Abstract
Increasing evidence suggests that regular exercise improves brain health and promotes synaptic plasticity and hippocampal neurogenesis. Exercise improves learning, but specific mechanisms of information processing influenced by physical activity are unknown. Here, we report that voluntary running enhanced the ability of adult (3 months old) male C57BL/6 mice to discriminate between the locations of two adjacent identical stimuli. Improved spatial pattern separation in adult runners was tightly correlated with increased neurogenesis. In contrast, very aged (22 months old) mice had impaired spatial discrimination and low basal cell genesis that was refractory to running. These findings suggest that the addition of newly born neurons may bolster dentate gyrus-mediated encoding of fine spatial distinctions.
Keywords: exercise, hippocampus, learning, neurogenesis, pattern separation
Human and animal studies have shown that exercise has profound benefits for cognitive function. In children and young adults, there is a positive correlation between physical activity and learning (1, 2). In addition, the aging-associated decline in human memory function (3) and associated atrophy of gray matter volume can be attenuated by exercise (2, 4). In animal studies, both voluntary and forced running paradigms enhance learning and memory (5–7). Research pertaining to the underlying mechanisms has revealed significant central physiological and structural changes resulting from exercise. In particular, in the hippocampus, a brain region important for learning and memory, running increases neurotrophin gene expression (8, 9), vascularization (10, 11), dendritic spine density (12, 13), and synaptic plasticity (6, 9). These exercise-induced changes are associated with a robust increase in dentate gyrus neurogenesis in young rodents as well as a reversal of the aging-related decline in cell genesis (6, 7, 10, 14). The positive association between running and neurogenesis has raised the hypothesis that newly born hippocampal neurons may mediate, in part, improved spatial learning associated with exercise.
Although adult hippocampal neurogenesis (15, 16) is a well-established phenomenon (17, 18), the precise functional relevance of newly born neurons has remained unclear (19). In a recent study, it was reported that adult neurogenesis may play an important role in dentate gyrus-mediated pattern separation (20), the mnemonic representation of inputs with high temporal and spatial similarity (21, 22). The anatomical basis of pattern separation rests on the low probability that any two CA3 neurons will receive mossy-fiber inputs from the same granule cells (23, 24). Furthermore, physiological experiments have shown that slight environmental differences elicit unique firing rate patterns in each environment in a small number of dentate granule cells (21). Interestingly, ablation of adult neurogenesis by x-irradiation or injection of lentivirus expressing dominant-negative Wnt (25, 26) impaired performance in the radial arm maze only when the rewarded arm was in close proximity to the sample arm (20). In addition, in a nonnavigational touch-screen system (27), irradiated mice could not distinguish between small differences (20). Although these are important observations, the potential effects of increased neurogenesis on pattern separation have not been researched. A relation between enhanced neurogenesis and improved pattern separation ability would provide additional evidence for a role of neurogenesis in pattern separation. Thus, in the present study, running adult and very aged mice were tested in spatial pattern separation tasks using a touch-screen system.
Here, we show that running improved discrimination between stimuli presented close together on a touch screen in adult animals. There was no effect of exercise with a larger separation between the stimuli, suggesting that running-associated cellular changes are not required when inputs are obviously distinct. Indeed, very old mice with low basal cell genesis that was refractory to running acquired the task in the larger separation condition only. Thus, exercise alone, without enhanced neurogenesis, is not sufficient to improve fine pattern separation ability. Furthermore, when stimuli were narrowly separated, neurogenesis and performance of individual adult mice were closely correlated. These findings indicate that exercise enhances distinct encoding of spatial information and that increased neurogenesis may contribute to the observed functional improvement.
Results
Running Wheel Activity.
Both adult and aged mice made use of the running wheel, averaging a distance of 23.5 ± 1.79 km (adult mice) or 5.4 ± 0.68 km (aged mice) per day. This is more than reported in previous studies (6, 10). The increase in distance traveled may have been attributable to the use of a saucer-shaped wheel with low resistance (Fig. 1).
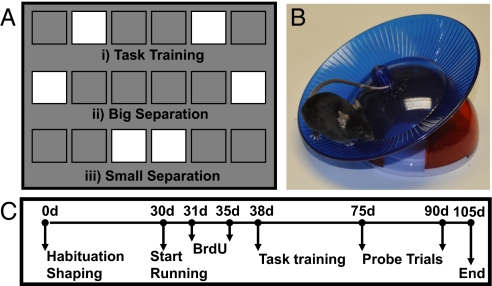
Fig. 1.
