Abstract
Community-acquired infections caused by methicillin-resistant Staphylococcus aureus (MRSA) expressing the Panton-Valentine leukocidin (PVL) are rampant, but the contribution of PVL to bacterial virulence remains controversial. While PVL is usually viewed as a cytotoxin, at sublytic amounts it activates protective innate immune responses. A leukotoxic effect might predominate in high inoculum studies, whereas protective proinflammatory properties might predominate in settings with lower bacterial inocula that more closely mimic what initially occurs in humans. However, these protective effects might possibly be neutralized by antibodies to PVL, which are found in normal human sera and at increased levels following PVL+ S. aureus infections. In a low-inoculum murine skin abscess model including a foreign body at the infection site, strains deleted for the pvl genes replicated more efficiently within abscesses than isogenic PVL+ strains. Coinfection of mice at separate sites with isogenic PVL+ and PVL- MRSA abrogated the differences in bacterial burdens, indicating a systemic effect on host innate immunity from production of PVL. Mice given antibody to PVL and then infected with seven different PVL+ strains also had significantly higher bacterial counts in abscesses compared with mice given nonimmune serum. Antibody to PVL had no effect on MRSA strains that did not produce PVL. In vitro, antibody to PVL incapacitated PVL-mediated activation of PMNs, indicating that virulence of PVL+ MRSA is enhanced by the interference of PVL-activated innate immune responses. Given the high rates of primary and recurring MRSA infections in humans, it appears that antibodies to PVL might contribute to host susceptibility to infection.
Keywords: bacterial pathogenesis, immunity, Panton-Valentine leukocidin, MRSA
Infection with methicillin-resistant Staphylococcus aureus (MRSA) strains in otherwise healthy individuals has become a serious public health issue (1–3). Community-acquired MRSA (CA-MRSA) causes primarily skin and soft tissue infections (SSTIs) (2, 4), but also can cause severe necrotizing pneumonias, usually secondary to a viral respiratory tract infection (1, 5). Production of the Panton-Valentine leukocidin (PVL) is a characteristic of CA-MRSA strains (4), but PVL’s contribution to pathogenesis of S. aureus is controversial (6–9). PVL is a bicomponent pore-forming toxin composed of the LukF and LukS proteins encoded by the corresponding genes present in tandem on a bacteriophage lysogenized within the S. aureus chromosome (10). Previous work with these types of toxins has shown that they can lyse polymorphonuclear neutrophils (PMNs) and monocytes of the white blood cell lineage (11, 12); however, importantly, at sublytic levels, staphylococcal leukocidins also have a strong proinflammatory effect on granulocytes (12). Whereas dissimilar outcomes from different investigators analyzing the contribution of PVL to virulence in experimental settings can be attributed to the use of different S. aureus strains and different infection systems for analysis of virulence, as well as different mouse strains, key factors related to human infections have not been incorporated into these previous evaluations. Regarding SSTIs, many infections likely contain particulate matter introduced into the site of infection, essentially introducing a “foreign body,” which is well known to enhance the virulence of S. aureus (13, 14). In addition, most humans, but not laboratory mice, have naturally acquired antibodies reactive with PVL (15), which could neutralize either its toxic or proinflammatory effects, in either case having a possibly profound effect on the course of infection with PVL-producing S. aureus. A leukotoxic effect might be dominant in virulence studies that use high bacterial inocula, whereas a positive effect from the proinflammatory properties of PVL might predominate in settings with lower bacterial inocula, likely more closely mimicking what occurs in many human infections, especially in the early stages.
Consequently, we postulated that PVL elaboration could affect SSTI outcome in a positive manner by activating inflammatory responses, leading to stronger innate immunity, but that in the presence of antibody, neutralization of this activity would then increase the bacterial burden in infected tissues. To test this hypothesis, we compared the ability of multiple strains of PVL-positive S. aureus and isogenic ∆pvl strains for virulence in a low-inoculum, foreign body–enhanced model of SSTI, and also examined the effect of antibodies to PVL on the outcome of infections.
