C). Moreover, endogenous CD25+ CD4+ Tregs in FcRn−/− mice failed to expand substantially following injections of IL-2/mAbCD25 complexes. With FcRn being crucial for the prolonged serum half-life of IgG (30), this finding strongly suggests that mAb-induced extension of IL-2 lifespan is a major mechanism for the strong biological activity of IL-2/mAb complexes.
IL-2/mAb Complexes Display an Extended in Vivo Lifespan.
In the absence of FcRn, serum half-life of IgG decreases to 1–1.5 days, compared to approximately 6–8 days under normal conditions (27). Therefore, the above findings on the role of FcRn could indicate that mAb-induced prolongation of in vivo IL-2 lifespan is an important mechanism for the potent activity of IL-2/mAb complexes (14–16, 24), especially in the case of IL-2/mAbCD25 complexes.
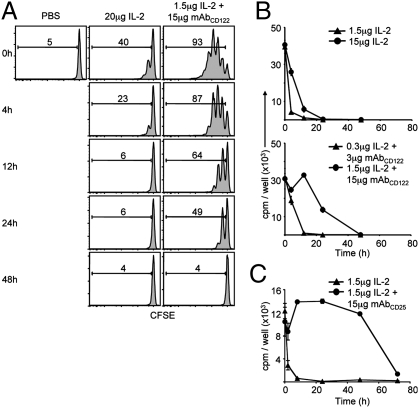
To investigate this idea, the in vivo lifespan of IL-2/mAb complexes was compared to IL-2 in WT hosts by two complementary approaches. First, a bolus of a high dose of IL-2 (20 μg) or a standard dose of IL-2/mAbCD122 complexes (1.5 μg/15 μg) was administered to WT mice at various intervals before injection of carboxyfluorescein succinimidyl ester (CFSE)-labeled MP CD8+ T cells. Measuring the proliferation of donor T cells 3 days later revealed that the activity of IL-2 alone tapered off rapidly after 4 h, whereas the activity of IL-2/mAbCD122 complexes was still evident even after 24 h (Fig. 2A).
Fig. 2.
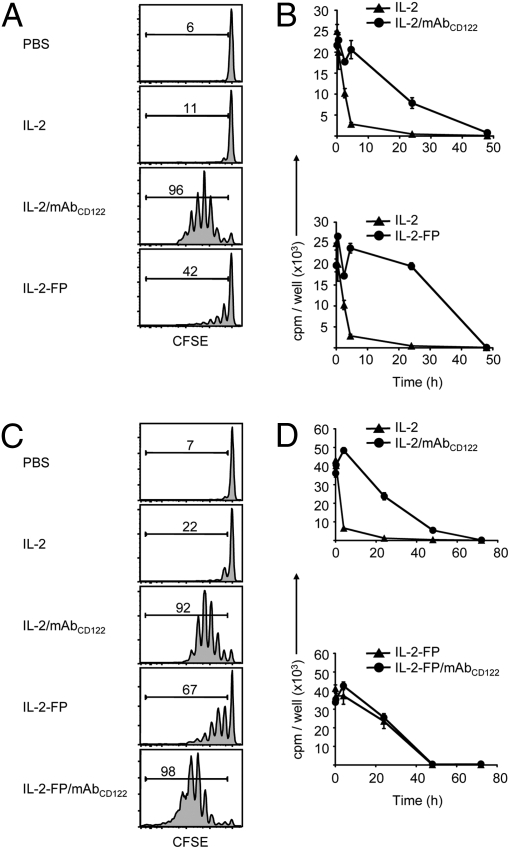
IL-2/mAbCD122 complexes have a prolonged in vivo half-life. (A) Host WT mice received a single injection of PBS, 20 μg rhIL-2 or 1.5 μg rhIL-2 plus 15 μg MAB602 anti-hIL-2 mAbCD122 at the indicated time-points before adoptive transfer of CFSE-labeled Thy1.1+ MP CD8+ T cells. Host spleens were analyzed by flow cytometry 3 days after adoptive transfer. Histograms shown are gated on Thy1.1+ CD8+ donor cells. Numbers indicate percentage of divided cells. (B and C) WT mice received a single injection at indicated doses of rhIL-2 (▲, 1.5 μg; •, 15 μg) or rhIL-2 plus MAB602 anti-hIL-2 mAbCD122 (▲, 0.3 μg plus 3 μg; •, 1.5 μg plus 15 μg) (B) or rhIL-2 plus 5344 anti-hIL-2 mAbCD25 (▲, 1.5 μg IL-2; •, 1.5 μg plus 15 μg) (C), and blood samples were collected at the indicated time-points after injection. Serum was assayed on CTLL-2 cells. Proliferation was determined by measuring incorporation of [3H]-thymidine. The data are representative of at least three different experiments, with each profile representing one of at least two mice.
