Abstract
Climate change and habitat destruction have been linked to global declines in vertebrate biodiversity, including mammals, amphibians, birds, and fishes. However, invertebrates make up the vast majority of global species richness, and the combined effects of climate change and land use on invertebrates remain poorly understood. Here we present 35 years of data on 159 species of butterflies from 10 sites along an elevational gradient spanning 0–2,775 m in a biodiversity hotspot, the Sierra Nevada Mountains of Northern California. Species richness has declined at half of the sites, with the most severe reductions at the lowest elevations, where habitat destruction is greatest. At higher elevations, we observed clear upward shifts in the elevational ranges of species, consistent with the influence of global warming. Taken together, these long-term data reveal the interacting negative effects of human-induced changes on both the climate and habitat available to butterfly species in California. Furthermore, the decline of ruderal, disturbance-associated species indicates that the traditional focus of conservation efforts on more specialized and less dispersive species should be broadened to include entire faunas when estimating and predicting the effects of pervasive stressors.
Keywords: biodiversity, elevational gradient, global change, Lepidoptera, phenology
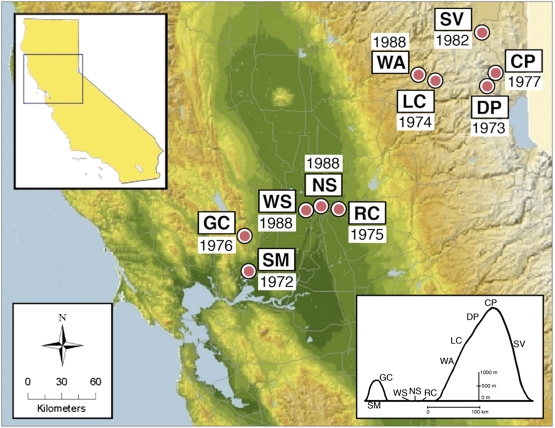
Contemporary and anticipated effects of climate change on biodiversity, including poleward shifts of latitudinal ranges, upslope shifts of elevational ranges, and possible extinctions, are clear and well-documented (1 –7). In addition, land-use change has been established as a leading cause of species endangerment (8 –10). Most studies to date have focused on vertebrate taxa, even though rates of extinction and range contractions estimated for vertebrate groups often underestimate the severity of current declines for invertebrate taxa (11, 12). Here we present an analysis of more than three decades of butterfly presence–absence data collected along an extensive elevational gradient (0–2,775 m) in western North America that has been sampled approximately every 2 weeks for between 19 and 35 years. The 10 study sites encompass much of the climatological, geologic, and floristic diversity of Northern California (Fig. 1 and Table S1). Because the data used here were collected by a single observer, the dataset is unusual among long-term ecological datasets in being free from sampling artifacts that can be introduced by multiple observers.
Fig. 1.
Map of study sites, abbreviations as follows: Suisun Marsh (SM), Gates Canyon (GC), West Sacramento (WS), North Sacramento (NS), Rancho Cordova (RC), Washington (WA), Lang Crossing (LC), Donner Pass (DP), Castle Peak (CP), and Sierra Valley (SV). Years near site labels indicate the year in which continuous sampling began at each site. The Inset in the Upper Left shows the location of the study area in Northern California. The Inset in the Lower Right is a cross-sectional schematic of Northern California, showing the elevational profile of the study sites.
We used this dataset to assess the extent to which climate change and land-use alteration have jointly influenced patterns of butterfly species richness in this system. Specifically, we test two predictions related to the effects of climate change and land-use alteration on biodiversity. First, we predict that any declines in richness will be most pronounced at the lowest elevations, consistent with global trends in the destruction of low-elevation habitat (13, 14). Second, we predict an upslope shift in elevational ranges, as warming temperatures cause envelopes of suitable climatic conditions to shift to higher elevations (2, 15–17). In conjunction with shifting elevational ranges, richness at the highest elevations should increase as species at mid-elevations find those habitats to be within acceptable abiotic limits (14). Other studies of invertebrates and global change have most often found that generalist species and those that are good dispersers are less sensitive to the impacts of climate and land-use change (1, 18, 19). We address this possibility by investigating the responses of butterflies in two natural history categories: ruderal and nonruderal species. Ruderal species tend to be generalists and good dispersers, whereas nonruderal species are specialists and poor dispersers (20, 21). In addition to addressing the major global-change predictions described above from the perspective of ruderal and nonruderal species, we investigate demographic connections between study sites for both ruderal and nonruderal species as a way to elucidate regional patterns of decline for these two groups.
