Abstract
The development of distinct regions in the amniote vertebral column results from somite formation and Hox gene expression, with the adult morphology displaying remarkable variation among lineages. Mammalian regionalization is reportedly very conservative or even constrained, but there has been no study investigating vertebral count variation across Amniota as a whole, undermining attempts to understand the phylogenetic, ecological, and developmental factors affecting vertebral column variation. Here, we show that the mammalian (synapsid) and reptilian lineages show early in their evolutionary histories clear divergences in axial developmental plasticity, in terms of both regionalization and meristic change, with basal synapsids sharing the conserved axial configuration of crown mammals, and basal reptiles demonstrating the plasticity of extant taxa. We conducted a comprehensive survey of presacral vertebral counts across 436 recent and extinct amniote taxa. Vertebral counts were mapped onto a generalized amniote phylogeny as well as individual ingroup trees, and ancestral states were reconstructed by using squared-change parsimony. We also calculated the relationship between presacral and cervical numbers to infer the relative influence of homeotic effects and meristic changes and found no correlation between somitogenesis and Hox-mediated regionalization. Although conservatism in presacral numbers characterized early synapsid lineages, in some cases reptiles and synapsids exhibit the same developmental innovations in response to similar selective pressures. Conversely, increases in body mass are not coupled with meristic or homeotic changes, but mostly occur in concert with postembryonic somatic growth. Our study highlights the importance of fossils in large-scale investigations of evolutionary developmental processes.
Keywords: constraint, development, Hox genes, segmentation, paleontology
Somitogenesis, somatic growth, and Hox gene expression are primary factors driving the formation of the vertebrate body axis (1, 2). Somitogenesis occurs via the rhythmic budding of embryonic somites from the anterior part of the presomitic mesoderm, until a point when the latter has shrunk to such a degree that no further somites can be formed (3). Periodic somite formation is controlled by a molecular oscillator or “segmentation clock” (4), whereas the speed of the clock is highly variable across different vertebrate lineages. For example, the segmentation clock in snakes ticks much faster than in a mouse, resulting in a higher number of relatively smaller somites (3). Because the ossified vertebrae are derived from the embryonic somites through resegmentation (1), vertebral numbers can provide insights into the speed of the segmentation clock and the pattern of somitogenesis. In addition, the relative size of vertebrae relative to the number of vertebrae provides information about the rate of somatic growth (5, 6).
Besides the marked variation in the number and sizes of vertebrae, the vertebrate body axis is also highly regionalized. In tetrapods, this regionalization is expressed in the formation of presacral, sacral, and caudal vertebral series, and the presacral portion can be further subdivided into cervical and dorsal series (with the latter subdivided still further into thoracic and lumbar vertebrae in mammals). These regional identities result from different Hox gene expressions along the vertebrate body axis (7, 8); consequently, the vertebral column can be viewed as a result of the transformation of repeated developmental modules into different evolutionary modules (9, 10). Hox genes are activated in the lateral somite precursors in the epiblast, so the timing of Hox activation during gastrulation largely determines the position of expression domains of Hox genes along the anteroposterior body axis, whereas the stem cell-derived medial somite precursors control segmentation (11). Because of the spatial dissociation between segmentation and axial regionalization, it has recently been suggested that the two processes are uncoupled (11, 12), despite some indication of crosstalk between the segmentation machinery and Hox patterning (11, 13).
The vertebral column has recently attracted the interest of many evolutionary and developmental biologists because the formative roles of somitogenesis, somatic growth, and Hox gene expression are coupled with easily observable adult phenotypes. For example, there have been investigations into vertebral count variation in numerous taxa, including fish, amphibians, reptiles, and mammals (5, 9, 14 –21). Most of these studies ask whether, and how, vertebral counts are affected by developmental constraints and modular evolution, and how changing vertebral counts effect body size evolution. Mammals in particular have attracted much attention because, with few exceptions, their precaudal vertebral counts are highly conserved, or constrained (15, 17). By contrast, reptiles (including birds) are more variable in their vertebral numbers; the most dramatic example are snakes, with some taxa possessing >300 precaudal vertebrae (5). However, all of these comparisons have suffered from the fact that there has, so far, been no comprehensive treatment of vertebral count variation across Amniota as a whole, and none that have taken full account of extinct taxa that include morphologies that are unrepresented in the modern biota.
Amniota is the tetrapod clade that is composed of Synapsida (crown mammals and their fossil relatives) and Reptilia (including birds), and is diagnosed by a suite of adaptations for terrestrial habits, including extraembryonic membranes (22). The oldest representatives of modern amniote crown clades have been recovered from Triassic deposits (23).
Here, we examine variation of presacral vertebral counts across all major amniote clades (SI Methods and SI Appendix), fossil and extant. We focus on the presacral portion of the body axis because it is regionalized in most amniotes, and because the caudal skeleton is a single, anatomically undifferentiated region whose segmentation is temporally disjunct from the presacral region and is derived by terminal addition at the tailbud, a different patterning than in the presacral region (9, 24). As a result, changes in caudal somite counts are uninformative with respect to the relative roles of both homeotic and meristic changes within specific regions of the axial skeleton. Additionally, the posterior parts of the vertebral column such as the sacral and caudal regions are often not preserved in fossils and therefore are difficult to study. By including fossils, we trace the evolutionary developmental history of extant taxa through the inclusion of their extinct relatives and examine developmental patterns in different ecologies within a comparative phylogenetic framework. In this study, we ask whether homeotic and somitogenetic patterns in the presacral region are correlated with each other as well as within and across major clades, whether similar ecologies and morphological specializations convergently release or constrain presacral patterning in different lineages, and whether changes in presacral patterning reflect changes in body size.