The second approach involved the use of IL-2-sensitive CTLL-2 cells for measuring IL-2 activity in the serum of mice at different time-points after injection of IL-2. This approach is reasonable as CTLL-2 cells did not respond to endogenous amounts of cytokines present in the normal mouse serum and, more importantly, displayed a comparable sensitivity to IL-2 and both forms of IL-2/mAb complexes (Fig. S2). Here, two different doses of IL-2 (15 μg vs. 1.5 μg) and of IL-2/mAbCD122 complexes (1.5 μg/15 μg vs. 0.3 μg/3 μg) were administered to assess the effect of cytokine concentration on in vivo lifespan. In line with above results using CFSE-labeled MP CD8+ T cells, serum half-life of IL-2 injected at a high dose (15 μg) was found to be approximately 6 h, whereas half-life of the standard dose (1.5 μg) of IL-2/mAbCD122 complexes was approximately 24 h, both for human and mouse IL-2 (Fig. 2B and Fig. S3). The half-life of IL-2 injected at the low dose (1.5 μg) appeared to be shorter (approximately 2 h); because this is the dose used to generate the standard dose of IL-2/mAbCD122 complexes, this result indicated that a 12-fold extension in IL-2 lifespan was achieved through the formation of IL-2/mAb complexes. The half-life measurement of IL-2/mAbCD122 complexes using CTLL-2 cells was therefore comparable to the direct in vivo measurement using MP CD8+ T cells (Fig. 2A).
Notably, in vivo half-life of the standard dose of IL-2/mAbCD25 complexes, as determined by the CTLL-2 approach, proved to be even longer than for IL-2/mAbCD122 complexes. Thus, IL-2 activity was detectable for up to 72 h for IL-2/mAbCD25 complexes with an estimated half-life of over 48 h (Fig. 2C and Figs. S3 and S4). Interestingly, half-life of IL-2/mAbCD122 complexes could be extended to resemble IL-2/mAbCD25 complexes by depletion of CD8+ T cells and NK cells in WT mice (Fig. S4), suggesting that consumption by these cell subsets is a limiting factor for the half-life of IL-2/mAbCD122 complexes.
Extended Half-Life Is Not Sufficient for Strong Activity of IL-2/mAbCD122 Complexes.
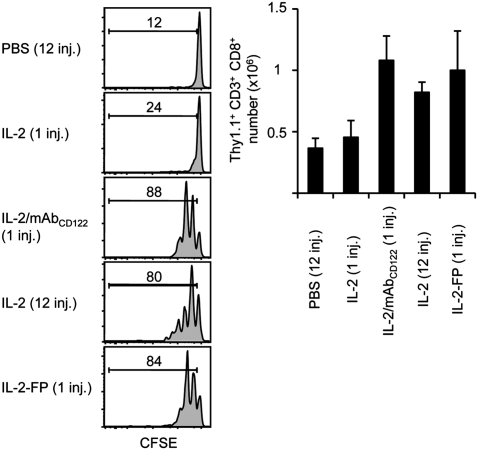
To further assess the role of extended in vivo IL-2 lifespan in mediating the potent activity of IL-2/mAbCD122 complexes, we tested whether repeated injections of IL-2 would mimic the strong activity of IL-2/mAbCD122 complexes. However, although repeated injections of IL-2 at 2 h intervals for the duration of 24 h displayed significantly more activity than one injection of IL-2, it was still considerably less effective in inducing proliferation of MP CD8+ T cells than one single injection of IL-2/mAbCD122 complexes (Fig. 3A). Alternatively, we determined whether repeated injections of IL-2/mAbCD122 complexes generated with F(ab’)2 fragments, which we have previously shown to display minimal activity (8), would restore the strong activity of intact IL-2/mAbCD122 complexes. Notably, repeated injections of IL-2/mAbCD122 complexes generated with F(ab’)2 fragments were as effective or even more effective than a single injection of intact IL-2/mAbCD122 complexes (Fig. 3B). Collectively, these results suggest the following two conclusions. First, because the half-life of F(ab’)2 fragment is approximately 6–12 h (31, 32), IL-2/F(ab’)2 complexes are ineffective mainly because of their short lifespan, thus indicating that extension of IL-2 half-life is an essential mechanism for in vivo activity of IL-2/mAb complexes. Second, and more importantly, repeated injections of IL-2/F(ab’)2 complexes, but not of IL-2, were able to mimic the potent activity of IL-2/mAbCD122 complexes, thus suggesting that the specific binding of anti-IL-2 mAb is crucial for their strong in vivo activity.
Fig. 3.