Results
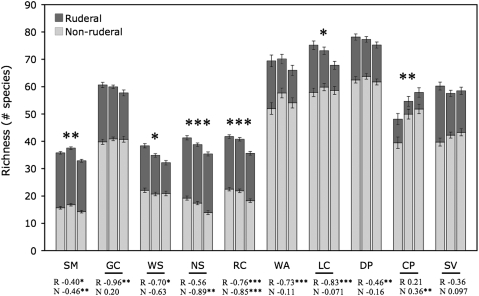
Richness has declined at 5 of the 10 sites since the initial surveys (Fig. 2 and Table S2), and the decrease has been greatest at the low-elevation sites. Richness has increased at only one site, the highest-elevation site (2,400–2,775 m) (Fig. 2). We found that temporal patterns of richness (increasing, decreasing, or static) differed considerably between ruderal and nonruderal species (Tables S3 and S4). Richness of both groups declined at the lower-elevation sites. However, at higher elevations, richness of ruderal species has declined, while richness of nonruderal species has either been stable or increased (Fig. 2). Ruderal and nonruderal species also differed in compositional turnover: ruderal species had lower turnover (i.e., species composition is similar from year to year), despite declines in overall ruderal richness at many sites, while nonruderal species had higher turnover (a greater fraction of these species have a more ephemeral presence, coming and going from year to year) (Table S5).
Fig. 2.
Temporal patterns of richness for all sites. Three bars are shown at each site, corresponding to the first, middle, and final third of the years at each site. Richness values are adjusted for sampling effort independently at each site; error bars are standard errors around sampling-adjusted estimates. Shading of bars indicates the number of species in two categories: ruderal and nonruderal. Asterisks above bars indicate a significant linear relationship between richness and years, considering all species at a site (both ruderal and nonruderal; Table S2). For sites marked with asterisks (*P < 0.05, **P < 0.01, ***P < 0.001), richness has declined over time, with the exception of CP, where there has been an increase in richness. Below the graph, a bar under the name of a site indicates a significant interaction between category (ruderal and nonruderal) and years. Values under site names are the β coefficients from multiple regression models independently examining richness for ruderal (R) and nonruderal (N) species, with significance denoted by asterisks: Negative values indicate a decline in the richness of the corresponding group. The decline in nonruderal species at WS is significant at P = 0.035, which is not significant following table-wide correction for false discovery rate.
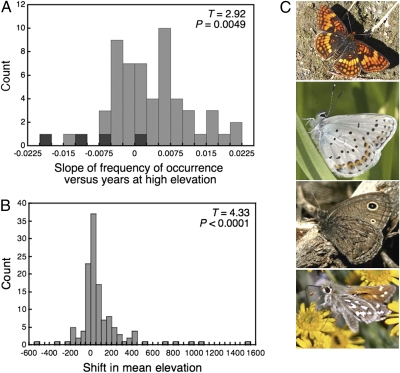
In addition to an increase in richness, abundance has also generally increased at the highest-elevation site (Figs. 2 and 3). However, two out of four alpine specialists, Cercyonis oetus and Hesperia nevada, at the highest-elevation site have become less abundant in recent years (a third alpine specialist, Oeneis chryxus ivallda, shows a negative, although nonsignificant, trend through time) (Fig. 3A). Along with the general increase in richness and abundance observed at the high-elevation site, there has been an upslope shift in elevational ranges along the west slope of the Sierra Nevada (Fig. 3B). Considering ruderal and nonruderal species separately, nonruderal species exhibited a significant upslope shift (T = 4.44, P < 0.0001), whereas ruderal species did not (T = 0.39, P = 0.70). An observed shift in mean elevation for a given species could be influenced by extirpation at lower elevations, rather than by an overall upward shift in elevation. An examination of the minimum and maximum elevations observed between early and late years (we focused on a comparison between the first and last 10-year blocks of time; SI Text) supports the conclusion that there has been an overall shift in elevational range: Across species, the minimum observed elevation did not change (T = 0.18, P = 0.43, one-tailed test), whereas the maximum observed elevation shifted upward (T = 1.69, P = 0.047, one-tailed test).