Results
Our analysis presents ancestral state reconstructions for presacral and cervical series counts across all major amniote clades (Figs. 1 and 2 and SI Appendix). We define cervical vertebrae as a rostrally situated subset of presacral elements that can be distinguished from more caudally situated vertebrae by either topological position (rostral to the shoulder girdle) or discrete morphology (25). We used the ratio between the number of cervical vertebrae to all presacral vertebrae as a reference for the relative proportions of different axial regions along the body axis (referred to as CP ratio hereafter). Because both the phylogenetic and developmental relationships between homeotic and somitogenetic variation are poorly constrained, we used phylogenetic independent contrasts (PIC) and phylogenetic generalized least-squares regression (PGLS) to test whether presacral vertebral numbers among amniotes are correlated with the CP ratio while minimizing the potential effects of phylogenetic autocorrelation (26, 27; see Methods). No significant correlation exists for any of the included amniote taxa, and neither PIC nor PGLS analyses resulted in a significant correlation between the two variables (r 2 = 0.06, correlation coefficient = −0.25) when autocorrelation was removed, arguing against the hypothesis that somitogenesis and regionalization are linked processes (see SI Appendix for details).
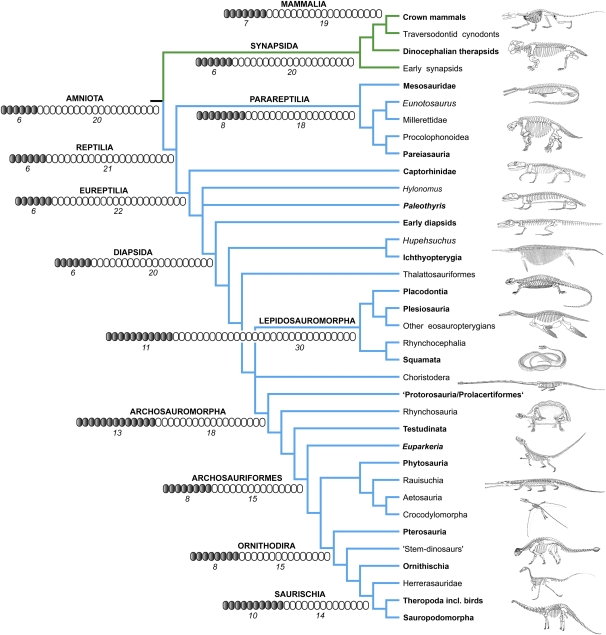
Fig. 1.
Phylogeny of the major amniote groups treated in this article, based on a compilation from several sources (23, 53, 54). The position of turtles follows current hypotheses (50, 51). See SI Appendix for a detailed phylogeny of all taxa treated in this article. Circles at major nodes illustrate ancestral states for cervical (gray) and dorsal (white) vertebrae, based on rounded numbers (in italics) derived from the ancestral reconstructions. The skeletal reconstructions of taxa in bold are modified from several sources (SI Appendix) and serve to illustrate the diversity of skeletal structures and the associated vertebral numbers. The figure is not to scale.
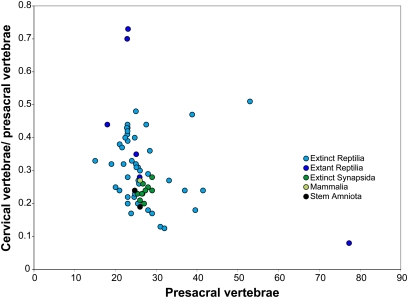
Fig. 2.
Diagram showing the cervical/presacral ratio (y axis) vs. presacral number (x axis) for the major amniote clades. Mammals, extinct synapsids, and stem amniotes (light green, green, and black circles) show much less variability than extinct and extant reptiles (light blue and blue circles). See section 7 of SI Appendix for taxa and values.
In Synapsida, only moderate deviations from the ancestral amniote condition (CP = 0.23) were recorded, remaining roughly in the range of ±0.04. The lowest ratio was found in the basal synapsid Haptodus (CP = 0.2), whereas the highest ratios occur in dinocephalian therapsids (CP = 0.275), crown mammals (CP = 0.27), and the closely related traversodontid cynodonts (CP = 0.27).
We recorded a remarkable amount of variation in the CP ratio among reptile lineages. In parareptiles, there is both a decrease in ratio relative to the ancestral amniote condition, as well as an increase. The strongest decrease can be seen in millerettids (CP = 0.17) and Macroleter (CP = 0.195). The strongest increase in ratio was recorded for mesosaurs (CP = 0.36), as well as Eunotosaurus and Nyctiphruretus (CP = 0.33 in both cases).
