Abstract
As the result of genetic alterations and tumor hypoxia, many cancer cells avidly take up glucose and generate lactate through lactate dehydrogenase A (LDHA), which is encoded by a target gene of c-Myc and hypoxia-inducible factor (HIF-1). Previous studies with reduction of LDHA expression indicate that LDHA is involved in tumor initiation, but its role in tumor maintenance and progression has not been established. Furthermore, how reduction of LDHA expression by interference or antisense RNA inhibits tumorigenesis is not well understood. Here, we report that reduction of LDHA by siRNA or its inhibition by a small-molecule inhibitor (FX11 [3-dihydroxy-6-methyl-7-(phenylmethyl)-4-propylnaphthalene-1-carboxylic acid]) reduced ATP levels and induced significant oxidative stress and cell death that could be partially reversed by the antioxidant N-acetylcysteine. Furthermore, we document that FX11 inhibited the progression of sizable human lymphoma and pancreatic cancer xenografts. When used in combination with the NAD+ synthesis inhibitor FK866, FX11 induced lymphoma regression. Hence, inhibition of LDHA with FX11 is an achievable and tolerable treatment for LDHA-dependent tumors. Our studies document a therapeutical approach to the Warburg effect and demonstrate that oxidative stress and metabolic phenotyping of cancers are critical aspects of cancer biology to consider for the therapeutical targeting of cancer energy metabolism.
Keywords: glycolysis, lymphoma, pancreatic cancer, redox stress, xenograft models
The propensity of cancer cells to take up glucose avidly and convert it to lactate, even under experimental conditions with adequate oxygen, has been called the Warburg effect or aerobic glycolysis (1, 2). The Warburg effect has been directly linked to the activation of oncogenes, such as MYC, Ras, and Akt, and loss of tumor suppressor genes, which result in the deregulated conversion of glucose to lactate (3–9). As a result of the pervasive presence of hypoxia in tumors, hypoxia-inducible factor (HIF-1) is commonly increased. HIF-1 levels can also be increased downstream of signal transduction pathways and by loss of the von Hippel-Lindau (VHL) tumor suppressor (10). HIF-1 is a critical transcription factor for hypoxic adaptation with its activation of glycolytic enzyme genes, including lactate dehydrogenase A (LDHA), which converts pyruvate to lactate coupled with the recycling of NAD+ (11).
Lactate dehydrogenase is a tetrameric enzyme comprising two major subunits A and/or B, resulting in five isozymes (A4, A3B1, A2B2, A1B3, and B4) that can catalyze the forward and backward conversion of pyruvate to lactate. LDHA (LDH-5, M-LDH, or A4), which is the predominant form in skeletal muscle, kinetically favors the conversion of pyruvate to lactate. LDHB (LDH-1, H-LDH, or B4), which is found in heart muscle, converts lactate to pyruvate that is further oxidized. It has long been known that many human cancers have higher LDHA levels than normal tissues (12), but the link between oncogenes and glycolysis was poorly understood. LDHA was identified as a direct target gene of the c-Myc oncogenic transcription factor (13, 14). Because HIF also activates LDHA (11, 15), we reasoned that reduction of LDHA expression would inhibit cellular transformation and in vivo tumorigenesis, because tumor tissues are hypoxic and normal tissues are neither profoundly hypoxic nor have activated MYC (14). Studies now document that reduction of LDHA reduced cellular transformation and markedly delayed tumor formation, indicating that LDHA is important for tumor initiation (16, 17). These studies provide proof-of-concept that LDHA is a tractable therapeutical target but do not establish whether LDHA is required for tumor progression. Furthermore, the mechanisms by which LDHA inhibition leads to inhibition of tumorigenesis are poorly understood.
Here, we report that reduction of LDHA causes bioenergetic and oxidative stress leading to cell death. Furthermore, using a drug-like small molecule (FX11 [3-dihydroxy-6-methyl-7-(phenylmethyl)-4-propylnaphthalene-1-carboxylic acid]), we document that LDHA is required not only for tumor initiation but for tumor maintenance and progression. When combined with another metabolic inhibitor, FK866 (APO866), that inhibits NAD+ synthesis through direct inhibition of nicotinamide phosphoribosyltransferase (NAMPT) (18, 19), FX11 is able to induce lymphoma regression. These studies indicate that targeting cancer metabolism through small drug-like molecules is achievable, and hence paves the way for the development of unique classes of anticancer drugs.
Results and Discussion
Reduction of LDHA Induces Oxidative Stress and Cell Death.
