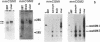Abstract
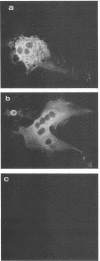
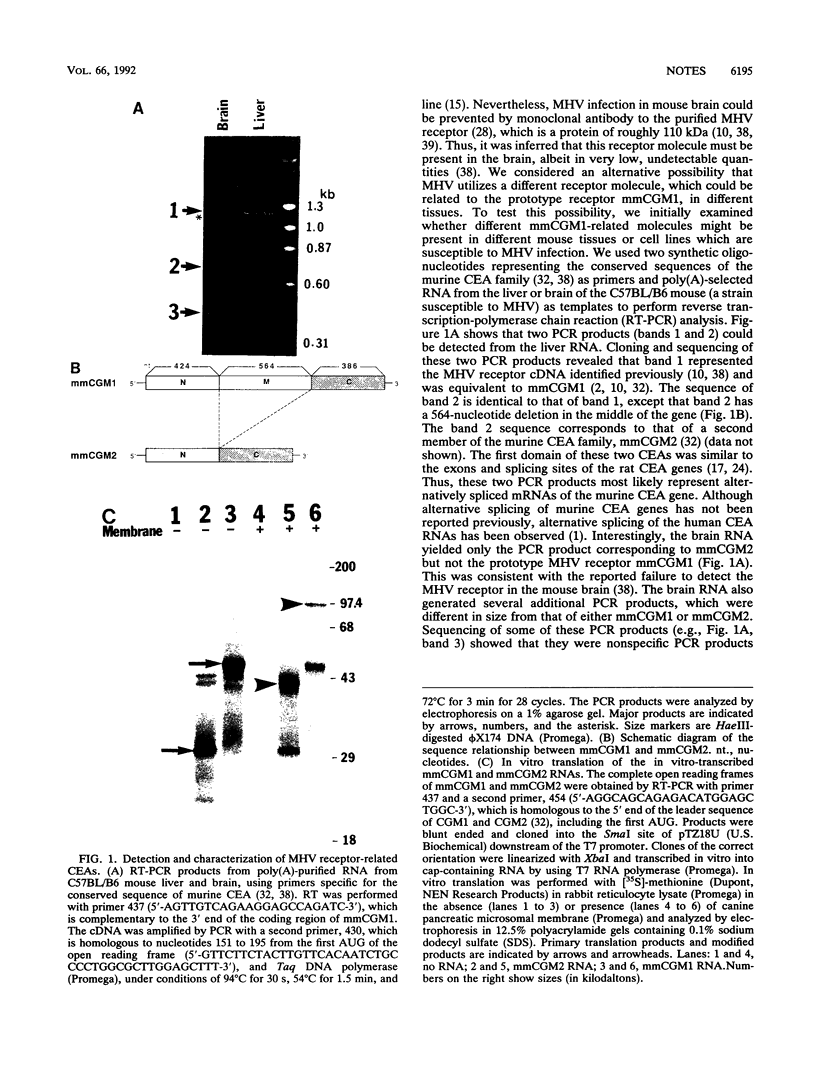
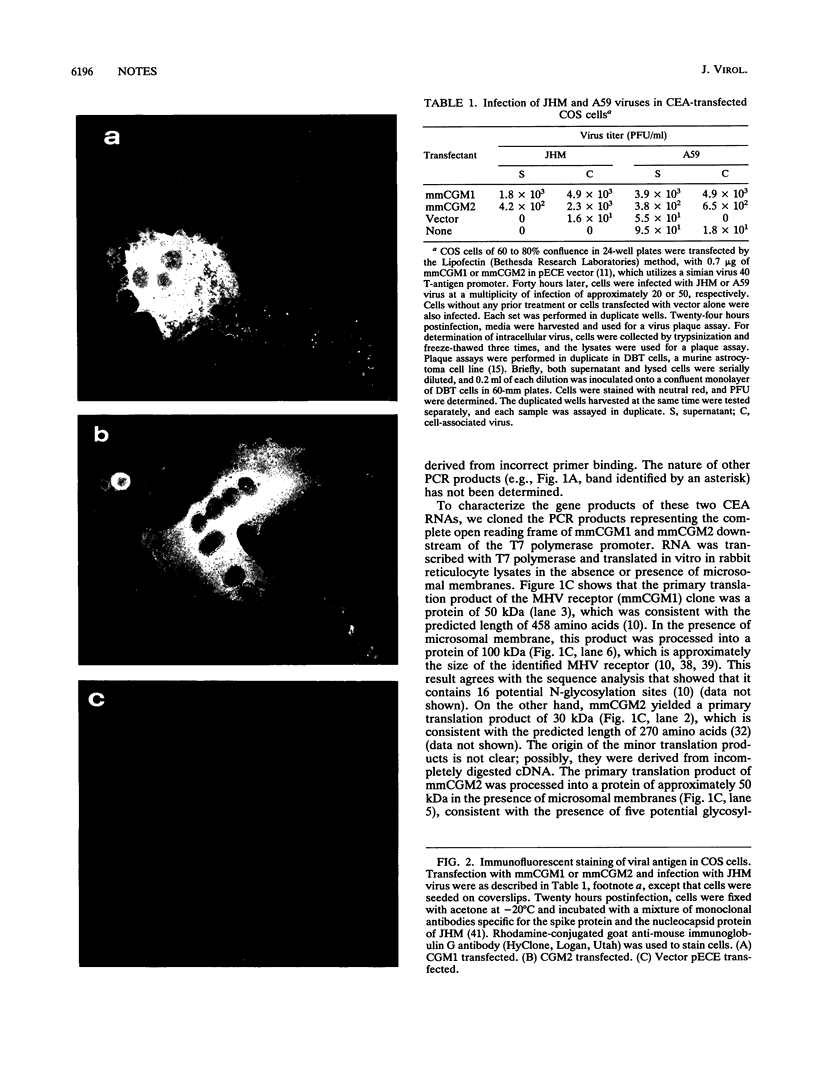
The cellular receptor for the murine coronavirus mouse hepatitis virus (MHV) has been identified as a member of the murine carcinoembryonic antigen (CEA) family (R. K. Williams, G. S. Jiang, and K. V. Holmes, Proc. Natl. Acad. Sci. USA 88:5533-5536, 1991). However, the receptor protein was not detected in some of the susceptible mouse tissues. We therefore examined whether other types of MHV receptor might exist. By polymerase chain reaction with the conserved sequences of murine CEA gene family members (mmCGM) as primers, we detected two CEA-encoding RNAs in the mouse liver. One of them (1.3 kb) encodes mmCGM1, which has previously been identified as the receptor for MHV, and the other one (0.8 kb) was shown to encode another member of mouse CGM, mmCGM2. The sequence analysis showed that mmCGM2 lacks 564 nucleotides in the middle of the gene compared with mmCGM1. These two CEA transcripts are probably derived from the same gene by an alternative splicing mechanism. Expression of either of these cDNA clones in COS-7 cells rendered these cells susceptible to MHV infection, suggesting that not only mmCGM1 but also mmCGM2 serves as a receptor for MHV. The mmCGM2 was the major CEA species in the mouse brain, which is a main target organ for the neurotropic strains of MHV. Very little mmCGM1 was detected in the mouse brain or in cells of the susceptible mouse astrocytoma cell line DBT. This result indicates that MHV may utilize different CEA molecules as the major receptor in the mouse brain and in the liver. This is a first identification of multiple receptors for a single virus. The presence of different receptors in different tissues may explain the target cell specificity of certain MHVs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett T. R., Kretschmer A., Austen D. A., Goebel S. J., Hart J. T., Elting J. J., Kamarck M. E. Carcinoembryonic antigens: alternative splicing accounts for the multiple mRNAs that code for novel members of the carcinoembryonic antigen family. J Cell Biol. 1989 Feb;108(2):267–276. doi: 10.1083/jcb.108.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin N., Turbide C., Afar D., Bell J., Raymond M., Stanners C. P., Fuks A. A mouse analogue of the human carcinoembryonic antigen. Cancer Res. 1989 Apr 15;49(8):2017–2021. [PubMed] [Google Scholar]
