Abstract
Plant introductions and subsequent community shifts are known to affect nutrient cycling, but most such studies have focused on nutrient enrichment effects. The nature of plant-driven nutrient depletions and the mechanisms by which these might occur are relatively poorly understood. In this study we demonstrate that the proliferation of the commonly introduced coconut palm, Cocos nucifera, interrupts the flow of allochthonous marine subsidies to terrestrial ecosystems via an indirect effect: impact on birds. Birds avoid nesting or roosting in C. nucifera, thus reducing the critical nutrient inputs they bring from the marine environment. These decreases in marine subsidies then lead to reductions in available soil nutrients, decreases in leaf nutrient quality, diminished leaf palatability, and reduced herbivory. This nutrient depletion pathway contrasts the more typical patterns of nutrient enrichment that follow plant species introductions. Research on the effects of spatial subsidy disruptions on ecosystems has not yet examined interruptions driven by changes within the recipient community, such as plant community shifts. The ubiquity of coconut palm introductions across the tropics and subtropics makes these observations particularly noteworthy. Equally important, the case of C. nucifera provides a strong demonstration of how plant community changes can dramatically impact the supply of allochthonous nutrients and thereby reshape energy flow in ecosystems.
Keywords: Cocos nucifera, community shifts, indirect effects, seabird, tropical islands
Allochthonous nutrient subsidies shape the dynamics of a broad range of ecosystems by stimulating bottom-up productivity (1, 2). This increase in productivity can then trigger a vast array of cascading changes in recipient food webs (3 –5). Several recent articles have demonstrated that introduced predators operating near the top of food webs can initiate these kinds of cascading effects on ecosystems by impacting the vectors of these subsidies, such as birds, triggering whole-scale shifts in ecosystem structure and function (6, 7). Yet top-down effects may not be the only mechanism by which spatial subsidies are disrupted. Here, we demonstrate that the proliferation of the coconut palm, Cocos nucifera, causes similar nutrient depletions with higher order effects by creating poor habitat for birds. Given the global proliferation of this plant in the tropics, our observations have a wide application. Even more broadly, this observation suggests that allochthonous subsidies may be blocked by the characteristics of species in the recipient system, making the recipient system a more active player in subsidy movement than has previously been acknowledged.
It is well established that the biological invasion of one species can have cascading effects across the invaded ecosystem, often through alteration of nutrient cycling (8, 9). However, in the majority of case studies where introduced plants have altered the nutrient cycle, the cascading effects have been as a result of increased inputs to the soil from the plants (either from increased litterfall or direct nitrogen fixation) (10, 11). The presumption is that introduced species generally profit from nutrient enrichment (12). However, recent work documenting that some invasive plants perform well in low-nutrient environments (13) suggests that more research on introduced plants specializing in low-nutrient systems is needed.
C. nucifera likely originated in Southeast Asia and then radiated regionally from this point of origin both via natural (water) and anthropogenic dispersal (14). Near monodominant stands of Cocos are now commonplace in many island and coastal forests around the tropics and subtropics of the world (see Cocos nucifera: History and Current Status at Palmyra in the SI Text). Working across a gradient of C. nucifera dominance at Palmyra atoll, this study examined the impact of C. nucifera proliferation on ecosystem ecology. We first examined habitat preferences of birds between C. nucifera and the common native tree species, Pisonia grandis and Tournefortia argentea, both on transect- and atoll-wide scales. We then examined the effects of C. nucifera dominance on levels of soil and foliar nutrients, and the consequent effects of changes in foliar nutrients on leaf palatability and herbivory. Finally, to document that high C. nucifera abundance is a cause rather than an effect of low soil nutrients (because palms are known to be able to persist in low-nutrient soils), we compared nutrient levels between islets made from dredge fill and natural islets containing different forest types. This comparison also allowed us to constrain the maximum time over which nutrient changes occur. Our results indicate that C. nucifera can dramatically impact allochthonous nutrients supply, with higher order effects on these ecosystems. We suggest that plant-driven alterations of spatial subsidies of the kind we observed at Palmyra are probably widespread, and can substantially shape nutrient flow across ecosystems and trigger cascading ecosystem changes.
Results
Vegetation Preference by Birds.
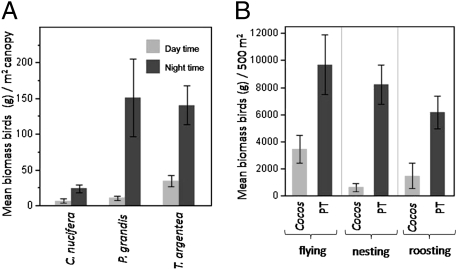
Data collected in atoll-wide surveys indicate that birds exhibit a strong preference for the trees P. grandis and T. argentea over C. nucifera, even when standardized by bird biomass and estimated canopy area per tree (Fig. 1A) (ANOVA F 2,66 = 30.2. P < 0.0001; T. argentea vs. C. nucifera: P < 0.0001; P. grandis vs. C. nucifera: P < 0.0001; P. grandis vs. T. argentea: P = 0.23).
Fig. 1.
Bird habitat preference by vegetation type. (A) Mean biomass (g) of birds per m2 of projected canopy coverage during day and night time surveys by tree species. (B) Mean biomass of birds (g) observed flying, nesting, and roosting along 500m2 coastal transects in Pisonia/Tournefortia (PT) and Cocos nucifera (Cocos) forest types. Data are means (± 1 SE) pooled by islet (n = 10).
