Abstract
The voltage sensor domain (VSD) is the key module for voltage sensing in voltage-gated ion channels and voltage-sensing phosphatases. Structurally, both the VSD and the recently discovered voltage-gated proton channels (Hv channels) voltage sensor only protein (VSOP) and Hv1 contain four transmembrane segments. The fourth transmembrane segment (S4) of Hv channels contains three periodically aligned arginines (R1, R2, R3). It remains unknown where protons permeate or how voltage sensing is coupled to ion permeation in Hv channels. Here we report that Hv channels truncated just downstream of R2 in the S4 segment retain most channel properties. Two assays, site-directed cysteine-scanning using accessibility of maleimide-reagent as detected by Western blotting and insertion into dog pancreas microsomes, both showed that S4 inserts into the membrane, even if it is truncated between the R2 and R3 positions. These findings provide important clues to the molecular mechanism underlying voltage sensing and proton permeation in Hv channels.
Keywords: ion conduction, membrane topology, membrane insertion, voltage sensor
Voltage sensor domain (VSD) is a protein module that senses transmembrane voltage and regulates ion permeation through the pore (1–3) and phosphoinositide turnover by the phosphatase (4). The fourth transmembrane segment (S4) of VSD contains periodically aligned positively charged residues (arginine and lysine), which play a critical role in voltage sensing (1, 2). Recent analyses of the crystal structures of voltage-gated potassium channels (5, 6) showed that basic residues in S4 form salt bridges with negatively charged residues from other helices, and these salt bridges are formed at both sides of a hydrophobic residue such as phenylalanine. This suggests a focused electric field within a crevice surrounded by transmembrane helices (6), which would enable extracellular and intracellular aqueous environments to enter deeply into the membrane through a narrow crevice. One line of functional evidence for this narrow crevice is that mutation within the VSD creates an ion permeation pathway that is activated upon hyperpolarization (7−9). Such ion conduction is called an “omega-current” (9) or “gating pore current” (10). Similar conductances through the VSD are elicited in a mutated form of human voltage-gated sodium channels (SCN4a) found in patients with periodic paralysis (11, 12) and in flatworm potassium channels (13).
Recently, the protein, which consists solely of a VSD, was identified in tunicate, mouse (14) and human (15) and called voltage sensor only protein (VSOP) or Hv1. Mouse VSOP (mVSOP) is found within the phagosomes of white blood cells, and is essential for robust production of superoxide anions (16, 17) and maintenance of intracellular pH (18) in phagocytes. Despite the lack of a pore domain, these proteins function as voltage-gated proton channels (Hv channels) in heterologous expression systems (14, 15) and when reconstituted into artificial membranes (19). S4 of both mVSOP and Hv1 contain three arginines (R201, R204, and R207 in mVSOP, which are equivalent to R205, R208, and R211 in Hv1) that correspond to positively charged amino acids in S4 of voltage-gated ion channels. Moreover, the area called the “paddle region,” which is comprised of parts of S3 and S4 in Hv1, can replace the corresponding region in voltage-gated potassium channels without affecting voltage-dependent ion channel activity, indicating that a paddle motif-like structure is conserved among Hv and voltage-gated potassium channels (20).
Earlier studies by ourselves and others showed that Hv channels form dimers via their cytoplasmic regions (21−23), although monomeric Hv channels lacking their cytoplasmic regions remain functional and exhibit robust Hv channel activity (21, 22). This motivated us to further define the minimum unit for Hv channel activity.
In this study, we constructed a set of mutants with deletion of the cytoplasmic region up to S4. Surprisingly, the protein truncated between the second arginine (R204) and the third arginine (R207) of S4 continued to function as a proton channel. Cysteine-scanning experiments with “PEGylation-protection” assays of the accessibility of a maleimide-conjugated reagent (24) showed that the truncated end of A206stop was water-accessible, whereas its upstream region was inaccessible to water, which suggests the truncated S4 is inserted in membrane. This was confirmed by a glycosylation assay in dog pancreas microsomes (25). These findings argue against a model in which the region downstream of the third arginine in S4 constitutes the critical proton permeation pathway (22). Such information on the membrane topology of S4 will be important for understanding the mechanism of proton permeation and voltage sensing in Hv channels.
Results and Discussion
Truncation Between R2 and R3 Does Not Eliminate Proton Channel Currents.