Mice were trained to distinguish between arrays of stimuli displayed on a touch screen in an operant chamber. (A) Three stimulus arrays were used during the experiments. (i) Mice were initially trained with an intermediate separation between stimuli for task training. Thereafter, animals started probe sessions with big (ii) and small (iii) stimulus separation conditions. (B) Mice were housed with or without a running wheel. (C) Timeline of the study.
Touch-Screen Testing.
Task training (intermediate separation between stimuli).
Following 1 month of shaping to the touch-screen box, the adult mice were housed with or without running wheels, were injected with BrdU over 5 days, and then began task training on day 38 of the study (Fig. 1C). The average number of days to complete the task training [i.e., ability of a mouse to complete acquisition (seven of eight correct choices) and one reversal with an intermediate separation (Fig. 1A) between the stimuli in one session of 60 trials for three of a total of four consecutive sessions] did not differ between the control and running groups. Thus, there was no significant difference between the groups in days taken to complete task training [control: 17.22 ± 2.49 days, runner: 14 ± 6.51 days; t(18) = 0.86, P > 0.39].
Probe trials (big and small separation between stimuli).
After completion of task training, mice were presented with either small or big separations between the stimuli. Mice were trained to reach a criterion of seven of eight trials correct before reversal of the position of the stimuli. ANOVA of only the acquisition data (before the first reversal) with repeated measures (group X separation) of trials to criterion revealed a significant effect of exercise [F(1,18) = 5.30, P < 0.03], a significant effect of separation [F(1,18) = 34.47, P < 0.0001], and a nonsignificant interaction between group and separation [F(1,18) = 2.54, P > 0.12]. As we predicted differences in the small but not the big condition, planned comparisons using posthoc Fisher’s predicted least-square difference (PLSD) t tests were carried out and revealed a significant difference between control and running mice on the small separation (P < 0.05) but no difference between the groups when there was a big separation between stimuli (P > 0.24). These findings suggest that the capacity to make fine distinctions between similar inputs was enhanced in the running mice. In addition, a trend toward a correlation (P = 0.13) was observed between small separation acquisition performance and newly born neuron density (Fig. 2).
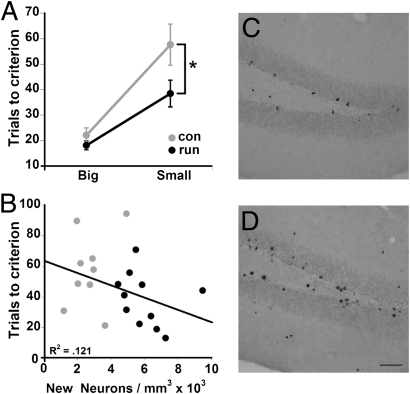
Fig. 2.
Acquisition of big and small separation between stimuli. Mice were trained to reach a criterion of seven of eight trials correct. (A) Runners (run) performed better than controls (con) in acquisition in the small separation condition (*P < 0.05) but not in the big separation condition (P > 0.24). (B) Trend toward a correlation between trials to acquisition of the small separation and newly born neuron density was observed (P = 0.13). Photomicrographs of BrdU-positive cells in control (C) and runner (D) dentate gyrus 10 weeks after the last injection. (Scale bar: 50 μm)
After mice acquired the ability to distinguish between the two stimuli (in either the big or small condition), the position of the stimuli was reversed and mice were trained to reach criterion again. The average number of reversals was analyzed for the big and small stimulus separations. ANOVA with repeated measures (group X session) showed a significant interaction for the small separation condition [F(2,18) = 8.48, P < 0.001] but not for the big separation condition [F(2,18) = 0.26, P > 0.77]. Specific comparisons between groups in the small separation sessions showed that running mice performed significantly better than control animals in session 2 (P < 0.002), indicating that runners learned the task faster than controls. Thereafter, control performance increased to the same level as the runners. Moreover, regression analysis revealed a significant correlation between reversal performance and newly born neuron density (P < 0.03). These findings show that the mice with the greatest increase in the number of newly born neurons performed best on the most challenging aspect of the test, reversals when the separation between locations was small (Fig. 3).