Fig. 3.
Montane shifts in frequency of occurrence and elevational range, with images of some of the species showing altered patterns of occurrence at the highest elevation. (A) The histogram shows the temporal trends for individual species at the highest site, CP. Values summarized are the slopes of regressions of occurrence versus years: Positive values indicate a species that has been more frequently observed over time at CP. Dark shading corresponds to slopes associated with four species that are considered alpine specialists. T and P values reported in each graph are the results of t tests asking whether the mean of the overall distribution differs from zero. (B) The histogram shows the distribution of elevational shifts by individual species along the west slope of the Sierra Nevada between early (1977–1986) and late (1988–2007) years, calculated from records at four sites, RC, LC, DP, and CP. The mean shift in elevation is 93.35 m (SE ± 21.56). (C) Chlosyne hoffmanni (Top) and Lycaeides idas (second from the Top) have been observed more frequently at CP, whereas Cercyonis oetus (third from the Top) and Hesperia nevada (Bottom) have been observed less frequently at CP in recent years (the latter two species are specialists of the alpine habitat at CP).
Climate across the transect has changed over the past three decades. Both average daily maximum temperatures and average daily minimum temperatures have increased across the majority of sites (Table S6), although precipitation has not varied systematically. Maximum temperatures, minimum temperatures, and precipitation all had consistent but generally weak associations with richness (Fig. S1 and Tables S7 and S8). Specifically, maximum daily temperatures had a positive association with richness, while minimum daily temperatures had a negative association (the effect of precipitation was more idiosyncratic) (Fig. S1).
Currently, data are available to address patterns of land use only at a coarse scale: Census data in 2-year blocks at the county level, going back to the mid-1980s. The conversion of land from open spaces to developed areas has been most pronounced around sites at the lowest, most heavily populated elevations (Table S9). There has been little or no development around the mid- and high-elevation sites, and land use in general (including grazing) has been relatively static at these sites (SI Text). Despite the coarse-grained land-use data, correlations between the extent of development and butterfly species richness are significantly negative for two of the Central Valley sites (WS and RC) and moderately significant (and also negative) for the third site (P = 0.067) (Table S10).
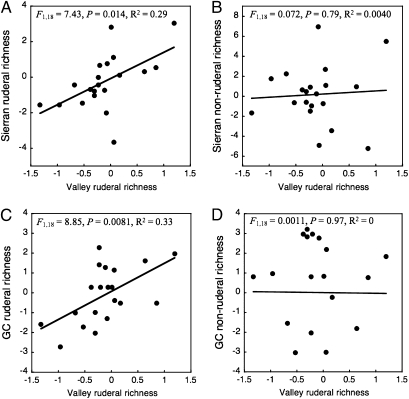
Finally, we addressed connections between species richness at valley and montane sites. In particular, we examined the possibility that the decline of ruderal species at montane sites (Fig. 1) could be explained by declines at lower elevations, which might act as demographic sources for dispersive, ruderal species colonizing from low to high elevations on a yearly basis. Consistent with this hypothesis, the richness of ruderal species in the valley predicts the richness of ruderal species in the mountains (both for the Sierran sites and for the Coast Range) (Fig. 4 A and C). However, valley ruderal richness does not predict montane nonruderal richness (Fig. 4 B and D); similarly, the richness of nonruderal fauna at low elevations does not predict either ruderal or nonruderal richness at higher elevations. The connection that we have detected between valley and montane richness is a within-year phenomenon: Correlations across years (i.e., incorporating a possible lag effect) are not significant, and there appears to be no time lag (SI Text).
Fig. 4.