We sought to understand the mechanisms of cell death following LDHA reduction by short interfering RNA (siLDHA), which has been shown to inhibit tumorigenesis. First, we determined the effect of reduced LDHA expression on oxygen consumption by human Panc (P) 493 B-lymphoid cells, because reduction of LDHA would favor the entry of pyruvate into mitochondria for oxidative phosphorylation, thereby enhancing oxygen consumption. Reduction of LDHA expression by siRNA in P493 cells resulted in an increase in oxygen consumption (Fig. 1 A and B). Oxygen consumption was similarly increased in a human pancreatic cancer line treated with siLDHA (Fig. S1 A and B).
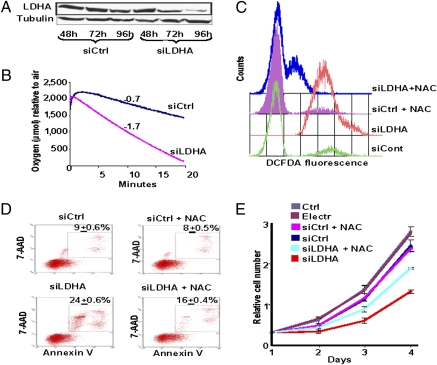
Fig. 1.
Reduction of LDHA expression by siRNA leads to increased oxygen consumption and oxidative stress-induced cell death of P493 human lymphoma B cells. siRNAs targeting human LDHA (SMARTpool) were transfected via electroporation to knock down the LDHA expression transiently. (A) Immunoblotting was performed on whole-cell lysates, probed with rabbit monoclonal anti-LDHA, and reprobed with anti-α-tubulin as a loading control. (B) Oxygen consumption of P493 cells was determined by the use of a Clark-type oxygen electrode at 72 h posttransfection with siLDHA (slope = −1.7) or siControl (slope = −0.7). (C) Intracellular ROS production was detected with DCFDA fluorescence and monitored by flow cytometry at 72 h posttransfection with siLDHA or siControl in the presence or absence of NAC. (D) Cell death was determined by flow cytometry of annexin V- and 7-AAD-stained cells at 96 h posttransfection with siLDHA or siControl in the presence or absence of NAC (20 mM added 24 h after transfection). The number in each figure represents the average percentage (±SEM) of dead cells. The number of dead cells treated with siLDHA compared with the control group has a P value of 0.0002 using the Student’s t test; those treated with siLDHA and NAC compared with the siLDHA group have a P value of 0.001. (E) Cell population growth of siControl cells compared with cells treated with siLDHA grown in the presence or absence 5 mM NAC added daily starting 24 h after transfection. Relative cell numbers at day 4 for siControl vs. siLDHA and siLDHA vs. siLDHA + NAC have a P value of 0.008 and 0.004, respectively. For siControl vs. siControl + NAC, the P value is 0.63.
Enhanced oxygen consumption through reduction of glycolysis by siLDHA was expected to increase the production of mitochondrial reactive oxygen species (ROS), particularly because glycolysis, which diverts pyruvate to lactate, diminishes cellular oxidative stress (20). We therefore determined the production of ROS by 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (DCFDA) fluorescence as measured by flow cytometry (Fig. 1C). We found that treatment of cells with siLDHA induced significant ROS. Hence, we exposed cells to N-acetylcysteine (NAC), a well-known antioxidant, and observed a significant reduction in ROS from siLDHA-treated cells (Fig. 1C).
We then sought to determine whether cell death associated with increased ROS and reduced LDHA expression could be diminished by the antioxidant NAC. We found that reduction of LDHA expression with siRNA markedly increased necrosis or late cell death, which is characterized by enhanced labeling of both 7-amino-actinomycin (7-AAD) and annexin V (Fig. 1D). Treatment with NAC at 24 h after transfection partially reduced cell death, which was also accompanied by partial rescue of cell proliferation (Fig. 1E). We noted that daily addition of 5 mM NAC resulted in a better rescue of cell proliferation than 20 mM NAC added once after transfection, reflecting the short lifetime of active NAC (Fig. 1E). The daily addition of 10 or 20 mM NAC, however, was toxic to the siRNA-transfected and control cells.
Having observed that reduction of LDHA by siRNA could induce oxidative stress and cell death, we sought a small-molecule inhibitor of LDHA as a drug-like tool to study tumor metabolism. We evaluated a series of compounds generated by Vander Jagt and co-workers (21, 22), who were specifically interested in targeting malarial LDH (pLDH), and found among the analogues of gossypol, which itself is a toxic inhibitor of LDHA, two dihydroxynapthoates: 11f (FX11; Pubchem ID: 10498042) and 11e [E; 2,3-dihydroxy-6-methyl-7-(methyl)-4-propylnaphthalene-1-carboxylic acid; Pubchem ID: 10265351]. We selected FX11 as a candidate small molecule for inhibiting human LDHA because it preferentially inhibited LDHA as opposed to LDHB or pLDH (21, 22). Compound E was selected for comparison because it had much lower inhibitory activity than FX11.
