Abstract
Inability to form new memories is an early clinical sign of Alzheimer’s disease (AD). There is ample evidence that the amyloid-β (Aβ) peptide plays a key role in the pathogenesis of this disorder. Soluble, bio-derived oligomers of Aβ are proposed as the key mediators of synaptic and cognitive dysfunction, but more tractable models of Aβ−mediated cognitive impairment are needed. Here we report that, in mice, acute intracerebroventricular injections of synthetic Aβ1–42 oligomers impaired consolidation of the long-term recognition memory, whereas mature Aβ1–42 fibrils and freshly dissolved peptide did not. The deficit induced by oligomers was reversible and was prevented by an anti-Aβ antibody. It has been suggested that the cellular prion protein (PrPC) mediates the impairment of synaptic plasticity induced by Aβ. We confirmed that Aβ1–42 oligomers interact with PrPC, with nanomolar affinity. However, PrP-expressing and PrP knock-out mice were equally susceptible to this impairment. These data suggest that Aβ1–42 oligomers are responsible for cognitive impairment in AD and that PrPC is not required.
Keywords: Alzheimer, neurotoxicity, object recognition test, surface plasmon resonance, protein aggregation
Alzheimer’s disease (AD) is the most common neurodegenerative disorder, and the major cause of dementia in the elderly. It causes synaptic dysfunction, progressive cognitive impairment, and accumulation of extracellular amyloid plaques and intraneuronal neurofibrillary tangles in the brain. Genetic, biochemical, and experimental evidence converge to associate AD pathogenesis with the accumulation of amyloid-β (Aβ) deriving from the metabolism of amyloid precursor protein (APP) through the serial activity of β- and γ-secretases. In the last decade, soluble oligomers of Aβ have been proposed as the key mediators of synaptic and cognitive dysfunction, because of stronger correlation between cortical levels of soluble Aβ species and synaptic loss than with plaque burden in AD patients (1, 2). In vitro and in vivo studies have now indicated that soluble Aβ oligomers impair synaptic plasticity, inhibiting hippocampal long-term potentiation (LTP), the electrophysiological correlate of learning and memory (3 –6). Memory impairment and LTP inhibition have also been detected in AD mouse models before plaque deposition in the brain parenchyma (7, 8).
Thus far, there are only a few reports of the in vivo involvement of Aβ oligomers in memory impairment in rats (9 –12). Several types of Aβ aggregate isolated from biological sources have been used in these studies. The mechanism through which Aβ oligomers act remains uncertain, but interactions have been reported with several receptors such as nicotinic, insulinic, and glutamatergic receptors, leading to detrimental effects on synaptic plasticity and spine formation (12 –16). Recently, the cellular prion protein (PrPC) has been proposed as another additional possible mediator of oligomer action. PrPC binds synthetic Aβ oligomers with high affinity and plays a role in the oligomer-mediated inhibition of LTP (17).
To determine which Aβ assemblies are responsible for memory deficit, we injected well-characterized oligomers or fibrils of synthetic Aβ1–42 into the lateral ventricle of C57BL/6 mice and assessed their performance in the novel-object recognition task, which is widely used for evaluating memory in AD mouse models (18 –21) and is based on spontaneous animal behavior, without the need of stressor elements. In addition, the use of defined synthetic Aβ preparations eliminates unknown factors in cell and brain extracts or cerebrospinal fluid that could mask or exacerbate their effects. This in vivo model was used to investigate whether Aβ oligomers interfere with either the encoding/consolidation or retrieval of memory, an important aspect distinguishing early from advanced clinical stages of AD (22). Finally, we investigated the ability of PrPC to bind Aβ oligomers and its involvement in their actions.
Results
Synthetic Aβ1–42 Oligomers Induce Reversible Memory Impairment, Preventable by Pretreatment with an Anti-Aβ Antibody.
