Abstract
Because GABAA receptors containing α2 subunits are highly represented in areas of the brain, such as nucleus accumbens (NAcc), frontal cortex, and amygdala, regions intimately involved in signaling motivation and reward, we hypothesized that manipulations of this receptor subtype would influence processing of rewards. Voltage-clamp recordings from NAcc medium spiny neurons of mice with α2 gene deletion showed reduced synaptic GABAA receptor-mediated responses. Behaviorally, the deletion abolished cocaine’s ability to potentiate behaviors conditioned to rewards (conditioned reinforcement), and to support behavioral sensitization. In mice with a point mutation in the benzodiazepine binding pocket of α2-GABAA receptors (α2H101R), GABAergic neurotransmission in medium spiny neurons was identical to that of WT (i.e., the mutation was silent), but importantly, receptor function was now facilitated by the atypical benzodiazepine Ro 15-4513 (ethyl 8-amido-5,6-dihydro-5-methyl-6-oxo-4H-imidazo [1,5-a] [1,4] benzodiazepine-3-carboxylate). In α2H101R, but not WT mice, Ro 15-4513 administered directly into the NAcc-stimulated locomotor activity, and when given systemically and repeatedly, induced behavioral sensitization. These data indicate that activation of α2−GABAA receptors (most likely in NAcc) is both necessary and sufficient for behavioral sensitization. Consistent with a role of these receptors in addiction, we found specific markers and haplotypes of the GABRA2 gene to be associated with human cocaine addiction.
Keywords: GABRA2, behavioral sensitization, nucleus accumbens, mutant mouse, human genetics
The nucleus accumbens (NAcc) is centrally implicated in the actions of drugs of abuse and in mediating the effects of environmental cues conditioned to drug reward in motivating drug-seeking behavior (1). GABAergic medium spiny neurons (MSNs) are the major neuronal type within the accumbens, their activity being regulated by dopaminergic and glutamatergic inputs. Local GABAergic interneurons and collateral projections (2) between neighboring MSNs may serve to sharpen competition between MSNs for access to the major output pathways. Intra-accumbal GABAergic systems may thus play an important role in regulating incentive motivational outputs, leading to compulsive behaviors, such as drug abuse. We therefore predicted that manipulation of GABAergic transmission within the NAcc will alter the ability of the NAcc to respond to psychostimulant drugs and to incentives, such as reward-predictive cues.
GABA mediates “fast” neural inhibition by activating ionotropic GABAA receptors, leading to the transmembrane flux of chloride and bicarbonate ions and a decrease in neuronal input resistance. The majority of these oligomeric protein complexes are made up of subunits from major subunit families, α, β, and γ. Given the established role of the NAcc in mediating the behavioral effects of both reward-associated cues, and of cocaine, determining the GABAA receptor subtypes that mediate the signal generated in this brain region by abused drugs may offer an approach to designing treatment strategies and identifying genetic risk for cocaine addiction. GABAA receptors containing α2 subunits are densely expressed in NAcc (3) but are absent, or only modestly represented, in the pallidal and ventral tegmental area output targets, respectively (4). Thus, this subtype of receptor may play an important role in these processes.
To test this hypothesis, we used mice with genetic manipulations of the Gabra2 gene. Gene deletion (α2−/−) reduced responses mediated by synaptic GABAA receptors in the MSNs of NAcc, and abolished cocaine’s ability to enhance behaviors conditioned to rewards (conditioned reinforcement) and to support behavioral sensitization. As gene-deletion experiments may be complicated by compensation, we additionally used mice in which their α2 subunit was genetically engineered (α2H101R) to selectively change the pharmacology of α2-GABAA receptors, but not their physiological function. Importantly, the mutation did not influence NAcc GABAergic transmission, but selectively made α2-GABAA receptors sensitive to the GABA-enhancing effects of Ro 15-4513, an atypical benzodiazepine. Remarkably, infusion of Ro 15-4513 into the NAcc of α2H101R but not WT mice enhanced locomotor activity, and in common with cocaine, repeated systemic treatment resulted in maintained sensitization. α2 receptors are also located in other brain areas involved in addictive behavior; however, by selecting behaviors known to be mediated by NAcc core, and by using local infusions of drugs into NAcc, we were able to provide some anatomical specificity to our findings. Importantly, we report that haplotypes of the GABRA2 gene are associated with cocaine addiction in humans.
Results
Expression of GABAA Subunits in α2−/− Mice.
RT-PCR analysis of expression of other GABAA receptor subunits, including α1, α3, α4, α5, α6, β1, β2, and γ1 and γ2 in punches obtained from NAcc, showed unchanged expression in the knockout mice (Fig. S1).
Electrophysiology in MSNs of α2−/− Mice.