Hv channels truncated at V216, and thus lacking most of their C-terminal cytoplasmic region, exhibited reduced dimerization but robust proton channel activity (21). To determine how much of the C-terminal side of the protein is necessary to retain Hv channel function, we first made three deletion constructs: I213stop, G211stop, and I209stop (Fig. 1A). All three exhibited Hv channel activity in tsA201 cells (Fig.1B), although its I-V curve showed that the activation threshold was significantly shifted rightward in I209stop. Current magnitudes did not differ between I213stop and G211stop, whereas I209stop showed lower current density. When we then deleted the region up to A206, one amino acid upstream of the third arginine (R3), voltage-dependent currents were still elicited, but the current density was significantly lower than with I213stop or G211stop (Fig. 1C) and slightly lower than with I209stop. In addition, some gating properties of A206stop differed from full-length mVSOP. The kinetics of rising phase were not strongly voltage dependent (Fig. 2 D and E). The two time constants required to fit the rising phase of the outward currents exhibited little voltage dependence (Fig. S1), but the contribution of each component changed with voltage. In addition, the I-V relationship was shifted rightward, as was seen with I209stop.
Fig. 1.
Proton currents through mVSOPs deleting the C-terminal side at distinct position in tsA201 cells. (A) Amino acid alignment of the S4 segment in the Hv channel, voltage-sensing phosphatase (Ci-VSP) and voltage-gated potassium channel (Shaker). The last amino acid of deletion mutants is indicated by an arrow. Stars on the three arginines indicate the positions of R1, R2, and R3. (B) Raw current traces (Left) and their I-V curves (Right). From a holding potential of −60 mV, 500-ms test pulses to membrane potentials of −60–150 mV were applied in 10-mV increments. pHin and pHout were 6.0 and 7.0, respectively. (C) Current density at a membrane potential of 150 mV in cells expressing the indicated deletion mutant. The numbers of cells were 3, 5, 6, 5, 6, and 3 for the wild-type, I213stop, G211stop, I209stop, A206stop, and L200stop, respectively. Values (in pA/pF) were 226 ± 98.9 for wild-type, 166.1 ± 45.7 for I213stop, 130.0 ± 52.5 for G211stop, 37.4 ± 16.0 for I209stop, 23.8 ± 11.6, and 3.0 ± 0.5 for A206stop (as mean ± SD).
Fig. 2.
A206stop retains hallmark properties of Hv channels. (A) Depolarization-induced increases pHin in tsA201 cells transfected with A206stop. BCECF ratio images recorded at the times indicated by the arrows are shown at the top. BCECF ratios were calibrated as described in (Fig. S4. pHin/pHout was 6.0/7.0). The timing of the test pulse is indicated below. (B) Zinc sensitivity of A206stop. 500-ms test pulses were applied from 0 mV to 150 mV in 10-mV increments. The current density at 130 mV was significantly reduced with in the presence of 100 μM zinc (20.5 ± 4.8 pA/pF vs. 1.9 ± 0.1 pA/pF, n = 3, mean ± SD). pHin/pHout was 6.0/7.0. Similar zinc sensitivity was observed in I209stop (Fig. S5). (C) Ion selectivity tested with 20 mM Na+ or K+ in the external solution. The external solution was changed in the sequence shown in the figure. Depolarizing step was applied to 100 mV from a holding potential of −80 mV. Tail currents were outward throughout this series of experiments, indicating that Na+ and K+ do not permeate. Outward tail currents were not observed when pHout was 6.5 (bottom). pHin/pHout was 5.3/8.0. (D) pHout dependence of the activation kinetics of A206stop current is shown by plotting t1/2 values for outward currents elicited by depolarizing steps (500 ms) against membrane potential. Activation times were compared between pHout 8.0 and 7.0 (n = 5). The pH of the pipette solution (pHin) was 6.0. (E) pHin dependence of the activation kinetics of A206stop currents. t1/2 values during 2-s depolarizing steps were compared between pHin 6.5 and 5.3. pHout was fixed to 7.5.
The lower current density seen with A206stop could reflect a lower level of protein expression. We therefore used Western blotting (Fig. S2A) to compare the expression of A206stop with the other proteins under study. There was no significant difference in expression level among full-length mVSOP and the four deletion mutants. Moreover, biotinylation of surface proteins followed by Western blotting (Fig. S2B) and immunostaining (Fig. S2C) both showed that surface expression of A206stop was indistinguishable from that of the full-length protein. Thus the diminished density of A206stop-derived currents was not due to misexpression or a lower level of expression. In addition, untransfected cells did not exhibit such currents. Further, L200stop, which lacked all three arginines, exhibited no outward currents (Fig. 1B). These findings negate the possibility that currents observed upon transfection of A206stop were derived from endogenous Hv channels.
To know whether A206stop retains the properties of Hv channels, we assessed several hallmark properties of Hv channel currents, including proton permeation, zinc sensitivity, and pH-dependent gating (26, 27). We initially examined proton-exporting activity by measuring intracellular pH (pHin) using a pH-sensitive ratiometric dye, BCECF, under whole-cell patch clamp. Depolarization applied from the patch pipette induced a clear increase in pHin, and repolarization caused the opposite change in pHin (Fig.2A, Fig. S3, and Movie S1).