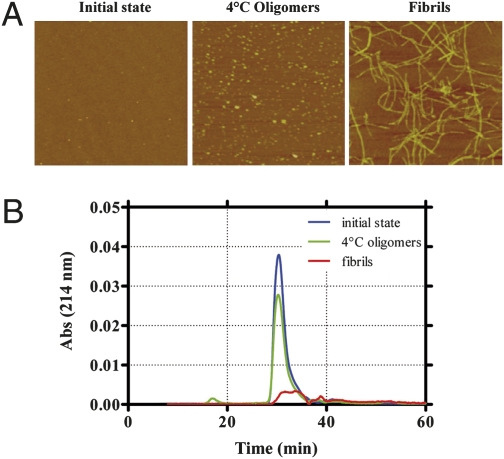
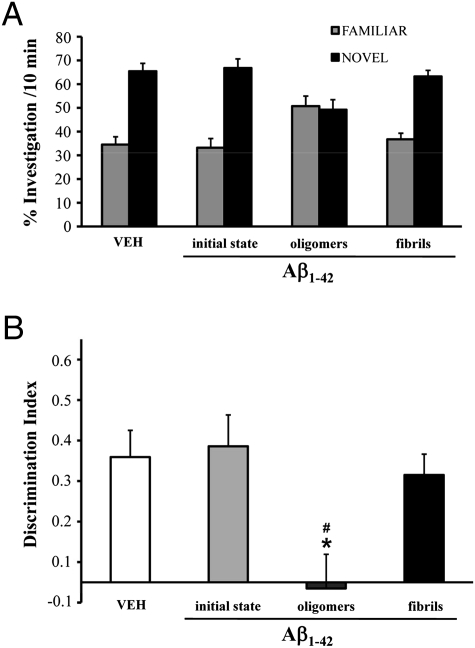
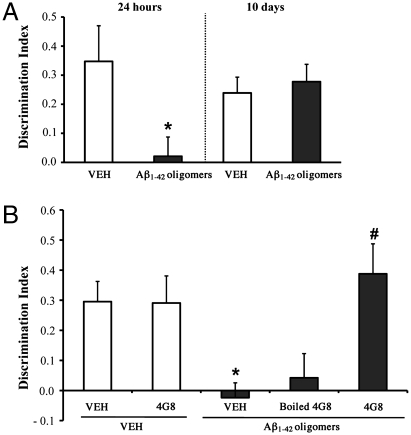
C57BL/6 male mice 7–8 weeks old received acute i.c.v. injections of either synthetic Aβ1–42 monomer, oligomer-containing solution or fibril-enriched solution and were subsequently tested in the novel-object recognition task. Oligomers and fibrils were obtained by incubating Aβ1–42 for 24 at 4 °C, pH 7.4 (3), or for 24 h at 37 °C, pH 2 (23), respectively. These preparations, and freshly dissolved Aβ1−42 (hereafter referred to as “initial state”), were characterized by atomic force microscopy (AFM) and size exclusion chromatography (SEC) before behavioral investigation. Only a few small Aβ particles were detected in the initial state, whereas the oligomer preparation contained spherical particles of 2–3 nm diameter (Fig. 1A) appearing in the SEC void volume (>75 kDa; Fig. 1B). On the basis of SEC, we estimated the actual oligomer concentration in this sample as 10–50 nM. After 24 h incubation at pH 2, Aβ1–42 assembled into structured fibrils of 3–4 nm diameter (Fig. 1A), which were blocked by the filter at the top of the SEC column (Fig. 1B). The Aβ1–42 preparations were injected (7.5 μL of 1 μM nominal Aβ solution) into the lateral ventricle of C57BL/6 mice 2 h before training in an arena containing two objects that they could explore freely (familiarization phase). Twenty-four hours later, the mice were reinjected and 2 h later exposed to one familiar and one new object (test phase). Aβ oligomer–injected mice were unable to distinguish the new object, with no significant difference in the percentage of time spent investigating the two (Fig. 2A), and a discrimination index significantly lower than vehicle-injected mice (Fig. 2B). Neither Aβ in the initial state nor the fibrils affected memory (Fig. 2 A and B). To establish whether the memory deficit was reversible, mice were injected with Aβ oligomers and tested first according to the protocol described above and a second time 10 days later. After 10 days, with no further Aβ injection, the memory deficit had fully recovered (Fig. 3A). This indicates that Aβ oligomer-mediated memory impairment does not depend on a persistent neurodegenerative phenomenon and can be rescued, suggesting that targeting Aβ oligomers might lead to recovery of cognitive functions (10).
Fig. 1.
Atomic force microscopy (AFM) and size exclusion chromatography (SEC) of different Aβ1–42 preparations. (A) AFM characterization of the Aβ1–42 preparations used in vivo: the “initial state” corresponds to the freshly dissolved peptide kept at 4 °C; the oligomers were formed after 24 h incubation at 4 °C, pH 7.4, and the fibrils after 24 h of incubation at 37 °C, pH 2 (scan size 2 μm × 2 μm). (B) Initial state (blue), 4 °C Aβ1–42 oligomers (green), and fibrils (red) analyzed by SEC, monitoring absorbance at 214 nm.
Fig. 2.
Aβ1–42 oligomers impair recognition memory in mice. (A) Effect of Aβ initial state, oligomers, and fibrils on memory was investigated in C57BL/6 male mice in the object recognition task after two i.c.v. injections (7.5 μL; 1.0 μM). Histograms indicate percentage (mean ± SEM) of exploration of the familiar and novel objects. Vehicle-injected mice (VEH; PBS 5 mM; n = 7) spent significantly more time investigating the novel object. Performance was comparable in mice given initial state Aβ (n = 10) and fibrils (n = 10). The Aβ oligomers significantly impaired memory, as shown by the inability of the mice to recognize the familiar object (n = 13) and spending equal time investigating both objects. (B) Histograms show the corresponding discrimination index (mean ± SEM) for the data shown in A (one-way ANOVA, F 3,36 = 5.76; P = 0.002; *P < 0.05 vs. VEH and fibrils; # P < 0.01 vs. initial state; Tukey’s post hoc test).
Fig. 3.