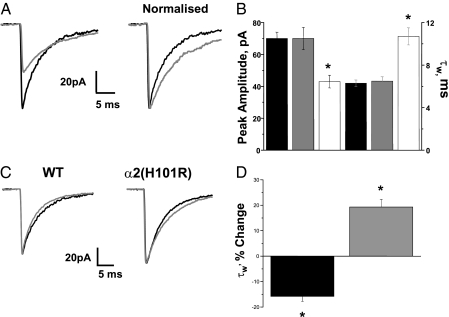
Deletion of the α2 subunit produced an ≈33% decrease in miniature inhibitory postsynaptic current (mIPSC) amplitude and a clear prolongation of the mIPSC decay (Fig. 1 A and B and Table S1). The frequency of mIPSCs did not differ from that recorded for WT neurons (Table S1).
Fig. 1.
Receptors incorporating the α2 subunit are expressed at GABAergic synapses in NAcc MSNs. (A) Representative ensemble averages of GABAA receptor mIPSCs recorded from neurons of the NAcc of WT (WT) (black) and α2−/− (gray) mice. These events are shown normalized to the WT amplitude on the right to aid comparison of the decay kinetics. (B) Bar chart comparing the average peak amplitude (Left y axis) and decay time constant τw (Right y axis) of GABAA receptor mediated mIPSCs of neurons from WT (black), α2H101R (gray), and α2−/− (white) mice. (C) Representative ensemble averages of GABAA receptor-mediated mIPSCs recorded from exemplar neurons of WT [i.e., four of eight neurons showing decrease decay time (see text)] and α2H101R mice in the absence (black) and presence (gray) of 10-μM Ro15-4513. (D) Effects of 10-μM Ro15-4513 on the decay kinetics (i.e., τw, expressed as % of change) of mIPSCs of WT (black) and α2H101R (gray) neurons of the NAcc. Data were obtained from 4 to 19 (WT), 8 to 11 (α2H101R), and 37 (α2−/−) neurons. Error bars indicate SEM. *, P < 0.01 vs. WT or control (unpaired Student’s t test and one-way RMA, respectively).
Cocaine Effects in α2−/− Mice.
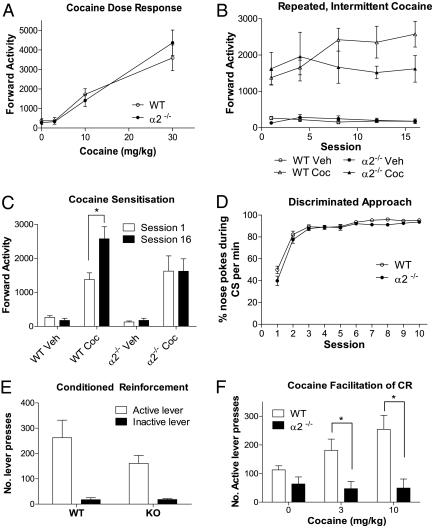
α2−/− mice displayed a stimulant response to cocaine, indistinguishable from that of WT mice (Fig. 2A). However, in contrast to WT, α2−/− mice did not develop behavioral sensitization when given cocaine repeatedly at 2- to 3-day intervals (Fig. 2B), so that even though the WTs showed an enhanced response to cocaine following repeated treatments, the α2−/− mice did not (Fig. 2C). Because the stimulant response of α2−/− mice to cocaine was normal, this deficit in sensitization was not attributable to altered drug sensitivity. Behavioral sensitization depends on the test context, and one possibility is that the α2−/− mice fail to form conditioned associations between the cocaine effect and the test environment. However, in a test of place-preference conditioning, the α2−/− mice were unimpaired in forming drug-environment associations (Fig. S2).
Fig. 2.
Effects of cocaine on conditioned behavior of α2−/− mice. (A) Effects of acute cocaine on locomotor activity during 60 min in WT (n = 8) and α2−/− mice (n = 8). Cocaine increased activity equally in both genotypes (dose by genotype interaction; F (3,39) = 0.729, NS). (B) Behavioral sensitization to cocaine (10 mg/kg) in WT (n = 9) but not in α2−/− (n = 7) mice compared with control groups of WT (n = 9) and α2−/− (n = 9) treated with saline. Data show mean (± SEM) number of beam breaks. Analysis was carried out for all 16 sessions but, for clarity of presentation, data from every fourth session are displayed. Locomotor activity increased over sessions for WT mice but not for α2−/− mice (Session by genotype by drug interaction; F (15,450) = 3.096, P < 0.05, ε = 0.291). (C) Locomotor activity on sessions 1 and 16 of repeated intermittent cocaine (session by genotype by drug interaction, F (1,30) = −0.650, P < 0.05). Askterisk denotes significance between sessions 1 and 16 (paired samples t test; P < 0.005). (D) Acquisition of Pavlovian conditioning in WT (n = 10) and α2−/− (n = 10) mice. Data show percentage of conditioned stimulus (light + tone) presentations eliciting nose pokes into the food delivery magazine over 15 sessions. No difference was evident between genotypes (session by genotype interaction; F (9,162) = 1.401, NS; effect of genotype; F (1,18) = 3.753, NS). (E) Active and inactive lever presses in tests of conditioned reinforcement in WT (n = 5) and α2−/− (n = 6) mice. Significant effect of lever (F (1,9) = 28.562, P < 0.001) demonstrates conditioned reinforcement, which did not differ between genotypes (lever by genotype interaction; F (1,9) = 1.968, NS). (F) Cocaine facilitation of conditioned reinforcement in WT (n = 5) and α2−/− (n = 6) mice. Responding on the active lever for CS presentation differs between genotypes (dose by genotype interaction, F (2,18) = 8.816, P < 0.05). WT mice showed an increase in responding (main effect of dose, F (3,12) = 3.284, P = 0.05), whereas α2−/− mice showed a decrease (main effect of dose, F (3.15) = 19.564, P < 0.001) after administration of increasing doses of cocaine. Asterisk indicates significant difference between WT and α2−/− lever-pressing rates using independent samples t test (3 mg/kg: t(9) = 3.093, P < 0.05; 10 mg/kg: t(9) = 3.087, P < 0.05).