Bird density increases at night for all tree species; we find this means a 4-fold increase of bird biomass from day to night on C. nucifera, 16-fold increase on P. grandis, and 4-fold increase on T. argentea (Fig. 1A). Including nighttime preferences for vegetation by birds further entrenches the observed preferences by birds for native species T. argentea and P. grandis (ANOVA F 2,24= 9.8, P < 0.001; T. argentea vs. C. nucifera: P < 0.01, P. grandis vs. C. nucifera: P < 0.01, P. grandis vs. T. argentea: P = 0.78).
The same trends across forest types can be seen in analysis of vegetation transects, where vegetation is more precisely quantified and cryptic bird species are censused (but where night surveys could not be conducted). Here again the biomass of birds flying (t =2.59, P = 0.01) nesting (t =5.16, P < 0.0001), and roosting (t = 3.07, P = 0.005) decline in Cocos forest (see Methods), compared to sites dominated by P. grandis and T.argentea (Fig. 1B) (“PT forest” hereafter; see Methods ). Analysis at the islet level (using pooled mean basal area and bird abundances from all transect surveys on the islet) demonstrate that nesting and roosting biomass declines approximately 8-fold (t = 2.73, P = 0.04) and flying biomass declines approximately 5-fold (t = 3.46, P = 0.01) in Cocos forests as compared to the PT forest.
Effect of Forest Type on Soil Nutrients.
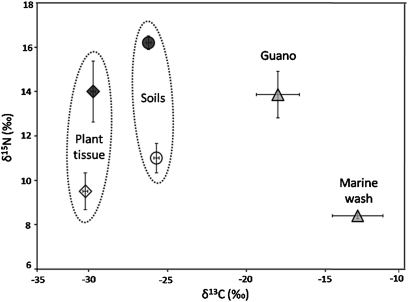
Measurements of all analyzed soil nutrients were significantly lower in Cocos as compared to PT forests (Table 1). Soil pH was higher in Cocos forests and C:N ratios were lower. Measurements of nitrogen isotopes showed that δ15N was significantly higher in soils from PT forests, indicating that the origin of these additional nutrients was from a high trophic-level source (Fig. 2) (t = 7.01, P < 0.0001). We found no significant difference in δ13C values (t = 1.6, P = 0.12). There were no significant effects of islet size on any soil nutrients.
Table 1.
Soil and foliar nutrients by forest type
| Cocos forests | PT forests | ||||
| Mean ± SE | Mean ± SE | t (df) | P | ||
| Soils | |||||
| Nitrate (μg/g) | 8.04 ± 2.31 | 100.74 ± 26.10 | 6.4 (57) | <0.0001** | |
| Ammonium (μg/g) | 39.59 ± 6.21 | 65.09 ± 5.23 | 3.5 (57) | 0.001** | |
| Phosphate (μg/g) | 1.63 ± 0.31 | 6.06 ± 0.90 | 5.4 (54) | <0.0001** | |
| pH | 7.57 ± 0.16 | 6.98 ± 0.16 | 2.9 (41) | 0.008** | |
| %N | 0.78 ± 0.13 | 1.32 ± 0.14 | 3 (46) | 0.008** | |
| %C | 14.84 ± 0.58 | 11.19 ± 0.30 | 5.5 (41) | <0.0001** | |
| Leaves | |||||
| C. nucifera | %N | 0.85 ± 0.02 | 1.00 ± 0.04 | 3.6 (29) | 0.001** |
| %P | 0.25 ± 0.02 | 0.23 ± 0.01 | 0.8 (34) | 0.45 | |
| P. grandis | %N | 2.26 ± 0.13 | 2.49 ± 0.04 | 2.3 (6) | 0.06* |
| %P | 0.34 ± 0.05 | 0.38 ± 0.02 | 0.7 (5) | 0.50 | |
| T. argentea | %N | 2.86 ± 0.02 | 3.56 ± 0.01 | 3.5 (22) | 0.002** |
| %P | 0.39 ± 0.02 | 0.45 ± 0.02 | 1.8 (25) | 0.07* | |
| C:N | 16.13 ± 0.77 | 13.1 ± 0.51 | 3.3 (20) | 0.004** |
Soil nutrients are from transects in forests dominated (>75% basal area) by Cocos nucifera (Cocos forests) or by Pisonia grandis and Tournefortia argentea (PT forests). Significant results, determined using Welch’s two-sample T-tests (after subsequent Bonferroni corrections) are in marked with **; near significant results are marked with *.
Fig. 2.
Differences in soil and plant tissue stable isotope values (means ± 1 SE) measured in Cocos-dominated forests (open) and Pisonia grandis and Tournefortia argentea (PT) dominated forests (solid black). Enriched δ15N values in PT forests are more similar to δ15N of bird guano than marine wash (both gray), indicating that birds are major vectors of nutrient subsidies into these forests. SE bars are depicted for all sample types, but are not visible in some cases because of scaling.
When soil nutrients and pH were examined by the source of the islet material (“fill” or “natural”), we found no significant differences in nutrients in PT sites; however, we detected significantly lower soil nutrients in Cocos forests that were formed from artificial fill compared to those formed from natural material (Table 2).
Table 2.