We next tested whether the strict proton selectivity characteristic of Hv channels is retained in A206stop. Because of the small amplitude and higher voltage threshold for activation of A206stop-derived currents, we were unable to determine reversal potentials by measuring tail currents. Instead, the shapes of the tail currents were compared while cells were perfused with NMDG-, sodium-, or potassium-containing extracellular solution under an extremely asymmetrical pH condition (pHout/pHin = 8.0/5.3) that made outward tail currents discernible. Tail currents were outward in the presence of sodium- or potassium-rich solution (Fig. 2C), as well as in NMDG-rich solution, consistent with the idea that the major charge carriers for the currents were protons. Changing pHout from 8.0 to 6.5 markedly reduced the outward tail currents. In addition, time-dependent increases in outward currents were sometimes observed, irrespective of the extracellular solution (Fig. 2C).
Another hallmark feature of Hv channels is zinc-sensitivity (26, 28, 29). Consistent with that feature, outward currents of A206stop were reversibly suppressed by 100 μM zinc in all cases (n = 3) (Fig. 2B). I209stop also showed similar zinc sensitivity (Fig. S4).
Voltage-dependent gating of Hv channels is known to be sensitive to both pHin and pHout (26, 30, 31). We therefore compared the current kinetics and current-voltage relationships in the same A206stop-expressing cells at pHout 7.0 and 8.0. The current-voltage relationship was shifted leftward with an increase in pHout (Fig. S5). In addition, the activation kinetics were slower at pHout 7.0 than at 8.0, and the times to half of the peak (t1/2) were significantly longer at pHout 7.0 than at pHout 8.0 over a wide range of membrane potentials (Fig. 2D). The effect of pHin on the gating of A206stop-derived currents was tested by measurements with three distinct pH values of the intracellular (pipette) solution. Superimposition of currents measured during 2-s depolarizations of the membrane potential to 90 mV showed that currents were made mildly faster as the intracellular solution became more acidic (pH 5.3, 6.0, and 6.5) (Fig. S6). This is indicated in the plot of t1/2 for activation at different voltage levels for pHin 6.5 and 5.3 (Fig. 2E). Taken together, these findings indicate that A206stop operates as Hv channels retaining most of the major hallmark properties of Hv channels.
N210R Is Proton-Conductive.
S4 of mVSOP or Hv1 contains an asparagine (N210 in VSOP, N214 in Hv1) 3-residue downstream of R3 site (Fig. 1A). It was previously shown that substitution of this asparagine with cysteine followed by the addition of MTSET, a cysteine-targeting substance similar in size to an arginine residue, blocks proton permeation (22). This raises the possibility that the region surrounding this asparagine forms the proton-conductive pathway upon depolarization (22). Our finding that A206stop remains proton-conductive argues against a model in which the asparagine (N210) replacing arginine at the putative R4 position determines proton selectivity. To test this further, we studied the effects of N210R mutation of mVSOP (equivalent to N214R of Hv1). Clear voltage-dependent ion currents were obtained from N210R despite its small current density, 7.63 ± 2.43 pA/pF (n = 5). Notably, the activation kinetics were significantly slower than the wild-type currents, and the tail currents were extremely slow (Fig. 3A). Moreover, the G-V curve (Fig. 3B) was shifted slightly leftward, as compared to the wild-type current (14). Reversal potentials measured while varying pHout confirmed that the charge carriers were protons (Fig. 3C). An N210K mutant of mVSOP (Fig. S7) and a N214R mutant of Hv1 (Fig. S8) exhibited currents with properties similar to N210R currents.
Fig. 3.
N210R shows proton channel activity. (A) Raw traces of currents through the N210R mutant. Both pHin and pHout were 7.0. From the holding potential of −80 mV, 1-s test pulses to membrane potentials of −80 mV to 150 mV were applied in 10-mV increments with 10-s intervals. (B) Conductance-voltage (G-V) curve. Curves were fitted by the Boltzmann equation. Valence (Z) was 1.1 ± 0.2, and the midpoint voltage, V1/2, was 67.8 ± 12.8 mV (mean ± SD, n = 6). (C) Reversal potentials at the indicated pHs. Reversal potential was determined as the voltage at which the direction of the current flow reversed during 200-ms ramp pulses (80 mV to −100 mV) following a 1-s depolarizing step. The line indicates the potentials predicted by the Nernst equation. Values were obtained from 6 cells at each pH. The pH of the intracellular solution was 7.0.
Topology of S4 of the Voltage-Gated Proton Channel.