Nevertheless, even if α2−/− mice are capable of forming environment-cocaine associations, and the acute response to cocaine is unaltered, the ability of cocaine to facilitate the conditioned response (5) may be attenuated in α2−/− mice. We tested this possibility using a measure of conditioned reinforcement known to be facilitated by psychomotor stimulants (6). Conditioned reinforcers (i.e., discrete environmental cues conditioned to a primary reward) are important in maintaining motivation to seek drug rewards when the drug itself is not immediately available, and thus contribute both to maintenance of drug habits and to relapse in abstaining addicts (7). Mildly food-deprived mice were first trained to associate delivery of food pellets with illumination of two cue lights flashing at 1 Hz and simultaneous presentation of a 2.9-kHz tone for 10 s. α2−/− and WT mice learned this association equally readily (Fig. 2D) again showing that the deletion has little effect on conditioning. Next, two levers were introduced into the apparatus, operation of one lever giving rise to cue presentation (but no food delivery); operation of the alternative lever had no programmed consequences. Both genotypes readily learned to operate the lever to obtain access to the cues (Fig. 2E), indicating that they had acquired conditioned reinforcing properties. However, although cocaine facilitated lever-pressing for the conditioned reinforcer in a dose-dependent fashion in the WT mice, it failed to do so in the α2−/− mice (Fig. 2F), indicating a loss of cocaine’s ability to facilitate conditioned reinforcement.
α2H101R Mutant Mice.
GABAA receptors containing α1, α2, α3, or α5 subunits possess binding sites for classical benzodiazepines, such as diazepam, whereas those containing α4 or α6 subunits do not. α4 and α6 subunits differ from benzodiazepine-sensitive subunits in possessing an arginine (R) instead of a histidine (H) residue in the benzodiazepine binding pocket (8). This single amino acid difference also changes the pharmacology of agents like Ro 15-4513 that normally act at the benzodiazepine binding site as negative allosteric modulators of GABAergic inhibition. Thus, whereas the atypical benzodiazepine derivative, Ro 15-4513, acts as an “inverse agonist” (i.e., decreases GABA-evoked currents) at “H” (α1-, α2-, α3-, and α5-containing) GABAA receptors, it acts as an “agonist” (i.e., facilitates GABA-evoked currents) at “R” (α4− and α6−containing) receptors (9, 10). Importantly, the point mutation does not alter the sensitivity of the receptor to its natural ligand, GABA. As selective ligands for α2-containing receptors are not available, we used this knowledge to differentially facilitate transmission at α2-containing receptors, using knock-in mice (11, 12) in which the normal H at position 101 within the benzodiazepine binding pocket of the α2 protein was replaced by R (α2H101R mice). In such mutant mice, Ro 15-4513 will continue to act as an inverse agonist at α1−, α3−, and α5-containing receptors, and as an agonist at α4- and α6-containing receptors, so that the only difference between WT and mutant mice will be the direction of Ro 15–4513s action at α2-containing receptors.
Electrophysiology in MSNs of α2H101R Mice.