Results
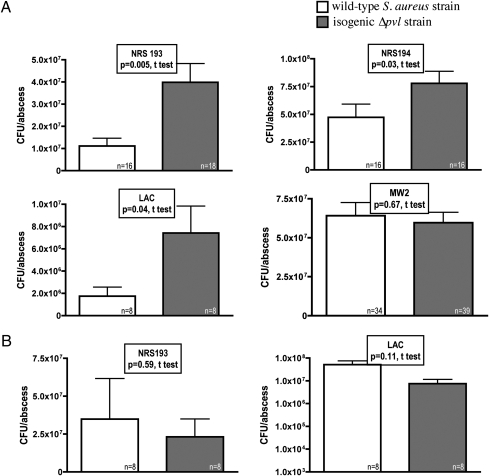
We initially compared the number of bacterial colony-forming units (cfu) per abscess recovered 3 days after s.c. infection of the flanks of mice with four different PVL-positive and isogenic ∆pvl CA-MRSA strains, including three USA400 CA-MRSA strains (NRS193, NRS194, and MW2) and one USA300 CA-MRSA strain (LAC). For three of the four strains tested, bacterial counts recovered from the abscesses of mice infected with the ∆pvl mutant were significantly higher (between 4 × 106 and 3 × 107 more cfu/abscess on average) than those of their corresponding wild-type parental strains except strain MW2 (Fig. 1A). To determine whether the loss of PVL production associated with increased virulence was due to local effects or to systemic effects, mice were simultaneously infected in the lateral flank with either a wild-type or an isogenic ∆pvl S. aureus strain. In this setting, there were no differences in the bacterial counts of the strains recovered from the two different sites in the same animal (Fig. 1B). This suggests that when PVL is produced by a wild-type strain, it apparently triggers a systemic activation of the innate immune response. This negates the difference in bacterial abscess counts between the wild-type and isogenic ∆pvl strains seen when these strains are inoculated separately into different mice. Notably, we found that inoculating separate groups of mice with higher doses of either PVL-producing or isogenic ∆pvl S. aureus did not lead to any differences in the bacterial burden in abscesses (Fig. S1). This suggests that PVL causes a systemic activation of protective host innate immunity in the early stages of infection when bacterial levels are low that is not apparent when high initial inocula are used in animal infections.
Fig. 1.
Bacterial counts from mouse abscesses induced with PVL+ and isogenic ∆pvl S. aureus strains. (A) Comparison of the bacterial counts in 72-h-old abscesses in the skin of mice induced by four wild-type MRSA strains (NRS193, NRS194, LAC, and MW2) with their respective isogenic ∆pvl counterparts. (B) Comparison of bacterial counts from mouse abscesses induced with two wild-type S. aureus strains (NRS193 and LAC) and their isogenic ∆pvl strains at different skin sites within the same mouse. P values are from unpaired t tests. Bars represent means; error bars represent SEM.
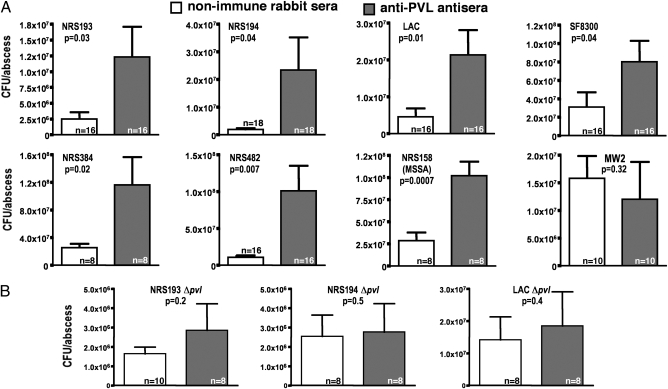
We hypothesized that if PVL were functioning in this setting to activate innate immunity and promote control of microbial levels, then antibodies specific to PVL would neutralize this activity and increase the levels of bacteria within abscesses in the SSTI model. We used recombinant LukF and LukS proteins to raise antibodies to each component in rabbits (Fig. S2). The cytotoxin-neutralizing activity of the sera was confirmed by the findings that the antibodies inhibited lysis of human PMNs exposed to a lytic dose of PVL, whereas nonimmune rabbit serum could not neutralize the cytotoxic activity of PVL (Fig. S3). We then injected by the i.p. route equal amounts of each antiserum into mice 48 h and 24 h before initiating a s.c. infection with eight different PVL-producing strains of S. aureus. Again, with the exception of strain MW2, bacterial counts from abscesses of mice given antisera to PVL were consistently higher than those from mice given nonimmune sera, including both MRSA and a PVL+ methicillin-sensitive strain, NRS158 (P values ranged from .007 to .04; Fig. 2A). These results suggest that antibody to PVL interferes with host defenses that contribute to controlling bacterial levels, allowing PVL-producing S. aureus strains to replicate more efficiently within mouse abscesses. Because antisera to S. aureus leukocidins are cross-reactive (10), and because the ∆pvl S. aureus strains may make additional leukocidins, such as LukD/LukE and gamma hemolysin, we next examined whether the effect of the antiserum to PVL was specific to this microbial factor by injecting mice with either nonimmune or PVL-immune rabbit antisera before infection with ∆pvl S. aureus strains. In this setting, there were no differences in the cfu counts recovered form the abscesses of mice given nonimmune or PVL-immune antisera (Fig. 2B), indicating that the activity of the antibody to PVL has a specific effect on PVL in terms of interfering with activation of host innate immunity to SSTIs.
