Abstract
Background
Various measures of arterial stiffness and wave reflection have been proposed as cardiovascular risk markers. Prior studies have not assessed relations of a comprehensive panel of stiffness measures to prognosis in the community.
Methods and Results
We used proportional hazards models to analyze first-onset major cardiovascular disease (CVD) events (myocardial infarction, unstable angina, heart failure or stroke) in relation to arterial stiffness (pulse wave velocity, PWV), wave reflection (augmentation index, carotid-brachial pressure amplification) and central pulse pressure in 2232 participants (mean age 63 years, 58% women) in the Framingham Heart Study. During median follow-up of 7.8 (range 0.2 to 8.9) years, 151 of 2232 participants (6.8%) had an event. In multivariable models adjusting for age, sex, systolic blood pressure, use of antihypertensive therapy, total and HDL cholesterol concentrations, smoking and presence of diabetes, higher aortic PWV was associated with a 48% increase in CVD risk (95% CI, 1.16 to 1.91 per SD, P=0.002). After adding PWV to a standard risk factor model, integrated discrimination improvement was 0.7% (95% CI, 0.05 to 1.3%, P<0.05). In contrast, augmentation index, central pulse pressure and pulse pressure amplification were not related to CVD outcomes in multivariable models.
Conclusions
Higher aortic stiffness assessed by PWV is associated with increased risk for a first cardiovascular event. Aortic PWV improves risk prediction when added to standard risk factors and may represent a valuable biomarker of CVD risk in the community.
Keywords: aorta, arterial stiffness, pulse wave velocity, cardiovascular disease, prognosis
Numerous studies performed over the past decade have identified peripheral pulse pressure, an indirect but widely available measure of arterial stiffness, as a novel cardiovascular disease (CVD) risk factor, although the close correlation between systolic and pulse pressure hinders efforts to distinguish these two hemodynamic indices.1–4 Interest has now shifted to more direct measures of arterial stiffness and central pulsatile hemodynamic load, such as carotid-femoral pulse wave velocity (PWV), central pulse pressure, and augmentation index.5 Recent studies have demonstrated that carotid-femoral PWV, a direct measure of stiffness of the thoracic and abdominal aorta, is associated with higher CVD event rates in high risk6–9 and community-based samples.10–12 However, several key questions remain. Studies that have evaluated PWV have not applied newer metrics of clinical utility, such as global model fit, discrimination, calibration and reclassification in models that adjust for standard CVD risk factors. Furthermore, since the heart and brain are exposed to central rather than peripheral pulse pressure, which may differ, central pulse pressure or measures of wave reflection, such as carotid-brachial pressure amplification and augmentation index, may represent incremental or superior measures of vascular risk compared to peripheral pulse pressure or PWV.13–17 To our knowledge, no prior community-based study has simultaneously compared the utility of PWV, central pulse pressure, and augmentation index for predicting cardiovascular risk. As a result, the relative contributions of these differing pulsatile hemodynamic measures to risk stratification in the community remain incompletely understood. The present analysis sought to clarify these questions.
Methods
Participants
The design and selection criteria for the Framingham Original and Offspring cohorts have been detailed previously.18;19 Participants attending the seventh examination of the Offspring cohort (N=3539; 1998–2001) or the 26th examination of the Original cohort (N=310; 1999–2001) were eligible for the present investigation. Tonometry measurements were implemented beginning in February 1999 as described previously.20 Participants were excluded from the present analysis for the following reasons: attended the visit prior to implementation of tonometry (N=879), bad or incomplete tonometry data (N=209), prior CVD (N=196) or missing covariate or follow-up information (N=333), resulting in a sample of 2232 participants (1299 [58%] women). All protocols were approved by Boston University Medical Center’s Institutional Review Board and participants provided written informed consent.
Clinical Evaluation and Definitions
Medical history, physical examination, and electrocardiography were performed routinely at each Framingham Heart Study examination.19;21 Blood pressures represent the average of two auscultatory blood pressures obtained by the physician on seated participants at the time of each Framingham clinic examination using a standardized measurement protocol. Peripheral pulse pressure was calculated as the difference between systolic and diastolic pressure. Body mass index (BMI) was calculated by dividing weight in kilograms by the square of the height in meters. Criteria for diabetes mellitus were a fasting glucose level of 126 mg/dL (7.0 mmol/L) or greater, or use of medications used to treat hyperglycemia.
Outcomes
Major CVD events were defined as fatal or nonfatal myocardial infarction, unstable angina (prolonged ischemic episode with documented reversible ST segment changes), heart failure, and ischemic or hemorrhagic stroke. Medical records were obtained for all hospitalizations and physician visits related to CVD during follow-up and were reviewed by a committee of 3 investigators; events were adjudicated following written guidelines. Criteria for these cardiovascular events have been described previously.22;23 Follow-up evaluations were performed on data acquired through December 31, 2007.