Aβ1–42 oligomer-mediated memory impairment is reversible and is prevented by pretreatment with the anti-Aβ 4G8 antibody. To investigate whether the Aβ oligomer-mediated memory impairment was reversible, mice were injected with oligomers and tested in the object recognition task 24 h or 10 days later. (A) Memory impairment induced by Aβ1–42 oligomers after 24 h (t 12 = -2.34; P = 0.03; *P < 0.05 Student’s t test; n = 7, mean ± SEM) had completely recovered 10 days after the injection (t 12 = 0.48; P = 0.64; Student’s t test). (B) To test whether the deficit was prevented by an anti-Aβ antibody, mice were treated 5 min before Aβ oligomer injection with 0.25 μg of monoclonal antibody 4G8. Analysis of variance indicated a significant interaction (4G8 x Aβ oligomers F 1,20 = 6.5; P = 0.01, ANOVA 2 × 2 test). The antibody alone had no effect, as the memory performance of 4G8-injected mice (n = 5) was comparable to that of vehicle-injected mice (n = 6). Aβ oligomers (n = 6) induced significantly impaired memory (*P < 0.05 vs. VEH or 4G8 alone, Bonferroni’s post hoc test), but this memory impairment was completely rescued by 4G8 pretreatment (n = 7; #P < 0.01 vs. Aβ oligomers, Bonferroni’s post hoc test). Pretreatment with the heat-denatured 4G8 antibody (n = 7) did not restore memory.
We then assessed whether i.c.v. infusion of 4G8, a monoclonal antibody directed to the midregion of Aβ, prevented the memory impairment induced by Aβ1–42 oligomers. 4G8 abrogates the disruption of synaptic plasticity induced by cell-derived Aβ oligomers (24). An i.c.v. injection of 0.25 μg/2 μL of 4G8, 5 min before the Aβ oligomers, completely prevented the memory impairment (Fig. 3B). Mice injected with Aβ oligomers did not discriminate between the familiar and novel object, but the 4G8 pretreatment fully prevented this memory impairment. Heat-denatured antibody, unable to bind Aβ, could not antagonize the effect of Aβ oligomers. An i.c.v. injection of 4G8 alone did not affect memory.
PrPC Binds to Aβ1–42 Oligomers but Does Not Govern Their Detrimental Effect on Memory.
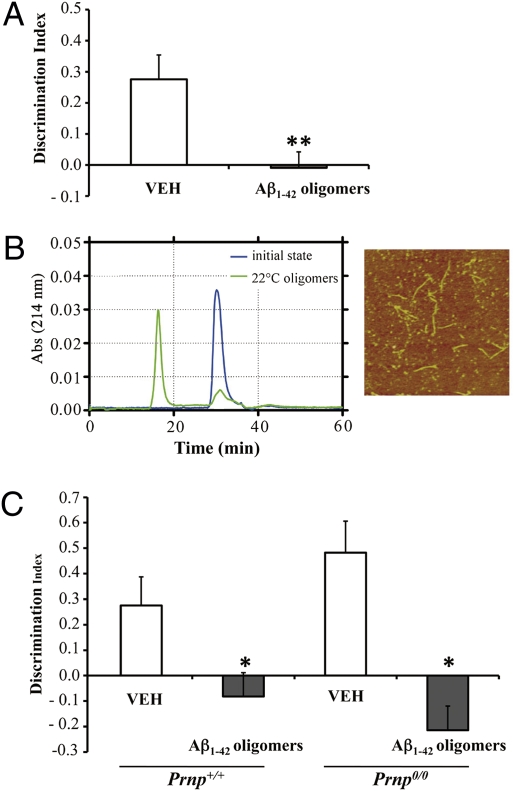
It has been proposed that the cellular prion protein (PrPC) is the Aβ oligomer-receptor governing Aβ-induced synaptic dysfunction (17). Aβ oligomers bound to PrPC on the neuronal surface and inhibited long-term potentiation (LTP) in hippocampal slices of wild-type (Prnp +/+) but not PrP knockout (Prnp 0/0) mice. Because recognition memory is dependent on the medial temporal lobe including the hippocampus (25), we examined whether oligomer-mediated memory impairment was also related to PrPC expression. We found that Prnp 0/0 mice were as susceptible as Prnp +/+ mice to oligomer-induced memory impairment (Figs. 2B and 4A). This suggests that PrPC is not required for the oligomer-mediated memory impairment. The performance of vehicle-treated Prnp 0/0 and vehicle-treated Prnp +/+ mice was similar (Figs. 2B and 4 A), indicating that lack of PrPC did not affect recognition memory per se.
Fig. 4.
Aβ1–42 oligomers impair recognition memory independently of PrPC. (A) Prnp 0/0 mice given an i.c.v. injection of Aβ oligomers prepared at 4 °C showed significant memory impairment (t 9 = −3,57; **P < 0.01 Student’s t test; VEH n = 5; Aβ1–42 Oligomers n = 6; mean ± SEM). (B) SEC of the 22 °C oligomer preparation (green), initial state (blue). AFM pictures of the oligomeric preparations are shown on the right of the SEC panel (scan size, 2 μm × 2 μm). (C) Oligomeric assemblies prepared at 22 °C significantly affected recognition memory in wild-type mice (Prnp +/+) (t 11 = −2.5; P = 0.03; Student’s t test; VEH n = 6; Aβ1–42 oligomers n = 7) and Prnp 0/0 mice (t 8 = −4.5; P = 0.02; Student’s t test; VEH n = 5; Aβ1–42 oligomers n = 5).