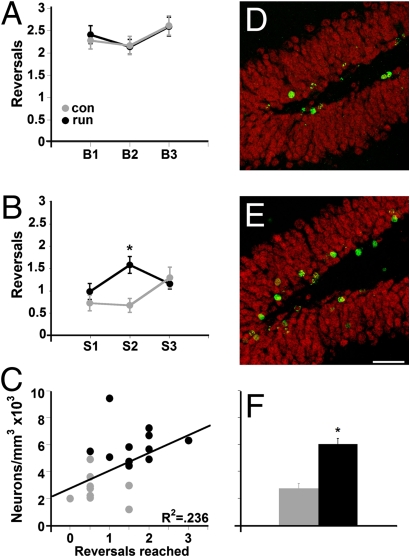
Fig. 3.
Reversal of stimulus reinforcement in the big and small conditions. The reinforcement of the stimuli was reversed each time a mouse reached criterion so that the previously correct location became the incorrect location and vice versa. (A) There was no difference between the groups in the big separation condition in the number of reversals. con, control; run, runner. (B) Runners performed better than controls in the reversal task when the separation between stimuli was small (*P < 0.002). (C) Significant correlation between task performance in the small condition and newly born neuron density was observed (P < 0.03). Confocal images of BrdU-positive cells in the dentate gyrus of sections derived from control (D) and runner (E) mice 10 weeks after the last injection. Sections were immunofluorescent double-labeled for BrdU (green) and NeuN (red). (Scale bar: 50 μm) (F) Newly born neuron density was increased in runners as compared with controls (*P < 0.0001).
Aged mice.
The very old mice exhibited impaired shaping to the touch screen (Fig. 1C), as evidenced by a failure to complete 30 trials in which the animal must initiate the next trial by an additional nose poke to the pellet receptacle for 2 consecutive days in 1 h at 70% correct (Methods, “must initiate” stage). Although the adult mice averaged 77.31% (±1.80%) correct across all their days of training, the very old mice only reached 43.03% (±1.01%). Therefore, they underwent modified testing, with 10 days of intermediate pattern separation in sedentary conditions, followed by 10 days of testing on the same task after housing with an exercise wheel (SI Text, training and testing of aged mice on the intermediate pattern separation task).
Acquisition.
Average trials to complete acquisition on the intermediate pattern separation task in block 1 (average score over 10 days) and block 2 (average score over 10 days) were analyzed by a repeated-measures ANOVA (block × group). There was a significant interaction between block and group [F(1,6) = 6.851, P < 0.04]. Specific comparisons showed that there was no significant difference between the groups within block 1 [t(6) = 1.28, P > 0.25] and block 2 [t(6) = 1.52, P > 0.18]. However, paired comparisons indicated that aged animals housed with running wheels after completing block 1 needed fewer trials to complete acquisition [29.4 ± 2.77 to 17.23 ± 1.9, block 1 to block 2, respectively; t(3) = 4.0, P < 0.03], whereas controls did not improve between block 1 and block 2 [22.13 ± 2.38 and 24.08 ± 2.57, block 1 to block 2, respectively; t(3) = 0.44, P > 0.69]. These findings suggest that exercise has a mild beneficial effect on intermediate pattern separation in very aged mice (Fig. 4A).
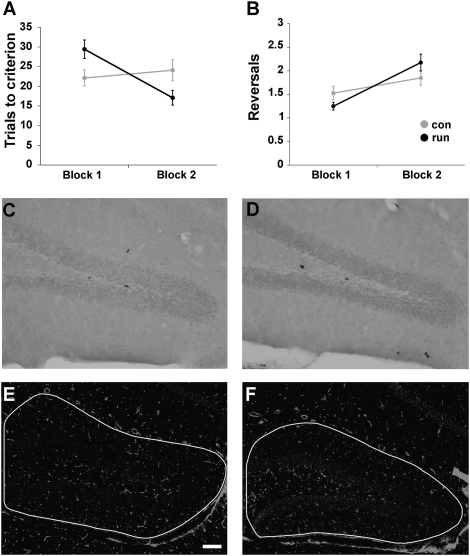
Fig. 4.
Intermediate spatial pattern separation in aged mice. Mice (n = 8) were trained for 10 days under sedentary conditions (block 1). Thereafter, mice were divided into sedentary (n = 4) and runner (n = 4) groups, injected with BrdU over 5 days, and trained for an additional 10 days on the same task (block 2). (A) Running improved performance on acquisition of the task in block 2 as compared with block 1 (P < 0.03). (B) Reversal performance was not influenced by exercise. con, control; run, runner. The number of BrdU-labeled cells did not differ between controls (C) and runners (D) at 17 days. (E and F) Lectin-stained vessel density was quantified using imaging software. There was no change in dentate gyrus vasculature in running aged mice (F) as compared with controls (E). (Scale bar: 100 μm.)