Fig. 2.
Bacterial counts from mice injected with nonimmune rabbit sera (NIS) or PVL-immune rabbit antisera followed by induction of abscesses with different S. aureus strains. (A) CFU/abscess for eight different PVL+ S. aureus strains determined in mice given NIS or antibody to PVL. (B) CFU/abscess for three strains deleted for the pvl genes following injection with NIS or antibody to PVL. P values are from t tests. Bars represent means; error bars represent SEM.
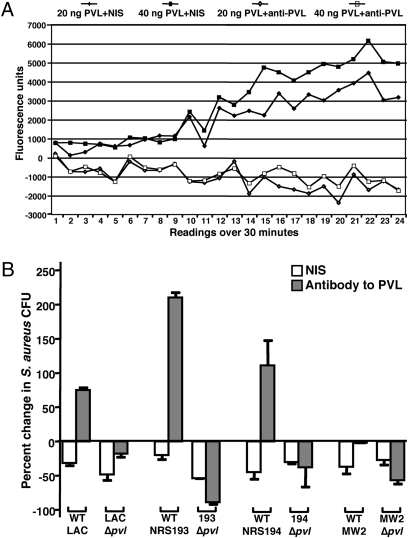
Previous studies have found that the induction of proinflammatory cytokine release by PMNs exposed to sublytic levels of PVL results from the opening of calcium ion (Ca2+) channels, which is a necessary step in PMN activation (16). We next explored whether antibodies to PVL affected PVL-mediated Ca2+ influx into PMNs by measuring the emission of fluorescence by cells preloaded with a calcium indicator. In the presence of nonimmune rabbit sera, PMNs exposed to sublytic amounts of purified PVL (20 and 40 ng) displayed Ca2+ channel opening, whereas antibody to PVL inhibited Ca2+ channel opening (Fig. 3A). These results suggest that antibodies to PVL may prevent PVL-induced activation of PMNs and subsequent innate immune responses to S. aureus infection.
Fig. 3.
Modulation of PMN activation and antibacterial effects on S. aureus by antibody to PVL. (A) Influx of calcium into PMNs exposed to sublytic amounts of purified PVL in the presence of NIS or antibody to PVL. (B) Susceptibility of PVL+ and isogenic ∆pvl S. aureus strains to the antimicrobial activity of PMNs. Bars represent means; error bars represent SEM. the percent change in S. aureus CFU in the presence of antibody to PVL was significantly higher in strains LAC, NRS193, and NRS194 producing PVL compared with cultures incubated with NIS (P <.05, t test). No significant differences were seen with the ∆pvl strains or with strain MW2.
To explore whether antibody-mediated augmentation of the virulence of PVL-producing S. aureus strains is indeed due to an effect on PMN-mediated host immunity, we analyzed the effect of antibodies to PVL on S. aureus survival in the presence of human PMNs in vitro. We found increased survival of three of four PVL-producing S. aureus strains in the presence of immune, but not nonimmune, sera to PVL, the exception again being strain MW2 (Fig. 3B). Antibody to PVL had no effect on survival of the isogenic ∆pvl S. aureus strains. Viability of PMNs were comparable when incubated with PVL+ or ∆pvl strains in the presence of either nonimmune or immune sera. Thus, the effect of antibody to PVL on PMN responses to PVL-producing S. aureus appears to result in interference with the antibacterial activity of the PMNs.
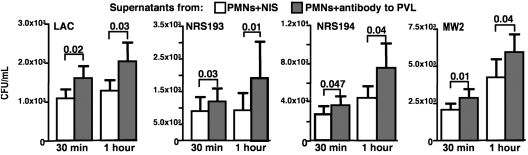
Most normal (i.e., nonimmunized) human or animal sera have little to no natural antibodies that mediate opsonic killing of S. aureus (17–19), a process that also requires a significant contribution from complement (20, 21), which was determined to be present at ∼5% of serum levels in the abscesses of infected mice. We thus hypothesized that control of this microbe within abscesses possibly could be due to a secreted antimicrobial factor. Supernatants from PMNs exposed to PVL+ strain NRS194 plus nonimmune rabbit sera inhibited the growth of 4 S. aureus strains to a greater degree than did supernatants from PMNs exposed to NRS194 and antisera to PVL (Fig. 4), indicating lower levels of antimicrobial factors in the PMN supernatants containing antibody to PVL. Interestingly, the PVL+ MW2 strain, which did not demonstrate the same effects in mice on deletion of pvl or in the presence of antibodies to PVL as other PVL+ strains tested, nonetheless was more susceptible to the antimicrobial activity in supernatants from PMNs incubated with PVL+ strain NRS194 and nonimmune sera compared with supernatants from PMNs incubated with the same strain and PVL-immune sera (Fig. 4). Thus, S. aureus strains that do not demonstrate enhanced virulence in the presence of antibody to PVL nonetheless seem to be equally susceptible to the antibacterial factors released by activated PMNs in response to PVL.