Soil nutrients by source of island material and forest type
| Original material | Fill material | |||
| Mean ± SE | Mean ± SE | t (df) | P | |
| Cocos forests | ||||
| Nitrate (μg/g) | 6.9 ± 1.5 | 3.3 ± 0.38 | 2.64 (30) | 0.01** |
| Ammonium (μg/g) | 53.6 ± 9.3 | 32.5 ± 9.2 | 2.25 (24) | 0.03** |
| Phosphate (μg/g) | 3.2 ± 0.57 | 0.6 ± 0.17 | 5.74 (26) | <0.0001** |
| pH | 7.2 ± 0.23 | 7.9 ± 0.04 | 3.17 (16) | 0.006** |
| PT forests | ||||
| Nitrate (μg/g) | 138.6 ± 43.7 | 70.8 ± 28.7 | 0.79 (21) | 0.44 |
| Ammonium (μg/g) | 65.4 ± 6.6 | 68.3 ± 3.9 | 0.38 (24) | 0.70 |
| Phosphate (μg/g) | 7.4 ± 1.4 | 4.3 ± 0.9 | 1.67 (20) | 0.11 |
| pH | 6.9 ± 0.27 | 7.1 ± 0.2 | 0.46 (18) | 0.65 |
Soil nutrients from transects on islets naturally formed or created by fill material from dredge spoilings. Results are separated by forest type as either dominated (>75% basal area) by Cocos nucifera (Cocos) or by Pisonia grandis and Tournefortia argentea (PT forests). Significant results, determined using Welch's two-sample T-tests (after subsequent Bonferroni corrections) are marked with **.
Effects of Forest Type on Leaf Nutrients.
Leaf nitrogen in T. argentea, P. grandis, and C. nucifera declined in Cocos forests (Table 1), although this decrease was not significant for P. grandis. Foliar phosphorus also declined in Cocos forests, but for T. argentea only (Table 1). The δ15N and δ13C values in T. argentea (the only tree species common enough across sites to allow for robust comparisons) were significantly lower in Cocos forests than in PT forests (Fig. 2) (δ15 N t = 3.0, P = 0.008; δ13C t = 2.2, P = 0.04), also implying a lower trophic level source of foliar nitrogen in Cocos forests.
Stable isotopic composition of δ15N for the two abundant allochthonous subsidies to this system ranges from 13.9 ‰ for bird guano (n = 18) to 8.4 ‰ for marine wash (predominantly algae) (n = 23).
Herbivory and Palatability of Leaves.
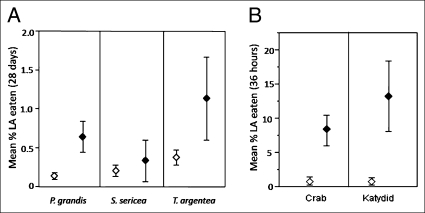
Herbivory was higher on all species in PT forests, as compared to Cocos forests; this difference was significant for three of the four species analyzed (Fig. 3A) (Wilcoxon tests: P. grandis Z90,78 = 2.5, P = 0.01; T. argentea Z 69,72 = −2.2, P = 0.03; Pearson C. nucifera χ2 1 = 0.037, P = 0.04)
Fig. 3.
Measured differences in herbivory and leaf palatability between Cocos nucifera (Cocos) and Pisonia grandis/Tournefortia argentea (PT) dominated forests. (A) Mean herbivory [percent leaf area (LA) removed over 28 days, pooled by tree] for three tree species in Cocos (open) and PT (solid black) forests (± 1 SE). (B) Median, quartiles, and range of percent leaf area eaten by insect herbivore Conocephalus saltator and crab omnivore Coenocephalus perlatus in paired palatability assays of P. grandis leaves collected from PT (solid black) and Cocos (open) forests. Herbivory was generally higher in PT forests, likely because of the higher nutrient content of their leaves.
Palatability trials conducted with herbivores in the lab indicate that both the katydid Conocephalus saltator and the crab Coenobita perlatus exhibited strong preferences for leaves of P. grandis from PT forests over those from Cocos forests (paired Student’s t-tests: C. perlatus, t = 3.8, P = 0.002; C. saltator, t = 2.6, P = 0.03) consuming 12 and 23 times more leaf area, respectively, from PT forest foliage than Cocos forests foliage (Fig. 3B).
Discussion
The majority of the birds at Palmyra prefer to nest and roost in the native trees T. argentea and P. grandis over the coconut palm. This behavior creates a stark gradient of bird abundance across the islets of the atoll, with birds ≈5 to 10 times denser at sites where T. argentea and P. grandis are dominant. The reason for this preference was not examined but is likely because of (i) the architecture of these palms, which do not provide stable large platforms for these colonially nesting birds, and (ii) rats that may nest and feed in Cocos and also predate or harass seabirds.
This impact of Cocos on bird abundance profoundly interrupts the valuable flow of allochthonous subsidies that birds provide to these impoverished terrestrial communities from the marine environment. This stark reduction in nutrient subsidies is significant. We estimate that birds vector to PT forests between 261 and 653 kg of N ha−1 y−1 and 42 to 105 kg of P ha−1 y−1 from guano, compared to between 23 and 34 kg of N ha−1 y−1 and 4 to 6 kg of P ha−1 y−1 in Cocos forests (see Guano Inputs at Palmyra in the SI Text). As reference, intensive agriculture fertilizer additions range between 60 and 400 kg N ha−1 y−1, and rates of agricultural P addition above 55 kg P ha−1 y−1 are considered a taxable pollutant in some countries (15, 16).