In coronal brain slices prepared from WT and α2H101R mice, a comparison of mIPSCs from NAcc core neurons revealed no significant difference in their properties (Table S1), confirming that basal GABAergic transmission is unaltered by the mutation. Ro 15-4513 enhances GABA-evoked responses by the mutated α2-containing receptor expressed in heterologous cell systems (10). Correspondingly, Ro15-4513 (10 μM) significantly prolonged the mIPSC decay in all accumbal neurons (n = 8) tested from α2H101R mice (Figs. 1 C and D), while it accelerated the mIPSC decay phase in four out of eight WT accumbal cells tested (Kolmogorov-Smirnov test, P < 0.01), and either had no effect (two cells, Kolmogorov-Smirnov test, P > 0.01), or prolonged the decay time constant τw ∼20% (two cells, Kolmogorov-Smirnov test, P < 0.01) in others. The expression in some WT spiny neurons of synaptic receptors incorporating the α4 subunit provides a parsimonious explanation for the heterogeneous effects of Ro 15-4513. These observations reinforce the α2−/− studies in confirming that a significant proportion of synaptic GABAA receptors in the NAcc core incorporate the α2 subunit, and reveal that Ro 15-4513 facilitates the function of the mutated receptor.
Behavior of α2H101R Mice.
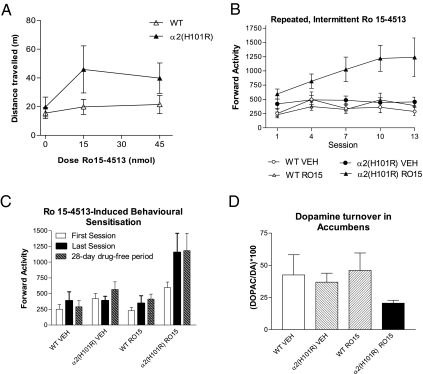
In keeping with its altered action at NAcc α2-GABAA receptors, local bilateral infusion of Ro 15-4513 (15 or 45 nM in 0.5 μL) into the NAcc of α2H101R, but not WT mice, gave rise to an increase in locomotor activity (Fig. 3A).
Fig. 3.
Effects of Ro 15-4513 in α2H101R mice. (A) Bilateral infusion of Ro 15-4513 (15 and 45 nmol in 0.5 μL) into NAcc increased locomotor activity during 30 min after infusion in mutant mice (n = 7) but not in WT mice (n = 9) (Ro 14-4513 × genotype interaction, F 2,32= 3.98; P = 0.029). Bars indicate SEM. (B) Repeated, intermittent Ro 15-4513 (10 mg/kg, i.p.) treatment increased locomotion in α2H101R but not WT (WT) mice (Ro 15-4513 × genotype interaction, F (1,60) = 7.17, P < 0.05). The Ro 15-4513-induced stimulant effect in α2H101R mice increased over sessions (main effect of session, F (13,390) = 3.13, P < 0.05, ε = 0.206). For clarity, data are presented for every third day. (C) Ro 15-4513–induced behavioral sensitization (relative to Session 1) in α2H101R mice was still apparent after a 28-day drug-free interval (greater Ro 15-4513–induced activity levels at 28-days [t(14) = −2.65, P < 0.05]. There were no differences between the final day of sensitization and day 28. (D) Dopamine turnover in the NAcc of WT and α2H101R mice treated with vehicle or 10 mg/kg Ro 15-4513. There were no significant main effects of treatment or genotype, or interaction terms, on the DOPAC/dopamine ratio, or on levels of dopamine or DOPAC (SI Text).
Behavioral Sensitization to Ro 15-4513.
A stimulant effect was also seen when Ro 15-4513 was given i.p. in the H101R mutant, whereas it had no effect in WT mice. Moreover, the stimulant effect in the mutants increased with repeated treatment (Fig. 3B), indicating behavioral sensitization that persisted for at least 28 days following the final treatment (Fig. 3C). Because the action of Ro 15-4513 at all other GABAA receptors would not differ between WT and α2H101R mutant mice, we deduce that facilitation of GABAergic transmission at α2-containing receptors, as shown in the electrophysiological experiments, resulted in a form of behavioral sensitization that resembles that induced by drugs of abuse.
Dopamine Turnover Following Ro 15-4513.
The locomotor stimulant properties of psychostimulants, such as cocaine, depend on their ability to increase dopamine turnover in the NAcc, cocaine-sensitized animals showing facilitated cocaine-induced dopamine release (13). Administration of Ro15-4513 to α2H101R-mutant mice did not increase dopamine turnover, measured as the ratio of dopamine to its metabolite, DOPAC, in NAcc (Fig. 3D and Fig. S3). Thus, consistent with a location downstream from dopamine synapses, stimulant effects resulting from activation of GABAA α2-containing receptors may not depend upon activation of dopamine release. That a benzodiazepine, midazolam, was able acutely to potentiate the stimulant effects of cocaine in WT, but not α2H101R mice (14), further supports an intimate involvement of α2-containing GABAA receptors in expression of cocaine’s effects.
Variations in the GABRA2 Gene in Human Cocaine Addicts.
We hypothesized that in human populations, variations in this gene might also confer susceptibility to cocaine addiction. We analyzed a sample from São Paulo, Brazil, which consisted predominantly of individuals of Caucasian origin, with some admixture of African and Native American ancestry. Adjustment for ethnicity was performed using ADMIXMAP (15) (SI Text) and key findings were verified by parallel analyses in Caucasians, the main ethnic group, defined according to both self-reported European ancestry (n = 876) and >70% European ancestry (n = 823) in ADMIXMAP (Table S2).