Noninvasive hemodynamic data acquisition
Participants were studied in the supine position after resting for approximately five minutes. Supine brachial systolic and diastolic blood pressures were obtained using an oscillometric device. Arterial tonometry with simultaneous electrocardiography was obtained from brachial, radial, femoral and carotid arteries using a commercially available tonometer (SPT-301, Millar Instruments, Houston, TX). All recordings were performed on the right side of the body. Transit distances were assessed by body surface measurements from the suprasternal notch to each pulse recording site. Tonometry and electrocardiographic data were digitized (1000 Hz) during the primary acquisition and transferred to the core laboratory (Cardiovascular Engineering, Inc., Norwood, MA) for analyses that were performed blinded to clinical data.
Tonometry data analysis
Tonometry waveforms were signal-averaged using the electrocardiographic R-wave as a fiducial point. Systolic and diastolic cuff blood pressures obtained at the time of the tonometry acquisition were used to calibrate the peak and trough of the signal-averaged brachial pressure waveform. Diastolic and integrated mean brachial pressures were used to calibrate carotid pressure tracings.24 Calibrated carotid pressure was used as a surrogate for central pressure.24 Central pulse pressure was defined as the difference between the peak and trough of the calibrated carotid pressure waveform. Carotid-brachial pulse pressure amplification was defined as brachial pulse pressure divided by central pulse pressure. Augmentation index was computed from the carotid pressure waveform as previously described.25 Carotid-femoral (aortic) and carotid-radial (muscular artery) pulse wave velocities were calculated from tonometry waveforms and body surface measurements, which were adjusted for parallel transmission in the brachiocephalic artery and aortic arch by using the suprasternal notch as a fiducial point.26 The carotid-femoral transit path spans the aorta, making carotid-femoral PWV a measure of aortic stiffness. In contrast, the carotid-radial transit path spans the subclavian, brachial and radial arteries, making carotid-radial PWV a measure of muscular artery stiffness.
Statistical analysis
The primary goal of the analyses was to determine whether key pulsatile hemodynamic measures were individually or jointly associated with increased risk of a first major CVD event in a model that adjusted for standard CVD risk factors. Baseline characteristics for the entire study sample were tabulated. Aortic PWV was right skewed and was inverse transformed to normalize its variance.
We examined the association between pulsatile hemodynamic measures and time to a first major CVD event by using Cox proportional hazards regression, after confirming that the assumption of proportionality was met. Covariates were selected based on components of the Framingham risk score27 and included the following at the baseline examination: age, sex, systolic blood pressure, use of antihypertensive therapy, total and high density lipoprotein cholesterol concentrations, regular use of cigarettes in the prior year, and presence of diabetes mellitus.
Pulsatile hemodynamic variables that showed significant relations with events in multivariable Cox models were evaluated further to assess prognostic importance. Cumulative probability curves were constructed by using the Kaplan–Meier method, with participant groups segregated according to quartiles of the hemodynamic variable of interest. Predictive value was assessed by using the likelihood ratio test, the Akaike information criterion, and the Schwartz’s Bayesian information criterion; the latter two tests carry a penalty for the number of variables used in the model and therefore can be compared directly across models with differing numbers of variables.28 To assess discrimination of events, we compared the C statistic, which is analogous to the area under the receiver operating characteristics curve, for models with and without pulsatile hemodynamic variables included. We also computed net reclassification improvement and integrated discrimination improvement.29 For the reclassification analysis, we did not use standard clinical cutpoints based on 10-year risk because we had only 7.8 years of follow-up. Instead, we computed predicted risk for all participants using a Cox model that included only the standard risk factors. Using predicted risk from this model, we defined cut points for risk groups based on quartiles of predicted risk in participants who experienced an event within 8 years, resulting in a uniform distribution of events across risk categories. We cross-classified categories of risk based on a model that included standard risk factors against those based on a model that added individual pulsatile hemodynamic variables. Cross-classification was assessed separately in participants who did or did not experience an event. Integrated discrimination improvement is equivalent to the difference in discrimination slopes between new and old models and is analogous to reclassification. However, the calculation is based on continuous rather than empirically thresholded differences in predicted risk in new and old models in individual cases and controls. Thus, integrated discrimination improvement is free of the dependence on choice of risk categories that is inherent in reclassification tables and may be used as an objective indicator of reclassification improvement. To assess calibration, we used the Hosmer-Lemeshow test, which compares observed and predicted risk according to deciles of predicted risk for various models.
To determine whether relations between aortic PWV and CVD events were mediated in part by associated abnormalities in peripheral or central pulse pressure or wave reflection, we additionally adjusted the aortic PWV model for brachial pulse pressure, central pulse pressure, and pulse pressure amplification. In order to determine whether the relation between hemodynamic measures and CVD events differed in older as compared to younger participants (segregated by median age) or in men as compared to women, we included interaction terms for these variables in separate models that also adjusted for standard risk factors. All analyses were performed with SAS version 9.1. A 2-sided P<0.05 was considered statistically significant.