We recharacterized FX11 and E using purified human liver LDHA and found Kis of 8 and >90 μM, respectively (Fig. S2 A and B). FX11 is a competitive inhibitor of LDHA with respect to NADH in the conversion of pyruvate to lactate by LDHA, whereby NADH is converted to NAD+. To document the selective binding of FX11 vs. E to LDHA further, we performed affinity chromatography with P493 cell lysate using FX11 or E immobilized on sepharose beads. Equal amounts of cell lysate were loaded onto FX11 or E affinity beads and extensively washed, and the bound LDHA was eluted with 1 mM NADH. The FX11 affinity beads yielded 4-fold more LDHA activity than the beads with immobilized E (Fig. S2C). Collectively, these results indicate that FX11 can bind and inhibit human LDHA enzyme activity. Because GAPDH is another pivotal glycolytic enzyme that converts NAD+ to NADH, we also sought to determine whether FX11 can inhibit its NAD+-dependent conversion of glyceraldehyde-3-phosphate to bis-phosphoglycerate. We found that even at 74 μM FX11, GAPDH activity was not inhibited. Through formal Michaelis–Menten kinetics, the estimated Ki is >>300 μM for GAPDH, indicating that FX11 was selective for LDHA among glycolytic enzymes that use the cofactor NAD.
As observed with siRNA-mediated reduction of LDHA, inhibition of LDHA by FX11 also resulted in increased oxygen consumption, ROS production, and cell death (Fig. 2 A–C). We found that the NAMPT inhibitor of NAD+ synthesis, FK866, also increased ROS production (Fig. 2B). Because the increase in ROS levels might result from lowered NADPH production attributable to the possible inhibition of glucose transport (and hence diminished hexose monophosphate shunt activity, which produces NADPH) by an indirect effect of FX11 and FK866, we measured and found that glucose uptake, as measured by NBD-glucose [2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose], was not significantly diminished and that NADPH levels were virtually unaltered by treatment with FX11 or FK866 (Fig. S3). NAC could partially rescue the diminished proliferation of P493 cells treated with either FX11 or FK866 (Fig. 2 D and E), indicating that oxidative stress contributed partially to the inhibition of cell proliferation. Given the significant effect of FX11 on the proliferation of P493 cells, which are dependent on Myc, we ruled out the trivial possibility that FX11 could inhibit Myc expression itself (Fig. S1C). In aggregate, these studies document that reduction of LDHA levels or activity triggers oxidative stress and cell death.
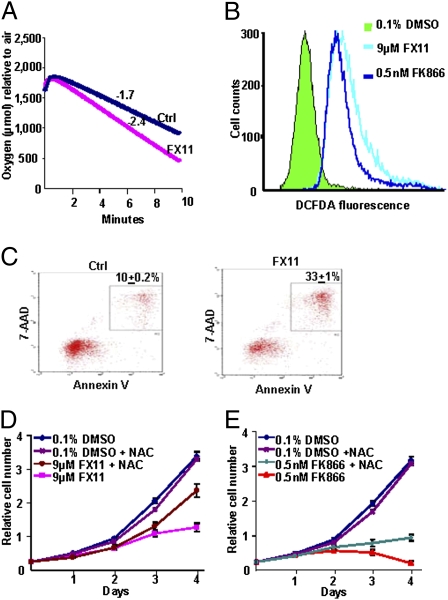
Fig. 2.
Inhibition of LDHA by FX11 resulted in increased oxygen consumption, ROS production, and cell death. (A) Oxygen consumption of P493 cells was determined by a Clark-type oxygen electrode in the presence (slope = −2.4) and absence (slope = −1.7) of FX11. Data are representative of duplicate experiments. (B) ROS levels were determined by DCFDA fluorescence in P493 cells treated with FX11 or FK866. Data are representative of triplicate samples of two separate experiments. (C) Cell death was determined by flow cytometry of Annexin V- and 7-AAD-stained cells after 24 h of FX11 treatment as compared with control. The number in each figure represents the average percentage of dead cells. The FX11-treated cells compared with the control group have a P value of 8.92e-06. (D and E) Cell population growth of control cells compared with cells treated with FX11 or FK866 in the presence or absence of 20 mM NAC. All cells were grown at 1 × 105 cells/mL. Cell counts were performed in triplicate and shown as mean ± SD, and the entire experiment was replicated, with similar results.
FX11 Inhibits Glycolysis and Alters Cellular Energy Metabolism.