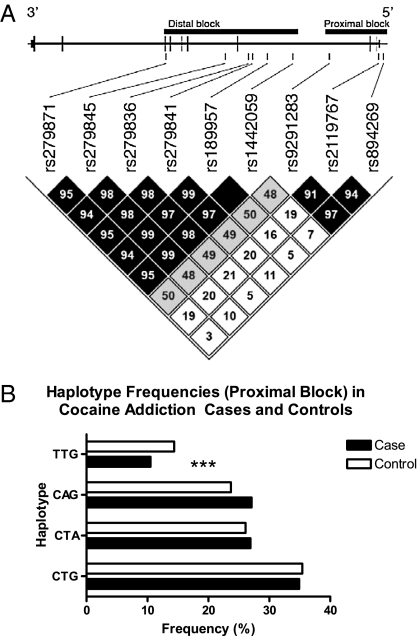
Nine haplotype-tagging SNPs tagging 80% of the Hapmap SNPs in the gene and the promoter region at an average r 2 > 0.9 were genotyped (Table S2 and Fig. 4A), revealing a linkage disequilibrium block structure with two distinct blocks of (i) 5′ SNPs rs894269, rs2119767, rs9291283, and (ii) the remaining downstream markers (Fig. 4A).
Fig. 4.
Haplotypes of GABRA2. (A) Pair-wise scaled SNP linkage (D’) of the 9 SNPs studied and an illustration of their position within the genomic sequence of the GABRA2 gene, which is encoded on the reverse genomic strand (5′ to 3′ in relation to the gene). The SNPs all fall within introns or the 5′ promotor region (thick vertical bars represent coding exons, and thin bars represent untranslated regions of the gene). These SNPs fall into two linkage disequilibrium (LD) blocks (black) consisting of six and three SNPs, respectively, with D’ > 90% (the first three SNPs of the first block comprise the Edenberg three SNP haplotype). (B) Haplotype frequencies in cases and controls. Significantly different haplotypes are indicated by three stars (P = 0.0014).
We tested both blocks and found that the promoter block is associated with cocaine addiction. Specifically, we identified a protective haplotype (TTG; 10.5% in cases, 14.4% in controls, P = 1.5 x10−3, P = 0.005 after permutation) (Fig. 4B). We did not find an association with the downstream haplotype block. An odds ratio of ∼0.7 (95% confidence interval or CI 0.56–0.87) for cocaine addiction for the protective haplotype compared with all other haplotypes, indicates that individuals carrying this form are about one-third less likely to develop cocaine addiction. Analyses in the Caucasian subsamples confirmed a similar association in both groups (odds ratios 0.68 and 0.69, respectively, 95% CI of 0.48–0.84), whereas other haplotypes did not (Table S2).
SNP rs894269 has the strongest association in the complete sample (Table S3) as well as both Caucasian subsamples. Its T allele reduces the risk by a factor of about 1.5 (odds ratio ∼0.68), suggesting a major contribution of SNP rs894269 to the observed protective effect on cocaine addiction. Bioinformatic sequence analysis revealed that rs894269 lies in an ultraconserved region ∼9 kb from the transcription start point, where the C allele interrupts an evolutionarily conserved cis-enhancer region predicted by ESPERR analysis (16), while the T allele conserves it.
Additional associations of several genetic variations further downstream in the GABRA2 gene with cocaine addiction were also found, as reported in studies of alcohol dependence (17, 18). Specifically, we observed significant association with three markers comprising a haplotype (rs279871, rs279845, and rs279836) defined by Edenberg et al. (18) (Table S3). Alcoholism was an exclusion criterion in our sample and alcohol consumption data were available in cases (although not controls). Alcohol consumption showed no association with GABRA2 genotype (regression models with unit consumption: beta 0.004 for TT genotype of rs894269 vs. others; P = 0.92).
Discussion
Overall, our observations indicate a central role for GABAergic systems employing α2-GABAA receptors in cocaine-induced behavioral plasticity and addiction across species. Although human studies indicate an association of GABRA2 with human addiction, the mouse studies pinpoint a possible mechanism. The mouse studies implicate α2-receptors in a neural circuitry downstream of dopaminergic pathways, and demonstrate that their activation is a prerequisite for dopaminergic systems to achieve their effects in facilitating conditioned behavior. Because deletion of α2 subunits prevented the ability of cocaine to facilitate conditioned reinforcement, we hypothesize that the importance of the system lies in mediating cocaine’s ability to facilitate behaviors elicited by environmental cues associated with rewards (including drug reward).