Both Fc and Fab contribute by distinct mechanisms to in vivo activity of IL-2/mAbCD122 complexes. (A) CFSE-labeled Thy1.1+ MP CD8+ T cells were adoptively transferred to WT mice. Host mice then received a single injection of rhIL-2, rhIL-2 plus MAB602 anti-hIL-2 mAbCD122, or 12 injections of PBS or rhIL-2, every 2 h for 24 h. Host spleens were analyzed by flow cytometry 3 days after adoptive transfer for CFSE dilution and cell recovery of Thy1.1+ CD8+ donor cells. Numbers indicate percentage of divided cells. (B) CFSE-labeled Thy1.1+ MP CD8+ T cells were adoptively transferred to WT mice. Host mice then received a single injection of rmIL-2 plus S4B6 anti-mIL-2 mAbCD122, rmIL-2 plus F(ab’)2 fragments of S4B6 anti-mIL-2 mAbCD122, or 12 injections of PBS or rmIL-2 plus S4B6 F(ab’)2, every 2 h for 24 h. CFSE dilution and cell recovery of Thy1.1+ CD8+ donor cells in lymph nodes was analyzed by flow cytometry 7 days after adoptive transfer. Numbers indicate percentage of divided cells. The data are representative of three different experiments, with each profile representing one of at least two mice.
IL-2-IgG Fusion Proteins Have a Long Half-Life but Low Biological Activity.
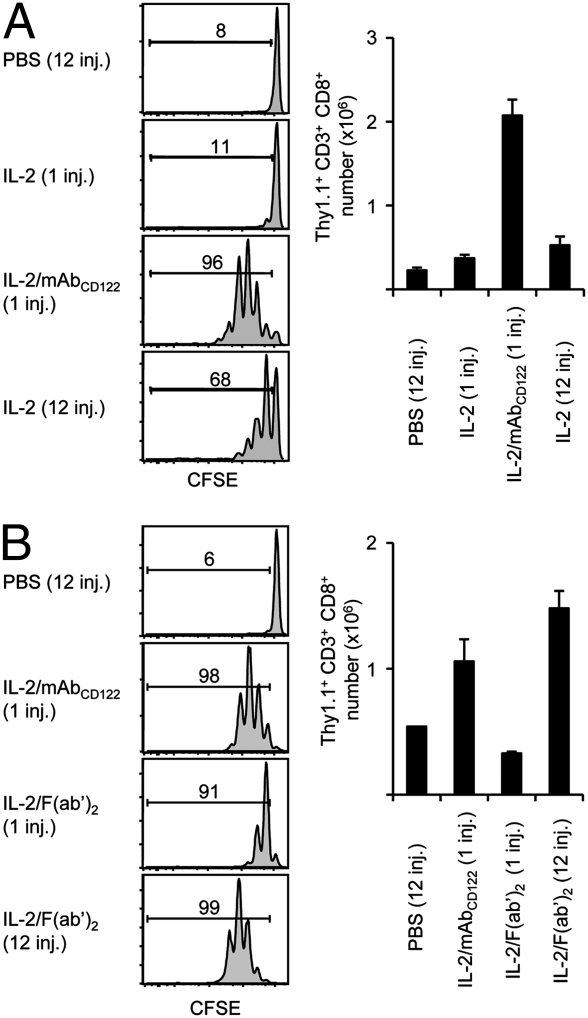
To further investigate the role of extended IL-2 half-life in contributing to IL-2/mAbCD122 activity in vivo, IL-2/mAbCD122 complexes were compared to a recombinant IL-2-IgG fusion protein (IL-2-FP), designated chTNT-3/IL-2 and comprised of hIL-2 covalently linked to a chimeric anti-nuclear IgG (33). At an equivalent IL-2 dose, IL-2-FP was found to be more potent than IL-2 alone, but considerably less effective than IL-2/mAbCD122 complexes in inducing expansion of MP CD8+ T cells (Fig. 4A and Fig. S5). Interestingly, this was the case despite the fact that IL-2-FP exhibited similar or even longer in vivo lifespan as IL-2/mAbCD122 complexes (Fig. 4B). Although these findings support the view that multiple factors are involved in mediating the activity of IL-2/mAbCD122 complexes, these results could also be due to structural differences between these reagents. For instance, unlike IL-2/mAbCD122 complexes, IL-2 in the IL-2-FP is covalently attached to IgG and cannot dissociate.
Fig. 4.