In addition to oxidative stress induced by inhibition of LDHA, we sought to determine how FX11 affects cellular bioenergetics. First, we observed that both FX11 and FK866 decreased mitochondrial membrane potential and that the combination accentuated the abnormality (Fig. 3A). In this regard, the combination of FX11 and FK866 was more toxic to P493 cells than either one alone, causing a more profound inhibition of cell proliferation (Fig. 3B). A dose–response study using a combination of FX11 and FK866 doses revealed a combination index (CI-50) of 0.78, suggesting a slightly synergistic effect of the combination on cell proliferation (Fig. S4A). After 20 h of exposure to FX11 or FK866, a decrease in ATP levels was accompanied by activation of AMP kinase and phosphorylation of its substrate acetyl-CoA carboxylase (Fig. 3 C and D and Fig. S1D), suggesting that, in addition to induction of oxidative stress, these agents depleted cellular energy levels. Decreased ATP levels, despite an increase in cellular oxygen consumption, suggests that FX11 treatment increased nonproductive mitochondrial respiration as reported with shRNA-mediated knock-down of LDHA (16). Because inhibition of LDHA decreases NAD+ recycling, treatment of P493 cells with FX11 was associated with increases in the NADH/NAD+ ratio (Fig. 3E) and cellular autofluorescence, reflective of elevated cellular NADH levels (Fig. S4 B and C). In contrast to FK866, FX11 significantly diminished but did not completely inhibit cellular production of lactate (Fig. 3F). These observations suggest that FX11 targets LDHA, inhibits glycolysis, and shunts pyruvate into the mitochondrion.
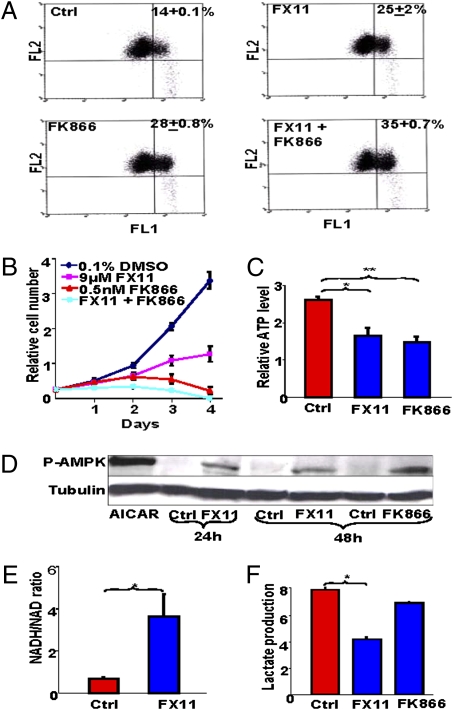
Fig. 3.
(A) FK866 enhances FX11-induced loss of mitochondrial membrane potential. P493 cells treated with control vehicle, FK866, FX11, or both inhibitors were stained with JC-1 and subjected to flow cytometric analysis, with FL2 representing red fluorescence intensity and FL1 representing green fluorescence intensity, which is reflective of cells with decreased mitochondrial membrane. The average percentage (±SEM) of cells with decreased membrane potential is indicated in each panel. The P values were 0.03, 0.002, and 0.0008 when comparing FX11-treated cells, FK866-treated cells, or cells treated with both, respectively, with the control group. (B) Effect of FK866 and FX11 on P493-6 cell proliferation. Live cells were counted using trypan blue dye exclusion. Data are shown as the mean ± SD of triplicate samples. (C) Effect of FX11 or FK866 on ATP levels. P493 cells were treated with 9 μM FX11 or 0.5 nM FK866 for 20 h and counted. ATP levels (mean ± SEM, n = 5 experiments) were determined by luciferin–luciferase-based assay on aliquots containing an equal number of live cells. *P = 0.008; **P = 0.003. (D) Immunoblot of phosphor-AMP kinase (PAMPK) in lysates of cell treated with FX11 or FK866. Tubulin serves as a loading control. AICAR, an AMP analogue that activates AMPK, was used to treat the cells as a positive control. (E) FX11 increases the NADH/NAD+ ratio. NADH/NAD+ ratio in P493 cells treated with 9 μM FX11 for 24 h as compared with vehicle control. *P = 0.028. (F) FX11 inhibits lactate production. Lactate levels in the media of P493 human B cells treated with 9 μM FX11 or 0.5 nM FK866 for 24 h as compared with control. Control RPMI contained 10.7 mmol/L glucose and no detectable lactate. *P = 6.9E-06.
FX11 Inhibits Cells that Are Dependent on Glycolysis.