Reversals.
To determine whether the number of reversals in the intermediate pattern separation task increased with running between block 1 and block 2, ANOVA with repeated measures (block × group) was carried out. There was no interaction between block and group [F(1,6) = 1.80, P > 0.23], suggesting that running did not improve performance on the more difficult stimulus reversal task in very old mice (Fig. 4B).
Cell Counts and Phenotype Analysis.
BrdU-positive cells in the dentate gyrus were counted. In adult mice, wheel running significantly increased the number [t(18) = 5.04, P < 0.0001] and density [t(18) = 5.34, P < 0.0001] of BrdU-labeled cells, confirming previous studies (6, 7, 9, 10, 14). In addition, 30 cells per dentate gyrus were analyzed for coexpression of BrdU and NeuN for neuronal phenotype. There was no difference between the groups in percentage of BrdU/NeuN-positive cells [t(18) = 1.09, P > 0.29], but, overall, the runner group had significantly more newly born neurons than control animals [t(18) = 5.16, P < 0.0001; Table 1 and Fig. 3F]. In the very aged mice, running did not increase the number of BrdU-labeled cells [control: 25.3 ± 5.63, runner: 29 ± 4.1; t(6) = 0.54, P > 0.61; Fig. 4 C and D].
Table 1.
Survival of BrdU+ cells and neurogenesis
| Control | Runner | |
| BrdU+ cells | 1,286 (144) | 2,956 (274)* |
| BrdU+/mm3 | 3,303 (357) | 7,153 (555)* |
| % neurons | 81 (2.5) | 85 (2.1) |
| Total neurons | 1,062 (143) | 2,483 (219)* |
| Neurons/mm3 | 2,731 (362) | 6,009 (432)* |
| Volume, mm3 | 0.45 (0.0082) | 0.47 (0.0087) |
Adult C57BL/6 mice (n = 20) were assigned to either the control (n = 9) or runner (n = 11) group after completion of shaping and before the onset of task training in the touch-screen system. Mice received five daily BrdU (50 mg/kg) injections. Survival, density of BrdU-labeled cells, and volume of the dentate gyrus were determined 10 weeks after the last BrdU injection. Phenotype of the surviving cells was determined by immunofluorescent double-labeling for BrdU and NeuN (neurons). The density and percentage of BrdU-positive cells double-labeled for NeuN are presented. All data are presented as means (SE).
*Significantly different from controls (P < 0.0001).
Vasculature.
Previous work has shown that exercise affects dentate gyrus vasculature (10, 28). Coronal sections from the adult and aged groups were stained with tomato lectin. Blood vessel density was analyzed in the dentate gyrus, including the molecular layer, using a semiautomatic analysis (Slidebook; Intelligent Imaging Innovations Inc.). There was a significant increase in dentate gyrus angiogenesis in adult running mice [t(18) = 2.14, P < 0.05] (Fig. S1). No change was observed in vasculature in aged runners [t(6) = 0.47, P > 0.66] (Fig. 4 E and F).
Discussion
To study the effect of exercise on dentate gyrus-mediated pattern separation, adult and aged mice were tested on a spatial discrimination task (29) carried out in an automated touch-screen testing system (27, 30). Running selectively enhanced spatial touch-screen performance when stimuli were presented in close proximity in adult mice. There was no difference between the groups when the separation between locations to be discriminated was large. In addition, task performance and neurogenesis were positively correlated in adult mice. A physiological model of newly born cell ablation, the natural decline of neurogenesis to very low levels in old mice, revealed only a very modest enhancement on the intermediate pattern separation task in runners that was not accompanied by alterations in angiogenesis or cell genesis. Taken together, these findings provide evidence that newly born neurons may contribute to fine pattern separation.
It has long been known that the hippocampus is important for the acquisition of memories (31, 32). However, the contributions of the different hippocampal subfields have only been investigated more recently. Area CA1 is thought to encode memories (33), area CA3 is thought to mediate pattern completion (34, 35), and the dentate gyrus is considered important for spatial pattern separation (21, 22, 36). Evidence from computer modeling (37) studies suggesting that granule cells mediate processing of spatial distinctions was supported by behavioral evidence from lesion studies (36, 38) and transgenic animals with a selective knockout of NMDA receptor NR1 (22) as well as in vivo recordings in different environments (21). However, it has not been previously investigated whether physical activity can enhance the function of a task mediated by a specific subfield, the dentate gyrus, and whether newly born granule cells may play a role. In the present study, it was shown that exercise produced improvements in the touch-screen task, providing evidence that physical activity, and possibly exercise-induced neurogenesis, is important for discrimination between similar locations.