In eureptiles, a remarkable amount of plasticity is evident at the base of the clade. In the nondiapsid taxa, low CP ratios, reaching as low as 0.125 (Paleothyris), are frequently observed. Much higher ratios relative to the ancestral amniote condition are also found in basal diapsids, with the highest values in Claudiosaurus (CP = 0.33), Coelurosauravus (CP = 0.38), and drepanosaurids (CP = 0.33). The highly derived marine ichthyopterygians/Hupehsuchus and thalattosaurs show at their base only marginally higher ratios relative to the ancestral condition (CP = 0.24 and 0.27, respectively). Eosauropterygians, which are marine too, possess a CP ratio of 0.47, whereas placodonts, the sister taxon to plesiosaurs and other eosauropterygians, exhibit a CP ratio of 0.32.
Lepidosauromorpha includes the taxa with the strongest deviations from the ancestral amniote condition; the CP ratios found include the lowest among all amniotes. Squamata as a whole has an ancestral CP value of 0.08. In rhynchocephalians, the aquatic pleurosaurids have a CP ratio of 0.18, whereas the terrestrial species such as the tuatara remain comparatively close to the ancestral amniote condition (CP = 0.28).
The aquatic choristoderes have a high CP ratio of 0.43, and so do archosauromorphs, with a CP ratio of 0.41. The values remain high throughout the various archosauromorph lineages, which also applies to turtles (CP = 0.44) despite their very low count of only 18 presacrals. The paraphyletic prolacertiforms differ strongly from each other, with some showing comparatively low CP ratios of ≈0.3; however, this is still much higher than the ancestral amniote count. The highest ratios among prolacertiforms are found in Tanystropheus (CP = 0.48) and Dinocephalosaurus (CP = 0.51), although it should be noted that these values are only marginally lower than those in many other archosauromorph clades. Also in archosauriforms, the ratios remain rather constant and approximate the ancestral archosauromorph condition. However, in Ornithodira a relatively higher presacral count is recorded, in both pterosaurs and dinosaurs. In the large-bodied and long-necked sauropodomorphs the CP ratio is initially 0.4 and thus almost identical to the ancestral archosauromorph pattern, whereas in the more derived sauropod clades Macronaria and Diplodocoidea the CP ratios increase to 0.52 and 0.54, respectively. Birds have among the highest ratios of all archosaurs (CP = 0.64).
Discussion
Somitogenesis vs. Regionalization.
Our PIC and PGLS analyses did not provide any statistical support for a correlation of homeotic regionalization with somitogenesis. Developmental connections between regionalization and segmentation are controversial. Experimental grafts of Fgf8 beads in developing embryos reveal that Hox expression domains track segment boundaries when the size and number of somites change, suggesting crosstalk between the processes (11), and the effects of notch signaling on both Hoxd expression and segmentation suggest a common coordinating mechanism (13). Conversely, Hox gene mutations do not impact somite number, and changes in somite numbers do not demonstrate corresponding changes in the size or extent of axial expression domains, suggesting independence of the processes (3, 11, 12, 28). Our results indicate that segmentation and homeotic regionalization act separately at higher-order phylogenetic levels, and that if transcription mechanisms exist that link the processes in model taxa, then they are decoupled in the evolution of new body plans.
Independence of the two processes allows us to use the CP ratio in relation to the presacral number to evaluate if the observed variation in vertebral counts is due to either homeotic change or alteration of somitogenesis. For example, an increased rate of somitogenesis in the absence of any modified homeotic effect should result in a higher number of cervical and dorsal vertebrae, but the relative proportions of axial regions formed from the different Hox gene expressions along the body axis should remain the same. If the CP ratio equals the ancestral condition but the overall presacral count is increased, then changes in somitogenesis, with no change in Hox gene expressions, were most likely responsible. By contrast, a decrease in ratio with no change in total presacral count would indicate that homeotic changes must have been involved in the trunk region; however, if the presacral count is increased, then both homeotic and somitogenetic changes must have been in effect. Lastly, an increase in this ratio would occur either if the relative number of cervical vertebrae increased because of homeotic changes (with the total presacral count remaining constant) or if the presacral count decreased because of altered somitogenesis but with no corresponding (homeotic) effects on the cervical region.
In synapsid evolution leading to living mammals, we found a remarkable conservatism relative to the ancestral amniote condition. In fact, the only observable changes in the developmental program are a slight decrease in cervical number in the basal synapsid Haptodus and the evolution of a slightly longer neck in dinocephalian therapsids, traversodontid cynodonts, and crown mammals. All of these modifications were possibly caused by moderate homeotic effects rather than by meristic change.
By contrast, reptiles display a much higher degree of plasticity (Fig. 2). Among parareptiles, indications for changes in either somitogenesis or Hox expression domains can be observed throughout the clade. For example, the marine mesosaurs have a high cervical number but overall show only a slight increase in the presacral count, indicating largely homeotic modifications with only minor meristic changes. At the same time, the enigmatic Eunotosaurus has the lowest presacral count among all amniote taxa considered in this study, which obviously must be largely due to changes in somitogenesis, whereas, interestingly, the cervical count is practically unaffected. Eureptiles behave even more plastic: at the origin of the clade in the Carboniferous, eureptiles already show concerted changes in somitogenesis and regionalization, resulting in higher presacral counts but a relatively lower number of cervicals relative to the ancestral condition. Among early diapsid reptiles, several taxa possess relatively longer necks without major changes in presacral counts, which suggests largely homeotic modifications. The marine ichthyopterygians and thalattosaurs show ancestrally mostly modifications in meristic counts rather than homeotic changes, which in the later evolution of ichthyosaurs becomes even more apparent (29). The likewise marine eosauropterygians are different in that they display significant modifications in both meristic counts and regionalization, resulting in higher presacral counts and a much longer neck. However, the sister taxon Placodontia conversely shows a reduction in presacral number and an only moderately elongated neck. In more derived placodonts, presacral counts are further reduced to 19–20, coupled with a shortening of the neck, indicating meristic changes with concerted Hox gene expressions.