Fig. 4.
Effect of antibody to PVL on production of an extracellular antibacterial factor by human PMNs exposed to PVL-positive S. aureus. S. aureus strains LAC, NRS193, NRS194, and MW2 were grown to midlogarithmic stage, diluted, and incubated with supernatants from PMNs exposed to S. aureus NRS194 in the presence of NIS or antibody to PVL. Samples were obtained after 30 min and 1 h for enumeration of viable S. aureus. Four separate experiments were conducted and analyzed. P values are from unpaired t tests. Bars represent means; error bars represent SEM.
Discussion
Our findings indicate that under conditions of relatively low bacterial inocula and the presence of a particulate foreign body, three of the four PVL-producing S. aureus strains tested had a reduced ability to replicate and survive in skin abscesses compared with isogenic strains deleted for production of this leukocidin. Importantly, in this setting, antibody to PVL appeared to decrease the ability of PMNs to control the proliferation of PVL-producing S. aureus, possibly by impairing the process through which these immune cells are activated. These findings likely are highly applicable to human infections with MRSA, given that a 30%–50% recurrence rate within 18 months of MRSA infection has been reported in previously infected humans, usually with the same strain (22–25), and that individuals with PVL-positive MRSA infections mount potent immune responses to PVL following primary infections (26, 27). This finding suggests that humans with recurrent PVL+ MRSA infections likely have very high levels of antibody at the onset of infection that nonetheless does nothing to prevent recurrent infection and might even promote reinfection. These findings also raise concerns as to whether a recently initiated phase 1 human trial of monovalent and bivalent vaccines containing the LukS component of PVL (http://www.clinicaltrials.gov/ct2/show/NCT01011335?term=NABI&rank=5) might enhance the susceptibility to infection. Overall, our experimental results suggest that the emergence of PVL-producing CA-MRSA in human SSTIs, and perhaps other infectious settings, may be due not to a direct leukotoxic effect of PVL, but rather to the presence of antibody to PVL, which delays the host’s ability to detect and respond to infection, particularly at early stages when bacterial levels are low.
Our conclusions are also consistent with previous results demonstrating that concentrations of PVL below the level needed to lyse PMNs induce the release of proinflammatory factors histamine, beta-glucuronidase, leukotriene B4, and interleukin-8 from granulocytes (12, 28, 29). Among the family of related S. aureus two-component toxins, PVL has the most potent activity in terms of activating proinflammatory mediators from granulocytes (12, 28, 29). Above a certain threshold, PVL multimerizes to form pores in membranes of PMNs, resulting in PMN lysis (11), which might contribute to virulence when bacterial levels increase in the presence of insufficient neutralizing antibody.
Apparently contradictory results regarding the role of PVL in CA-MRSA virulence, as defined by mouse models of pneumonia and skin infections, likely reflect experimental conditions that mask some important biologic effects of PVL and also do not take into account the contribution of antibody to the infectious process. At the much higher challenge doses of PVL-positive S. aureus used by other investigators, the positive, proinflammatory effects of PVL would not be apparent, although we do note that some findings were consistent with ours in regard to the enhanced virulence of pvl-deleted strains (7). Our use of challenge doses in the presence of a foreign body that allows infections with 3 logs fewer bacteria than those used by other investigators (107cfu/abscess of S. aureus LAC compared with our inoculum of 104cfu/abscess) (8, 30) likely is relevant to our ability to show that the loss of PVL increases virulence as measured by higher bacterial loads in abscesses. Using an inocula of 107 cfu, Voyich et al. (8) found no appreciable difference in abscess volume or dermanecrosis between isogenic PVL+ and PVL- S. aureus LAC. In contrast, Brown et al. (31), using the same LAC strains and also at an inoculum of 107cfu/abscess, found that the PVL+ LAC strain replicated more within abscesses than did the isogenic ∆pvl mutant. The different strains of mice used by Voyich et al. (8) and Brown et al. (31) (Crl:SKH1-hBR hairless vs. Balb/C mice) may explain the different results, but overall these high challenge doses likely masked any potential effects from PVL elaboration that would be manifest in human infections, which most likely start out initially with much lower infectious inocula along with particulate matter introduced into wounded or abraded tissues.