Our finding that Aβ oligomers impair memory in Prnp 0/0 mice contrasts with the reported normal LTP in oligomer-treated Prnp 0/0 hippocampal slices (17). To rule out the possibility that the different effect on memory was due to different oligomer preparations, we repeated the behavioral test using Aβ1–42 oligomers prepared at 22 °C according to the Lauren at al. procedure (17). AFM confirmed the presence of spherical species and protofibrils, whereas SEC indicated that most peptide was converted to high-molecular-weight aggregates (>75kDa; Fig. 4B). The 22 °C-Aβ oligomers impaired recognition memory in both Prnp +/+ and Prnp 0/0 mice (Fig. 4C). Prnp 0/0 mice spent slightly more time on the familiar object, but the difference was not significant. A slight preference for the familiar object was also reported in APP transgenic mice (20).
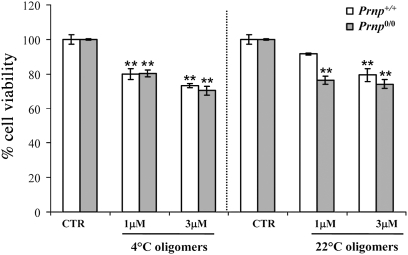
We also tested the involvement of PrPC in mediating Aβ oligomer toxicity in vitro, by investigating the effect on survival of primary hippocampal neurons from wild type or Prnp0/0 cells. After 72 h of treatment with 4 °C or 22 °C Aβ oligomers (1–3 μM), cell survival was measured by MTT assay. Oligomers were toxic to both Prnp +/+ and Prnp 0/0 hippocampal cells, consistent with the conclusion that their adverse effects are independent of PrPC (Fig. 5).
Fig. 5.
Vulnerability of hippocampal neurons to Aβ1–42 oligomers is independent of PrPC. Histograms show percentage cell survival in MTT test after exposure to 4 °C and 22 °C oligomers (mean ± SEM); 72-h treatment with Aβ1–42 oligomers (1 and 3 μM) caused similar death of hippocampal neurons from Prnp +/+ and Prnp 0/0 mice. Two-way ANOVA for 4 °C oligomers revealed a nonsignificant interaction transgene (tg) × treatment (F 1,12 = 0.29; P = 0.7) and a significant interaction tg × treatment for 22 °C oligomers (F 1,12 = 5.1; P = 0.02), **P < 0.01; Tukey’s test vs. VEH group) .
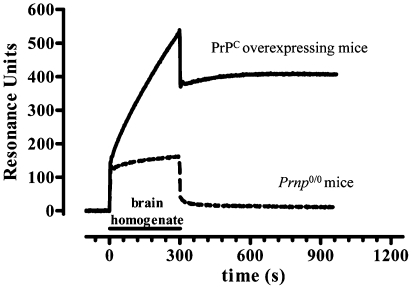
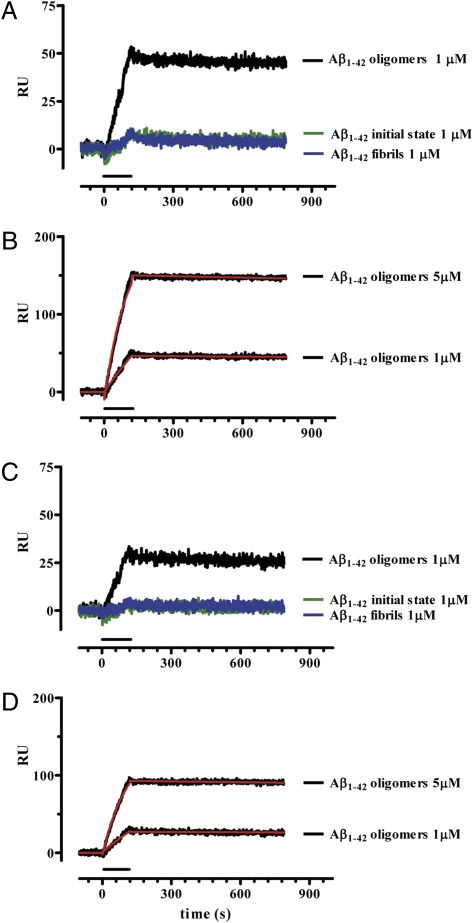
Although PrPC does not influence Aβ oligomer-induced memory dysfunction, surface plasmon resonance (SPR) detected a high-affinity interaction between Aβ oligomers and PrPC. PrPC from mouse brain homogenates was captured on the sensor surface of SPR chips by either 3F4 or 94B4, two anti-PrPC antibodies. Preliminary data confirmed that the captured protein is actually PrPC, as no capture was detected when flowing brain homogenate from Prnp 0/0 mice (Fig. 6). Moreover, PrPC captured by both 94B4 and 3F4 maintains the ability to bind 6D11, an anti-PrP antibody directed against the epitope 93–109, i.e., the region suggested to be involved in the interaction with Aβ oligomers. When Aβ initial state, oligomers or fibrils were assayed for their binding to PrPC, only Aβ oligomers bound PrPC specifically, and Aβ initial state and fibrils did not (Fig. 7 A and C). The binding was dose dependent, with a dissociation constant (K d) of ≤20 nM monomer equivalent (Fig. 7 B and D). Thus, although Aβ oligomers interact with PrPC with high affinity, they do not act together to induce memory derangement.
Fig. 6.