Palmyra’s coral-derived soils are broadly nutrient-deficient, and the loss of bird-derived nutrient inputs in Cocos forests is readily evidenced in the soil nutrients of these forests, with reductions ranging from 0.5- to 12.5-fold. The smallest depletion (a 50% reduction in NH4 +) is likely muted because of NH4 + nitrification (leading to particularly high changes in NO3 − pool). Because of high rainfall at Palmyra (4,500 mm per year), these direct soil measurements of nutrients almost certainly underestimate larger changes to nutrient throughflow and nutrient availability to plants. This trend for increasing soil nutrients near areas of high bird density is well established for temperate and polar zones (17, 18). However, there is considerably less understanding of the effects of these subsidies in ecosystems where rainfall is relatively high, coastal marine waters are relatively oligotrophic, and bird densities are relatively low; despite the fact that these conditions typify most of the coastal zones in the tropics (19, 20). While physical sources of subsidies (i.e., marine wash) would likely be low in these systems, inputs from highly mobile birds, which can draw nutrients from dispersed or distant but locally aggregated oceanic sources, appear to be important. The magnitude of nutrient alterations observed at Palmyra also suggest that the effects of palm proliferation might be even stronger in drier environments, where higher changes in nutrient pools have been observed (21, 22).
This trend for reductions in soil nutrients in areas where abundance of C. nucifera is high is mirrored, in a more subdued fashion, in foliar nutrients. We observed a 15 to 20% reduction in average foliar N, and 13% declines in foliar P for T. argenta only (Table 1). The magnitude of these changes in foliar nutrients is comparable with, or greater than, shifts reported in other studies conducted at sites with strong gradients in soil nutrient abundance (19, 21), although it is lower than some of the extreme changes in foliar nutrients seen in sites with higher bird densities or lower rainfall (22). Because plants use soil nutrients to advance myriad physiological processes, particularly growth, it is natural that differences in foliar nutrients between forest types are not as pronounced as differences in soil nutrients.
The elevated δ15 N levels in both soils and plants in PT forests identify bird-derived nutrient subsidies as the most parsimonious explanation for these intersite differences in soil and plant nutrient quality. As high trophic-level marine predators, seabird δ15N values are enriched, compared to most other inputs to the system, because of trophic fractionation of δ15N (23). The δ15N values we observe in soils and plants in PT forests closely match the elevated values we measured directly in bird guano. In contrast, the δ15N values measured in Cocos forests are well below that of bird guano and closer to other potential N sources measured onsite (marine wash) or obtained from the literature (e.g., rain) (24, 25). δ15N is known to increase in sites with more available nitrogen; still, the high δ15N in PT sites is too high to be explained by this mechanism alone (26). There are no other viable, nonguano-related, alternative explanations for this δ15N shift: there are no nitrogen-fixing terrestrial plants on the islets we surveyed at Palmyra (27), lightning is rare (personal observation), wind patterns in the region are not likely to deposit large quantities of transcontinental dust, and typical δ15N values of surrounding seawater are low (25). There were no differences in δ13C in soils. The small but significant difference in foliar δ13C is most likely a result of different levels of canopy closure and light between forest types (24).
The differences we observed in the nutrient content of soils and trees in the two different forest types are reflected in processes of herbivory. Herbivores are known to preferentially select more nutrient-rich food sources (23, 28), and in our palatability trials, generalist herbivores exhibited very strong preferences for plant material derived from PT forests over material derived from the same species in Cocos forests. Likewise, standing herbivory was three to five times higher in PT forests than Cocos forests for multiple species. These observed declines in herbivory are primary indicators that the loss of bird-derived nutrients is having cascading effects on the ecology of these forests.
The cause of varying densities of C. nucifera at Palmyra is unknown, but likely the result of some combination of historical chance (where palms arrived or were planted) and incomplete gradual spread from these sites via competitive exclusion. This is an aspect that warrants further research. Further evidence that C. nucifera dominance drives the observed nutrient differences via changes in bird abundance, rather than responding to low nutrients or to unmeasured intrinsic site differences, was found in comparing nutrient levels on Palmyra’s naturally produced islets to its recent man-made islets. This provides a large-scale natural experiment on the effect of forest change. All nutrients on C. nucifera-dominated islets created in the 1940s are lower than those in C. nucifera-dominated islets of natural origin, suggesting that (i) dredge materials are of low nutrient quality, (ii) little nutrient enrichment has occurred at these sites in the past 70 years, and (iii) nutrient levels on natural islets dominated by C. nucifera may yet fall further, especially those in which C. nucifera establishment was recent. The case of PT forest islets provides a sharp contrast. Man-made PT forest islets are nutrient rich and are indistinguishable from PT-dominated islets of natural origin. This finding suggests that 70 years is ample time for nutrient-poor man-made islets to be converted to nutrient-rich ecosystems when they host PT forests, which facilitate the import of bird-derived subsidies. Given the rates of bird inputs into PT forests, it is likely that this occurs much more quickly.