Prolonging half-life of IL-2 is not sufficient to mimic IL-2/mAbCD122 complexes. (A and C) CFSE-labeled Thy1.1+ MP CD8+ T cells were adoptively transferred to WT mice. On days 1 and 3, host mice received injections of PBS, rhIL-2, rhIL-2 plus MAB602 anti-hIL-2 mAbCD122, or chTNT-3/IL-2 hIL-2 fusion protein (IL-2-FP), as well as (C) IL-2-FP plus MAB602 anti-hIL-2 mAbCD122 (IL-2-FP/mAbCD122). CFSE dilution of cells recovered from the host spleen was analyzed by flow cytometry 6 days after adoptive transfer. Histograms shown are gated on Thy1.1+ CD8+ donor cells. Numbers indicate percentage of divided cells. (B and D) WT mice received a single injection of rhIL-2 (▲, IL-2), rhIL-2 plus MAB602 anti-hIL-2 mAbCD122 or IL-2-FP (both displayed as •), as well as (D) IL-2-FP plus MAB602 anti-hIL-2 mAbCD122 (•, IL-2-FP/mAbCD122). Blood samples were collected at the indicated time-points, and serum was assayed for proliferation of CTLL-2 cells by measuring incorporation of [3H]-thymidine. The data are representative of three different experiments, with each profile representing one of at least two mice.
Thus, we tested whether binding of anti-IL-2 mAb to IL-2-FP might boost the activity of IL-2-FP. Interestingly, complexes of IL-2-FP bound to anti-IL-2 mAb MAB602, designated IL-2-FP/mAbCD122 complexes, were found to induce much stronger expansion of MP CD8+ T cells than IL-2-FP and even surpassed IL-2/mAbCD122 complexes (Fig. 4C and Fig. S5). Surprisingly, binding of IL-2-FP to mAbCD122 did not alter the lifespan of IL-2-FP (Fig. 4D), thus indicating that a significant enhancement of IL-2 activity can be achieved without further increasing the half-life of IL-2.
IL-2/mAbCD122 Complexes Are Protected from Interaction with CD25.
Recently, we found that, in addition to immune cells such as Tregs, nonimmune cells express CD25. Therefore, ubiquitous expression of CD25 may lead to a decrease in IL-2 availability to T cells and NK cells (1, 2). Accordingly, an additional mechanism by which IL-2/mAbCD122 complexes, that are recognized by βγ IL-2R irrespective of CD25 expression, display strong activity could be that they avoid interaction with these CD25+ cells. To test this idea, we took advantage of the blocking anti-CD25 mAb PC61. Concomitant injection of IL-2 and anti-CD25 mAb only minimally increased the activity of soluble IL-2 (Fig. 5A). However, coadministration of anti-CD25 mAb greatly increased the activity of IL-2-FP, leading to proliferation that was almost as intense as with IL-2/mAbCD122 complexes at an equivalent IL-2 dose (Fig. 5A). Moreover, with repeated IL-2 injections every 2 h, use of anti-CD25 mAb also markedly enhanced the effects of IL-2 (Fig. 5B).
Fig. 5.
IL-2 requires prolonged half-life and CD25 blockade to mimic the activity of IL-2/mAbCD122 complexes. (A) CFSE-labeled Thy1.1+ MP CD8+ T cells were adoptively transferred to WT mice. On days 1 and 3, host mice received injections of PBS, rhIL-2, rhIL-2 plus MAB602 anti-hIL-2 mAbCD122, or chTNT-3/IL-2 IL-2-FP. Where indicated, mice also received daily injections of 200 μg of anti-CD25 mAb. CFSE dilution and cell recovery of Thy1.1+ CD8+ donor cells in spleen was analyzed by flow cytometry 6 days after adoptive transfer. (B) CFSE-labeled Thy1.1+ MP CD8+ T cells were adoptively transferred to WT mice. Host mice then received a single injection of rhIL-2 or rhIL-2 plus MAB602 anti-hIL-2 mAbCD122, or 12 injections of PBS or rhIL-2 every 2 h for 24 h. Where indicated, mice also received daily injections of 200 μg anti-CD25 mAb. Host spleens were analyzed by flow cytometry 3 days after adoptive transfer for CFSE dilution and cell recovery of Thy1.1+ CD8+ donor cells. Numbers in A and B indicate percentage of divided cells. The data are representative of three different experiments, with each profile representing one of at least two mice.
The above results strongly suggest that preventing interaction of IL-2 with CD25 is a second major mechanism to explain how IL-2/mAbCD122 complexes mediate their strong activity. Nevertheless, it is notable that the activity of IL-2-FP, and also IL-2 given repeatedly in the presence of blocking anti-CD25 mAb, was still slightly lower than that of IL-2/mAbCD122 complexes (Fig. 5). To determine whether this difference could be due to an inability of the anti-CD25 mAb to completely neutralize IL-2 interaction with CD25, the activity of IL-2-FP and IL-2/mAbCD122 complexes was directly compared in CD25−/− mice. One complicating feature of CD25−/− mice is that their high serum levels of IL-2 are able to induce spontaneous proliferation of adoptively-transferred donor MP CD8+ T cells (34, 35). To circumvent this problem, the activity of IL-2-FP and IL-2/mAbCD122 complexes was measured in these hosts 3 days after injection of donor T cells, just before the time it takes for donor T cells to undergo cell division in response to host IL-2. Significantly, as assessed by expansion of donor MP CD8+ T cells, the activity of IL-2/mAbCD122 complexes, repeated IL-2 injections, and IL-2-FP were all virtually identical in CD25−/− hosts (Fig. 6).