Specific capture of PrPC by 3F4 antibody immobilized on the sensor chip. 3F4 was immobilized on the sensor chip using amine-coupling chemistry, with final immobilization levels of ∼6,000 resonance units, RU. After 90° rotation of the fluid system, brain homogenates from PrPC overexpressing mice or Prnp 0/0 mice were injected in parallel.
Fig. 7.
Surface plasmon resonance shows selective, high-affinity binding of Aβ1−42 oligomers to PrPC. The Aβ1–42 species were perfused for 2 min on sensor surfaces on which PrPC had been captured by 3F4 (A and B) or 94B4 (C and D) monoclonal antibodies. The nonspecific binding on sensor surfaces immobilizing the antibodies alone was subtracted. Sensorgrams show the time course of the Aβ−dependent SPR signal in resonance units (RU). Only Aβ oligomers bound PrPC specifically, whereas the initial state and fibrils did not (A and C). The sensorgrams obtained with 1- and 5-μM Aβ1−42 oligomers were analyzed by the Langmuir equation, modeling a simple bimolecular interaction (B and D). Fitting is shown in red. Parameters of Aβ oligomer binding to (3F4)-PrPC were as follows: K on: 2.1 × 103 M-1s−1; K off: 4.0 × 10−5 s−1; K d: 19.5 nM; Rmax: 211 RU; for binding to (94B4)-PrPC: K on: 1.8 × 103 M-1s−1; K off: 4.0 × 10−5 s−1; K d: 22.6 nM; Rmax: 143 RU.
Aβ1–42 Oligomers Impair Memory Encoding/Consolidation.
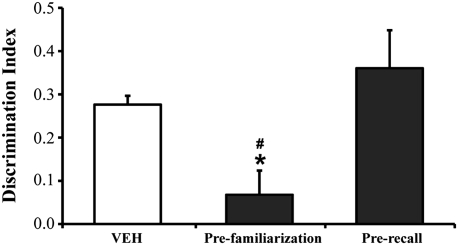
The behavioral protocol adopted in the experiments described above could not clarify whether oligomers affected memory encoding/consolidation or recall (26, 27). To gain a clearer understanding of the mechanism of oligomer action, we tested the mouse’s memory after a single oligomer injection before either the familiarization or test phase. Mice injected 2 h before familiarization were unable to remember the object previously investigated, whereas mice injected 2 h before the test phase recalled the familiar object investigated the day before (Fig. 8). These data indicate that Aβ oligomers acutely disrupt anterograde memory storage but do not interfere with its retrieval when the information has been properly stored. This suggests that the memory deficit in our murine model mimics the situation in early-stage AD patients who are unable to store newly acquired information but preserve old memories (22).
Fig. 8.
Aβ oligomers acutely disrupt memory storage but not memory retrieval. To clarify the Aβ oligomers’ action on memory formation and recall, mice were given a single i.c.v. injection of oligomers either before familiarization or before memory recall evaluation. One-way ANOVA revealed a significant effect of treatment (F 2,22 = 7.05; P = 0.043). The memory impairment was observed only in animals receiving Aβ oligomers before the familiarization phase (prefamiliarization; n = 10), which were unable to distinguish between the two objects (*P < 0.05 vs. VEH; # P < 0.01 vs. oligomers prerecall; Tukey’s posthoc test). No effect was detectable when mice were treated with either vehicle (n = 8) or oligomers before memory recall evaluation (oligomer prerecall; n = 7).
Discussion
Several recent reports indicate that natural Aβ oligomers are the main toxic Aβ assembly responsible for memory disruption. These studies used soluble Aβ oligomers from biological sources, arguing against the use of synthetic Aβ because of the high concentrations required to detect detrimental effects. In previous studies, in fact, intracerebral injections of synthetic Aβ, that included mixtures of Aβ fibrils, protofibrils, oligomers, and monomers in unknown proportions, had deleterious effects on learned behavior in rats. These deficits were detectable a long time after the postinjection and with total amounts of Aβ several orders of magnitude higher than those of the natural oligomers (28 –32).
Here we demonstrated that well-characterized synthetic Aβ oligomers were responsible for an immediate memory impairment in mice injected i.c.v. and tested in the novel-object recognition task. The effect was detectable at a nanomolar concentration of Aβ oligomers (10–50 nM). Proof that Aβ oligomers are the active amyloid-β species was the lack of effect of either the freshly solubilized Aβ (initial state) or fibrils. We also found that the memory deficit was transient, as 10 days after the injection, the memory performance was normal. This suggests that the oligomer-mediated memory impairment might be therapeutically rescued.
Learning and memory depend on a complex process involving information encoding, consolidation, storage, and retrieval (26, 27). LTP is a widely used experimental paradigm that measures synaptic plasticity and is a correlate of learning and memory (33). Because of controversial findings from electrophysiological (5) and behavioral studies (4) on the action of oligomers on LTP/memory induction or expression, we investigated the effects of Aβ oligomers on memory encoding/consolidation or retrieval. Aβ oligomers inhibited the encoding/consolidation of information, without affecting its retrieval if properly stored. Aβ oligomers injected i.c.v. before acquisition of the information (familiarization phase) prevented the information being either encoded or consolidated. In contrast, when the oligomers were injected 24 h after the information had been processed, no deficit was detected, suggesting that Aβ oligomers do not abolish the retrieval of stabilized information but do prevent its encoding or consolidation. Memory processing requires NMDA receptor activation and intracellular signaling leading to AMPA receptor trafficking, synthesis of new proteins, and formation of dendritic spines (34, 35). All of these processes are affected by Aβ oligomers in vitro, using primary neuronal cultures (12, 15, 36, 37).