Coconut palms have rapidly expanded their range in the last several millennia to achieve their current pantropical distribution; for the most part, perceptions of this expansion are neutral or positive (see SI Text). The scale of nutrient depletions and higher order ecosystem changes that we have observed to be underway at Palmyra as a result of the expansion of this species suggest that the implications of the global expansion of the coconut should be more closely monitored and considered.
Broad-scale changes to the resource budgets of ecosystems, such as we observed, are well documented for introduced or range-expanding plants that increase soil nutrients (29, 30). However, this study provides an interesting example of how an expanding plant population may create and thrive in nutrient-poor conditions. We suggest that examples of introduced plants reducing available nutrients in this fashion may be more abundant than have been previously documented (30).
These results also expand our understanding of the range of mechanisms that can interrupt allochthonous subsidy transfers. Numerous cases are available, demonstrating how predators may reduce densities of birds or other animal vectors of nutrients and cause large-scale ecosystem alterations as a result of nutrient loss from these animal vectors (6, 7). We provide here an example of how a plant can play the same role, with equally important consequences for ecosystem function. In bird systems alone, there are multiple examples of plants that deter birds, suggesting that our observations at Palmyra have wide-reaching importance (31, 32). However the implications of these results extend far beyond bird-dominated systems. Given the ubiquitous importance of spatial subsidies across a wide breadth of scales and system types (33, 34), we contend that more attention should be paid to the role plants play in governing transfers of spatial subsidies between ecosystems.
Methods
Study Site.
We studied the effects of Cocos expansion on the ecology of wet tropical forests at Palmyra Atoll (162°05’ W, 5°53’ N; 4,500 mm annual rainfall) in the Northern Line Island Chain in the Central Pacific. Currently administered by the United States Fish and Wildlife Service as a National Wildlife Refuge, Palmyra has no indigenous human population and a limited history of human habitation (World War II military occupation). The atoll is composed of a ring of coral-derived islets, which encircle a centralized saltwater lagoon system. Some islets at Palmyra are not natural, having been created in the 1940s from dredged coral sand and rock. Two forest types dominate islets in Palmyra: forests generally consisting of tall P. grandis trees in the interior and fringing T. argentea along the coast, and forests of C. nucifera. Both forest types can include low densities of six other tree species. For most analyses, we define forest type as either a “PT forest” (>75% basal of P. grandis and T. argentea combined) or a “Cocos forest” (>75% basal area of C. nucifera). Mixed species sites (with <75% basal area of either Cocos or P. grandis and T. argentea) are not included in these analyses. The avifauna on Palmyra is comprised entirely of seabirds and shorebirds. Boobies (Sula spp.) make up the largest proportion of the bird biomass on the atoll, but smaller terns (Sterna fuscata and Gygis alba) and noddies (Anous spp.) are also abundant, as well as multiple less common species. Open areas on Palmyra also provide excellent habitat for seabirds; however, they must be actively maintained or they revert to forest.
Vegetation and Soil Surveys.
To compare forest and soil characteristics to bird density, we conducted detailed sampling of vegetation and soils on 14 islets free from any contemporary human activity. We only sampled islets that were separated by distances ≥ 250 m or by deep seawater channels, considering such islets independent from one another.
Forest type was characterized using 50 × 2 m belt transects (n = 77). In each transect we measured and identified all plants >1 cm diameter at breast height and calculated basal area from the diameter-at-breast height measurements (35). Transects were spaced evenly around the coast of each islet (maximum of 10, minimum of 2 transects per islet; scaled to islet size). When possible, transects were arranged in pairs: one coastal transect 5 m in from the high-tide line parallel to the coast, and one interior transect laid 50 m inland and parallel to the coastal transect. If the islet was ≤ 100 m in width, no interior transect was conducted. Each pair of transects was at least 250 m from the next nearest pair.
On each transect we took six soil samples: one sample at 0 to 5 cm depth and a second one at 10 to 15 cm depth at three evenly spaced positions along the transects. Soils from the same transect at the same depth were homogenized before analysis. We collected samples of mature, nonsenescent leaves in full sun, from three individual trees of T. argentea, P. grandis, and Cocos when they were available on each transect. The leaf samples from the three individuals of each species were homogenized before analysis. We classed each sampled islet as a natural or artificial fill (created from military dredge spoilings) site. These designations were made by overlaying a map of Palmyra generated in 1874 (36) with a current map of the atoll; sites that completely overlaid land masses in the 1874 map were deemed to be “natural” (n = 35; 18 Cocos-dominated, 17 PT-dominated), while sites that fell completely or partially in water in the 1874 map were deemed to be of “artificial fill” origin (n = 28; 16 Cocos-dominated, 12 PT-dominated).
Bird Surveys and Nutrient Inputs.
Bird communities were surveyed at two spatial scales: at the atoll level and at the transect level. For the atoll-level surveys we counted all nesting and roosting birds visible from the coast, within 10 m of the high-tide line, around the perimeter of all islets in the atoll. To examine bird preferences for vegetation, we identified the tree species upon which each bird was observed nesting/roosting, counted all adult plants (>2 m height) that did not have birds, and determined the species and breeding status (i.e., nesting or only roosting) of every bird censused. We complemented atoll-wide coastal surveys with 32 randomly located belt transects (10 m in width) that ran across the interior of each islet, beginning 10 m from the high-tide line. Densities of birds calculated on these interior transects were used to extrapolate overall interior densities of birds across each islet. For bird surveys conducted at the vegetation/soil transect level, we counted all birds nesting or roosting within a 50 × 10 m band directly overlaying each vegetation/soil transect, as well as the number of birds observed flying directly over the transect area in a 3 min period. All bird surveys were conducted over a 2-week period (Aug 2007) during daytime hours (1000–1700). In sum, 8,641 trees were surveyed, including 6,224 C. nucifera, 1,671 T. argentea, 356 P. grandis, and 390 of all other species combined.