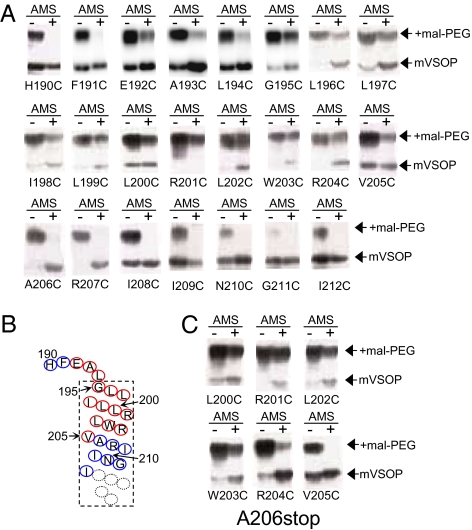
The results of the electrophysiological studies suggest that the region downstream of the second arginine (R2) in S4 is not required for determining proton selectivity. We then wondered which region of S4 faces the aqueous environment and whether the portion of S4 remaining in A206stop is inserted into the membrane. We performed site-directed cysteine-scanning of mVSOP proteins heterologously expressed in tsA201 cells in which the accessibility of cysteines to maleimide-containing reagent was determined by Western blotting. This “PEGylation-protection” assay was originally developed by Deutsch and colleagues (24) and used to study the topology and folding of Shaker-like potassium channels in microsomes and the KvAP channel in the bacterial membrane (32). With this method, target proteins are exposed to two maleimide-containing reagents, 4-acetamido-4’-maleimidylstilbene-2,2’-disulfonic acid (AMS) and maleimide-conjugated polyethylene glycol (mal-PEG). The binding of the latter can be detected as a band shift on Western blots, reflecting its large molecular size; AMS is smaller than mal-PEG and does not cause a band shift on Western blots. Furthermore, when applied to cultured cells first, AMS protects any cysteines exposed to the aqueous environment from subsequently added mal-PEG. Consequently, after solubilization to remove the lipids from an AMS treated sample, AMS-inaccessible cysteines are able to bind mal-PEG. mVSOP has two cysteines, C245 and C103. In pilot studies, five sites (Fig. S9A)—including P125C (S1–S2 linker), H190C (S3–S4 linker), and N269C (C terminus), as well as C245 (C), and C103 S1—were individually tested for their accessibility to AMS based on protection from mal-PEG binding. For P125C, H190C, and N269C, the two native cysteines were substituted with serine to obtain a cysteine-less background. Four of the sites (P125C, H190C, C245, and N269C) were predicted to be in aqueous environment, and all showed protection from band shift in the presence of AMS (Fig. S9B). C103 in S1 was predicted to be in the membrane, and it exhibited a clear band shift irrespective of AMS (Fig. S9). AMS accessibility to the intracellular sites C245 and N269C suggested that AMS permeates cell membrane under this condition, as described before with prolonged incubation with a high concentration of AMS (24).
AMS accessibility to individual sites in S4 was then tested using a cysteine-less background. On the N-terminal side of S4, H190C and F191C were accessible to AMS, as indicated by the absence of band shift (Fig. 4 A and B), whereas E192C, A193C, L194C showed mild band shift, suggesting these sites are partially shielded from the aqueous environment. Incomplete protection in these sites could reflect the presence of a mixture of protein populations with distinct conformations, as membrane potential and pHin were not controlled and signals were derived from both the plasma membrane and intracellular membrane fractions in this assay. Additional inner sites, between G195C and R204C, showed clear band shifts, suggesting these sites are AMS-inaccessible. V205C showed mild protection, whereas A206C, R207C, and I208C showed no trace of a band shift (Fig. 4A), indicating V205 may be partially accessible and A206 to I208 are fully accessible to AMS. When we performed a similar experiment with A206stop, we found that L200C, R201C, L202C, and W203C were all inaccessible to AMS, as is the case of full-length mVSOP (Fig. 4C). Unlike the full-length protein, however, V205C was fully accessible to AMS. Partial protection from the band shift at R204C suggests that R204C is slightly accessible to AMS in the truncated protein, which is in contrast to the full-length protein. These results indicate that S4 of A206stop is inserted into the membrane, but differences in the pattern of accessibility between A206stop and full-length mVSOP suggest the structure of S4 is slightly altered in A206stop or that the aqueous crevice is reshaped.
Fig. 4.
(A) Western blots for PEGylation-protection assays with a set of mutants in which the amino acids within S4 were substituted, one at a time, with cysteine in tsA201 cells. (B) Summary of the membrane topology of S4 in the full-length clone. A site with a clear upper band (corresponding to the mal-PEG-bound component) in the presence of AMS was classified as an AMS-inaccessible site and shown in red. Blue residue denotes an AMS-accessible position. (C) Western blots obtained when amino acids in A206stop were substituted with cysteine as in panel A). The same experiment was repeated two to three times for each site and similar results were obtained.