We also note that Tseng et al. (32) claimed that antibody to PVL blocked muscle injury by two PVL-producing S. aureus strains in a skin infection model using CD1 mice when infections were established with 109 cfu. Interestingly, these investigators did not observe differences in muscle injury between PVL-producing and PVL− strains at lower inocula of 107 or 108, or when SKH1 or C57BL-6 mouse strains were infected with 109 cfu. At that inoculum, PVL-producing strains caused larger muscle lesions than PVL− strains in CD1 and Balb/C mice, although similar bacterial levels of both strains were recovered from abscesses. Similarly, Brown et al. (31) claimed that antibody to PVL induced by s.c., but not mucosal, vaccination protected against skin infection with a single strain of USA300, which might be relevant to their high-dose inoculum model. But they reported only weight changes, not bacterial loads, and they reported no control experiment infecting immunized mice with the Δpvl strain, calling into question the specificity of the observed protection. Moreover, the foregoing studies reported no difference in outcomes following intranasal challenge between mice immunized s.c. with LukS and/or LukF and adjuvant alone.
The lack of an effect of antibody to PVL on the virulence of the USA400 strain MW2 suggests that other factors produced by S. aureus, such as phenol-soluble modulins and α-toxin (30), might modify the effects of PVL, emphasizing a well-established concept regarding S. aureus pathogenesis that the virulence of a strain may not be dependent on the elaboration of a single factor, but rather that the overall properties of the strain are the key for virulence. Strain MW2 seems to elaborate more of the golden carotenoid pigments, which imparts antioxidant properties (Fig. S4) (33) and hence resistance to PMN-mediated antimicrobial effects, potentially masking any effects of PVL activation of granulocytes on this strain. Overall, a variety of factors need to be considered to gain more insight into the role of PVL in the emergence of the CA-MRSA epidemic. These factors include the amount of PVL produced, which might be beneficial at low amounts but harmful to the host at higher amounts, as well as the immune status of the infected individual, particularly the presence of antibody to PVL. Of note, other infectious diseases, such as Dengue hemorrhagic fever, have been proposed to have an antibody-dependent enhancement of pathogenesis (34). The findings from our animal studies, along with the high recurrence rate of MRSA infections in humans, often involving the same strain (22–25, 35), and the increased antibody levels to PVL reported after a primary infection (26, 27), suggest the need for caution when considering the value of immunization against PVL, due to the potential of antibody to enhance virulence in at least some common settings of S. aureus infection.
Materials and Methods
Bacterial Strains.
S. aureus strains MW2 (NRS123), NRS158 (MSSA), NRS193, NRS194, NRS384, and NRS482 were obtained from the Network on Antibiotic Resistance in S. aureus (NARSA). Isogenic ∆pvl versions of strains MW2, NRS193, and NRS194 were generated by allelic replacement with PVL-encoding genes that were disrupted by a cassette conferring resistance to erythromycin (∆pvl::erm). The lukSF ORFs, with at least a 0.5-kb flanking upstream and downstream sequence, were amplified by PCR (upstream primer, BamHIuplukSF2: gga tcc caa ata aga ggt gta aca cct cg; downstream primer, BamHIdownlukFR2: gga tcc ctt tta aac ata gct cat cac cc; BamHI sequences in bold) and cloned into pCR4-TOPO (Invitrogen). Outward primers with 5′ XhoI tails were designed to amplify lukSF-pCR4-TOPO outward from the cloned lukSF ORFs, leaving <100 bp of the 5′ end of lukS and the 3′ end of lukF (XhoIlukSdeleteF: ggg ctc gag tac atc aat tta tga agt tga ttg gg; XhoIlukFdeleteR: ggg ctc gag ttg cag cta ata gtc ttt ttt tga cc; XhoI sequences in bold). XhoI excised the TN917 erythromycin-resistance gene (ermR) from pTLV1 (36) and was ligated with the preceding PCR product, yielding ∆pvl::ermR-pCR4-TOPO. The ∆pvl::ermR construct was excised with BamHI, followed by ligation into BamHI-digested pMAD plasmid, a temperature-sensitive Eschericha coli–S. aureus shuttle vector (37). Plasmid ∆pvl::ermR-pMAD was transformed into protoplasts of S. aureus strain RN4220 by electroporation (38) and then phage-transduced into target S. aureus strains (MW2, NRS193, and NRS194) using phage 85 (39). A shift to a nonpermissive temperature induced homogolous recombination of ∆pvl::ermR-pMAD into the chromosome. Strains that underwent a second homologous crossover event, replacing the wild-type lukSF genes with ∆pvl::ermR that also were cured of the pMAD plasmid, were identified by blue-white selection due to the loss of the β-galactosidase gene encoded on pMAD. Selection of ∆pvl::ermR constructs were done on LB agar plates with 100 μg ampicillin/mL for E. coli and 3 μg erythromycin/mL for S. aureus. Successful deletion of the pvl genes (∆pvl) was confirmed by PCR and Western blot analysis for protein production (Fig. S5); the lower protein band that remains in ∆pvl samples can be attributed to cross-reactive staphylococcal leukocidin(s) (10). The SF8300 strain, along with LAC and its isogenic ∆pvl counterpart (LAC ∆pvl), were provided by M. Otto. Strains MW2, NRS193, and NRS194 belong to sequence type USA400, whereas strains NRS384, NRS482, LAC, and SF8300 belong to sequence type USA300.