Bird abundances increase at night when birds return from foraging. To determine if nighttime preferences differed from diurnal habitat preferences, we conducted 10 additional surveys in which we counted the number and location of birds present in a given area every 4 h for a 24 h period using spotlights. We analyzed relative nighttime bird abundance per tree species for the three tree species that composed more than 5% of basal area across all vegetation surveys (C. nucifera, P. grandis, and T. argentea).
Bird numbers were converted to biomass using established conversion factors from species in this region (37). To account for interspecific variability in tree-canopy size, the number of trees were converted to average canopy coverage by measuring the horizontal length of the crown of 30 individuals of each species and assuming each tree covered a roughly circular area of ground. Thus, the final metric used for analysis of bird density was bird biomass per square meter of projected canopy coverage.
To quantify isotopic values of nonbird-derived marine inputs to terrestrial soil and vegetation, we collected and homogenized organic marine wash (primarily algae), a known contributor to other island nutrient cycles, from randomly located 10 m beach transects (38).
Laboratory Analyses.
Field moist-soil samples were sieved (<2 mm) and extracted in KCl for ammonium and nitrate immediately after collection (39). Phosphates were extracted from soils using resin bags (40). Ammonium, nitrate, and phosphate extractions were analyzed using a discrete analyzer (Westco SmartChem 200). The pH of soils was measured from a 1:1 mixture of soil and water with a pH electrode. The %N, %-organic C content, δ15N, and δ13C of soils were measured using oven-dried, ball-milled samples in an elemental analyzer coupled with a mass spectrometer at the Stanford Stable Isotope Biogeochemistry Laboratory (Thermo Finnegan Delta-Plus IRMS). These samples were each run twice: once unadulterated for δ15 N and %N, and once after repeated acidifications to remove CaCO3 for analyses of δ13C and % organic C. Leaf samples (washed, oven dried, and milled) were Kjeldahl-digested and then analyzed for %N and %P on a continuous flow autoanalyzer (Alpkem Flow Solution IV). Leaf powder from T. argentea (the most widely distributed tree species), the homogenized bird guano, and marine wash powders, were also analyzed for %C, δ15N, and δ13C at the Stanford Stable Isotope Biogeochemistry Laboratory.
Higher-Order Effects: Herbivory Measurements and Foliage Palatability.
We performed field herbivory measurements and laboratory palatability trials to assay the biological significance of any observed differences in nutrient quality of leaves in different forest types. To measure herbivory in the field, we marked 10 undamaged leaves (from same phylotaxis position) or leaflets (for Cocos) on 10 P. grandis, T. argentea, C. nucifera, and Scaevola sericea growing in PT forests and 10 individuals of the same species growing in Cocos forests (total 800 leaves). We collected the individual leaves after 1 month and assessed cumulative percent-area eaten from scanned images of leaves using the program Sigma Scan (41). Herbivory on C. nucifera was very low and dominated by damage from leaf-mining insects. This damage was not readily quantifiable using scanning software, so all C. nucifera leaflets were instead visually scored in blind trials for the presence or absence of herbivory. For analysis, leaves were pooled by tree, with statistics conducted on mean herbivory per tree.
For laboratory palatability trials, we collected P. grandis leaves from trees growing in Cocos and PT forests (each leaf from a different tree). We then presented these leaves as pairs (one from each forest type) in cafeteria-type assays (42) to the generalist herbivore Conocephalus saltator (Orthoptera) (n = 10 replicates), and the omnivorous hermit crab, Coenobita perlatus (n = 14 replicates). Before the experiments, the animals were fed ad libitum with lettuce for 3 h, and then starved for 10 h. We then offered one P. grandis leaf square from each forest type (1 × 1 cm for C. saltator, 8 × 8 cm for C. perlatus) to individual animals. Leaf squares were left with herbivores for 12 (C. perlatus) or 36 h (C. saltator), after which we measured the leaf area consumed from each pair.
Statistical Analyses.
All statistics were computed in JMP 7 (SAS Institute). Datasets were tested for normality using a Shapiro-Wilk test. When necessary, data were Box-Cox transformed to normalize distributions. Comparisons were made using Welch’s t-test or analysis of variance (ANOVA) with subsequent Tukey-Kramer posthoc pairwise comparisons. Data from herbivory trials could not be normalized and were analyzed using a Wilcoxon ranked-sum test. For linear regression analyses, residuals were checked for conformation to normality. When multiple dependent variables (i.e., various metrics of bird abundance, soil quality, or leaf quality) were analyzed against the same independent variable (e.g., forest type), sequential Bonferroni corrections were conducted. All graphs and tables depict untransformed data and all mean values are shown with ± 1 SE.