Physical activity has many functional benefits ranging from memory to mood and is accompanied by changes in neurotransmitters, growth factors, spine density, synaptic plasticity, and vascularization (11–13, 39). Similar to previous research, wheel running enhanced angiogenesis in the adult but not aged dentate gyrus (10). Thus, although we cannot definitively rule out an explanation of enhanced performance of the adult animals in terms of angiogenesis, findings in aged runners indicate that exercise alone, without angiogenesis or neurogenesis, is insufficient to improve small pattern separation. Indeed, among the cellular effects of voluntary exercise in the hippocampus, enhanced dentate gyrus neurogenesis is a striking structural alteration. Previous studies have reported a positive correlation between physical activity, neurogenesis, and performance in less specific hippocampus-dependent spatial maze tasks (6, 7, 10). However, studies in which newly born cells were ablated in runners did not always have consistent results. For example, some researchers found that irradiation of newly born cells in running mice selectively blocked improvement in a general test of hippocampal function, water maze learning (40), but not in contextual fear conditioning (40, 41), whereas others reported opposite findings (42). We propose that these discrepant findings might be explained in terms of differences in the requirements for spatial pattern separation. Because this factor was not manipulated explicitly in these previous studies and many other factors differ between these tests, such a suggestion must remain as conjecture. However, support for this idea comes from the finding in the present study that running improves performance in the condition with a high but not a low requirement for pattern separation when other factors are held constant across the two conditions. Furthermore, previous studies may have been confounded by possible changes in motor skills. In the present study, a touch-screen method was used, which has minimal motor requirements (27, 30).
All animals were sedentary during the habituation and shaping portion of the experiment in which mice were trained to retrieve a reward pellet and to initiate experiments by touching the screen. Adult mice were housed with or without wheels once task training began. There was no effect of running on the average number of days to master the task when there was an intermediate separation between the stimuli [i.e., the ability of a mouse to make a series of correct choices in the touch-screen box (reinforcement of a square on the screen) and to achieve one reversal for 3 of 4 consecutive days]. Effects of physical activity on pattern separation performance in adult mice became apparent during probe trials, when stimuli were presented in close proximity. Runners performed better than controls when the separation between stimuli was small but did not differ from controls in the large separation condition. Both acquisition of the task and the ability to learn that the stimulus was reversed in location, were enhanced in the small separation condition. The small separation task is difficult for the mice to acquire and likely places a high demand on the dentate gyrus. Additional neurons generated following exercise could benefit performance. It is of interest that probe trials were carried out 1.5–2 months after the onset of running, corresponding to the time frame in which newly born neurons exhibit the greatest amount of synaptic plasticity (43, 44). Given the difficulty of the task (no mouse achieved more than three reversals in the small separation condition), the doubling of the number of highly plastic newly born neurons in runners appears beneficial. Furthermore, a significant correlation between newly born cell number and task performance in individual mice was observed. Moreover, these findings are consistent with recent work suggesting that neurogenesis mediates dentate gyrus pattern separation (20).
In previous studies, researchers have attempted to correlate spatial memory of individual animals with newly born cell number, with varied outcomes. During aging, some researchers reported a positive correlation between a decline in learning and a decreased number of newly born cells (45), whereas others showed an opposite link between cell genesis and performance (46). In the present study, a positive correlation between the number of newly born neurons and task performance in individual mice was observed. In the acquisition phase of the small separation task, there was a trend toward a correlation between performance and neurogenesis. However, the association was significant only for the most difficult aspect of the study, the ability of the mouse to distinguish between small separations that were reversed in location each time the mouse reached criterion. This relation was observed without a phenotype shift in the percentage of newly born neurons between control and runner groups, in agreement with some (47, 48) but not other (10, 14, 49) reports. Differentiation may depend on exercise intensity (50) and use of a wheel with low resistance in the current study may account for the observed difference. The correlation between performance in the small condition and neurogenesis in individual adult animals suggests that newly born neurons may become incrementally important as the requirement for pattern separation increases.