Growth of S. aureus Strains for Mouse Infections.
S. aureus strains were grown in yeast extract–casamino acid–sodium pyruvate (YCP) broth at 37 °C with gyratory shaking to mid-late logarithmic phase (optical density at 650 nm of ∼0.8–0.9), washed twice, and suspended in 1/100 volume of YCP broth. Aliquots were frozen at −80 °C until ready for use in mouse infections, at which time they were thawed and diluted in YCP broth to the desired inocula. The actual cfu injected was confirmed by plate counts of the inocula.
Mouse Model of S. aureus Skin Abscess Infection.
Swiss Webster mice age 3–5 weeks were purchased from Harlan Laboratories. S. aureus inocula were mixed with an equal volume of sterile cytodex microcarrier beads (131–220 μM; Sigma-Aldrich), and 100 μL of the mixture was injected intradermally within the shaved midback flank region of the mouse. Each abscess was induced with an inoculum of 104 cfu (S. aureus LAC, NRS384, and NRS482) or 105 cfu (MW2, NRS158, NRS193, NRS194, and SF8300). Two injections were administered to each mouse, one on each side. After 3 days, the mice were euthanized, and the abscesses were harvested for bacterial enumeration. The ∆pvl strains were plated on antibiotic-selective media. In experiments comparing the effects of nonimmune and PVL-immune sera for the outcome of S. aureus abscess infection, 200 μL of sera was administered i.p. 48 h and 24 h before infection was established. Animal experiments were conducted in accordance with guidelines of and under a study approved by the Harvard Medical Area Institutional Animal Care and Use Committee.
Production of Antibody to PVL.
The genes encoding the two components of the PVL toxin, lukS and lukF, were cloned separately into a maltose-binding protein fusion vector (pMAL-c2×; New England Biolabs), as described elsewhere (15), and purified according to the manufacturer’s instructions. Two New Zealand White rabbits (Millbrook Breeding Labs) were used for antibody production, with one rabbit immunized with the LukS protein component of the toxin and the other immunized with LukF. The rabbits were first immunized three times via the s.c. route with 10 μg of each protein mixed in an equal volume of incomplete Freund’s adjuvant 1 week apart. Three follow-up booster immunizations via the i.v. route were given during the following week.
Statistical Analysis.
Data were analyzed by either the parametric or nonparametric unpaired or paired t test using the Prism 4 software package.
Preparation of Human PMNs.
PMNs were purified from fresh human blood from healthy adult volunteers who provided informed consent under a protocol approved by the Partner’s Healthcare Institutional Review Board using gradient centrifugation with Polymorphprep (Axis-Shield).
Calcium Assays of Human PMNs.
A Fluo-4 Direct Calcium Assay Kit (Molecular Probes) was used to compare human PMN uptake of extracellular calcium ions when incubated with purified PVL in the presence of either nonimmune rabbit sera or PVL-immune rabbit antisera. Purified PMNs were suspended in the Fluo-4 Direct calcium assay buffer to ∼6.25 × 106 cells/mL, after which the cells were loaded with the Fluo-4 Direct calcium assay reagent following the manufacturer’s protocol. Rabbit sera (10 μL undiluted, heat-inactivated at 56 °C for 30 min) and purified PVL (20 or 40 ng/10 μL) were added to the loaded PMNs immediately before fluorescence measurements. The approximate fluorescence excitation and emission maxima of 485 and 528 nm were used. Readings were taken approximately every 1.5 min over a 30-min period.
Antimicrobial Activity of Human PMNs Against S. aureus.