Drug addiction is frequently conceptualized in terms of maladaptive associative learning, allowing environmental cues associated with the drug to initiate drug taking by activating incentive processes or stimulus-response habits (1). Such cues come to activate incentive motivational pathways that depend upon interactions between basolateral amygdala and NAcc (19). Learning the cue-reward association depends on synaptic plasticity within the basolateral amygdala, and genetic manipulations of glutamatergic systems that impair or facilitate plasticity correspondingly impair and facilitate the development of conditioned reinforcement (20, 21). That α2−/− mice showed normal conditioned reinforcement indicates that learning processes within the basolateral amygdala were unaffected by the deletion, although α2-GABAA receptors are heavily expressed in this structure (3).
On the other hand, the ability of psychostimulants to facilitate conditioned reinforcement is attributable to their action in facilitating dopaminergic transmission within the NAcc (6, 19). The failure of cocaine to facilitate conditioned reinforcement in the α2−/− mice thus strongly suggests altered neuronal function within the accumbens. Within NAcc, dopamine acts on MSNs to modulate sensitivity to glutamatergic signals from basolateral amygdala and other corticolimbic areas (22). By mediating a phasic inhibitory input into MSNs, α2-GABAA receptors are likely to interact with such systems to determine MSN output.
During behavioral sensitization, the stimulant effects of addictive drugs increase with repeated administration. Sensitization is strongly influenced by the formation of associations between environmental cues and the drug effect (5, 23). Such learning depends upon neuronal plasticity in NAcc pathways (24), providing a simple model of drug incentive learning underlying the development of addictions (5, 25). The lack of sensitization to cocaine in the α2−/− mice is thus also consistent with a role of α2-GABAA receptors in pathways necessary for cocaine-induced facilitation of conditioned activity (9).
α2-GABAA receptors are not directly involved in signaling reward or in the animal’s ability to form conditioned associations with rewarding events, as neither conditioned place preference nor conditioned reinforcement was impaired in the α2−/− mice. In agreement, data from primate self-administration studies (26, 27) suggest that compounds with some selectivity for α2-GABAA receptors may possess lower abuse potential than drugs with selectivity for α1-receptors. Rather, α2-receptors appear to mediate dopamine’s ability to act as a “gain amplifier” so that, in their absence, facilitation of dopamine transmission by cocaine is no longer effective in strengthening behavior directed by conditioned cues.
Both of our mouse models employed constitutive mutants, so that changes in receptor function will have occurred throughout the brain and during development. Nevertheless, it is highly unlikely that our findings reflect developmental changes as a result of the genetic manipulation. First, we found no evidence for altered expression of other GABAA receptor subtypes in the α2−/− mice, indicating a lack of compensation in subunit expression, at least in the adult mouse, although we cannot exclude changes to other receptors/channels.
Second, the α2H101R mouse provided persuasive evidence to further implicate α2-GABAA receptors in the absence of compensation. Importantly, as revealed here for the NAcc, the arginine to histidine manipulation does not affect GABAergic transmission mediated by the mutant receptor (28, 29). Therefore, in the absence of a benzodiazepine, the mutant receptor is physiologically identical to WT, providing no pressure for compensation. However, as the mutation selectively imparts a GABA facilitatory action to Ro15-4513, this drug was used to explore the role of α2-GABAA receptors. Importantly, Ro15-4513 enhanced locomotion and induced sensitization in α2H101R, but not WT mice.
The behavioral effects we observe are unlikely to depend on GABA directly regulating ventral tegmental area dopamine neurons, where the α2 subunit is absent (3, 30) or sparse (4), nor the ventral pallidum projection area of the MSNs, where the α1 subunit predominates (3, 4, 30). Instead, these behaviors are probably attributable to α2-GABAA receptors in the NAcc. Our electrophysiological studies revealed their expression within inhibitory synapses of this brain region. Furthermore, in α2H101R mice, the acute stimulant effect of Ro 15-4513 was mimicked by direct administration of the drug into the NAcc.
Collectively, our findings indicate an important role for α2-GABAA receptors in modulating activity of GABAergic MSNs, thus contributing to the incentive effects of drugs of abuse. This mechanism provides a potential explanation for the association we observed between human cocaine addiction and haplotypes of the GABRA2 gene encoding α2 subunits.
Drug-associated cues are well known to trigger drug seeking and to induce relapse in abstaining addicts, so that facilitation of their action by cocaine represents a double jeopardy for relapse. Our finding that α2-GABAA receptors are necessary for such facilitation points to a possible target for substances that might act to prevent “lapses” (i.e., single drug use) in abstaining addicts from developing into binge use of cocaine (i.e., return to addictive patterns of consumption).
Materials and Methods
Animals.