Aged animals were only tested in the intermediate pattern separation task because they did not achieve the set criterion for testing in the small separation condition. Exercise resulted in a modest improvement in task acquisition upon comparison before and after running. There was no effect of physical activity on the ability to distinguish between intermediate separations that were reversed in location. Exercise did not enhance cell genesis in these very aged mice, which differs from previous observations in 18-month-old male C57BL/6 runners (10). This suggests that at very advanced age, cell genesis becomes refractory to running, possibly attributable to loss of plasticity of rapid amplifying type 2 progenitor cells (18). In addition, there was no change in dentate gyrus angiogenesis in the aged mice, similar to previous observations (10). It remains unclear what may mediate the slight improvement in performance. However, this physiological model of virtually ablated cell genesis supports the hypothesis that neurogenesis is important for spatial pattern separation.
In summary, the present study shows that running increases neurogenesis and promotes the ability to make fine spatial distinctions on a touch-screen task that is not dependent on motor skills. In addition, an exercise-induced increase in the number of newly born neurons was correlated with enhanced performance, suggesting that neurogenesis may play a role in spatial pattern separation. Furthermore, very old mice with low basal cell genesis that was refractory to running were able to acquire only the larger separation task, showing that running in the absence of cellular and vascular plasticity is not sufficient to improve pattern separation ability. Taken together, our findings indicate that exercise-induced neurogenesis improves dentate gyrus-mediated encoding of distinct spatial representations.
Methods
Subjects.
Twenty 3-month-old and eight 22-month-old male C57/BL6 mice (Jackson Laboratories) were individually housed in a temperature-controlled (22 °C) room with a 12-h light/dark cycle (lights on 7:00 AM to 7:00 PM). Mice were given ad libitum access to water and were food-deprived to 85% free feeding weight throughout the study. Following pretraining in the touch-screen system, mice were housed under control (adult: n = 9, aged: n = 4) or exercise (adult: n = 11, aged: n = 4) conditions for the remainder of the study.
Mouse Touch Screen.
The testing apparatus consisted of a sound-attenuating box containing a standard modular chamber (Med Associates; height = 23 cm, width = 30 cm, depth = 25 cm) with clear plexiglas walls, a metal frame, and a floor consisting of metal bars spaced 1 cm apart. The operant chamber was fitted with an infrared touch screen (Craft Data Ltd.), a pellet receptacle with light illumination and head entry detectors, a 14-mg pellet dispenser, a 3-W house light, and a tone generator (Med Associates). The infrared sensors eliminated the need for force for nose poke detection. A plexiglas “mask” containing six windows (2.5 × 2.5 cm, equally spaced 0.5 cm apart) ≈1.6 cm from the floor of the chamber positioned in front of the touch screen allowed the presentation of stimuli on the touch screen to be spatially localized and prevented the mouse from accidentally triggering the touch screen (e.g., with its tail) (Fig. 1A).
Shaping.
Habituation.
On the first 2 days of shaping, animals were placed in operant boxes with the screen switched off, the house light on, and the magazine filled with food pellets for 15 min each day to become accustomed to the box.
Pavlovian training.
On day 3, animals underwent Pavlovian conditioning. A white square stimulus was presented randomly in one of five possible locations on the screen (all locations were on the bottom of the screen and were aligned horizontally). Each pellet delivery was combined with a tone. The intertrial interval (ITI) was 30,000 ms. The screen did not need to be touched, but if the mouse touched any part of the screen, including a nonlit area, a tone was generated and a pellet was given. Mice were given 30 automated Pavlovian training trials for 1 day.
“Must touch” stage.
Once Pavlovian training was complete, animals moved on to the “must touch” stage. Each trial began with the presentation of one white square stimulus displayed randomly in one of five possible locations. The mouse was rewarded for touching the white stimulus part of the screen; in such case, a tone was presented and a reward was given. A touch to a location that did not contain a square was scored as incorrect; no tone was generated, no reward was given, and the stimulus remained on the screen until it was touched. The next trial began after the food pellet was eaten (i.e., when an exit from the pellet receptacle was detected). The ITI was 5,000 ms. Mice continued on to the next stage of training when they completed a criterion of 30 trials in 1 h at 70% correct.
“Must initiate” stage.
Stimuli were presented in the same manner as in the previous stage. A reward was only given for correct stimulus touches. After reward collection, the animal was required to initiate the next trial by an additional nose poke to the pellet receptacle. Mice continued training until each animal completed 30 trials in 1 h at 70% correct for 2 consecutive days. The adult mice averaged 77.31 ± 1.80% across all their days of training. The very old mice only reached an average of 43.03 ± 1.01%. Therefore, the aged mice were tested in a modified version of the pattern separation task as described in SI Text (training and testing of aged mice on the intermediate pattern separation task).