We also carried out in vitro translation in the presence of dog pancreas rough microsomes of constructs where S4s were inserted into the Lep model proteins and either flanked or preceded by two engineered glycosylation sites (Fig. 5 A and B) (25). With this method, the membrane-insertion efficiency of S4 can be determined by quantification of the relative amounts of diglycosylated molecules (25).
Fig. 5.
Membrane insertion assayed based on glycosylation in microsomes. (A Top) setup for membrane insertion assay with a LepP2 domain and two glycosylation sites flanking S4. Middle and bottom panels: SDS/PAGE gels of in vitro transcribed/translated products and the corresponding calculated insertion efficiencies. (B Upper) Setup for the membrane insertion assay with two glycosylation sites upstream of S4s. (Middle and Bottom) SDS-gels of in vitro transcribed/translated products and the corresponding calculated insertion efficiencies. Data represents the average of three independent experiments with standard deviations.
Insertion of S4 was first tested using the construct where S4 is placed in the middle of the large P2 domain (33) (Fig. 5A). In this design, the ratio of mono- (inserted) to diglycosylated (noninserted) molecules can be converted to an apparent free energy of membrane insertion (25). Interestingly, the Hv channel-derived S4 was inserted with much higher efficiency (81% or −0.83 kcal/mol) than S4 from voltage-gated potassium (Kv) channels, such as Kv1.3 (50% or 0.0 kcal/mol) (34), KvAP (0.5 kcal/mol) (35), KAT1 (1.04 kcal/mol) (36), Shaker (1.23 kcal/mol) (36) channels. Truncation up to I209 or A206 gradually reduced the insertion efficiency (Fig. 5A), whereas truncation up to L200 completely blocked insertion (10% insertion is the background in the assay). That the A206 truncated fragment inserted into the membrane to a lesser extent than the full S4 segment is consistent with electrophysiological results (Fig. 1B).
To examine the possibility that parts of the LepP2 domain downstream of S4 affect insertion of the truncated S4s, another setup was used in which a stop codon followed S4, and insertion was monitored by the absence of glycosylation of an NST site placed only three residues upstream from the predicted start of S4, at residue L184 (Fig. 5B) (33). Previous work showed that the Asn in the NST glycosylation consensus site must be at least 14 residues away from the membrane interface for glycosylation to occur (37). This assay is thus not only useful for measuring membrane insertion efficiency, it can also be used as a “molecular ruler” to estimate the exact location of the membrane-embedded part of a given transmembrane segment. Fifteen percent or less glycosylation of the second glycosylation site was seen in the I209 and A206 truncation constructs, whereas the L200 truncation construct was 92% diglycosylated (Fig. 5B), showing that both the I209 and A206 S4 are situated within the membrane. Thus both glycosylation assays demonstrate that S4 truncated at A206 inserts at least to some extent into the ER membrane. The quantitative differences between the two assays may reflect the different mechanisms employed to prevent glycosylation: in the first assay, the test segment must span the membrane to prevent translocation of the LepP2 domain to the lumen during protein synthesis in the ER, whereas “dipping” into the membrane may be sufficient to inhibit glycosylation of an upstream NST site in the second assay. The latter is supported by the observation that truncation of the flanking residues from I209 to Q229 increases the insertion efficiency from 79 to 93%.
A206stop, which lacks the entire C-terminal region downstream of the R2 position, retains functionality as an Hv channel. Glycosylation assays showed that S4, upstream of R3, can be inserted into the membrane. How do these findings relate to the structure and dynamics of S4 in Kv channels resolved by x-ray crystallography (6) and biophysical approaches (1–3, 9)? Alignment of amino acids of the Hv and Kv channels (Fig. 1A) revealed that the N-terminal flanking region of R1 (E192 to L198) corresponds to the region M356 to R362 of the Shaker Kv channel, which is exposed to the extracellular environment in the resting state (38). By contrast, our PEGylation protection assay showed that the N-terminal flanking region of R1 (E192 to L198) of mVSOP is shielded from the aqueous environment. This raises the possibility that S4 of VSOP starts several residues more N-terminal than the predicted site shown in Fig. 1A. If similar numbers of amino acids span the cell membrane in the Hv and Kv channels, a region downstream of R2 containing N210 (equivalent of N214 in Hv1) likely faces the intracellular environment or even protrudes out of the membrane. Tombola et al. (22) hypothesized that N214 in Hv1 (equivalent of N210 in mVSOP) corresponds to the fourth arginine of S4 in the Kv channel and changes its location upon depolarization to the open proton-conductive pore via a mechanism similar to that mediating the omega or gating pore current (8, 9). This hypothesis was supported by the findings that a N214R Hv1 mutant was nonconductive and a N214C Hv1 mutant became nonconductive upon conjugation with MTSET (22). However, our study showed that a N210R mVSOP mutant (equivalent to N214R) still operates as an Hv channel. It is possible that MTSET binding silences the activity of the N214C mutant by stabilizing the closed state of the channel, not by occluding the proton permeation pathway. The significantly slowed gating of the N210R mutant and the positive shift in the threshold voltage of A206stop seen in our study suggest that a segment downstream of R2 is involved in regulating gating rather than in proton permeation, although it is also possible that N210 is located close to an entry site for protons and thereby influences conductance, for example through altering the local electrostatic potential. Our results are more consistent with a model that a region of S4 upstream of N210 (equivalent to N214 in Hv1) is located in the center of membrane and contributes to proton selectivity.