All components of the assay were diluted or resuspended in MEM (Gibco) containing 1% BSA. Overnight YCP-broth grown cultures of S. aureus (37 °C) were diluted to an OD650 of 0.4, followed by further dilution of 1 in 200, to achieve ∼2 × 106 cfu/mL Purified PMNs were suspended to ∼6.25 × 106 cells/mL. Nonimmune and PVL-immune antisera were heat-inactivated, diluted 1 in 5, and adsorbed twice with a turbid suspension (∼1010 cfu in 1 mL) of the target ∆pvl strain, to remove non-PVL specific antibodies. The 100 μL of each component was added to each assay, and MEM+1% BSA was added to achieve a final volume of 400 μL. The tubes were incubated at 37 °C for 6 h with end-over-end rotation, and surviving cfu was determined by serial dilution and plating. The reactions were centrifuged, and the supernatant was filter-sterilized and stored −20 °C until needed.
Evaluation of Antimicrobial Activity of Supernatants from PMN Activated by S. aureus in the Presence of Antibody
S. aureus strains to be tested were diluted from an overnight 37 °C culture into YCP medium to an OD650 of 0.1, followed by growth at 37 °C with aeration until an OD650 of 0.4 was reached. Each strain was then diluted 1:5,000 in MEM+1% BSA (∼2 × 104 cfu/mL). Then 10 μL of diluted S. aureus was added to 100 μL of the PMN antimicrobial assay supernatant to be tested, within a well of a microtiter plate. The microtiter plate was shaken at 37 °C, and samples were plated at timed intervals for bacterial count comparisons.
Supplementary Material
Acknowledgments
We thank the Network on Antibiotic Resistance in S. aureus (NARSA) and M. Otto for providing the staphylococcal strains. The following isolates were obtained through the NARSA program, supported under National Institute of Allergy and Infectious Diseases/National Institutes of Health (NIH) Contract HHSN272200700055C: NRS123 (MW2), NRS158, NRS193, NRS194, NRS384, and NRS482. This work was supported by NIH Grant AI46706.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.J.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910344107/DCSupplemental.
References
- 1.Francis JS, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100–107. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- 2.Gorak EJ, Yamada SM, Brown JD. Community-acquired methicillin-resistant Staphylococcus aureus in hospitalized adults and children without known risk factors. Clin Infect Dis. 1999;29:797–800. doi: 10.1086/520437. [DOI] [PubMed] [Google Scholar]
- 3.Herold BC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 4.Naimi TS, et al. Comparison of community- and health care–associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976–2984. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 5.Gillet Y, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 6.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. Poring over pores: α-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 7.Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis. 2008;198:1166–1170. doi: 10.1086/592053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voyich JM, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–1770. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 9.Labandeira-Rey M, et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1130–1133. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko J, Kamio Y. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: Structures, pore-forming mechanism, and organization of the genes. Biosci Biotechnol Biochem. 2004;68:981–1003. doi: 10.1271/bbb.68.981. [DOI] [PubMed] [Google Scholar]
- 11.Finck-Barbançon V, Duportail G, Meunier O, Colin DA. Pore formation by a two-component leukocidin from Staphylococcus aureus within the membrane of human polymorphonuclear leukocytes. Biochim Biophys Acta. 1993;1182:275–282. doi: 10.1016/0925-4439(93)90069-d. [DOI] [PubMed] [Google Scholar]
- 12.Hensler T, et al. Leukotriene B4 generation and DNA fragmentation induced by leukocidin from Staphylococcus aureus: Protective role of granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF for human neutrophils. Infect Immun. 1994;62:2529–2535. doi: 10.1128/iai.62.6.2529-2535.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Begier EM, et al. Connecticut Bioterrorism Field Epidemiology Response Team. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39:1446–1453. doi: 10.1086/425313. [DOI] [PubMed] [Google Scholar]
- 14.Ford CW, Hamel JC, Stapert D, Yancey RJ. Establishment of an experimental model of a Staphylococcus aureus abscess in mice by use of dextran and gelatin microcarriers. J Med Microbiol. 1989;28:259–266. doi: 10.1099/00222615-28-4-259. [DOI] [PubMed] [Google Scholar]
- 15.Gauduchon V, et al. Neutralization of Staphylococcus aureus Panton Valentine leukocidin by intravenous immunoglobulin in vitro. J Infect Dis. 2004;189:346–353. doi: 10.1086/380909. [DOI] [PubMed] [Google Scholar]