- Boyle J. F., Weismiller D. G., Holmes K. V. Genetic resistance to mouse hepatitis virus correlates with absence of virus-binding activity on target tissues. J Virol. 1987 Jan;61(1):185–189. doi: 10.1128/jvi.61.1.185-189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M. J., Lewicki H. A., Talbot P. J., Knobler R. L. Murine hepatitis virus-4 (strain JHM)-induced neurologic disease is modulated in vivo by monoclonal antibody. Virology. 1984 Jan 30;132(2):261–270. doi: 10.1016/0042-6822(84)90033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham P. R., Weber J. N., Whitby D., McIntosh K., Dalgleish A. G., Maddon P. J., Deen K. C., Sweet R. W., Weiss R. A. Soluble CD4 blocks the infectivity of diverse strains of HIV and SIV for T cells and monocytes but not for brain and muscle cells. Nature. 1989 Jan 26;337(6205):368–370. doi: 10.1038/337368a0. [DOI] [PubMed] [Google Scholar]
- Collins A. R., Knobler R. L., Powell H., Buchmeier M. J. Monoclonal antibodies to murine hepatitis virus-4 (strain JHM) define the viral glycoprotein responsible for attachment and cell--cell fusion. Virology. 1982 Jun;119(2):358–371. doi: 10.1016/0042-6822(82)90095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel R. G., Lampert P. W., Talbot P. J., Buchmeier M. J. Site-specific alteration of murine hepatitis virus type 4 peplomer glycoprotein E2 results in reduced neurovirulence. J Virol. 1986 Aug;59(2):463–471. doi: 10.1128/jvi.59.2.463-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rossi A., Roncella S., Calabro M. L., D'Andrea E., Pasti M., Panozzo M., Mammano F., Ferrarini M., Chieco-Bianchi L. Infection of Epstein-Barr virus-transformed lymphoblastoid B cells by the human immunodeficiency virus: evidence for a persistent and productive infection leading to B cell phenotypic changes. Eur J Immunol. 1990 Sep;20(9):2041–2049. doi: 10.1002/eji.1830200924. [DOI] [PubMed] [Google Scholar]
- Dveksler G. S., Pensiero M. N., Cardellichio C. B., Williams R. K., Jiang G. S., Holmes K. V., Dieffenbach C. W. Cloning of the mouse hepatitis virus (MHV) receptor: expression in human and hamster cell lines confers susceptibility to MHV. J Virol. 1991 Dec;65(12):6881–6891. doi: 10.1128/jvi.65.12.6881-6891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Fleming J. O., Trousdale M. D., el-Zaatari F. A., Stohlman S. A., Weiner L. P. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J Virol. 1986 Jun;58(3):869–875. doi: 10.1128/jvi.58.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freistadt M. S., Kaplan G., Racaniello V. R. Heterogeneous expression of poliovirus receptor-related proteins in human cells and tissues. Mol Cell Biol. 1990 Nov;10(11):5700–5706. doi: 10.1128/mcb.10.11.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Hirano N., Fujiwara K., Hino S., Matumoto M. Replication and plaque formation of mouse hepatitis virus (MHV-2) in mouse cell line DBT culture. Arch Gesamte Virusforsch. 