Materials and Methods
Plasmids and Heterologous Expression of mVSOP.
All studies were done with mVSOP (14). cDNA fragments encoding C terminus-deleted mVSOP (L200stop, I213stop, G211stop, I209stop, and A206stop) were generated by PCR amplification from mVSOP, and subcloned into the PstI and SacII sites of pIRES2-EGFP (Invitrogen). Transfection into HEK tsA201 cells was accomplished using Polyfect (Qiagen).
Electrophysiological Analysis.
Whole-cell patch recording was performed as described previously (14). The pipettes had resistances ranging from 5 to 24 MΩ. The most commonly used solution contained: 75 or 65 mM N-methyl D-glucamine (NMDG), 1 mM CaCl2, 1 mM MgCl2, and 100 or 150 mM hepes for pH 7.0 or 7.5, or 96 mM CHES for pH 8.0. Sodium or potassium external solutions contained: 80 mM hepes, 45 mM NMDG, 1 mM CaCl2, 1 mM MgCl2, and 20 mM NaOH or KOH (pH 8.0). Intracellular solutions contained: 75 or 65 mM NMDG, 1 or 3 mM MgCl2, 1 mM EGTA, and 183 mM hepes for pH 7.0 or 100 mM Mes for pH 5.3, 6.0, or 6.5. Osmolarity and pH were adjusted using glucose (50-100 mM) and methanesulfonate, respectively. Experiments were carried out at room temperature. The holding potential was set to −60 mV unless specifically noted otherwise. Junction potentials were not adjusted. In some cases (data shown in Figs. S1, S5 and S6), leak subtraction was performed with the P/5 protocol. The conductance curve for the N210R mutant (Fig. 3) was fitted with the Boltzmann equation, G= 1/(1+ exp[ze(V − V1/2)/kT]), where k is the Boltzmann constant, e is the elementary electric charge, T is temperature, and z is the valence. Values are presented as means ± SE unless specifically stated otherwise.
pH Imaging.
Ratio imaging of intracellular BCECF (Molecular Probes) was performed using a method similar to that described previously (39). tsA201 cells were cultured on plastic dishes and imaged using an inverted microscope (IX71; Olympus) with a 40× objective lens (NA:0.55)(Olympus). The intracellular solution contained 65 mM NMDG, 3 mM MgCl2, 1 mM EDTA, 140 mM glucose, 80 μM BCECF, and 10 mM Mes (pH 6.0). The pH of the extracellular solution was 7.0. Excitation of BCECF at 470–495 nm and at 420–440 nm was alternated using a computer-controlled shutter with a filter wheel assembly (Ludl Electronic Products Ltd.). The fluorescent emission was directed onto a 536/40 nm filter (Semrock) and imaged using an EM-CCD digital camera C9100 (Hamamatsu Photonics) at 15,660 pixel for each frame. To estimate absolute values of pHin, calibration plots were generated in separate experiments (shown in Fig. S3). Fluorescence images were recorded from cells in the whole cell patch-clamp condition with the intracellular solution at one of three pHs (pH 6.0: 10 mM Mes, pH 7.0: 10mM hepes, pH 8.0: 10 mM CHES). Ratio values were obtained from multiple cells at each pHin.
PEGylation-Protection Assays.