Most analyses presented compare Cocos vs. PT forests. Such a classification is simplistic, as actual forests span a gradient of C. nucifera abundance and include some intermediate sites that fit neither of these designations (14 of 77 sampled transects were omitted in this type of analyses). Still, this analysis was deemed preferable to regression by basal area of C. nucifera, because intermediate sites often contained a high abundances of species uncommon elsewhere on the atoll, and basal area of C. nucifera may overestimate canopy importance of C. nucifera, because these palms have particularly high basal area-to-canopy cover relationships. However, to document that C. nucifera has a roughly linear effect on bird abundance and nutrient levels, we also analyzed the relationship between dependent variables of interest and differing densities of C. nucifera basal area using linear regressions; such analyses encompassed data from all sampled transects, including intermediate sites. For all variables, the direction and the significance (using α of 0.05) of the effects (not presented) were consistent with other (Welch’s t-test or ANOVA) analyses.
Supplementary Material
Acknowledgments
We thank J. Estes and M. Power for reviewing a previous draft of this paper and providing constructive feedback; J. Collen, F. Micheli, T. Raab, and P. Vitousek for their advice throughout; D. Mucciarone and D. Turner for invaluable analytical assistance; A. Briggs, G. Carroll, P. de Salles, Z. Drozdz, C. Hanson, L. Love-Anderegg, W. Love-Anderegg, J. McCallen, L. Palumbi, T. Robbins, and J. Tam for assistance in the field; and the United States Fish and Wildlife Service and the Nature Conservancy for logistical and scientific support. This work was supported in part by the National Science Foundation, the National Geographic Society (Grant for Research and Exploration), and the Stanford Gabilan Graduate Fellowship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914169107/DCSupplemental.
References
- 1.Polis GA, Anderson WB, Holt RD. Toward an integration of landscape and foodweb ecology: The dynamics of spatially subsidized foodwebs. Annu Rev Ecol Syst. 1997;28:289–316. [Google Scholar]
- 2.Marczak LB, Thompson RM, Richardson JS. Meta-analysis: Trophic level, habitat, and productivity shape the food web effects of resource subsidies. Ecology. 2007;88:140–148. doi: 10.1890/0012-9658(2007)88[140:mtlhap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Duggins DO, Simenstead CA, Estes JA. Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science. 1989;245:170–173. doi: 10.1126/science.245.4914.170. [DOI] [PubMed] [Google Scholar]
- 4.Nakano S, Murakami M. Reciprocal subsidies: Dynamic interdependence between terrestrial and aquatic food webs. Proc Natl Acad Sci USA. 2001;98:166–170. doi: 10.1073/pnas.98.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabo JL, Power ME. River-watershed exchange: effects of riverine subsidies on riparian lizards and their terrestrial prey. Ecology. 2002;83:1860–1869. [Google Scholar]
- 6.Croll DA, Maron JL, Estes JA, Danner EM, Byrd GV. Introduced predators transform subarctic islands from grassland to tundra. Science. 2005;307:1959–1961. doi: 10.1126/science.1108485. [DOI] [PubMed] [Google Scholar]
- 7.Fukami T, et al. Above- and below-ground impacts of introduced predators in bird-dominated island ecosystems. Ecol Lett. 2006;9:1299–1307. doi: 10.1111/j.1461-0248.2006.00983.x. [DOI] [PubMed] [Google Scholar]
- 8.Chapin FS, et al. Consequences of changing biodiversity. Nature. 2000;405:234–242. doi: 10.1038/35012241. [DOI] [PubMed] [Google Scholar]
- 9.Vitousek P, et al. Introduced species: A significant component of human-caused global change. N Z J Ecol. 1997;21:1–16. [Google Scholar]
- 10.Vitousek P, Walker LR. Biological invasion by Myrica faya in Hawai’i: plant demography, nitrogen fixation, ecosystem effects. Ecol Monogr. 1989;59:247–265. [Google Scholar]
- 11.Allison SD, Vitousek P. Rapid nutrient cycling in leaf litter from invasive plants in Hawai’i. Oecologia. 2004;141:612–619. doi: 10.1007/s00442-004-1679-z. [DOI] [PubMed] [Google Scholar]
- 12.Davis MA, Grime JP, Thompson K. Fluctuating resources in plant communities: a general theory of invasability. J Ecol. 2000;88:528–534. [Google Scholar]
- 13.Funk J, Vitousek P. Resource-use efficiency and plant invasion in low-resources systems. Nature. 2007;446:1079–1081. doi: 10.1038/nature05719. [DOI] [PubMed] [Google Scholar]
- 14.Maloney BK. Palaeoecology and the origin of the coconut. GeoJournal. 1993;31:355–362. [Google Scholar]
- 15.Pearson J, Stewart GR. Tansley Review no. 56. The deposition of atmospheric ammonia and its effect on plants. New Phytol. 1993;125:283–305. doi: 10.1111/j.1469-8137.1993.tb03882.x. [DOI] [PubMed] [Google Scholar]