Discussion
Collectively, these findings suggest that IL-2/mAbCD122 complexes function by extending the half-life of IL-2 and by preventing the interaction of IL-2 with CD25, thus leading to a 40-fold higher in vivo biological activity compared to soluble IL-2. A notable difference between the two distinct IL-2/mAb complexes is that IL-2/mAbCD25 acutely rely on FcRn, whereas FcRn play only a minimal role for IL-2/mAbCD122. Because the half-life of IL-2/mAbCD122 complexes could be extended to resemble the half-life of IL-2/mAbCD25 complexes by depleting CD8+ T and NK cells, it could be hypothesized that consumption by rapidly-dividing MP CD8+ T and NK cells limits the half-life of IL-2/mAbCD122 to approximately 24 h. In contrast, IL-2/mAbCD25 complexes bind selectively to the relatively small population of CD25-expressing cells, mostly CD25+ CD4+ T cells, resulting in a lifespan of about 72 h for these complexes. Thus, the differential consumption of IL-2/mAb complexes might explain their half-life and level of FcRn dependence. This notion is further supported by the observation that the half-life of IL-2/mAbCD25 complexes is significantly reduced in FcRn−/− mice, whereas the half-life of IL-2/mAbCD122 complexes in FcRn−/− mice remains largely unaffected. It should be noted that FcRn become important for serum IgG half-life only after 24–36 h (27).
According to the literature, IL-2 homeostasis is believed to depend on IL-2 production by T cells and IL-2 elimination by renal metabolism. As mentioned above, IL-2 is mainly produced by activated CD4+ T cells, with a minor contribution by activated CD8+ cells, NK cells, NK T cells, and DCs that have been stimulated by microbial stimuli (1, 2, 9). Accordingly, the vast majority of IL-2 production in vivo will take place in the secondary lymphoid organs, notably the lymph nodes, and will also be consumed there by proliferating T cells. Hence, under normal conditions, it is unlikely that IL-2 leaks out into the blood and is then eliminated by the kidneys. Thus, normal IL-2 homeostasis might not depend largely on renal elimination but more on absorption by CD25+ cells. Evidence for this idea is provided in this study where repeated i.p. injections of IL-2 led to some proliferation of memory CD8+ T cells, which was further enhanced by concomitant CD25 blockade.
Other evidence for CD25 in IL-2 homeostasis comes from studies using CD25−/− animals. CD25-deficient mice contain highly elevated serum IL-2 levels (34, 35). This may be due to the presence of strongly-activated T cells, but the majority of T cells in CD25−/− mice are resting, slowly-proliferating cells. High serum IL-2 levels in CD25−/− mice might be due to a lack of CD25+ CD4+ Tregs, thus leading to diminished utilization of IL-2. In line with this idea is the observation that CD25+ CD4+ Tregs are able to deprive effector T cells from cytokines (36), including IL-2, and that transfer of purified CD25+ CD4+ Tregs to CD25−/− mice led to a reduction of serum IL-2 levels (37). Apart from its presence on Tregs, CD25 on other lymphocytes or on nonimmune cells might also play a role in IL-2 homeostasis. In this context, we found significant CD25 expression on mouse nonimmune cells in vivo. Also, fibroblasts have been reported to express CD25 (2, 38, 39). Interestingly, injection of IL-2-FP plus anti-CD4 mAb with the aim of depleting CD25+ CD4+ Tregs was not as efficient in stimulating memory CD8+ T cells in vivo as IL-2-FP plus anti-CD25 mAb.
Finally, cytokines can also interact with components of the extracellular matrix (ECM), most notably heparan sulfate proteoglycans, which have been reported to bind various cytokines, including IL-1α, IL-1β, IL-2, IL-3, IL-6, IL-7, GM-CSF, and TGF-β1, in addition to chemokines and growth factors (40 –44). Notably, anti-IL-2 mAbs might mediate some of their effects by influencing the binding of IL-2 to the ECM.
Materials and Methods
Mice.
C57BL/6 (referred to as WT) and CD25−/− mice, on a C57BL/6 background, were obtained from Charles River and The Jackson Laboratory, respectively. IL-7 tg mice on a C57BL/6 background, which express the IL-7 transgene under the control of the mouse MHC class II Eα promoter (45), were a kind gift of Drs. Daniela Finke (Center for Biomedicine, Basel, Switzerland) and Rod Ceredig (National University of Ireland, Galway, Ireland) and were bred onto a Thy1.1-congenic background. FcRI−/−FcRγ−/−FcRIIB−/− and FcRn−/− were a kind gift of Dr. Jeffrey Ravetch (The Rockefeller University, New York, NY) and Dr. Derry Roopenian (The Jackson Laboratory, Bar Harbor, ME), respectively. All animals were maintained under specific pathogen-free conditions and used at 6–8 weeks of age. Experiments were conducted in accordance to Swiss Federal Veterinary Office guidelines and to National Institutes of Health guidelines and were approved by the Cantonal Veterinary Office and the Institutional Animal Care and Use Committee at The Scripps Research Institute.