- 16.Schaff UY, et al. Calcium flux in neutrophils synchronizes beta2 integrin adhesive and signaling events that guide inflammatory recruitment. Ann Biomed Eng. 2008;36:632–646. doi: 10.1007/s10439-008-9453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fattom A, et al. Safety and immunogenicity of a booster dose of Staphylococcus aureus types 5 and 8 capsular polysaccharide conjugate vaccine (StaphVAX) in hemodialysis patients. Vaccine. 2004;23:656–663. doi: 10.1016/j.vaccine.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 18.Kelly-Quintos C, Cavacini LA, Posner MR, Goldmann D, Pier GB. Characterization of the opsonic and protective activity against Staphylococcus aureus of fully human monoclonal antibodies specific for the bacterial surface polysaccharide poly-N-acetylglucosamine. Infect Immun. 2006;74:2742–2750. doi: 10.1128/IAI.74.5.2742-2750.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maira-Litrán T, Kropec A, Goldmann DA, Pier GB. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated Staphylococcal Poly-N-acetyl-β-(1-6)-glucosamine. Infect Immun. 2005;73:6752–6762. doi: 10.1128/IAI.73.10.6752-6762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunnion KM, Lee JC, Frank MM. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect Immun. 2001;69:6796–6803. doi: 10.1128/IAI.69.11.6796-6803.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunnion KM, Zhang HM, Frank MM. Availability of complement bound to Staphylococcus aureus to interact with membrane complement receptors influences efficiency of phagocytosis. Infect Immun. 2003;71:656–662. doi: 10.1128/IAI.71.2.656-662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang SS, et al. Strain-relatedness of methicillin-resistant Staphylococcus aureus isolates recovered from patients with repeated infection. Clin Infect Dis. 2008;46:1241–1247. doi: 10.1086/529381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller LG, et al. A prospective investigation of outcomes after hospital discharge for endemic, community-acquired methicillin-resistant and -susceptible Staphylococcus aureus skin infection. Clin Infect Dis. 2007;44:483–492. doi: 10.1086/511041. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen DM, Mascola L, Brancoft E. Recurring methicillin-resistant Staphylococcus aureus infections in a football team. Emerg Infect Dis. 2005;11:526–532. doi: 10.3201/eid1104.041094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skiest D, et al. Community-onset methicillin-resistant Staphylococcus aureus in an urban HIV clinic. HIV Med. 2006;7:361–368. doi: 10.1111/j.1468-1293.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- 26.Brown EL, et al. Pediatric antibody response to community-acquired Staphylococcus aureus infection is directed to Panton-Valentine leukocidin. Clin Vaccine Immunol. 2009;16:139–141. doi: 10.1128/CVI.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Croze M, et al. Serum antibodies against Panton-Valentine leukocidin in a normal population and during Staphylococcus aureus infection. Clin Microbiol Infect. 2009;15:144–148. doi: 10.1111/j.1469-0691.2008.02650.x. [DOI] [PubMed] [Google Scholar]
- 28.König B, et al. Activation of human effector cells by different bacterial toxins (leukocidin, alveolysin, and erythrogenic toxin A): Generation of interleukin-8. Infect Immun. 1994;62:4831–4837. doi: 10.1128/iai.62.11.4831-4837.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konig B, Prevost G, Piemont Y, Konig W. Effects of Staphylococcus aureus leukocidins on inflammatory mediator release from human granulocytes. J Infect Dis. 1995;171:607–613. doi: 10.1093/infdis/171.3.607. [DOI] [PubMed] [Google Scholar]
- 30.Li M, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci USA. 2009;106:5883–5888. doi: 10.1073/pnas.0900743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown EL, et al. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin Microbiol Infect. 2009;15:156–164. doi: 10.1111/j.1469-0691.2008.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tseng CW, et al. Staphylococcus aureus Panton-Valentine leukocidin contributes to inflammation and muscle tissue injury. PLoS One. 2009;4:e6387. doi: 10.1371/journal.pone.0006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu GY, et al. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med. 2005;202:209–215. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green S, Rothman A. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr Opin Infect Dis. 2006;19:429–436. doi: 10.1097/01.qco.0000244047.31135.fa. [DOI] [PubMed] [Google Scholar]
- 35.Huang SS, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis. 2003;36:281–285. doi: 10.1086/345955. [DOI] [PubMed] [Google Scholar]
- 36.Muller E, et al. Isolation and characterization of transposon mutants of Staphylococcus epidermidis deficient in capsular polysaccharide/adhesin and slime. Infect Immun. 1993;61:551–558. doi: 10.1128/iai.61.2.551-558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnaud M, Chastanet A, Débarbouillé M. New vector for efficient allelic replacement in naturally nontransformable, low–GC content, gram-positive bacteria. Appl Environ Microbiol. 2004;70:6887–6891. doi: 10.1128/AEM.70.11.6887-6891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J. In: Methods in Molecular Biology. Nickloloff JA, editor. Totowa, NJ: Humana Press Inc; 1993. pp. 209–212. [Google Scholar]
- 39.Foster TJ, Höök M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6:484–488. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.