It stands to reason that if FX11 targets LDHA, cells that depend on LDHA for glycolysis would be more susceptible to FX11 inhibition than those that primarily use oxidative phosphorylation. In this regard, we sought to determine whether metabolic phenotypes could affect the sensitivity of cancer cells to FX11. We used the human RCC4 renal cell carcinoma cell line and the RCC4 cell line reconstituted with VHL (RCC4-VHL). Loss of VHL in RCC4 rendered these cells constitutively glycolytic because of the stabilization and expression of HIF-1 and HIF-2. Reconstitution with VHL resulted in degradation of HIF-1α and HIF-2α and increased mitochondrial biogenesis and oxygen consumption (23). Given the metabolic differences in these isogenic cell lines, we expect a more significant influence of FX11 on the RCC4 cells as compared with the RCC4-VHL cells. Indeed, a dose–response study revealed that RCC4 is more sensitive to FX11 compared with RCC4-VHL (Fig. S5 A and B).
To corroborate these findings further, we studied the glycolytic MCF-7 and the oxidative MDA-MB-453 breast carcinoma cell lines (24). We confirmed that MCF-7 was more dependent on glucose, whereas MDA-MB-453 was more dependent on glutamine oxidation (Fig. S6 A and B), such that deprivation of glucose has a more profound growth inhibitory effect on MCF-7. A dose-dependent study further revealed that MCF-7 is more sensitive to FX11 (Fig. S5 C and D). Although there are many other differences between these cell lines, the correlation of FX11 sensitivity and glucose dependency of MCF-7 supports the notion that glycolysis predisposes cancer cells to growth inhibition by FX11.
We further tested whether the inhibition of human P493 B cells by FX11 depended on glucose or on LDHA. The growth of P493 was inhibited by about 60% when depleted of glucose as compared with growth in normal medium (Fig. S5E). Addition of FX11 could not inhibit P493 cells further in the absence of glucose, suggesting that the effect of FX11 on cell proliferation was glucose-dependent. Furthermore, knock-down of LDHA expression by two sequential electroporations with siRNA caused a markedly diminished proliferative rate that was not further slowed by FX11 (Fig. S5F). Although we noted that siControl cells had a modestly diminished proliferation rate as compared with untreated cells, the siLDHA treatment remarkably disabled cell proliferation contemporaneous with reduced LDHA expression (Fig. S6 C and D). These observations collectively indicate that the growth inhibitory effect of FX11 is consistent with its ability to inhibit LDHA.
We surmised that the human P493 B-lymphoma cells would be sensitive to FX11 because they express LDHA (25) but that the sensitivity would be heightened under hypoxia when glycolysis is favored. This is particularly important, because P493 cells depend on both glucose and glutamine metabolism when cultured at 20% (vol/vol) O2 (26). When P493 cells were subjected to a dose–response study with FX11, we found that growth inhibition by 9 μM FX11 was increased when the cells were cultured at 1% O2 (Fig. S7 A and B). Hypoxia also sensitized the human P198 pancreatic cancer cell line to inhibition by FX11 (Fig. S7 C and D), suggesting that reliance on LDHA for hypoxic metabolism caused cancer cells to be susceptible to the growth-inhibitory effects of LDHA inhibition by FX11.
FX11 Inhibits Tumorigenesis in Vivo.
Although the renewed interest in the Warburg effect is accompanied by a greater understanding of its molecular underpinnings, targeting it for therapeutical purposes remains a major challenge (27). By characterizing a small-molecule inhibitor of LDHA, we found that it is effective in inhibiting cellular growth and triggering cell death by both inducing ROS production and depleting ATP. We observed that hypoxia further sensitized human P493 lymphoma cells to LDHA inhibition by FX11. In this regard, the pervasive hypoxic tumor microenvironment, as compared with normal tissues, ought to force a further dependency of these lymphoma cells on glycolysis, and particularly on LDHA (Fig. 4A and Fig. S8A). Of note, primary human lymphomas have been documented to have elevated LDHA expression particularly in hypoxic regions (28). Hence, we sought to determine whether in vivo efficacy could be demonstrated with FX11 as an inhibitor of LDHA. We calculated a desired FX11 dose of 42 μg for daily i.p. injection. We expected an initial serum level of ∼100 μM, assuming a uniform and immediate distribution in the vascular system without accounting for the drug half-life or drug metabolism. It should be noted that solubility was a significant dose-limiting factor, because we could only double the dose further before reaching the limited solubility of FX11 in aqueous solution.
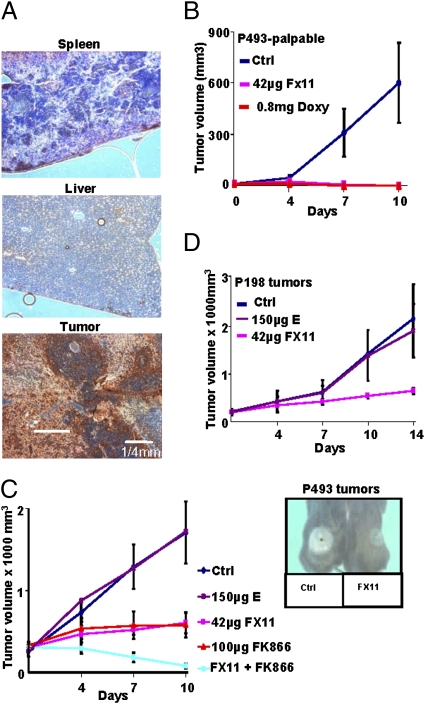
Fig. 4.