Exercise and BrdU Injections.
On completion of shaping, adult animals were divided into two groups: control (CON, n = 9) and exercise (RUN, n = 11) and running wheels were added to the cages of the exercise group. Wheels were linked to a bicycle computer (Sigma Sport USA) to measure daily running distance (51). One day thereafter, mice were given daily i.p. injections of BrdU, 50 mg/kg, over 5 consecutive days (Fig. 1 B and C).
Task Training.
Two days after the last BrdU injection, animals were tested on a spatial discrimination task (30). Animals were placed in the operant boxes with six-hole masks (Fig. 1A). White squares (the stimuli) were presented in two of the six possible locations, aligned in a row along the bottom of the screen. The two stimuli were in positions 2 and 5 if position 1 was the furthest left, and each position was numbered consecutively from left to right. Only one of the squares was reinforced with the tone and pellet, and the correct side was counterbalanced between mice. When the mouse had achieved the criterion of seven of eight trials correct (i.e., it had completed the acquisition phase), the reinforced stimulus was reversed (i.e., the previously incorrect location became the correct location, and vice versa). When the mouse completed this first reversal (by again getting seven of eight trials correct on the new side), the reward contingency was again reversed. This procedure continued for 60 trials, or a maximum of eight reversals. On the next day, the mouse was presented with the same stimuli, and the correct side was the same as the side that was last correct in the previous session; that is, if a mouse started with position 5 correct and achieved acquisition and completed two reversals to criterion (the session therefore ending during its third reversal), it would start with position 2 being the correct stimulus on the next day. Mice were given task training until they were able to complete to criterion with at least one reversal for 3 of 4 consecutive days. Thereafter, they continued to probe trials.
Probe Trials.
For probe trials, the mouse was presented with one of either a small or a big separation condition (counterbalanced so that half of the animals in each group were first presented with the big separation and the animals in the other half of the group were presented with small separations; Fig. 1A). In the big separation condition, the two stimuli were presented in positions 1 and 6, and in the small separation condition, stimuli were presented at positions 3 and 4. The same rules for criterion applied, except that probe trials were given for a finite number of days. Each mouse was given each separation (small or big) alternating every 2 days per block over 12 days of testing. For example, if they started with the big separation, they would be presented with that condition for 2 days, then the small condition for 2 days, and then the big condition again for 2 days until testing was complete.
Histology/Immunohistochemistry.
Animals were deeply anesthetized with sodium pentobarbital (Merial) and perfused transcardially (SI Text, histology/immunohistochemistry).
Quantity and phenotype of newly born cells.
Immunohistochemistry for BrdU and immunofluorescent double-labeling for BrdU and Neuronal Nuclei (Neun) were performed on free-floating 40-μm coronal sections that were pretreated for BrdU immunohistochemistry by denaturing DNA, as described previously (6, 14). BrdU-positive cells were counted in a 1-in-6 series of sections (240 μm apart) through a ×20 objective (BX51; Olympus) throughout the rostrocaudal extent of the granule cell layer (SI Text, quantity and phenotype of newly born cells). To analyze the phenotype of the newly born cells, a 1-in-12 series of sections (480 μm apart) was double-labeled with BrdU and NeuN, and was analyzed by confocal microscopy (Fluoview FV1000 Olympus). Thirty BrdU-positive cells per adult mouse were analyzed for the coexpression of BrdU and NeuN for neuronal phenotype. The ratio of BrdU-positive cells colabeling with NeuN was determined.
Vasculature.
Lectin staining (Lycopersicon esculentum; Vector) was used to visualize hippocampal blood vessels as described previously (10, 28) (SI Text, vasculature). For quantification of blood vessel density, the hippocampus was imaged at ×10 magnification using the confocal microscope with identical parameters for each image (Fluoview FV1000; Olympus). Specifically, eight 3-μm-thick optical sections through the hippocampus were taken from six equidistant sections (240 μm apart) from each brain. Thereafter, the z-stacks were merged using Slidebook (Intelligent Imaging Innovations, Inc.). Using this program, the outline of the dentate gyrus, including the hilus and molecular layer, was traced by the experimenter, who was blinded to the treatment groups. Subsequently, the density of lectin-stained vessels was calculated by the software.
Statistical Analysis.