Relationships between the richness of ruderal species at the Central Valley sites (WS, NS, RC) and the richness of both ruderal (A) and nonruderal (B) species in the Sierran sites (excluding SV) and both ruderal (C) and nonruderal (D) species in the Coast Range (GC). Values on both axes for all graphs are residuals from the relationship between richness and the number of visits per year (SI Text). Thus, these values have been corrected for variation in sampling effort among sites. Statistics reported in the upper portion of each graph are results of simple linear regressions. SV was excluded from these analyses, because the position of the site east of the Sierran crest (Fig. 1) makes it less likely to be connected via dispersal to the low elevations west of the Sierran crest.
Discussion
Butterfly communities along the elevational gradient examined here are temporally dynamic: Richness has generally declined at half of the 10 sites, while richness has increased at the highest-elevation site (Fig. 2 and Table S2). The declines in richness were centered at the lowest-elevation sites, which is consistent with the prediction that low-elevation biodiversity is disproportionately impacted by stressors associated with habitat destruction and climate change (14). At the highest elevation, there has been an increase in both richness (Fig. 2) and the abundance of individual species, with the exception of some alpine specialists that appear to be in decline (Fig. 3A). Demographic declines for species that specialize in the alpine environment are consistent with the warming and drying of that most extreme habitat (2, 22).
At intermediate elevations, dynamics have been complex: There has been a general upslope shift in elevational ranges (Fig. 3B), although temporal patterns of richness across sites differ between ruderal and nonruderal species. The ruderal taxa, which are both more dispersive and have more generalized habitat associations, have declined at the three intermediate-elevation sites (Fig. 2). These results present a contrast with those from Europe, where generalist butterflies have tended to be more resilient to both climatic and land-use changes (23 –25). This difference in the response of generalists, between our results and many European studies, is consistent with the possibility that European species are more resilient as a consequence of the longer evolutionary history these taxa have experienced with human development and land-use changes. We note, however, that it has been suggested that ongoing intensification of land use in Europe may now be having a greater impact on common, generalist species (26). The unusual pattern of more widespread decline of ruderal species at our sites appears to be a consequence of regional colonization dynamics: Low-elevation populations of ruderal species, which have been heavily impacted, act as sources for migrants that seasonally recolonize higher elevations. This link between valley and montane ruderal species is supported by significant linear relationships between ruderal richness at low and high elevations (Fig. 4). As the richness of ruderal species at low elevations has declined, richness at higher elevations has declined in parallel for these dispersive species.
Habitat destruction and shifting climatic regimes are both implicated in the temporal patterns of richness described here, and we can ask which of these two classes of stressors is having a greater impact. Both maximum and minimum temperatures have increased across sites (Table S6). However, these variables have contrasting effects on richness, as has been observed with other studies of Lepidoptera (27), which make these climatic factors unlikely to be the predominant forces shaping the observed temporal patterns of richness in our study (Fig. S1). Furthermore, if climate is predominantly responsible for the patterns of richness, then it should have a greater impact on ruderal species because they are in decline at 7 out of 10 sites, compared with nonruderal species, which are in decline at only 3 sites. However, of 30 potential interactions examining the effects of climate on ruderal and nonruderal species, in only 2 cases did interannual climate variation influence ruderal and nonruderal species differently (Table S8). Although climate does generally influence interannual variation in butterfly richness (Fig. S1), and is associated with shifting phenologies in the Central Valley (28), climatic variables do not have differential effects on ruderal and nonruderal species richness. This suggests that some factor in addition to climatic change has affected butterfly species richness along this gradient. We suggest habitat alteration at low elevations, which has likely destroyed habitat directly (potentially affecting both larval hosts and adult nectar resources) and reduced connectivity among habitats. The importance of habitat alteration is borne out by significant, negative correlations between development and richness at low elevations (described above). Habitat conversion also affects midelevation sites because it reduces the populations of species that colonize from lower elevations on an annual basis.
In summary, our results highlight the influence of both climate and land-use change on patterns of biodiversity, but these drivers influence ruderal and nonruderal species differentially. The contrasting dynamics for more generalized versus more specialized species highlight the value of regional datasets, such as ours, where demographic connections across sites and along elevational gradients can be seen. Climate change and habitat alteration clearly influence biodiversity (29). Understanding and predicting how these two drivers act independently and in concert (30, 31), and how their effects vary among taxa (32) and regions (15, 33), will be an important next step toward conserving biodiversity.