Several neuronal receptors have been proposed as mediating the effect of Aβ on synaptic plasticity and memory, including the α-7-nicotinic (16), glutamatergic (39–40), and insulin (14) receptors. Recently, a new receptor protein has been proposed as an important mediator of this detrimental action. In an elegant study Lauren et al. (17) reported that PrPC mediates the Aβ oligomer-induced hippocampal synaptic plasticity impairment. We confirmed that Aβ oligomers bind to PrPC with high affinity, but also found that PrPC is not required for oligomer-induced memory impairment and cytotoxicity. These observations do not support the contention that PrPC is involved in the toxic effects of Aβ. The difference may be due to the fact that object recognition memory is associated with the perirhinal cortex more than the hippocampus. However, some human and primate studies have shown that hippocampal lesions result in impaired object recognition (41, 42) and that, for the 24 h intertrial interval from familiarization to test phase used in our study, hippocampal activity is required (43). However, the high-affinity binding between Aβ oligomers and PrPC may indicate a functional link between the two proteins. PrPC has been involved in neurotrophic signaling (44, 45), and in the regulation of Aβ-production (46), suggesting that PrPC and Aβ may be part of a common molecular pathway governing neuronal differentiation. Further behavioral and biochemical investigations will be necessary to clarify the involvement of PrPC in the neuropathology of AD. One limitation of this study worth to be mentioned may be the use of oligomeric Aβ preparations which haven’t been proven to be identical to those found in the brain of AD patients. However, since there remains no consensus as to which brain-derived oligomeric species mediate cognitive deficits in AD, we choose the current approach to extend studies addressing the role of PrPC in mediating Aβ-oligomer’s effects on memory.
In conclusion, we describe a simple and reliable mouse model of Aβ-induced memory dysfunction. Unlike Aβ aggregates purified from biological sources, synthetic Aβ oligomers are chemically defined, and can be easily produced and biophysically characterized. The novel-object recognition task is simple and reproducible, it measures recognition memory, which is heavily impaired in AD, and relies on spontaneous animal behavior without the need for stressor elements such as food or water deprivation, electric foot-shock, or aversive environments like water (25). A single i.c.v. injection of a nanomolar concentration of synthetic Aβ1–42 oligomers impairs memory consolidation within 24 h, suggesting that oligomers rapidly interfere with the synaptic activity necessary for the stabilization of new memories.
This model could therefore be useful for studying the mechanisms through which Aβ oligomers disrupt memory storage, and to direct therapies for earlier stages of disease, when rescue is still possible. Using this model we demonstrated that Aβ oligomers induce in vivo memory impairment and bind PrPC with high affinity, but found no evidence that the two events are related.
Materials and Methods
Aβ1–42 Synthesis and Sample Preparation.
Depsi-peptide Aβ1–42 was synthesized as previously described (47, 48). At variance with the native peptide, the depsi-peptide is highly soluble and it has a much lower propensity to aggregate, thus preventing the spontaneous formation of seeds in the solution (49, 50). The native Aβ1–42 peptide was then obtained from the depsi-peptide by a “switching” procedure in basic conditions. The alkaline stock solution (300 μM) was diluted in PBS and used immediately (initial state solution) or, to obtain Aβ1–42 oligomers, it was diluted to 100 μM Aβ in 50 mM phosphate buffer, 150 mM NaCl, pH 7.4, and incubated for 24 h at either 4 °C (3) or 22 °C (17). Fibrils were produced by incubating 100 μM Aβ1–42 at acidic pH overnight at 37 °C (23). All Aβ1–42 preparations were diluted to 1 μM in PBS before intracerebroventricular injection (details in SI Text)
Size Exclusion Chromatography.
Size exclusion chromatography (SEC) was performed on an FPLC apparatus (Biologic FPLC System; Biorad) equipped with a precision column prepacked with Superdex 75 resin, with a separation range of 3–70 kDa (GE Healthcare) (details in SI Text).
Atomic Force Microscopy.
For atomic force microscopy (AFM) analysis, each sample was diluted to 10 μM with H2O and incubated for 0.5–2 min on a freshly cleaved mica disk. The disk was washed with H2O and dried under a gentle nitrogen stream. The sample was mounted onto a Multimode AFM with a NanoScope V system (Veeco/Digital Instruments) operating in Tapping Mode using standard phosphorus-doped silicium probes (Veeco).
Surface Plasmon Resonance.
Binding studies were done using the ProteOn XPR36 Protein Interaction Array system (Bio-Rad) (51). Anti-PrP monoclonal antibodies 3F4 (52) and 94B4 (53) were immobilized on the sensor chip by amine-coupling chemistry. PrPC was then captured by flowing a total brain homogenate (0.5 mg protein/mL prepared in PBS containing 0.5% Nonidet P-40 and 0.5% Na-deoxycholate) from Tg(WT-E1) mice overexpressing wild-type mouse PrP carrying an epitope tag for the monoclonal antibody 3F4 (54). The Aβ1–42 initial state, oligomer and fibril preparations were then injected. The resulting sensorgrams (time course of SPR signal) were fitted by the simplest 1:1 interaction model (ProteOn analysis software), to obtain the corresponding association and dissociation rate constants (details in SI Text ).