Flow Cytometry.
Cell suspensions of pooled LN (cervical, axillary, inguinal, and mesenteric) or spleen were prepared according to standard protocols and stained for flow cytometry analysis using PBS containing 4% FCS and 2.5mM EDTA (46). The following mAbs were used: PE-conjugated anti-CD3 (145-2C11, eBioscience), peridinin chlorophyll protein-cyanin 5.5-conjugated anti-CD8α (53-6.7, BD Biosciences), and allophycocyanin (APC)-conjugated anti-Thy1.1 (HIS51, eBioscience). Samples were acquired on a LSR II (BD Biosciences) and analyzed using FlowJo software (Tree Star).
Proliferation in Vivo.
CD44high CD122high MP CD8+ T cells were obtained from pooled LN and spleen of IL-7 tg mice on a Thy1.1-congenic background. CD4+ T cells were obtained from pooled LN and spleen of WT mice on a Thy1.1-congenic background. Isolation of CD8+ and CD4+ T cells was performed using the EasySep CD8+ and CD4+ T cell enrichment kit (Stem Cell Technologies), respectively, yielding routinely purities of >95%. Enriched MP CD8+ T cells or CD4+ T cell were injected i.v. at 2–3 × 106 cells/mouse.
Administration of Cytokines, Antibodies, and Fusion Proteins in Vivo.
Unless otherwise stated, mice received i.p. injections of 1.5 μg IL-2 or a mixture of 1.5 μg IL-2 plus 15 μg anti-IL-2 mAb. Recombinant mouse IL-2 (rmIL-2) was obtained from eBioscience. S4B6 anti-mIL-2 mAbCD122 was generated from culture supernatants of the S4B6.1 hybridoma and purified from either culture supernatant or ascites fluid on protein G column. F(ab’)2 fragments of S4B6 were prepared as previously described (8). JES6-1A12 anti-mIL-2 mAbCD25 was obtained from eBioscience. Recombinant human IL-2 (rhIL-2) and MAB602 (clone 5355) anti-hIL-2 mAbCD122 were purchased from R&D Systems. 5344 (clone 5344.111) anti-hIL-2 mAbCD25 was obtained from BD Biosciences. Where indicated, mice also received daily i.p. injections of 200 μg anti-CD25 (PC61, Bioexpress), anti-CD8 (YTS169, Bioexpress) and/or anti-NK1.1 mAb (PK136, Bioexpress). The hIL-2 fusion protein IL-2-FP (chTNT-3/IL-2) was a kind gift of Dr. Alan L. Epstein (University of Southern California, Los Angeles, CA) (33). Mice received i.p. injections of 15 μg IL-2-FP or a mixture of 15 μg IL-2-FP plus 15 μg anti-IL-2 mAb, where 15 μg of IL-2-FP equaled the proliferative capacity of 1.5 μg rhIL-2 as measured on CTLL-2 cells (ATCC) in vitro. All anti-IL-2 mAbs were incubated with IL-2 or IL-2-FP for 15 min at room temperature before injection.
Proliferation ex Vivo.
CTLL-2 cells were seeded at 5 × 104 cells/well in a 96-well plate, and 10 μL of mouse serum or control was added to each well. Cells were cultured under standard conditions (37 °C, 5% CO2) for 48 h. [3H]-thymidine was added for the last 24 h and cell proliferation was measured using [3H]-thymidine incorporation on a liquid scintillation counter (Beckman).
Supplementary Material
Acknowledgments
We thank Dr. A. Epstein for providing IL-2-FP, Drs. D. Finke and R. Ceredig for providing IL-7 tg mice, Drs. J. Ravetch, D. Roopenian, A. Shaw, and S. Akilesh (both from the Washington University School of Medicine, St. Louis, MO) for providing FcR−/− mice, the animal caretakers of the University Hospital of Lausanne for their technical help, and the National Cancer Institute’s Biological Resources Branch for providing hIL-2. This work was supported by the Swiss National Science Foundation Grant 320000-118471 (to O.B.).
Footnotes
Conflict of interest statement: O.B., C.D.S., and J.S. are shareholders of Nascent Biologics Inc.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909384107/DCSupplemental.