α2−/− mice (31) and α2H101R “knock-in” mice (11, 12) were generated as previously described. Mice were offspring of heterozygous pairings (α2−/− experiments) or F1 to F3 offspring of homozygous mutant or WT pairings bred at the Universities of Sussex or Dundee. In both cases, the background was mixed 50% C57BL/6J – 50% 129SvEv. All experiments were carried out under the United Kingdom Animal (Scientific Procedures) Act 1986, following ethical review by the University of Sussex Ethical Review Committee, or University of Dundee Ethical Review Committee.
Locomotor Activity.
Locomotor activity, and behavioral sensitization to cocaine (α2−/−) mice or Ro 15-4513 (α2H101R) mice were measured in circular runways, as previously described (32).
Conditioned Reinforcement.
Mice were trained in operant boxes to press a lever to activate a stimulus previously paired with free food delivery. Rates of lever pressing following i.p. cocaine (0, 3, 10 mg/kg) were assessed.
Intra-Accumbens Administration of Ro 15-4513.
Seven female α2H101R and 9 WT littermates were implanted stereotaxically under isofluorane anesthesia with guide cannulae (26 ga.) aimed at NAcc (coordinates AP1.34; L+/− 1.00; DV −3.20 in ref. 33), and locomotor activity measured after vehicle and Ro 15-4513 infusions. Location of cannulae was confirmed histologically.
Dopamine Turnover in the NAcc.
WT and α2H101R mice, males and females were dosed with vehicle or 10 mg/kg Ro 15-4513 (group size 7–12), killed 15 min later and NAcc dissected, immediately placed on dry ice, and stored at −80 °C until assay by HPLC with electrochemical detection.
Electrophysiology.
Coronal slices containing the NAcc were prepared from WT and α2H101R mice [postnatal day 17 (P17) to P24]. Whole-cell patch clamp recordings were made at 35°C from neurons of the NAcc core. See SI Materials and Methods for further information.
Human Genetic Association Studies.
Blood samples from 699 cocaine addiction patients were identified in outpatient inpatient units and 866 unpaid controls via the blood transfusion service of São Paulo, Brazil (34). Complete description of the methodology is provided in SI Materials and Methods. See also Table S4.
Supplementary Material
Acknowledgments
This research was carried out within the Medical Research Council Clusters “GABA” and “Biomarkers,” supported by Medical Research Council Grants G0600874 (to D.N.S.) and G0802715 (to S.L.K., D.B., J.J.L., and D.N.S.); European Community (EC) FP-6 Integrated Project IMAGEN (PL037286) (to G.S. and D.N.S.); EC-FP7 Project ADAMS (to G.S.); Tenovus and the Anonymous Trust (D.B. and J.J.L.); a Marie Curie Reintegration grant (to S.L.K.); a Biotechnology and Biological Sciences Research Council studentship and British Pharmacological Society training grant (to C.I.D.); Medical Research Council CASE studentship with Merck, Sharp and Dohme (H.V.M.); Wellcome Trust Value in People (G.B.); the United Kingdom Department of Health National Institute for Health Research–Biomedical Research Centre Mental Health (G.S., G.B., and S.D.); and São Paulo Research Foundation grants (to H.V.) sponsored the Brazilian sample collection.
Footnotes
The authors declare no conflict of interest.
The article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910117107/DCSupplemental.
References
- 1.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 2.van Dongen YC, Mailly P, Thierry AM, Groenewegen HJ, Deniau JM. Three-dimensional organization of dendrites and local axon collaterals of shell and core medium-sized spiny projection neurons of the rat nucleus accumbens. Brain Struct Funct. 2008;213:129–147. doi: 10.1007/s00429-008-0173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarzer C, et al. Distribution of the major gamma-aminobutyric acid(A) receptor subunits in the basal ganglia and associated limbic brain areas of the adult rat. J Comp Neurol. 2001;433:526–549. doi: 10.1002/cne.1158. [DOI] [PubMed] [Google Scholar]
- 4.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 5.Le Merrer J, Stephens DN. Food-induced behavioral sensitization, its cross-sensitization to cocaine and morphine, pharmacological blockade, and effect on food intake. J Neurosci. 2006;26:7163–7171. doi: 10.1523/JNEUROSCI.5345-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins TW, Watson BA, Gaskin M, Ennis C. Contrasting interactions of pipradrol, d-amphetamine, cocaine, cocaine analogues, apomorphine and other drugs with conditioned reinforcement. Psychopharmacology (Berl) 1983;80:113–119. doi: 10.1007/BF00427952. [DOI] [PubMed] [Google Scholar]
- 7.Di Ciano P, Everitt BJ. Neuropsychopharmacology of drug seeking: Insights from studies with second-order schedules of drug reinforcement. Eur J Pharmacol. 2005;526:186–198. doi: 10.1016/j.ejphar.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Wieland HA, Lüddens H, Seeburg PH. A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J Biol Chem. 1992;267:1426–1429. [PubMed] [Google Scholar]