HEK293 cells were washed with PBS, treated with or without 10 mM AMS for 30 min at room temperature, and homogenized. The cell homogenates were pelleted, washed twice in PBS, and solubilized in the buffer [2% (wt/vol) SDS, 6 M urea, and 15 mM Tris, pH 7.4]. Aliquots of the resultant solution were then incubated with 5 mM mal-PEG (Sigma-Aldrich) for 30 min at room temperature, after which the proteins were separated on 12.5% SDS/PAGE and electrophoretically transferred to Immobilon-P membranes (Millipore). A polyclonal rabbit anti-mVSOP antibody generated against the amino acids Met-1-Ala-72 in the N terminus of mVSOP was used for detection. Horseradish peroxidase-conjugated anti-rabbit IgG from donkey (Amersham Pharmacia) was used as the secondary antibody. Intracellular access of AMS in this assay was not due to the contamination by damaged cells, as our flow cytometric assessment of cell viability indicated that most of cells were intact.
Membrane Insertion in Dog Pancreas Microsomes.
All constructs used in the glycosylation assays were based on the pGEM1-Lep construct described previously (25). DNA encoding four different mVSOP-S4 H-segments flanked by GGPG….GPGG tetrapeptides (GGPGLLFKSHHFEALGLLILLRLWRVARIINGIIISVKTRSERQILRLKGPGG, GGPGLLFKSHHFEALGLLILGPGG, GGPGLLFKSHHFEALGLLILLRLWRVGPGG or GGPGLLFKSHHFEALGLLILLRLWRVARIGPGG) were inserted between codons 226–227 and codon 253 of the lep gene. In the Lep construct shown in Fig. 5B, which contains two glycosylation sites on the N-terminal side of S4, a NST glycosylation site followed by a GG flexible linker replaced the previous GGPG linker and stop codons replaced the codons for Q229, L200, A206, or I209. Constructs in pGEM1-Lep were transcribed and translated in a SP6 TnT Quick system (Promega) in the presence of dog pancreas microsomes as described (25).
Supplementary Material
Acknowledgments
We thank Dr. Mari Sasaki for help in generating the antibodies against the mVSOP protein. We also thank Dr. Carol Deutsch for helpful discussion on the PEGylation-protection assay and critical reading of the manuscript and Bernhard Dobberstein for providing dog pancreas microsomes. This work was supported by HFSP research grants to Y. Okamura and G.v.H.; a Takeda Science Foundation grant to Y. Okamura; grants from the Japan Ministry of Education, Culture, Sports, Science, and Technology to Y. Okamura, Y. Okochi, and T.K.; and a grant from the Lundbeck Foundation to M.H.H.N.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 1817.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911868107/DCSupplemental.
References
- 1.Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiol Rev. 2000;80:555–592. doi: 10.1152/physrev.2000.80.2.555. [DOI] [PubMed] [Google Scholar]
- 2.Horn R, Ding S, Gruber HJ. Immobilizing the moving parts of voltage-gated ion channels. J Gen Physiol. 2000;116:461–476. doi: 10.1085/jgp.116.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Männikkö R, Elinder F, Larsson HP. Voltage-sensing mechanism is conserved among ion channels gated by opposite voltages. Nature. 2002;419:837–841. doi: 10.1038/nature01038. [DOI] [PubMed] [Google Scholar]
- 4.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- 5.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 6.Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 7.Starace DM, Bezanilla F. Histidine scanning mutagenesis of basic residues of the S4 segment of the shaker k+ channel. J Gen Physiol. 2001;117:469–490. doi: 10.1085/jgp.117.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starace DM, Bezanilla F. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature. 2004;427:548–553. doi: 10.1038/nature02270. [DOI] [PubMed] [Google Scholar]
- 9.Tombola F, Pathak MM, Isacoff EY. Voltage-sensing arginines in a potassium channel permeate and occlude cation-selective pores. Neuron. 2005;45:379–388. doi: 10.1016/j.neuron.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 10.Sokolov S, Scheuer T, Catterall WA. Ion permeation through a voltage-sensitive gating pore in brain sodium channels having voltage sensor mutations. Neuron. 2005;47:183–189. doi: 10.1016/j.neuron.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Sokolov S, Scheuer T, Catterall WA. Gating pore current in an inherited ion channelopathy. Nature. 2007;446:76–78. doi: 10.1038/nature05598. [DOI] [PubMed] [Google Scholar]