Lepidosauria present the most dramatic deviations from the ancestral amniote condition. It seems that in squamates, largely meristic changes through altered somitogenesis have acted in concert with homeotic modifications affecting the trunk region. In snakes, there is an indication that at least in some taxa the cervical axial skeleton is absent posterior to the atlas-axis or first rib-bearing element because of anterior expansion of Hox expression domains that are associated with thoracic region identity despite the persistence of other cervical-coding genes (30, 31). In rhynchocephalians, marine taxa display axial patterning similar to what is ancestrally observed in squamates, whereas terrestrial taxa remain close to the ancestral amniote condition.
Interestingly, Archosauromorpha consistently possesses high CP ratios due to a relative elongation of the neck probably induced largely by homeotic changes, because the presacral number is, at least initially, not significantly increased. However, meristic changes may have played a larger role in turtle evolution, as they have a very low presacral count (18 vertebrae) with the overall CP ratio remaining similar to that of other archosauromorphs. Meristic changes are also apparent in the "prolacertiform" Dinocephalosaurus with a total presacral count of 53 and a cervical count of 27. Furthermore, in pterosaurs and dinosaurs (Ornithodira), a relatively higher presacral count is recorded. In this regard, the increased CP ratios in derived giant sauropods suggest that mostly homeotic changes were involved in the evolution of an elongated neck. Finally, birds expand on the ancestral archosauromorph pattern by increasing the amount of homeotic change in the anterior body axis, again resulting in fairly high cervical numbers.
Constraint vs. Selection.
Our analysis indicates that the hypothesized developmental constraints in the evolution of mammalian vertebral formulae (15) most likely characterized basal synapsids and significantly predated the origin of the mammalian crown group. This is in sharp contrast to the pattern recovered in Reptilia, in which a remarkable amount of plasticity is documented throughout the clade, suggesting the absence or release of constraints in the evolution of the reptilian axial skeleton. When comparing the CP ratios and the means of deviation from the ancestral presacral amniote count in synapsids and reptiles, a significant difference could be recorded for the CP ratios (P = 0.04) but not for the presacral numbers (P = 0.07). This indicates that patterns of homeotic change behave more plastically in reptiles, but that despite the high amount of meristic variation in this clade, there are no significant differences in somitogenesis at the origin of major lineages within it.
Selection may also act as the catalyst for an increase in plasticity (32). The earliest reptiles exhibit a much greater range of ecologies than coeval synapsids, a phenomenon that might be associated with the observed plasticity in their developmental mechanisms. For example, several reptile lineages acquired numerous adaptations to a marine life by the late Paleozoic/early Mesozoic [e.g., mesosaurs, eosauropterygians, ichthyosaurs, and placodonts (33)], and among archosauriforms, active flight evolved at least twice during the Mesozoic [in pterosaurs and birds (34)]. By contrast, the greatest range of ecological innovations in synapsids have occurred in Cenozoic placental mammals, which include taxa that are exclusively marine (whales, sirenians, and to some degree pinnipeds), fossorial (e.g., moles and golden moles), or volant (bats), although ecological breadth has been recently discovered in Mesozoic clades within or close to the crown clade Mammalia (35). By contrast, basal synapsid taxa were largely (although not exclusively) terrestrial (36). Support of a developmental constraint hypothesis is based on the reported conservatism of the mammalian vertebral formula despite the many different ecological adaptations in that group (15); however, it should also be noted that in cases in which mammals evolved extreme ecologies, as in marine species, changes in the vertebral formula did occur (see below). Thus, the developmental constraint in mammals is not insurmountable.
Ecology and Vertebral Formulae.
Our dataset allows us to test whether similar ecologies/morphological adaptations are correlated with similar developmental responses across different taxa. In marine reptiles, body elongation is frequent and overall results in significantly higher presacral numbers relative to terrestrial amniotes (P = 0.007). However, body elongation in marine taxa can be coupled with either a relative shortening or a lengthening of the neck. In cases of neck elongation, as in mesosaurs, eosauropterygians, or within thalattosaurs (Askeptosauridae), we infer neck elongation to be the result of homeotic changes, meaning that the total number of presacrals is conserved but the relative proportion of cervical vertebrae increases with neck length, although some meristic change may also occur either in concert or secondarily. Plesiosaurs present an impressive example of altered somitogenesis occurring after the establishment of their primary body plan, with some taxa possessing presacral counts of well over 70 vertebrae. In the exceptionally long-necked archosauromorph "prolacertiform" Tanystropheus (Fig. 1), homeotic change, whether ancestral or apomorphic, was also an initial driving factor but in this case was coupled with enhanced somatic growth. The prolacertiform Dinocephalosaurus seems to have expanded on the same pattern by additional meristic change. Conversely, marine reptiles with a short neck, such as ichthyopterygians, remain close to the ancestral cervical count and increase the trunk series through somitogenesis, as exemplified in Qianichthyosaurus zhoui from the Triassic of China, which possesses six cervicals but 63 presacrals (29). The same pattern is also observed in the short-necked thalattosaur Xinpusaurus, which has only 4–5 cervical vertebrae but up to 40 presacrals (37). A particular problem in contrasting cases of neck elongation is the unsolved discussion on the functional factors related to this phenomenon (38).