Methods
Presence/absence data were collected on an approximately biweekly basis for between 19 and 35 years (Fig. 1). Sites were chosen for the inclusion of maximal habitat diversity and number of butterfly species (Table S1). Sampling occurred during portions of the year when butterflies were flying: Nearly year-round at low-elevation sites, and for a more restricted window of time at higher elevations. Sampling followed the “Pollard walk” method, with a fixed route being walked and the presence of all butterfly species noted (34). Observations were entered into a Microsoft Access relational database, then migrated to an open-source MySQL database for online access at http://butterfly.ucdavis.edu.
We used a multiple regression approach to investigate temporal patterns in richness while controlling for sampling effort (we also investigated sampling using the nonparametric richness estimator Chao 2; SI Text). Specifically, at each site, richness was modeled with the number of species as the dependent variable, and the following independent variables: years and the number of visits per year. In some cases, the relationship between visits and observed richness is best fit with a polynomial relationship (thus the quadratic term for visits was included here and in other analyses when it was significant at P < 0.05; Figs. S2 and S3). All assumptions of multiple regression analysis were evaluated (SI Text). In addition to the multiple regression approach, we used an alternate analysis to visualize temporal patterns: Rather than treat years as a continuous variable, we divided the data for each site into thirds (early, middle, and late years) and used that as an ordered, categorical variable in ANOVA models including “years” (early, middle, and late) and the number of visits per year.
To investigate the possibility that ruderal and nonruderal species have exhibited different temporal patterns of richness, we repeated the analyses described above for ruderal and nonruderal groups separately, as well as in a combined analysis including “type” (ruderal or nonruderal) as a categorical factor and the interaction between type and years. The assignment of ruderal or nonruderal status to species was based on field observations and was made before the analyses reported here (20, 21). In addition to species richness, we also investigated community turnover for ruderal and nonruderal species as a descriptive index that is complementary to richness (SI Text).
We focused on one site, Castle Peak, to ask whether there have been changes in the frequency with which individual species have been observed over time at a high-elevation location. Although we do not have abundance data (as in absolute counts of individuals), we analyzed the fraction of days in which a species was observed in a given year out of the number of times the site was visited in that year. We calculated the fraction of days observed per year and regressed this value against years, and then extracted the slopes of these regressions as a measure of species-specific trends over time. The distribution of the slopes was analyzed to ask (using a single-sample t test) whether the distribution had a mean different from zero. We did not perform this analysis separately for ruderal and nonruderal species because only six ruderal species at Castle Peak had enough observations within the 27 years to be analyzed individually. See SI Text for a discussion of early years that were conservatively excluded from analyses due to insufficient sampling.
In addition to temporal patterns of richness at each site, we investigated the possibility that the presence of species has shifted through time along the primary elevational gradient represented in the data: the west slope of the Sierra Nevada. We focused on four sites (RC, LC, DP, and CP) that (i) had a long record of data, starting in the 1970s (Fig. 1), and (ii) were geographically relevant to potentially shifting elevational ranges on the west slope. Because an investigation of the elevational ranges of individual species is dependent on species-specific records, we could not remove the effect of sampling with the covariate approach that we used for richness (described above). Instead, we analyzed large blocks of years averaged together to reduce sampling-associated bias. Specifically, we compared elevational ranges (or occupancy) between two blocks of time, 1977–1986 and 1998–2007, representing early and late years from our data. For each block of time, we calculated the average elevation at which a given species was observed (see Table S1 for elevations). This was done by first averaging within years and across sites, and then within the two blocks of time. Finally, the difference between the average elevation in the two time blocks was calculated as the average shift in elevation for a given species. This was done both for all species considered together and separately for ruderal and nonruderal species; for each group a single-sample t test was used to ask whether the distribution of observed elevational-range shifts had a mean different from zero (with a positive value indicating an upslope shift). We also similarly analyzed the shift in the maximum and minimum elevational observations by species between the same two blocks of time.