- 12.Struyk AF, Markin VS, Francis D, Cannon SC. Gating pore currents in DIIS4 mutations of NaV1.4 associated with periodic paralysis: Saturation of ion flux and implications for disease pathogenesis. J Gen Physiol. 2008;132:447–464. doi: 10.1085/jgp.200809967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klassen TL, Spencer AN, Gallin WJ. A naturally occurring omega current in a Kv3 family potassium channel from a platyhelminth. BMC Neurosci. 2008;9:52. doi: 10.1186/1471-2202-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- 15.Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okochi Y, Sasaki M, Iwasaki H, Okamura Y. Voltage-gated proton channel is expressed on phagosomes. Biochem Biophys Res Commun. 2009;382:274–279. doi: 10.1016/j.bbrc.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 17.Ramsey IS, Ruchti E, Kaczmarek JS, Clapham DE. Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc Natl Acad Sci USA. 2009;106:7642–7647. doi: 10.1073/pnas.0902761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan D, et al. Voltage-gated proton channels maintain pH in human neutrophils during phagocytosis. Proc Natl Acad Sci USA. 2009;106:18022–18027. doi: 10.1073/pnas.0905565106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SY, Letts JA, MacKinnon R. Functional reconstitution of purified human Hv1 H+ channels. J Mol Biol. 2009;387:1055–1060. doi: 10.1016/j.jmb.2009.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alabi AA, Bahamonde MI, Jung HJ, Kim JI, Swartz KJ. Portability of paddle motif function and pharmacology in voltage sensors. Nature. 2007;450:370–375. doi: 10.1038/nature06266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch HP, et al. Multimeric nature of voltage-gated proton channels. Proc Natl Acad Sci USA. 2008;105:9111–9116. doi: 10.1073/pnas.0801553105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tombola F, Ulbrich MH, Isacoff EY. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron. 2008;58:546–556. doi: 10.1016/j.neuron.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SY, Letts JA, Mackinnon R. Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc Natl Acad Sci USA. 2008;105:7692–7695. doi: 10.1073/pnas.0803277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu J, Deutsch C. Pegylation: A method for assessing topological accessibilities in Kv1.3. Biochemistry. 2001;40:13288–13301. doi: 10.1021/bi0107647. [DOI] [PubMed] [Google Scholar]
- 25.Hessa T, et al. Recognition of transmembrane helices by the endoplasmic reticulum translocon. Nature. 2005;433:377–381. doi: 10.1038/nature03216. [DOI] [PubMed] [Google Scholar]
- 26.Decoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- 27.DeCoursey TE. Voltage-gated proton channels: What's next? J Physiol. 2008;586:5305–5324. doi: 10.1113/jphysiol.2008.161703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byerly L, Suen Y. Characterization of proton currents in neurones of the snail, Lymnaea stagnalis. J Physiol. 1989;413:75–89. doi: 10.1113/jphysiol.1989.sp017642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cherny VV, DeCoursey TE. pH-Dependent inhibition of voltage-gated H(+) currents in rat alveolar epithelial cells by Zn(2+) and other divalent cations. J Gen Physiol. 1999;114:819–838. doi: 10.1085/jgp.114.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapus A, Romanek R, Qu AY, Rotstein OD, Grinstein S. A pH-sensitive and voltage-dependent proton conductance in the plasma membrane of macrophages. J Gen Physiol. 1993;102:729–760. doi: 10.1085/jgp.102.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cherny VV, Markin VS, DeCoursey TE. The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J Gen Physiol. 1995;105:861–896. doi: 10.1085/jgp.105.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neale EJ, Rong H, Cockcroft CJ, Sivaprasadarao A. Mapping the membrane-aqueous border for the voltage-sensing domain of a potassium channel. J Biol Chem. 2007;282:37597–37604. doi: 10.1074/jbc.M706437200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hessa T, et al. Molecular code for transmembrane-helix recognition by the Sec61 translocon. Nature. 2007;450:1026–1030. doi: 10.1038/nature06387. [DOI] [PubMed] [Google Scholar]
- 34.Tu L, Wang J, Helm A, Skach WR, Deutsch C. Transmembrane biogenesis of Kv1.3. Biochemistry. 2000;39:824–836. doi: 10.1021/bi991740r. [DOI] [PubMed] [Google Scholar]
- 35.Hessa T, White SH, von Heijne G. Membrane insertion of a potassium-channel voltage sensor. Science. 2005;307:1427. doi: 10.1126/science.1109176. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, et al. Contribution of hydrophobic and electrostatic interactions to the membrane integration of the Shaker K+ channel voltage sensor domain. Proc Natl Acad Sci USA. 2007;104:8263–8268. doi: 10.1073/pnas.0611007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nilsson I, et al. Proline-induced disruption of a transmembrane alpha-helix in its natural environment. J Mol Biol. 1998;284:1165–1175. doi: 10.1006/jmbi.1998.2217. [DOI] [PubMed] [Google Scholar]
- 38.Larsson HP, Baker OS, Dhillon DS, Isacoff EY. Transmembrane movement of the shaker K+ channel S4. Neuron. 1996;16:387–397. doi: 10.1016/s0896-6273(00)80056-2. [DOI] [PubMed] [Google Scholar]
- 39.Demaurex N, Downey GP, Waddell TK, Grinstein S. Intracellular pH regulation during spreading of human neutrophils. J Cell Biol. 1996;133:1391–1402. doi: 10.1083/jcb.133.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.