Marine mammals, such as cetaceans and sirenians, have similar patterning of the axial skeleton relative to that seen in short-necked marine reptiles, in which the overall number of presacrals may increase but the ancestral cervical count is largely retained (17, 39). This suggests that although the cervical region of mammals may be interpreted as developmentally constrained (40), the dorsal region does appear to be able to respond to certain selective pressures in a similar way to those of reptiles with equivalent ecologies.
Pinnipeds, comprising the walruses, sea lions, and seals, are largely aquatic and do not possess any modifications of the ancestral carnivoran pattern, instead they retain the highly conserved number of 20 thoracolumbar vertebrae typical of other carnivores (41). The same conservatism is also observed in basal placodont reptiles, whose vertebral numbers are similar to the ancestral condition. Consequently, both of these clades indicate that either marine habits need not to be coupled with changes in vertebral number or that the basal taxa examined retained largely terrestrial habits. It is noteworthy, however, that derived placodonts possess a turtle-like carapace over the trunk, which correlates with a remarkable decrease in vertebral number. Other taxa with extensive dermal armor, a shell, or strongly modified thoracic ribs (bearing extensive plates or accessory ossifications) also possess low presacral counts, including turtles, the parareptile Eunotosaurus, and ankylosaurian dinosaurs. Although we did not find any statistical support for these observations, it is interesting that among placental mammals the armored armadillos also have a reduced presacral count (40, 42). In addition, it appears that altered somitogenesis always occurs in the trunk regions of these taxa, whereas their cervical series are unaffected. This example may be another indication that both mammals and reptiles rely on the same developmental responses under similar selective pressures.
A vertebral formula of 8 cervical, 10 trunk, and 2 sacral vertebrae (with a varying number of caudal vertebrae) is highly conserved among both fossil and recent turtles. The recently described basalmost turtle Odontochelys semitestacea (43), from the Late Triassic of China, departs from this general pattern in possessing only 9 trunk vertebrae. This low number probably represents a reduction from that present in the ancestor of turtles, assuming either archosauromorph affinities (Fig. 1) or an alternative, more basal position of turtles relative to living diapsids (44).
The aforementioned examples demonstrate that although taxon-specific variation is present in all amniote lineages, specific ecological/morphological adaptations are consistently coupled with similar underlying developmental mechanisms.
Size and Vertebral Formulae.
Increasing body size by increasing vertebral numbers within the axial skeleton (pleomerism) is a primary mechanism of body size increase in fish, some lissamphibians, and snakes (5, 21). The role of pleomerism in other amniote groups has not been investigated. Our observations clearly demonstrate that the dramatic increases in body size seen in many dinosaur taxa are not accompanied by an increase in vertebral number. All dinosaur taxa show a generally conservative presacral vertebral count that is relatively close to the ancestral amniote condition. Derived sauropods, the macronarians and diplodocoids, also fit this pattern despite the evolution of impressively long necks in these clades, which leads us to infer that within sauropods, neck elongation was not only due to elongation of individual vertebrae but also the result of homeotic effects, causing somewhat higher cervical and lower dorsal numbers than in many other dinosaurs (45). Additional meristic changes may occur in some individual sauropod species, as in Mamenchisaurus and Euhelopus, which both possess 30 presacral vertebrae, the highest number present among the sauropod taxa sampled in this study. This pattern is similar to that described for marine reptiles (see above), which indicates that whatever the respective ecology, high cervical counts in an elongated neck appear to be under the primary influence of homeotic changes. By contrast, body size per se seems to play only a marginal role (if any) in the evolution of sauropod vertebral counts. Similarly, giant snakes do not reach final body size through significant increases in vertebral number but through postembryonic somatic growth (5), as must also have been the case in dinosaurs.
Conversely, small amniotes often possess high vertebral counts. This is best exemplified in squamates, where numerous fossorial lineages including amphisbaenians, dibamids, and snakes attain adult body lengths as small as 100 mm (46, 47) but possess the highest vertebral counts among amniotes. This observation provides additional support for the observation that segmentation and body size are not positively correlated within amniote lineages. We therefore hypothesize that factors other than size, for example habits and locomotion, may be more important in driving the evolution of high or low vertebral counts in many amniote clades (21).
Conclusions.
Shortly after the origin of amniotes, reptiles and synapsids evolved very different degrees of plasticity in their vertebral numbers, with reptiles displaying much more variation. This discrepancy could be due to a developmental constraint in the synapsid lineage lacking in reptiles. Unfortunately, the current dearth of large-scale comparative ecological studies over extended temporal intervals prevents examination of the effects of these potentially different selective regimes on the evolutionary trajectories of these two lineages.
We demonstrate that “extreme ecologies” such as marine life habits seem to elicit the same developmental responses, independent of the phylogenetic position of the taxa involved. In addition, size does not appear to be a major factor in the evolution of vertebral counts.