In vivo efficacy of FX11 as an antitumor agent. (A) Immunohistochemical staining to detect hypoxic regions (dark brown) of spleen, liver, and P493 lymphoma by pimonidazole labeling. (B) Effect of FX11 on growth of palpable human P493 B-cell xenografts. Control animals were treated with daily i.p. injection of vehicle (2% (vol/vol) DMSO), and doxycycline (0.8 mg/day) was used as a positive control because it inhibits Myc expression and tumorigenesis in P493 cells. (C) Effect of FX11 and/or FK866 daily treatment as compared with control or compound E (a weak LDHA inhibitor) on established human lymphoma xenografts. (Inset) Photographs of representative animals treated with control vehicle or FX11. (D) FX11 inhibited P198 human pancreatic cancer xenografts as compared with compound E. For experiments in all panels, 2.0 × 107 P493 cells or 5 × 106 P198 cells were injected s.c. into SCID mice or athymic nude mice, respectively. When the tumor volume reached 200 mm3, 42 μg of FX11 and/or 100 μg of FK866 was injected i.p. daily and observed for 10–14 days. The tumor volumes were measured using digital calipers every 4 days and calculated using the following formula: [length (mm) × width (mm) × width (mm) × 0.52]. The results represent the average ± SEM.
First, we sought to determine whether FX11 could inhibit P493 tumor initiation after a palpable tumor developed. As controls, we injected animals with vehicle (2% (vol/vol) DMSO) or with 0.8 mg of doxycycline to inhibit MYC expression in these transformed human B cells. As expected, doxycycline profoundly inhibited palpable tumor xenograft growth as compared with vehicle-injected control animals (Fig. 4B). Intriguingly, daily i.p. injection of 42 μg of FX11 also resulted in a remarkable inhibition of tumor growth. It is notable that we have ruled out the trivial possibility that FX11 might directly inhibit Myc expression to mediate this profound effect (Fig. S1C). These observations indicate that LDHA is necessary for tumor initiation.
To test whether LDHA is required for tumor maintenance or progression, we challenged the ability of FX11 to inhibit tumor xenograft growth by treating P493 lymphomas or human P198 pancreatic tumors that reached the size of 200 mm3 before treatment commenced. For comparison, we treated animals with vehicle control or a compound related to FX11, called E, that lacks the benzyl group and has a Ki for LDHA of >90 μM, or more than 10-fold higher than that of FX11. We found that E had no antitumor activity as compared with vehicle. At this solubility-limiting dose, FX11 displayed a static but significant effect over 10 days (Fig. 4C). Intriguingly, we observed that the hypoxic regions associated with untreated control tumors were relatively diminished in the FX11-treated tumors (Fig. S8B). We speculated that hypoxic tumor cells are more dependent on glycolysis, and hence are diminished by FX11 relative to the nonhypoxic tumor cells. We also observed a significant response of human P198 tumor xenografts to FX11 as compared with E (Fig. 4D). Although a more aggressive human pancreatic tumor xenograft LZ10.7 grew significantly faster than P198, it was also sensitive to FX11 as a single agent (29) (Fig. S8C). The structure and activity relation of FX11 and E in vivo correlated with the inhibition and binding of LDHA by these compounds in vitro, further supporting the notion that FX11 targets LDHA. These results collectively indicate that LDHA plays a role in tumor progression and maintenance.
On the basis of our studies of cultured cells (Fig. 3B), we hypothesized that FX11 might accentuate the effect of FK866 in the treatment of the P493 human lymphomas. Hence, we selected a dose of 100 μg of FK866, which gave a static outcome, and, indeed, we found remarkable tumor regression when animals were treated with both FX11 and FK866 (Fig. 4C). These findings underscore the fact that targeting cancer metabolism is feasible and that LDHA is a significant candidate target for further development.
Given the significant effects of FX11 as an LDHA inhibitor in vivo, we studied a group of treated animals to begin to understand the potential side effects of FX11. It is notable that humans lacking LDHA develop normally but have been shown to display exertional myopathy (30). In this regard, although we did not formally exercise the animals to examine exertional tolerance, we did not note lethargy or the inability to eat and drink. In fact, animals treated with FX11 did not lose weight. In initial studies of the hematology and blood chemistry, we did not see cytopenia in animals treated with FX11 alone; however, two (of five studied) animals treated with FK866 did show mild thrombocytopenia (Fig. S9). The average leukocyte count in the control group was skewed upward by two animals that had leukocytosis with >15 K/μL (normal range: 1.8–to 10.7 K/μL). The blood chemistries did not reveal any evidence of kidney [blood urea nitrogen (BUN) or creatinine] or liver (aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase) toxicity in animals treated with FX11 or FK866 alone at doses that affected tumor growth in vivo (Fig. S9). However, the combination of FX11 and FK866, as compared with control, increased BUN.