Mice.
Male C57BL/6 mice were obtained from Charles River-Italy. Zürich I Prnp 0/0 mice (55) maintained on a pure C57BL/6 background were obtained from the European Mouse Mutant Archive (strain EM01723). Mice were 7–8 weeks of age (details in SI Text) .
Aβ1–42 Intracerebroventricular Injection and Object Recognition.
Mice were implanted with a stainless steel cannula by stereotaxic surgery (L ± 1.0; DV-3.0 from dura). Recognition memory was measured using an open-square gray arena and various objects of different sizes and materials. The task started with a habituation trial on day 1 followed by a familiarization trial (day 2) in which two identical objects were presented to the animals and the test trial (day 3), where one familiar object was substituted with a novel one, as detailed in SI Text .
Hippocampal Neuron Cultures and Determination of Aβ1–42 Oligomer Toxicity.
Primary hippocampal cultures were prepared from 2-day-old mice, as detailed in SI Text. Twelve days from the plating date, the neurons were treated with either 1 or 3 μM synthetic Aβ1–42 oligomers prepared at both 4 °C and 22 °C. After 72 h of Aβ treatment, cell survival was measured by MTT assay (details in SI Text).
Statistical Analysis.
Statistical analysis was performed using the StatView program. Object recognition data were analyzed using one- or two-way between-subject ANOVA as appropriate, followed by Student’s t test for comparisons of only two groups or Bonferroni’s or Tukey’s posthoc tests as appropriate.
Supplementary Material
Acknowledgments
We thank Richard Kascsak for the 3F4 antibody and Jan P. Langeveld for the 94B4 antibody. This work was supported by grants from Fondazione Cariplo (Nobel-Guard Project), Banca Intesa San Paolo, the European Community Network of Excellence NeuroPrion, the Italian Ministry of Health (to G.F.), and Telethon-Italy (TCR08005) (to R.C.). R.C. is an Associate Telethon Scientist (Dulbecco Telethon Institute, Fondazione Telethon).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.H.A. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911829107/DCSupplemental.
References
- 1.Lue LF, et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLean CA, et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Lambert MP, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shankar GM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: A potent role for trimers. J Physiol. 2006;572:477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang HW, et al. Soluble oligomers of beta amyloid (1-42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924:133–140. doi: 10.1016/s0006-8993(01)03058-x. [DOI] [PubMed] [Google Scholar]
- 7.Hsia AY, et al. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mucke L, et al. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: Synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleary JP, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 10.Lesné S, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 11.Poling A, et al. Oligomers of the amyloid-beta protein disrupt working memory: Confirmation with two behavioral procedures. Behav Brain Res. 2008;193:230–234. doi: 10.1016/j.bbr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shankar GM, et al. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Felice FG, et al. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 14.De Felice FG, et al. Protection of synapses against Alzheimer’s-linked toxins: Insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci USA. 2009;106:1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacor PN, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 17.Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang SM, et al. Neprilysin-sensitive synapse-associated amyloid-beta peptide oligomers impair neuronal plasticity and cognitive function. J Biol Chem. 2006;281:17941–17951. doi: 10.1074/jbc.M601372200. [DOI] [PubMed] [Google Scholar]
- 19.Mouri A, et al. Oral vaccination with a viral vector containing Abeta cDNA attenuates age-related Abeta accumulation and memory deficits without causing inflammation in a mouse Alzheimer model. FASEB J. 2007;21:2135–2148. doi: 10.1096/fj.06-7685com. [DOI] [PubMed] [Google Scholar]
- 20.Scholtzova H, et al. Memantine leads to behavioral improvement and amyloid reduction in Alzheimer’s-disease-model transgenic mice shown as by micromagnetic resonance imaging. J Neurosci Res. 2008;86:2784–2791. doi: 10.1002/jnr.21713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, et al. Learning-memory deficit with aging in APP transgenic mice of Alzheimer’s disease and intervention by using tetrahydroxystilbene glucoside. Behav Brain Res. 2006;173:246–254. doi: 10.1016/j.bbr.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 22.Greene JD, Baddeley AD, Hodges JR. Analysis of the episodic memory deficit in early Alzheimer’s disease: Evidence from the doors and people test. Neuropsychologia. 1996;34:537–551. doi: 10.1016/0028-3932(95)00151-4. [DOI] [PubMed] [Google Scholar]
- 23.Stine WB, Jr, Dahlgren KN, Krafft GA, LaDu MJ. In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. J Biol Chem. 2003;278:11612–11622. doi: 10.1074/jbc.M210207200. [DOI] [PubMed] [Google Scholar]