Finally, our study emphasizes the importance of fossils when interpreting developmental evolutionary morphologies. The fossil record helps to recognize "the pattern of evolutionary change we want to explain" (48, page 530), and as such is indispensable for large-scale interpretations of evolutionary processes. As we have illustrated in our study, the fossil record can provide insights into ancient developmental mechanisms even if the developmental transformation itself is no longer present in a living taxon. By examining also Paleozoic amniotes, we have explored developmental programs that are 20–100 million years older than the oldest fossils of the living amniote groups.
Although our sampling of fossil clades is very comprehensive, the taxa considered in the analysis represent only a small subsample of the amniote evolutionary tree, because of various preservational biases of other known species and extinction so far not recorded in the fossil record. For example, our study considered 96 nonavian dinosaur genera, whereas Wang and Dodson (49) estimated that ≈1,850 genera once existed. As such, as more information from the fossil record becomes available, this could be used to further explore the evolutionary and developmental patterns discussed in this article.
Methods
A total of 436 amniote taxa were considered in this study, including many recent taxa, dinosaurs, basal synapsids, and numerous stem members of recent and extinct clades. Data were obtained by critically reviewing the literature, supplemented with data from personal observations on original specimens (SI Appendix). In cases where a taxon possessed variable vertebral formulae, the mean value was used in the analysis. Identification of the cervicodorsal boundary was problematic in some taxa because of the transitional morphology that sometimes occurs in this region. Where personal observations of material were not possible, or where specimens were inadequately figured, information obtained from the primary literature was taken at face value; however, in most cases we were able to use several cues to establish the position of this boundary, including the position of the parapophysis relative to the neurocentral boundary, the shape of the ribs, or the presence/absence of ribs.
Data for each of the clades surveyed were supplied as follows: Synapsida (M.R.S.-V.); Ornithischia and nonavian Theropoda (P.M.B.); Aves (P.G.P.E.); Sauropodomorpha and Crurotarsi (D.P.); Squamata (J.J.H.); Parareptilia, basal Eureptilia, Thalattosauriformes (J.M.); Testudinata, Ichthyopterygia, Sauropterygia excluding Plesiosauria, basal Crurotarsi, basal Diapsida (together with J.M.), Choristodera, Pterosauria (T.M.S.); and Plesiosauria (I.W.).
Individual composite phylogenies were constructed for the major taxonomic groups on which the vertebral counts were mapped. These composite phylogenies are informal supertrees, representing an evaluation of the current evidence based on each person’s individual taxonomic expertise (SI Appendix). We also assembled a general tree of amniote relationships incorporating all of the major clades as well as basal, monospecific taxa representing independent lineages (Fig. 1). Perhaps the most controversial issue in the general amniote tree was the phylogenetic position of turtles (44). For the present study, we followed the latest molecular results (50, 51) and grouped turtles as the sister taxon to Archosauriformes.
Ancestral states for vertebral numbers were reconstructed by using squared-change parsimony, which minimizes the sum of squared changes along a rooted tree, in the software package Mesquite 2.6 (52). The ancestral states for the presacral and cervical counts of each taxonomic group were first reconstructed by using the individual phylogenies and then mapped onto the general amniote phylogeny, whereas the values for monospecific taxa were entered directly. Similar to the individual phylogenies, the ancestral states for the major clades were then reconstructed by using the squared-change parsimony algorithm.
Because we were interested primarily in vertebral numbers and different regionalization patterns, we did not take measurements of individual vertebrae. Furthermore, metric data for individual vertebrae in complete presacral series of fossils are rare. As such, our study cannot be used to evaluate different patterns of somatic growth.
PIC and PGLS were calculated by using the software package COMPARE 4.6b (27), with resolved branch lengths set to 1 and polytomies artificially resolved with branch lengths of 0.001, which is effectively zero but satisfies the algorithmic requirements of the program.
The comparison of the means of the CP ratios and the deviations from the ancestral presacral amniote count was performed by a two-tailed t test in Microsoft Excel.
Supplementary Material
Acknowledgments
We thank Fernando Abdala for advice, Heike Mewis for technical help, Florian Witzmann and P. David Polly for discussion, and three anonymous reviewers for suggestions that helped to much improve the work. This work was supported by the "Fonds zur Förderung des akademischen Nachwuchses (FAN) des Zürcher Universitätsvereins (ZUNIV)" (M.R.S.-V.) and Deutsche Forschungsgemeinschaft Grant Mu 1760/2-3 (to J.M.). J.J.H. was supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912622107/DCSupplemental.