All statistical analyses were carried out using Statview (Abacus Corporation) (SI Text, statistical analysis).
Supplementary Material
Acknowledgments
We thank Dr. Mark Mattson for comments on the manuscript, Linda Kitabayashi for help with figure preparation, Nicholas McCollum for image analysis advice, and Kriti Gandhi for technical assistance. This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911725107/DCSupplemental.
References
- 1.Sibley BA, Etnier JL. The relationship between physical activity and cognition in children: A meta-analysis. Pediatric Exercise Science. 2003;15:243–256. [Google Scholar]
- 2.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 3.Gazzaley A, Sheridan MA, Cooney JW, D’Esposito M. Age-related deficits in component processes of working memory. Neuropsychology. 2007;21:532–539. doi: 10.1037/0894-4105.21.5.532. [DOI] [PubMed] [Google Scholar]
- 4.Colcombe SJ, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 5.Fordyce DE, Farrar RP. Enhancement of spatial learning in F344 rats by physical activity and related learning-associated alterations in hippocampal and cortical cholinergic functioning. Behav Brain Res. 1991;46:123–133. doi: 10.1016/s0166-4328(05)80105-6. [DOI] [PubMed] [Google Scholar]
- 6.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Praag H. Neurogenesis and exercise: Past and future directions. NeuroMol Med. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- 8.Neeper SA, Gómez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 9.Farmer J, et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 10.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira AC, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 13.Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 15.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 17.Taupin P. Adult neurogenesis in the mammalian central nervous system: Functionality and potential clinical interest. Med Sci Monit. 2005;11:RA247–RA252. [PubMed] [Google Scholar]
- 18.Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- 19.Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 22.McHugh TJ, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 23.Amaral DG, Ishizuka N, Claiborne B. Neurons, numbers and the hippocampal network. Prog Brain Res. 1990;83:1–11. doi: 10.1016/s0079-6123(08)61237-6. [DOI] [PubMed] [Google Scholar]
- 24.Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Lie DC, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 26.Jessberger S, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morton AJ, Skillings E, Bussey TJ, Saksida LM. Measuring cognitive deficits in disabled mice using an automated interactive touchscreen system. Nat Methods. 2006;3:767. doi: 10.1038/nmeth1006-767. [DOI] [PubMed] [Google Scholar]
- 28.van Praag H, et al. Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci. 2007;27:5869–5878. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McTighe SM, Mar AC, Romberg C, Bussey TJ, Saksida LM. A new touchscreen test of pattern separation: Effect of hippocampal lesions. NeuroReport. 2009;20:881–885. doi: 10.1097/WNR.0b013e32832c5eb2. [DOI] [PubMed] [Google Scholar]
- 30.Brigman JL, et al. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn Mem. 2008;15:50–54. doi: 10.1101/lm.777308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 33.Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5:361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- 34.Marr D. Simple memory: A theory for archicortex. Proc R Soc Lond Ser B. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 35.Nakazawa K, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: Double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- 37.Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- 38.Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008;18:1064–1073. doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- 39.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Clark PJ, et al. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 41.Kitamura T, et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 42.Wojtowicz JM, Askew ML, Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur J Neurosci. 2008;27:1494–1502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- 43.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 45.Drapeau E, et al. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3:227–234. doi: 10.1111/j.1474-9728.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- 47.Kitamura T, Mishina M, Sugiyama H. Enhancement of neurogenesis by running wheel exercises is suppressed in mice lacking NMDA receptor epsilon 1 subunit. Neurosci Res (NY) 2003;47:55–63. doi: 10.1016/s0168-0102(03)00171-8. [DOI] [PubMed] [Google Scholar]
- 48.Koehl M, et al. Exercise-induced promotion of hippocampal cell proliferation requires beta-endorphin. FASEB J. 2008;22:2253–2262. doi: 10.1096/fj.07-099101. [DOI] [PubMed] [Google Scholar]
- 49.Fabel K, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 50.Lou SJ, Liu JY, Chang H, Chen PJ. Hippocampal neurogenesis and gene expression depend on exercise intensity in juvenile rats. Brain Res. 2008;1210:48–55. doi: 10.1016/j.brainres.2008.02.080. [DOI] [PubMed] [Google Scholar]
- 51.Bruestle DA, Cutler RG, Telljohann RS, Mattson MP. Decline in daily running distance presages disease onset in a mouse model of ALS. NeuroMol Med. 2009;11:58–62. doi: 10.1007/s12017-009-8064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.