Conclusions
Targeting cancer energy metabolism has been partly elusive because of our poor understanding of metabolic phenotypes of different cancers, compounded by the lack of understanding of cellular responses to inhibition of specific enzymes involved in energy metabolism. Here, we document that LDHA inhibition, which was previously shown through genetically engineered cells to reduce tumor initiation (16, 17), not only resulted in decreased ATP levels and reduced mitochondrial membrane potential but in a remarkable increase in oxidative stress that is linked to cell death. Furthermore, inhibition of LDHA by FX11 inhibited tumor xenograft progression, confirming that LDHA is required for tumor maintenance.
We found that similar to siRNA reduction of LDHA expression, FX11 could increase cellular oxygen consumption, increase ROS production, and induce cell death that could be partially rescued by the antioxidant NAC. Seemingly paradoxical to the antiapoptotic effect of NAC in vitro observed in the current work, we have documented previously that NAC has an antitumorigenic effect in vivo because of its inhibition of HIF-1 in tumors (31). The illusion of a conundrum with NAC treatment arises only when results of experiments performed in vitro under normoxic conditions are compared with the results of NAC effects on in vivo tumorigenesis when HIF-1 is induced within the hypoxic tumor microenvironment. Our current observations are corroborated by studies in Saccharomyces cerevisiae with enhanced or defective respiration (32). Genetic knock-down or glucose repression of respiration in yeast reduced apoptosis and enhanced clonogenic survival, whereas forced enhancement of respiration increased ROS production and reduced colony growth that could be partially rescued by the antioxidant glutathione. In this regard, a recent perspective on cancer energy metabolism emphasizes the importance of redox homeostasis in cancer cell survival (6). FX11 also reduced ATP levels, suggesting that inhibition of LDHA caused bioenergetic and oxidative stress, which, together, inhibits tumor xenograft maintenance and progression.
Because FX11 has a catechol moiety, it could hypothetically be converted in vivo to a dihydroquinone that is reactive and could cause effects other than inhibition of LDHA. Although the reactive dihydroquinone could also be produced from compound E, it had no detectable antitumor activity in vivo. Hence, it is unlikely that conversion of FX11 to a dihydroquinone could account for its antitumor activity. We were unable, however, to rule out whether other off-target effects of FX11 might contribute its biological activities in addition to the inhibition of LDHA. Notwithstanding this caveat, we found that tumor growth in both human B-lymphoma and pancreatic cancer xenograft models was effectively inhibited by FX11. The effectiveness of inhibiting LDHA in vivo may be further enhanced by the diminished production of lactate, which was documented recently by Sonveaux et al. (33) to be an energy substrate for aerobic cancer cells in an established tumor. At a very large tumor size (200 mm3 in SCID mice, which is equivalent to about a 1-kg tumor in an adult human), we found that FX11, together with FK866, which is an inhibitor of NAD+ synthesis, could induce tumor regression in a human lymphoma xenograft model. Collectively, our studies demonstrate that LDHA is required for tumor progression and that targeting cancer metabolism through small drug-like molecules is achievable to control tumor growth.
Experimental Procedures
Detailed materials and methods are available online in SI Experimental Procedures. Briefly, oxygen consumption was measured using a Clark-type oxygen electrode (Oxytherm System; Hansatech Instruments Ltd). The measurement of intracellular ROS production was measured by staining cells with carboxy-H2DCFDA (Molecular Probes) according to the manufacturer’s instructions. An annexin V–7-AAD apoptosis detection Kit I (BD Biosciences Pharmingen) was used according to the manufacturer’s instructions. The lipophilic cation dye [JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide; Invitrogen] was used to detect the loss of the mitochondrial membrane potential. ATP levels were determined by luciferin–luciferase-based assay (Promega). Lactate production was measured by the ABL700 Radiometer analyzer (Radiometer America, Inc.) according to the manufacturer’s instructions. The animal studies were performed according to the protocols approved by the Animal Care and Use Committee at The Johns Hopkins University.
Supplementary Material
Acknowledgments
We thank Dr. M. Mizuma for his help with the pancreatic cancer xenograft models; Dr. S. Sukumar for her gift of breast cancer cell lines, Dr. J. Kahn for his help with biostatistics; and L. Blosser and A. Tam for their expertise in flow cytometry. We thank Drs. P. Cole, L. Gardner, J. Isaacs, and M. Vuica-Ross for their comments. This work was funded by a Leukemia and Leukemia Lymphoma Foundation Translational Research grant and was partially supported by National Institutes of Health Grants R01CA113669, P01CA134292, R01CA051497, and R01CA57341.