References
- 1.Richardson MK, Allen SP, Wright GM, Raynaud A, Hanken J. Somite number and vertebrate evolution. Development. 1998;125:151–160. doi: 10.1242/dev.125.2.151. [DOI] [PubMed] [Google Scholar]
- 2.Carroll SB. Endless Forms Most Beautiful. New York: Norton; 2005. [Google Scholar]
- 3.Gomez C, et al. Control of segment number in vertebrate embryos. Nature. 2008;454:335–339. doi: 10.1038/nature07020. [DOI] [PubMed] [Google Scholar]
- 4.Dequéant ML, Pourquié O. Segmental patterning of the vertebrate embryonic axis. Nat Rev Genet. 2008;9:370–382. doi: 10.1038/nrg2320. [DOI] [PubMed] [Google Scholar]
- 5.Head JJ, David Polly P. Dissociation of somatic growth from segmentation drives gigantism in snakes. Biol Lett. 2007;3:296–298. doi: 10.1098/rsbl.2007.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert SF. Developmental Biology. 8th Ed. New York: Sinauer; 2008. [Google Scholar]
- 7.Burke AC, Nelson CE, Morgan BA, Tabin C. Hox genes and the evolution of vertebrate axial morphology. Development. 1995;121:333–346. doi: 10.1242/dev.121.2.333. [DOI] [PubMed] [Google Scholar]
- 8.Wellik DM, Capecchi MR. Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science. 2003;301:363–367. doi: 10.1126/science.1085672. [DOI] [PubMed] [Google Scholar]
- 9.Polly PD, Head JJ, Cohn MJ. In: Beyond Heterochrony: The Evolution of Development. Zelditch ML, editor. New York: Wiley; 2001. pp. 305–335. [Google Scholar]
- 10.Wagner GP, Mezey J, Calabretta R. In: Modularity. Understanding the Development and Evolution of Complex Natural Systems. Callebaut W, Rasskin-Gutman D, editors. Cambridge, MA: MIT Press; 2005. pp. 33–50. [Google Scholar]
- 11.Iimura T, Denans N, Pourquié O. Establishment of Hox vertebral identities in the embryonic spine precursors. Curr Top Dev Biol. 2009;88:201–234. doi: 10.1016/S0070-2153(09)88007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez C, Pourquié O. Developmental control of segment numbers in vertebrates. J Exp Zool B. 2009;312B:533–544. doi: 10.1002/jez.b.21305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zákány J, Kmita M, Alarcon P, de la Pompa JL, Duboule D. Localized and transient transcription of Hox genes suggests a link between patterning and the segmentation clock. Cell. 2001;106:207–217. doi: 10.1016/s0092-8674(01)00436-6. [DOI] [PubMed] [Google Scholar]
- 14.Morin-Kensicki EM, Melancon E, Eisen JS. Segmental relationship between somites and vertebral column in zebrafish. Development. 2002;129:3851–3860. doi: 10.1242/dev.129.16.3851. [DOI] [PubMed] [Google Scholar]
- 15.Narita Y, Kuratani S. Evolution of the vertebral formulae in mammals: a perspective on developmental constraints. J Exp Zool B. 2005;304B:91–106. doi: 10.1002/jez.b.21029. [DOI] [PubMed] [Google Scholar]
- 16.O’Keefe FR, Hiller N. Morphologic and ontogenetic patterns in elasmosaur neck length, with comments on the taxonomic utility of neck length variables. Paludicola. 2006;5:206–229. [Google Scholar]
- 17.Buchholtz EA, Booth AC, Webbnik KE. Vertebral anatomy in the Florida manatee, Trichechus manatus latirostris: a developmental and evolutionary analysis. Anat Rec. 2007;290:624–637. doi: 10.1002/ar.20534. [DOI] [PubMed] [Google Scholar]
- 18.Ward AB, Brainerd EL. Evolution of axial patterning in elongate fishes. Biol J Linn Soc. 2007;90:97–116. [Google Scholar]
- 19.Asher RJ, Lehmann T. Dental eruption in afrotherian mammals. BMC Biol. 2008;6:14. doi: 10.1186/1741-7007-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boisvert CA. Vertebral development of modern salamanders provides insights into a unique event of their evolutionary history. Jour Exp Zool B. 2009;312B:1–29. doi: 10.1002/jez.b.21238. [DOI] [PubMed] [Google Scholar]
- 21.Lindell LE. The evolution of vertebral number and body size in snakes. Funct Ecol. 1994;8:708–719. [Google Scholar]
- 22.Gauthier J, Kluge AG, Rowe T. Amniote phylogeny and the importance of fossils. Cladistics. 1988;4:105–209. doi: 10.1111/j.1096-0031.1988.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 23.Benton MJ. Paläontologie der Wirbeltiere. Munich: Dr. Friedrich Pfeil; 2007. p. 472. [German translation of Vertebrate Paleontology, 3rd Ed] [Google Scholar]
- 24.LeDouarin NM, Grapin-Botton A, Catala M. Patterning of the neural primordium in the avian embryo. Semin Cell Dev Biol. 1996;7:157–167. [Google Scholar]
- 25.Buchholtz EA, Stepien CC. Anatomical transformation in mammals: developmental origin of aberrant cervical anatomy in tree sloths. Evol Dev. 2009;11:69–79. doi: 10.1111/j.1525-142X.2008.00303.x. [DOI] [PubMed] [Google Scholar]
- 26.Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- 27.Martins EP. COMPARE, Computer programs for the statistical analysis of comparative data. Bloomington: Indiana Univ; 2004. Ver 4.6b. [Google Scholar]