References
- 1.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 2.Létourneau S, Krieg C, Pantaleo G, Boyman O. IL-2- and CD25-dependent immunoreg-ulatory mechanisms in the homeostasis of T-cell subsets. J Allergy Clin Immunol. 2009;123:758–762. doi: 10.1016/j.jaci.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Smith KA. Interleukin-2: Inception, impact, and implications. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 4.Waldmann TA. The multi-subunit interleukin-2 receptor. Annu Rev Biochem. 1989;58:875–911. doi: 10.1146/annurev.bi.58.070189.004303. [DOI] [PubMed] [Google Scholar]
- 5.Minami Y, Kono T, Miyazaki T, Taniguchi T. The IL-2 receptor complex: Its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura Y, et al. Heterodimerization of the IL-2 receptor beta- and gamma-chain cytoplasmic domains is required for signalling. Nature. 1994;369:330–333. doi: 10.1038/369330a0. [DOI] [PubMed] [Google Scholar]
- 7.Willerford DM, et al. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 8.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 9.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donohue JH, Rosenberg SA. The fate of interleukin-2 after in vivo administration. J Immunol. 1983;130:2203–2208. [PubMed] [Google Scholar]
- 11.Hu P, Mizokami M, Ruoff G, Khawli LA, Epstein AL. Generation of low-toxicity interleukin-2 fusion proteins devoid of vasopermeability activity. Blood. 2003;101:4853–4861. doi: 10.1182/blood-2002-10-3089. [DOI] [PubMed] [Google Scholar]
- 12.Boyman O, Surh CD, Sprent J. Potential use of IL-2/anti-IL-2 antibody immune complexes for the treatment of cancer and autoimmune disease. Expert Opin Biol Ther. 2006;6:1323–1331. doi: 10.1517/14712598.6.12.1323. [DOI] [PubMed] [Google Scholar]
- 13.Harvill ET, Fleming JM, Morrison SL. In vivo properties of an IgG3-IL-2 fusion protein. A general strategy for immune potentiation. J Immunol. 1996;157:3165–3170. [PubMed] [Google Scholar]
- 14.Jones AT, Ziltener HJ. Enhancement of the biologic effects of interleukin-3 in vivo by anti-interleukin-3 antibodies. Blood. 1993;82:1133–1141. [PubMed] [Google Scholar]
- 15.Finkelman FD, et al. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol. 1993;151:1235–1244. [PubMed] [Google Scholar]
- 16.Klein B, Brailly H. Cytokine-binding proteins: Stimulating antagonists. Immunol Today. 1995;16:216–220. doi: 10.1016/0167-5699(95)80161-8. [DOI] [PubMed] [Google Scholar]
- 17.Boyman O, Ramsey C, Kim DM, Sprent J, Surh CD. IL-7/anti-IL-7 mAb complexes restore T cell development and induce homeostatic T Cell expansion without lymphopenia. J Immunol. 2008;180:7265–7275. doi: 10.4049/jimmunol.180.11.7265. [DOI] [PubMed] [Google Scholar]
- 18.Mostböck S, et al. IL-2/anti-IL-2 antibody complex enhances vaccine-mediated antigen-specific CD8(+) T cell responses and increases the ratio of effector/memory CD8(+) T cells to regulatory T cells. J Immunol. 2008;180:5118–5129. doi: 10.4049/jimmunol.180.7.5118. [DOI] [PubMed] [Google Scholar]
- 19.Molloy MJ, Zhang W, Usherwood EJ. Cutting edge: IL-2 immune complexes as a therapy for persistent virus infection. J Immunol. 2009;182:4512–4515. doi: 10.4049/jimmunol.0804175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verdeil G, Marquardt K, Surh CD, Sherman LA. Adjuvants targeting innate and adaptive immunity synergize to enhance tumor immunotherapy. Proc Natl Acad Sci USA. 2008;105:16683–16688. doi: 10.1073/pnas.0805054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin GH, Hirano T, Murakami M. Combination treatment with IL-2 and anti-IL-2 mAbs reduces tumor metastasis via NK cell activation. Int Immunol. 2008;20:783–789. doi: 10.1093/intimm/dxn036. [DOI] [PubMed] [Google Scholar]
- 22.Wilson MS, et al. Suppression of murine allergic airway disease by IL-2:anti-IL-2 monoclonal antibody-induced regulatory T cells. J Immunol. 2008;181:6942–6954. doi: 10.4049/jimmunol.181.10.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webster KE, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: Induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phelan JD, Orekov T, Finkelman FD. Cutting edge: Mechanism of enhancement of in vivo cytokine effects by anti-cytokine monoclonal antibodies. J Immunol. 2008;180:44–48. doi: 10.4049/jimmunol.180.1.44. [DOI] [PubMed] [Google Scholar]