To investigate climatic patterns and associations with richness, we focused on three variables: average daily maximum temperatures, average daily minimum temperatures, and average daily precipitation totals. For all of these, values were averaged over the entire “biological” year: from fall of one year (starting September 1) through the end of summer the following year. We used simple linear regression models to ask whether there have been significant temporal trends in climatic variables across Northern California. For these analyses, we increased the breadth of our investigations by including climate data starting in 1970 (and going through 2007), rather than restricting analyses to years with butterfly data (see SI Text for a discussion of sources from which weather data were taken). Analyses of weather data and richness followed a multiple regression format as outlined above (see SI Text for more details).
Trends in land use were investigated using publicly available data, as detailed in SI Text. These data were used for two purposes: (i) we investigated trends through time in development, to quantify which regions have been developed the most rapidly in recent decades; and (ii) we asked whether county-wide patterns of development are correlated with patterns of butterfly richness (following a correction for variation in visits; SI Text).
Finally, we investigated the hypothesis that ruderal dynamics were demographically linked between low- and high-elevation sites by asking whether annual variation in the richness of ruderal species in the Central Valley sites (WS, NS, and RC) could predict, using linear regression, the richness of ruderal species at higher elevations, both in the Coast Range (GC) and in the Sierra Nevada. Variation in sampling effort was corrected for here as in other analyses above (SI Text).
Supplementary Material
Acknowledgments
This research was funded by National Science Foundation Grants DEB-9306721 to A.M.S. and DBI-0317483 to A.M.S. and J. F. Quinn. M.L.F. was supported by the University of Nevada, Reno. N.J.S. was supported by Department of Energy Program for Ecosystem Research Grant DE-FG02-08ER64510. J.A.F was supported by National Science Foundation Grant DEB-0614223. For discussion and comments we thank K. Anderson, G. Crutsinger, J. Grace, J. Hellmann, E. Runquist, and C. Thomas. Images of butterflies in Fig. 3 are used with the permission of E. Runquist (C. hoffmanni photo), K. and M. Strangeland (L. idas and C. oetus), and D. Nunnallee (H. nevada).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909686107/DCSupplemental.
References
- 1.González-Megías A, Menéndez R, Roy D, Brereton T, Thomas CD. Changes in the composition of British butterfly assemblages over two decades. Glob Change Biol. 2008;14:1464–1474. [Google Scholar]
- 2.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2006;37:637–669. [Google Scholar]
- 3.Pounds JA, Fogden MPL, Campbell JH. Biological response to climate change on a tropical mountain. Nature. 1999;398:611–615. [Google Scholar]
- 4.Root TL, et al. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- 5.Walther GR, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 6.Lenoir J, et al. A significant upward shift in plant species optimum elevation during the 20th century. Science. 2008;320:1768–1771. doi: 10.1126/science.1156831. [DOI] [PubMed] [Google Scholar]
- 7.Pöyry J, Luoto M, Heikkinen RK, Kuussaari M, Saarinen K. Species traits explain recent range shifts of Finnish butterflies. Glob Change Biol. 2009;15:732–743. [Google Scholar]
- 8.Czech B, Krausman PR. Distribution and causation of species endangerment in the United States. Science. 1997;277:1116–1117. [Google Scholar]
- 9.Pimm SL, Raven P. Biodiversity: Extinction by numbers. Nature. 2000;403:843–845. doi: 10.1038/35002708. [DOI] [PubMed] [Google Scholar]
- 10.Sala OE, et al. Biodiversity: Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- 11.Thomas JA, et al. Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science. 2004;303:1879–1881. doi: 10.1126/science.1095046. [DOI] [PubMed] [Google Scholar]
- 12.Conrad KF, Warren MS, Fox R, Parsons MS, Woiwod IP. Rapid declines of common, widespread British moths provide evidence of an insect biodiversity crisis. Biol Conserv. 2006;132:279–291. [Google Scholar]