- 16.De Haan C, Blackburn HD. Livestock and the Environment: Finding a Balance. UK: Wrenmedia, Fressingfield; 1997. [Google Scholar]
- 17.Sobey DG, Kenworthy JB. The relationship between Herring Gulls and the vegetation of their breeding colonies. J Ecol. 1979;67:469–496. [Google Scholar]
- 18.Mulder CPH, Keall SN. Burrowing birds and reptiles: impacts on seeds, seedlings and soil in an island forest in New Zealand. Oecologia. 2001;127:350–360. doi: 10.1007/s004420000600. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt S, Dennison WC, Moss GJ, Stewart GR. Nitrogen ecophysiology of Heron Island, a subtropical coral cay of the Great Barrier Reef, Australia. Funct Plant Biol. 2004;31:517–528. doi: 10.1071/FP04024. [DOI] [PubMed] [Google Scholar]
- 20.Onuf CP, Teal JM, Valiela I. Interactions of nutrients, plant growth and herbivory in a mangrove ecosystem. Ecology. 1977;58:514–526. [Google Scholar]
- 21.Ellis JC. Marine birds on land: a review of plant biomass, species richness, and community composition in bird colonies. Plant Ecol. 2005;181:227–241. [Google Scholar]
- 22.Anderson WB, Polis GA. Nutrient fluxes from water to land: birds affect plant nutrient status on Gulf of California Islands. Oecologia. 1999;118:324–332. doi: 10.1007/s004420050733. [DOI] [PubMed] [Google Scholar]
- 23.Mizutani E, Wada E. Nitrogen and carbon isotope ratios in bird rookeries and their ecological implications. J Ecol. 1988;69:340–349. [Google Scholar]
- 24.Buchmann N, Kao WY, Ehleringer J. Influence of stand structure on carbon-13 of vegetation, soils, and canopy air within deciduous and evergreen forests in Utah, United States. Oecologia. 1997;110:109–119. doi: 10.1007/s004420050139. [DOI] [PubMed] [Google Scholar]
- 25.Altabet MA. Nitrogen isotopic evidence for micronutrient control of fractional NO-3 utilization in the equatorial pacific. Limnol Oceanogr. 2001;46:368–380. [Google Scholar]
- 26.Martinelli LA, et al. Nitrogen stable isotopic composition of leaves and soil: Tropical versus temperate forests. Biogeochemistry. 1999;46:45–65. [Google Scholar]
- 27.Peterson BJ, Fry B. Stable isotopes in ecosystem studies. Annu Rev Ecol Syst. 1987;18:293–320. [Google Scholar]
- 28.Coley PD, Barone JA. Herbivory and plant defenses in tropical forests. Annu Rev Ecol Syst. 1996;27:305–335. [Google Scholar]
- 29.Vitousek P, Walker LR, Whiteaker LD, Mueller-Dombois D, Matson PA. Biological invasion by Myrica faya alters ecosystem development in Hawai’i. Science. 1987;238:802–804. doi: 10.1126/science.238.4828.802. [DOI] [PubMed] [Google Scholar]
- 30.Ehrenfeld JG. Effects of exotic plant invasion on soil nutrient cycling processes. Ecosystems (N Y, Print) 2003;6:503–523. [Google Scholar]
- 31.Faere CJ, Gill EL, Carty P, Carty H, Ayrton V. Habitat use by Seychelles sooty terns Sterna fuscata and implications for colony management. Biol Conserv. 1997;81:69–76. [Google Scholar]
- 32.Feenstra KR, Clements DR. Biology and impacts of Pacific island invasive species. 4. Verbesina encelioides, Golden Crownbeard (Magnoliopsida: Asteraceae) Pac Sci. 2008;62:161–176. [Google Scholar]
- 33.Power ME, et al. In: Food Webs in Landscapes. Polis GA, Power ME, Huxel GR, editors. New York: Chapman and Hall; 2002. pp. 217–240. [Google Scholar]
- 34.Pringle RM, Fox-Dobbs K. Coupling of canopy and understory food webs by ground-dwelling predators. Ecol Lett. 2008;11:1328–1337. doi: 10.1111/j.1461-0248.2008.01252.x. [DOI] [PubMed] [Google Scholar]
- 35.Trejo I, Dirzo R. Floristic diversity of Mexican seasonally dry tropical forests. Biodivers Conserv. 2002;11:2063–2084. [Google Scholar]
- 36.Collen JD, Garton DW, Gardner JP. Shoreline changes and sediment redistribution at Palmyra Atoll (equatorial Pacific Ocean): 1874 – present. J Coast Res. 2009;25:711–722. [Google Scholar]
- 37.Spear LB, Ainley DG, Walker WA. Foraging dynamics of seabirds in the eastern tropical Pacific Ocean. J Avian Biol. 2007;35:1–99. [Google Scholar]
- 38.Catenazzi A, Donnelly MA. The Ulva connection: marine algae subsidize terrestrial predators in coastal Peru. Oikos. 2007;116:75–86. [Google Scholar]
- 39.Keeney DR, Nelson DW. In: Agronomy, A Series of Monographs. Page AL, editor. Madison, WI: Soil Science Society of America; 1987. [Google Scholar]
- 40.Kuo S. In: Methods of Soil Analysis: Part 3, Chemical Methods. Sparks DL, editor. Madison, WI: Soil Science Society of America and American Society of Agronomy; 1996. pp. 898–899. [Google Scholar]
- 41.Coley PD. Effects of plant growth rate and leaf lifetime on the amount and type of anti-herbivore defense. Oecologia. 1988;74:531–536. doi: 10.1007/BF00380050. [DOI] [PubMed] [Google Scholar]
- 42.Dirzo R. Experimental studies on slug-plant interactions I. The acceptability of thirty plant species to the slug Agriolimax caruanae . J Ecol. 1980;68:981–988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.