- 24.Klyubin I, et al. Amyloid beta protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nat Med. 2005;11:556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- 25.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: A new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGaugh JL. Memory—a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 27.Sara SJ. Retrieval and reconsolidation: Toward a neurobiology of remembering. Learn Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- 28.Cleary J, Hittner JM, Semotuk M, Mantyh P, O’Hare E. Beta-amyloid(1-40) effects on behavior and memory. Brain Res. 1995;682:69–74. doi: 10.1016/0006-8993(95)00323-i. [DOI] [PubMed] [Google Scholar]
- 29.Frautschy SA, et al. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol Aging. 2001;22:993–1005. doi: 10.1016/s0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 30.McDonald MP, Dahl EE, Overmier JB, Mantyh P, Cleary J. Effects of an exogenous beta-amyloid peptide on retention for spatial learning. Behav Neural Biol. 1994;62:60–67. doi: 10.1016/s0163-1047(05)80059-7. [DOI] [PubMed] [Google Scholar]
- 31.O’Hare E, et al. Delayed behavioral effects following intrahippocampal injection of aggregated A beta (1-42) Brain Res. 1999;815:1–10. doi: 10.1016/s0006-8993(98)01002-6. [DOI] [PubMed] [Google Scholar]
- 32.Sweeney WA, Luedtke J, McDonald MP, Overmier JB. Intrahippocampal injections of exogenous beta-amyloid induce postdelay errors in an eight-arm radial maze. Neurobiol Learn Mem. 1997;68:97–101. doi: 10.1006/nlme.1997.3770. [DOI] [PubMed] [Google Scholar]
- 33.Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- 34.Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 35.Lüscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh H, et al. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacor PN, et al. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almeida CG, et al. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 39.De Felice FG, et al. Alzheimer’s disease-type neuronal tau hyperphosphorylation induced by A beta oligomers. Neurobiol Aging. 2008;29:1334–1347. doi: 10.1016/j.neurobiolaging.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Irvine GB, El-Agnaf OM, Shankar GM, Walsh DM. Protein aggregation in the brain: The molecular basis for Alzheimer’s and Parkinson’s diseases. Mol Med. 2008;14:451–464. doi: 10.2119/2007-00100.Irvine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reed JM, Squire LR. Impaired recognition memory in patients with lesions limited to the hippocampal formation. Behav Neurosci. 1997;111:667–675. doi: 10.1037//0735-7044.111.4.667. [DOI] [PubMed] [Google Scholar]
- 42.Zola SM, et al. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Kanaani J, Prusiner SB, Diacovo J, Baekkeskov S, Legname G. Recombinant prion protein induces rapid polarization and development of synapses in embryonic rat hippocampal neurons in vitro. J Neurochem. 2005;95:1373–1386. doi: 10.1111/j.1471-4159.2005.03469.x. [DOI] [PubMed] [Google Scholar]
- 45.Santuccione A, Sytnyk V, Leshchyns’ka I, Schachner M. Prion protein recruits its neuronal receptor NCAM to lipid rafts to activate p59fyn and to enhance neurite outgrowth. J Cell Biol. 2005;169:341–354. doi: 10.1083/jcb.200409127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parkin ET, et al. Cellular prion protein regulates beta-secretase cleavage of the Alzheimer’s amyloid precursor protein. Proc Natl Acad Sci USA. 2007;104:11062–11067. doi: 10.1073/pnas.0609621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sohma Y, Sasaki M, Hayashi Y, Kimura T, Kiso Y. Novel and efficient synthesis of difficult sequence-containing peptides through O-N intramolecular acyl migration reaction of O-acyl isopeptides. Chem Commun (Camb) 2004;(1):124–125. doi: 10.1039/b312129a. [DOI] [PubMed] [Google Scholar]
- 48.Coin I, Beyermann M, Bienert M. Solid-phase peptide synthesis: From standard procedures to the synthesis of difficult sequences. Nat Protoc. 2007;2:3247–3256. doi: 10.1038/nprot.2007.454. [DOI] [PubMed] [Google Scholar]
- 49.Taniguchi A, et al. “Click peptide”: pH-triggered in situ production and aggregation of monomer Abeta1-42. ChemBioChem. 2009;10:710–715. doi: 10.1002/cbic.200800765. [DOI] [PubMed] [Google Scholar]
- 50.Taniguchi A, et al. ‘O-Acyl isopeptide method’ for peptide synthesis: Solvent effects in the synthesis of Abeta1-42 isopeptide using ‘O-acyl isodipeptide unit’. J Pept Sci. 2007;13:868–874. doi: 10.1002/psc.905. [DOI] [PubMed] [Google Scholar]
- 51.Bravman T, et al. Exploring “one-shot” kinetics and small molecule analysis using the ProteOn XPR36 array biosensor. Anal Biochem. 2006;358:281–288. doi: 10.1016/j.ab.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Kascsak RJ, et al. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987;61:3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langeveld JP, et al. Rapid and discriminatory diagnosis of scrapie and BSE in retro-pharyngeal lymph nodes of sheep. BMC Vet Res. 2006;2:19–32. doi: 10.1186/1746-6148-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiesa R, Piccardo P, Ghetti B, Harris DA. Neurological illness in transgenic mice expressing a prion protein with an insertional mutation. Neuron. 1998;21:1339–1351. doi: 10.1016/s0896-6273(00)80653-4. [DOI] [PubMed] [Google Scholar]
- 55.Büeler H, et al. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.