- 13.Colwell RK, Brehm G, Cardelus CL, Gilman AC, Longino JT. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science. 2008;322:258–261. doi: 10.1126/science.1162547. [DOI] [PubMed] [Google Scholar]
- 14.Nogués-Bravo D, Araújo MB, Romdal T, Rahbek C. Scale effects and human impact on the elevational species richness gradients. Nature. 2008;453:216–219. doi: 10.1038/nature06812. [DOI] [PubMed] [Google Scholar]
- 15.Chen IC, et al. Elevation increases in moth assemblages over 42 years on a tropical mountain. Proc Natl Acad Sci USA. 2009;106:1479–1483. doi: 10.1073/pnas.0809320106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson RJ, Gutierrez D, Gutierrez J, Monserrat VJ. An elevational shift in butterfly species richness and composition accompanying recent climate change. Glob Change Biol. 2007;13:1873–1887. [Google Scholar]
- 17.Wilson RJ, et al. Changes to the elevational limits and extent of species range associated with climate change. Ecol Lett. 2005;8:1138–1146. doi: 10.1111/j.1461-0248.2005.00824.x. [DOI] [PubMed] [Google Scholar]
- 18.Kotiaho JS, Kaitala V, Komonen A, Paivinen J. Predicting the risk of extinction from shared ecological characteristics. Proc Natl Acad Sci USA. 2005;102:1963–1967. doi: 10.1073/pnas.0406718102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark PJ, Reed JM, Chew FS. Effects of urbanization on butterfly species richness, guild structure, and rarity. Urban Ecosyst. 2007;10:321–337. [Google Scholar]
- 20.Shapiro AM, Van Buskirk R, Kareofelas G, Patterson WD. Phenofaunistics: Seasonality as a property of butterfly faunas. In: Boggs CL, Watt WB, Ehrlich PR, editors. Butterflies: Ecology and Evolution Taking Flight. Chicago: University of Chicago Press; 2003. pp. 111–147. [Google Scholar]
- 21.Thorne JH, O’Brien J, Forister ML, Shapiro AM. Building phenological models from presence/absence data for a butterfly fauna. Ecol Appl. 2006;16:1842–1853. doi: 10.1890/1051-0761(2006)016[1842:bpmfad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Descimon H, Bachelard P, Boitier E, Pierrat V. Decline and extinction of Parnassius apollo populations in France—continued. In: Kuhn E, Feldman R, Settele J, editors. Studies on the Ecology and Conservation of Butterflies in Europe. Sofia, Bulgaria: Pensoft; 2006. [Google Scholar]
- 23.Warren MS, et al. Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature. 2001;414:65–69. doi: 10.1038/35102054. [DOI] [PubMed] [Google Scholar]
- 24.van Swaay C, Warren M, Lois G. Biotope use and trends of European butterflies. J Insect Conserv. 2006;10:189–209. [Google Scholar]
- 25.Menendez R, et al. Species richness changes lag behind climate change. Proc R Soc Lond B Biol Sci. 2006;273:1465–1470. doi: 10.1098/rspb.2006.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Dyck H, Van Strien AJ, Maes D, Van Swaay CAM. Declines in common, widespread butterflies in a landscape under intense human use. Conserv Biol. 2009;23:957–965. doi: 10.1111/j.1523-1739.2009.01175.x. [DOI] [PubMed] [Google Scholar]
- 27.Dennis RLH, Sparks TH. Climate signals are reflected in an 89 year series of British Lepidoptera records. Eur J Entomol. 2007;104:763–767. [Google Scholar]
- 28.Forister ML, Shapiro AM. Climatic trends and advancing spring flight of butterflies in lowland California. Glob Change Biol. 2003;9:1130–1135. [Google Scholar]
- 29.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 30.White P, Kerr JT. Contrasting spatial and temporal global change impacts on butterfly species richness during the 20th century. Ecography. 2006;29:908–918. [Google Scholar]
- 31.Brook BW, Sodhi NS, Bradshaw CJA. Synergies among extinction drivers under global change. Trends Ecol Evol. 2008;23:453–460. doi: 10.1016/j.tree.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Jetz W, Wilcove DS, Dobson AP. Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biol. 2007;5:1211–1219. doi: 10.1371/journal.pbio.0050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohlemüller R, et al. The coincidence of climatic and species rarity: High risk to small-range species from climate change. Biol Lett. 2008;4:568–572. doi: 10.1098/rsbl.2008.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollard E. Method for assessing changes in abundance of butterflies. Biol Conserv. 1977;12:115–134. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.