Footnotes
The authors declare no conflict of interest. C.V.D. is member of the Scientific Advisory Board of Agios Pharmaceuticals; there is no sponsored research or technology licensing activities involving the company. An invention relating to this work was reported to the Johns Hopkins Technology Transfer office.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914433107/DCSupplemental.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 3.Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: Metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Kroemer G, Pouyssegur J. Tumor cell metabolism: Cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinhouse S. Glycolysis, respiration, and anomalous gene expression in experimental hepatomas: G.H.A. Clowes memorial lecture. Cancer Res. 1972;32:2007–2016. [PubMed] [Google Scholar]
- 8.Frezza C, Gottlieb E. Mitochondria in cancer: Not just innocent bystanders. Semin Cancer Biol. 2009;19:4–11. doi: 10.1016/j.semcancer.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- 10.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 12.Goldman RD, Kaplan NO, Hall TC. Lactic dehydrogenase in human neoplastic tissues. Cancer Res. 1964;24:389–399. [PubMed] [Google Scholar]
- 13.Lewis BC, et al. Identification of putative c-Myc-responsive genes: Charac-terization of rcl, a novel growth-related gene. Mol Cell Biol. 1997;17:4967–4978. doi: 10.1128/mcb.17.9.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shim H, et al. c-Myc transactivation of LDH-A: Implications for tumor metabolism and growth. Proc Natl Acad Sci USA. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Firth JD, Ebert BL, Ratcliffe PJ. Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements. J Biol Chem. 1995;270:21021–21027. doi: 10.1074/jbc.270.36.21021. [DOI] [PubMed] [Google Scholar]
- 16.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link be-tween glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Xie H, et al. LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol Cancer Ther. 2009;8:626–635. doi: 10.1158/1535-7163.MCT-08-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–7442. [PubMed] [Google Scholar]
- 19.Nahimana A, et al. The NAD biosynthesis inhibitor APO866 has potent antitumor activity against hematologic malignancies. Blood. 2009;113:3276–3286. doi: 10.1182/blood-2008-08-173369. [DOI] [PubMed] [Google Scholar]
- 20.Brand KA, Hermfisse U. Aerobic glycolysis by proliferating cells: A protective strategy against reactive oxygen species. FASEB J. 1997;11:388–395. doi: 10.1096/fasebj.11.5.9141507. [DOI] [PubMed] [Google Scholar]
- 21.Deck LM, et al. Selective inhibitors of human lactate dehydrogenases and lactate dehydrogenase from the malarial parasite Plasmodium falciparum. J Med Chem. 1998;41:3879–3887. doi: 10.1021/jm980334n. [DOI] [PubMed] [Google Scholar]
- 22.Yu Y, et al. Selective active site inhibitors of human lactate dehydrogenases A4, B4, and C4. Biochem Pharmacol. 2001;62:81–89. doi: 10.1016/s0006-2952(01)00636-0. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Mazurek S, Michel A, Eigenbrodt E. Effect of extracellular AMP on cell proliferation and metabolism of breast cancer cell lines with high and low glycolytic rates. J Biol Chem. 1997;272:4941–4952. doi: 10.1074/jbc.272.8.4941. [DOI] [PubMed] [Google Scholar]
- 25.Kim JW, et al. Evaluation of myc E-box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Mol Cell Biol. 2004;24:5923–5936. doi: 10.1128/MCB.24.13.5923-5936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao P, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garber K. Energy deregulation: Licensing tumors to grow. Science. 2006;312:1158–1159. doi: 10.1126/science.312.5777.1158. [DOI] [PubMed] [Google Scholar]
- 28.Giatromanolaki A, et al. Lactate dehydrogenase 5 expression in non-Hodgkin B-cell lymphomas is associated with hypoxia regulated proteins. Leuk Lymphoma. 2008;49:2181–2186. doi: 10.1080/10428190802450629. [DOI] [PubMed] [Google Scholar]
- 29.Feldmann G, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: A new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanno T, et al. Lactate dehydrogenase M-subunit deficiency: A new type of hereditary exertional myopathy. Clin Chim Acta. 1988;173:89–98. doi: 10.1016/0009-8981(88)90359-2. [DOI] [PubMed] [Google Scholar]
- 31.Gao P, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12:230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruckenstuhl C, et al. The Warburg effect suppresses oxidative stress induced apoptosis in a yeast model for cancer. PLoS One. 2009;4:e4592. doi: 10.1371/journal.pone.0004592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonveaux P, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.