- 25.Kamimura D, Bevan MJ. Naive CD8+ T cells differentiate into protective memory-like cells after IL-2 anti IL-2 complex treatment in vivo. J Exp Med. 2007;204:1803–1812. doi: 10.1084/jem.20070543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes N, et al. FcgammaRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity. 2002;16:379–389. doi: 10.1016/s1074-7613(02)00287-x. [DOI] [PubMed] [Google Scholar]
- 27.Roopenian DC, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170:3528–3533. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- 28.Boyman O, Cho JH, Tan JT, Surh CD, Sprent J. A major histocompatibility complex class I-dependent subset of memory phenotype CD8+ cells. J Exp Med. 2006;203:1817–1825. doi: 10.1084/jem.20052495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kieper WC, et al. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J Exp Med. 2002;195:1533–1539. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roopenian DC, Akilesh S. FcRn: The neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 31.Iwahashi T, et al. Specific targeting and killing activities of anti-P-glycoprotein monoclonal antibody MRK16 directed against intrinsically multidrug-resistant human colorectal carcinoma cell lines in the nude mouse model. Cancer Res. 1993;53:5475–5482. [PubMed] [Google Scholar]
- 32.Buist MR, et al. Kinetics and tissue distribution of the radiolabeled chimeric monoclonal antibody MOv18 IgG and F(ab’)2 fragments in ovarian carcinoma patients. Cancer Res. 1993;53:5413–5418. [PubMed] [Google Scholar]
- 33.Hornick JL, et al. Pretreatment with a monoclonal antibody/interleukin-2 fusion protein directed against DNA enhances the delivery of therapeutic molecules to solid tumors. Clin Cancer Res. 1999;5:51–60. [PubMed] [Google Scholar]
- 34.Tsunobuchi H, et al. Memory-type CD8+ T cells protect IL-2 receptor alpha-deficient mice from systemic infection with herpes simplex virus type 2. J Immunol. 2000;165:4552–4560. doi: 10.4049/jimmunol.165.8.4552. [DOI] [PubMed] [Google Scholar]
- 35.Wakabayashi K, et al. IL-2 receptor alpha(−/−) mice and the development of primary biliary cirrhosis. Hepatology. 2006;44:1240–1249. doi: 10.1002/hep.21385. [DOI] [PubMed] [Google Scholar]
- 36.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 37.Sharma R, et al. A regulatory T cell-dependent novel function of CD25 (IL-2Ralpha) controlling memory CD8(+) T cell homeostasis. J Immunol. 2007;178:1251–1255. doi: 10.4049/jimmunol.178.3.1251. [DOI] [PubMed] [Google Scholar]
- 38.Plaisance S, et al. The IL-2 receptor present on human embryonic fibroblasts is functional in the absence of P64/IL-2R gamma chain. Int Immunol. 1993;5:843–848. doi: 10.1093/intimm/5.8.843. [DOI] [PubMed] [Google Scholar]
- 39.Gruss HJ, Scott C, Rollins BJ, Brach MA, Herrmann F. Human fibroblasts express functional IL-2 receptors formed by the IL-2R alpha- and beta-chain subunits: association of IL-2 binding with secretion of the monocyte chemoattractant protein-1. J Immunol. 1996;157:851–857. [PubMed] [Google Scholar]
- 40.Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol. 2006;6:633–643. doi: 10.1038/nri1918. [DOI] [PubMed] [Google Scholar]
- 41.Gordon MY, Riley GP, Watt SM, Greaves MF. Compartmentalization of a haematopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. Nature. 1987;326:403–405. doi: 10.1038/326403a0. [DOI] [PubMed] [Google Scholar]
- 42.Ramsden L, Rider CC. Selective and differential binding of interleukin (IL)-1 alpha, IL-1 beta, IL-2 and IL-6 to glycosaminoglycans. Eur J Immunol. 1992;22:3027–3031. doi: 10.1002/eji.1830221139. [DOI] [PubMed] [Google Scholar]
- 43.Clarke D, Katoh O, Gibbs RV, Griffiths SD, Gordon MY. Interaction of interleukin 7 (IL-7) with glycosaminoglycans and its biological relevance. Cytokine. 1995;7:325–330. doi: 10.1006/cyto.1995.0041. [DOI] [PubMed] [Google Scholar]
- 44.Borghesi LA, Yamashita Y, Kincade PW. Heparan sulfate proteoglycans mediate interleukin-7-dependent B lymphopoiesis. Blood. 1999;93:140–148. [PubMed] [Google Scholar]
- 45.Mertsching E, Burdet C, Ceredig R. IL-7 transgenic mice: Analysis of the role of IL-7 in the differentiation of thymocytes in vivo and in vitro. Int Immunol. 1995;7:401–414. doi: 10.1093/intimm/7.3.401. [DOI] [PubMed] [Google Scholar]
- 46.Krieg C, Boyman O, Fu YX, Kaye J. B and T lymphocyte attenuator regulates CD8+ T cell-intrinsic homeostasis and memory cell generation. Nat Immunol. 2007;8:162–171. doi: 10.1038/ni1418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.