1974;44(3):298–302. doi: 10.1007/BF01240618. [DOI] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Kodelja V., Lucas K., Barnert S., von Kleist S., Thompson J. A., Zimmermann W. Identification of a carcinoembryonic antigen gene family in the rat. Analysis of the N-terminal domains reveals immunoglobulin-like, hypervariable regions. J Biol Chem. 1989 Apr 25;264(12):6906–6912. [PubMed] [Google Scholar]
- Lai M. M. Coronavirus: organization, replication and expression of genome. Annu Rev Microbiol. 1990;44:303–333. doi: 10.1146/annurev.mi.44.100190.001511. [DOI] [PubMed] [Google Scholar]
- Lee H. J., Shieh C. K., Gorbalenya A. E., Koonin E. V., La Monica N., Tuler J., Bagdzhadzhyan A., Lai M. M. The complete sequence (22 kilobases) of murine coronavirus gene 1 encoding the putative proteases and RNA polymerase. Virology. 1991 Feb;180(2):567–582. doi: 10.1016/0042-6822(91)90071-I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Pachuk C. J., Bredenbeek P. J., Zoltick P. W., Spaan W. J., Weiss S. R. Molecular cloning of the gene encoding the putative polymerase of mouse hepatitis coronavirus, strain A59. Virology. 1989 Jul;171(1):141–148. doi: 10.1016/0042-6822(89)90520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasick J. M., Dales S. Infection by coronavirus JHM of rat neurons and oligodendrocyte-type-2 astrocyte lineage cells during distinct developmental stages. J Virol. 1991 Sep;65(9):5013–5028. doi: 10.1128/jvi.65.9.5013-5028.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebstock S., Lucas K., Thompson J. A., Zimmermann W. cDNA and gene analyses imply a novel structure for a rat carcinoembryonic antigen-related protein. J Biol Chem. 1990 May 15;265(14):7872–7879. [PubMed] [Google Scholar]
- Robb J. A., Bond C. W. Pathogenic murine coronaviruses. I. Characterization of biological behavior in vitro and virus-specific intracellular RNA of strongly neurotropic JHMV and weakly neurotropic A59V viruses. Virology. 1979 Apr 30;94(2):352–370. doi: 10.1016/0042-6822(79)90467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears A. E., McGwire B. S., Roizman B. Infection of polarized MDCK cells with herpes simplex virus 1: two asymmetrically distributed cell receptors interact with different viral proteins. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5087–5091. doi: 10.1073/pnas.88.12.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L., Cardellichio C. B., Winograd D. F., de Souza M. S., Barthold S. W., Holmes K. V. Monoclonal antibody to the receptor for murine coronavirus MHV-A59 inhibits viral replication in vivo. J Infect Dis. 1991 Apr;163(4):879–882. doi: 10.1093/infdis/163.