- 28.Wellik DM. Hox patterning of the vertebrate skeleton. Dev Dyn. 2007;236:2456–2463. doi: 10.1002/dvdy.21286. [DOI] [PubMed] [Google Scholar]
- 29.McGowan C, Motani R. Ichthyopterygia. Handbuch der Paläoherpetologie Part 8. Munich: Dr. Friedrich Pfeil; 2003. p. 175. [Google Scholar]
- 30.Cohn MJ, Tickle C. Developmental basis of limblessness and axial patterning in snakes. Nature. 1999;399:474–479. doi: 10.1038/20944. [DOI] [PubMed] [Google Scholar]
- 31.Woltering JM, et al. Axial patterning in snakes and caecilians: evidence for an alternative interpretation of the Hox code. Dev Biol. 2009;332:82–89. doi: 10.1016/j.ydbio.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 32.Schwenk K, Wagner GP. In: Key Words and Concepts in Evolutionary Developmental Biology. Hall BK, Olson WM, editors. Cambridge, MA: Harvard Univ Press; 2003. pp. 52–61. [Google Scholar]
- 33.Müller J. In: Recent Advances in the Origin and Early Radiation of Vertebrates. Arratia G, Cloutier R, Wilson VH, editors. Munich: Dr. Friedrich Pfeil; 2004. pp. 379–408. [Google Scholar]
- 34.Brochu CA. Progress and future directions in archosaur phylogenetics. J Paleontol. 2001;75:1185–1201. [Google Scholar]
- 35.Luo Z-X. Transformation and diversification in early mammal evolution. Nature. 2007;450:1011–1019. doi: 10.1038/nature06277. [DOI] [PubMed] [Google Scholar]
- 36.Kemp T. The Origin and Evolution of Mammals. Oxford: Oxford Univ Press; 2005. [Google Scholar]
- 37.Liu J, Rieppel O. The second thalattosaur from the Triassic of. Guizhou, China. Vertebrata Palasiatica. 2001;39:77–87. [Google Scholar]
- 38.McHenry CR, Cook AG, Wroe S. Bottom-feeding plesiosaurs. Science. 2005;310:75. doi: 10.1126/science.1117241. [DOI] [PubMed] [Google Scholar]
- 39.Tomilin AG. Mammals of the USSR and Adjacent Countries, Volume IX: Cetacea. Moscow: Acad Sci Publ House; 1957. [in Russian] [Google Scholar]
- 40.Galis F, et al. Extreme selection in humans against homeotic transformations of cervical vertebrae. Evolution. 2006;60:2643–2654. [PubMed] [Google Scholar]
- 41.Sánchez-Villagra MR, Narita Y, Kuratani S. Thoracolumbar vertebral number: the first skeletal synapomorphy for afrotherian mammals. Syst Biodivers. 2007;5:1–7. [Google Scholar]
- 42.Galliari FC, Carlini AC, Sánchez-Villagra MR. Evolution of the axial skeleton in armadillos (Mammalia, Dasypodidae) Mamm Biol. 2009 doi:10.1016/j.mambio.2009.03.014. [Google Scholar]
- 43.Li C, Wu X-C, Rieppel O, Wang L-T, Zhao L-J. An ancestral turtle from the Late Triassic of southwestern China. Nature. 2008;456:497–501. doi: 10.1038/nature07533. [DOI] [PubMed] [Google Scholar]
- 44.Werneburg I, Sánchez-Villagra MR. Timing of organogenesis support basal position of turtles in the amniote tree of life. BMC Evol Biol. 2009;9:82. doi: 10.1186/1471-2148-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson JA. Sauropod dinosaur phylogeny: critique and cladistic analysis. Zool J Linn Soc. 2002;136:217–276. [Google Scholar]
- 46.Hoffstetter R, Gasc J-P. In: in Biology of the Reptilia, Volume 1: Morphology. Gans C, editor. London: Academic; 1969. pp. 201–310. [Google Scholar]
- 47.Hedges SB. At the lower size limit in snakes: two new species of threadsnakes (Squamata: Leptotyphlopidae: Leptotyphlops) from the Lesser Antilles. Zootaxa. 2008;1841:1–30. [Google Scholar]
- 48.Wagner G. In: From Embryology to Evo-Devo: A History of Developmental Evolution. Laubichler MD, Maienschein J, editors. Cambridge, MA: MIT Press; 2007. [Google Scholar]
- 49.Wang SC, Dodson P. Estimating the diversity of dinosaurs. Proc Natl Acad Sci USA. 2006;103:13601–13605. doi: 10.1073/pnas.0606028103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyer A, Zardoya R. Recent advances in the (molecular) phylogeny of vertebrates. Annu Rev Ecol Evol Syst. 2003;34:311–338. [Google Scholar]
- 51.Iwabe N, et al. Sister group relationship of turtles to the bird-crocodilian clade revealed by nuclear DNA-coded proteins. Mol Biol Evol. 2005;22:810–813. doi: 10.1093/molbev/msi075. [DOI] [PubMed] [Google Scholar]
- 52.Maddison WP, Maddison DR. Mesquite: A Modular System for Evolutionary Analysis. Tucson: Univ of Arizona; 2004. Ver 2.6. [Google Scholar]
- 53.Müller J, Reisz RR. The phylogeny of early eureptiles: comparing parsimony and Bayesian approaches in the investigation of a basal fossil clade. Syst Biol. 2006;55:503–511. doi: 10.1080/10635150600755396. [DOI] [PubMed] [Google Scholar]
- 54.Tsuji LA, Müller J. Assembling the history of the Parareptilia: phylogeny, diversification, and a new definition of the clade. Fossil Rec. 2009;12:71–81. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.