- 9.Crestani F, Assandri R, Täuber M, Martin JR, Rudolph U. Contribution of the alpha1-GABA(A) receptor subtype to the pharmacological actions of benzodiazepine site inverse agonists. Neuropharmacology. 2002;43:679–684. doi: 10.1016/s0028-3908(02)00159-4. [DOI] [PubMed] [Google Scholar]
- 10.Benson JA, Löw K, Keist R, Mohler H, Rudolph U. Pharmacology of recombinant gamma-aminobutyric acidA receptors rendered diazepam-insensitive by point-mutated alpha-subunits. FEBS Lett. 1998;431:400–404. doi: 10.1016/s0014-5793(98)00803-5. [DOI] [PubMed] [Google Scholar]
- 11.Morris HV, Dawson GR, Reynolds DS, Atack JR, Stephens DN. Both alpha2 and alpha3 GABAA receptor subtypes mediate the anxiolytic properties of benzodiazepine site ligands in the conditioned emotional response paradigm. Eur J Neurosci. 2006;23:2495–2504. doi: 10.1111/j.1460-9568.2006.04775.x. [DOI] [PubMed] [Google Scholar]
- 12.Dias RS, et al. Evidence for a significant role of alpha 3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci. 2005;25:10682–10688. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalivas PW, Duffy P. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse. 1990;5:48–58. doi: 10.1002/syn.890050104. [DOI] [PubMed] [Google Scholar]
- 14.Morris HV, et al. Alpha2-containing GABA(A) receptors are involved in mediating stimulant effects of cocaine. Pharmacol Biochem Behav. 2008;90:9–18. doi: 10.1016/j.pbb.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Hoggart CJ, et al. Control of confounding of genetic associations in stratified populations. Am J Hum Genet. 2003;72:1492–1504. doi: 10.1086/375613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolbe D, et al. Regulatory potential scores from genome-wide three-way alignments of human, mouse, and rat. Genome Res. 2004;14:700–707. doi: 10.1101/gr.1976004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129B:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- 18.Edenberg HJ, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- 20.Mead AN, Stephens DN. Involvement of AMPA receptor GluR2 subunits in stimulus-reward learning: evidence from glutamate receptor gria2 knock-out mice. J Neurosci. 2003;23:9500–9507. doi: 10.1523/JNEUROSCI.23-29-09500.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mead AN, Stephens DN. Selective disruption of stimulus-reward learning in glutamate receptor gria1 knock-out mice. J Neurosci. 2003;23:1041–1048. doi: 10.1523/JNEUROSCI.23-03-01041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vezina P, Stewart J. Conditioning and place-specific sensitization of increases in activity induced by morphine in the VTA. Pharmacol Biochem Behav. 1984;20:925–934. doi: 10.1016/0091-3057(84)90018-2. [DOI] [PubMed] [Google Scholar]
- 24.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 25.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 26.Ator NA. Contributions of GABAA receptor subtype selectivity to abuse liability and dependence potential of pharmacological treatments for anxiety and sleep disorders. CNS Spectr. 2005;10:31–39. doi: 10.1017/s1092852900009883. [DOI] [PubMed] [Google Scholar]
- 27.Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci USA. 2005;102:915–920. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marowsky A, Fritschy JM, Vogt KE. Functional mapping of GABA A receptor subtypes in the amygdala. Eur J Neurosci. 2004;20:1281–1289. doi: 10.1111/j.1460-9568.2004.03574.x. [DOI] [PubMed] [Google Scholar]
- 29.Löw K, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 30.Marksteiner J, Kaufmann W, Pfaller K, Sieghart W, Saria A. Striatal efferents preferentially innervate neurons in the ventral pallidum containing GABAA receptor alpha 1 subunit-like immunoreactivity. Synapse. 1996;23:107–114. doi: 10.1002/(SICI)1098-2396(199606)23:2<107::AID-SYN6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 31.Dixon CI, Rosahl TW, Stephens DN. Targeted deletion of the GABRA2 gene encoding alpha2-subunits of GABA(A) receptors facilitates performance of a conditioned emotional response, and abolishes anxiolytic effects of benzodiazepines and barbiturates. Pharmacol Biochem Behav. 2008;90:1–8. doi: 10.1016/j.pbb.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Mead AN, Vasilaki A, Spyraki C, Duka T, Stephens DN. AMPA-receptor involvement in c-fos expression in the medial prefrontal cortex and amygdala dissociates neural substrates of conditioned activity and conditioned reward. Eur J Neurosci. 1999;11:4089–4098. doi: 10.1046/j.1460-9568.1999.00828.x. [DOI] [PubMed] [Google Scholar]
- 33.Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press, Boston; 2001. [Google Scholar]
- 34.Turchi MD, et al. Genetic diversity and HIV-1 incidence estimation among cocaine users in São Paulo, Brazil. J Acquir Immune Defic Syndr. 2002;30:527–532. doi: 10.1097/00126334-200208150-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.