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen O., Perry D., Dales S. In vivo and in vitro models of demyelinating diseases. III. JHM virus infection of rats. Arch Neurol. 1980 Aug;37(8):478–484. doi: 10.1001/archneur.1980.00500570026003. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Merluzzi V. J., Rothlein R., Barton R., Marlin S. D., Springer T. A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989 Mar 10;56(5):849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- Turbide C., Rojas M., Stanners C. P., Beauchemin N. A mouse carcinoembryonic antigen gene family member is a calcium-dependent cell adhesion molecule. J Biol Chem. 1991 Jan 5;266(1):309–315. [PubMed] [Google Scholar]
- Ubol S., Griffin D. E. Identification of a putative alphavirus receptor on mouse neural cells. J Virol. 1991 Dec;65(12):6913–6921. doi: 10.1128/jvi.65.12.6913-6921.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Kavanaugh M. P., North R. A., Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991 Aug 22;352(6337):729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- Wang K. S., Schmaljohn A. L., Kuhn R. J., Strauss J. H. Antiidiotypic antibodies as probes for the Sindbis virus receptor. Virology. 1991 Apr;181(2):694–702. doi: 10.1016/0042-6822(91)90903-o. [DOI] [PubMed] [Google Scholar]
- Weiner L. P. Pathogenesis of demyelination induced by a mouse hepatitis. Arch Neurol. 1973 May;28(5):298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]
- Weis J. J., Toothaker L. E., Smith J. A., Weis J. H., Fearon D. T. Structure of the human B lymphocyte receptor for C3d and the Epstein-Barr virus and relatedness to other members of the family of C3/C4 binding proteins. J Exp Med. 1988 Mar 1;167(3):1047–1066. doi: 10.1084/jem.167.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. K., Jiang G. S., Holmes K. V. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5533–5536. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. K., Jiang G. S., Snyder S. W., Frana M. F., Holmes K. V. Purification of the 110-kilodalton glycoprotein receptor for mouse hepatitis virus (MHV)-A59 from mouse liver and identification of a nonfunctional, homologous protein in MHV-resistant SJL/J mice. J Virol. 1990 Aug;64(8):3817–3823. doi: 10.1128/jvi.64.8.3817-3823.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. A., Dales S. In vivo and in vitro models of demyelinating disease: efficiency of virus spread and formation of infectious centers among glial cells is genetically determined by the murine host. J Virol. 1988 Sep;62(9):3371–3377. doi: 10.1128/jvi.62.9.3371-3377.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]