Results
Baseline characteristics of the study sample are presented in Table 1. During a median follow-up period of 7.8 (range 0.2 to 8.9) years, 151 of 2232 participants (6.8%; 77 women) had a first major CVD event comprised of 57 fatal or nonfatal myocardial infarctions, 4 episodes of coronary insufficiency, 57 episodes of new heart failure and 33 strokes.
Table 1.
Baseline characteristics of the sample (N=2232).
| Variable | Value |
|---|---|
| Clinical Measures | |
| Age, years | 63±12 |
| Women, N (%) | 1299 (58) |
| Height, cm | 167±10 |
| Weight, kg | 75±16 |
| Body mass index, kg/m2 | 27.2±4.6 |
| Blood pressure, mm Hg | |
| Systolic | 127±20 |
| Diastolic | 74±10 |
| Pulse | 54±17 |
| Heart rate, beats/min | 65±11 |
| Total cholesterol, mg/dl | 201±36 |
| HDL cholesterol, mg/dl | 56±17 |
| Triglycerides, ln(mg/dL) | 4.7±0.5 |
| Hypertension treatment, no. (%) | 717 (32) |
| Diabetes, N (%) | 179 (8) |
| Smoker, N (%) | 278 (12) |
| Vascular measures | |
| Carotid-femoral pulse wave velocity, m/s* | 9.3 (7.8, 11.8) |
| Inverse carotid-femoral pulse wave velocity, ms/m | 107±31 |
| Carotid-radial pulse wave velocity, m/s | 10.0±1.5 |
| Central pulse pressure, mm Hg | 52±17 |
| Carotid-brachial amplification ratio | 1.06±0.12 |
| Augmentation index, % | 15±13 |
All values are mean ± standard deviation except as noted.
Median (25th, 75th percentiles)
Cox proportional hazards models for individual pulsatile hemodynamic measures are presented in Table 2. In models that adjusted for standard risk factors, carotid-femoral (aortic) PWV was associated with increased risk for a first major CVD event with a hazard ratio (HR) of 1.48 (95% CI 1.16 to 1.91, P=0.002) per standard deviation lower inverse carotid-femoral PWV, which corresponds to higher aortic PWV. In contrast, carotid-radial (muscular artery) PWV, augmentation index, central pulse pressure and carotid-brachial pulse pressure amplification were not related to events in risk factor-adjusted models (Table 2). The relation between aortic PWV and events remained significant and essentially unaltered (HR 1.47–1.49, P<0.003) when the model was further adjusted for brachial pulse pressure, central pulse pressure or carotid-brachial pulse pressure amplification. There were no significant interactions between aortic PWV and median age (P=0.31), sex (P=0.38) or treatment for hypertension (P=0.15) when interaction terms were added to the risk factor-adjusted model. When the model including standard risk factors and aortic PWV was repeated after excluding 57 heart failure events, the relation between aortic PWV and events remained significant (HR 1.95, 95% CI 1.62 to 2.52, P=0.015).
Table 2.
Cox proportional hazards models for individual pulsatile hemodynamic measures as predictors of a major cardiovascular event during the follow-up period.
| Hemodynamic measure | Hazard Ratio (LCI,UCI) | P |
|---|---|---|
| Carotid-femoral (aortic) pulse wave velocity | 1.48 (1.16, 1.91) | 0.002 |
| Carotid-radial (muscular artery) pulse wave velocity | 1.07 (0.92, 1.25) | 0.77 |
| Augmentation index | 0.91 (0.77, 1.07) | 0.24 |
| Central pulse pressure | 1.00 (0.99, 1.01) | 0.98 |
| Pulse pressure amplification | 0.86 (0.19, 3.82) | 0.84 |
HR, hazard ratio; LCI, UCI, lower and upper 95% confidence intervals; CVD, cardiovascular disease. Hazard ratios expressed per 1 SD higher value, adjusted for age, sex, total cholesterol, HDL cholesterol, systolic blood pressure, smoking, diabetes and hypertension treatment. For carotid-femoral pulse wave velocity, the inverse transform was used and results were expressed per 1 SD lower inverse values (which corresponds to higher pulse wave velocity).
When aortic PWV was added to a base model that included age and sex, measures of model performance improved to a degree that was comparable to adding the full set of standard risk factors to the base model (Table 3). When aortic PWV was added to the risk factor-adjusted model, model fit was improved, as indicated by reductions in log likelihood, the Akaike information criterion and the Schwartz’s Bayesian information criterion. However, the C statistic was unchanged (0.796 to 0.800, P=0.3). The Hosmer-Lemeshow test demonstrated excellent calibration (Table 3). Addition of aortic PWV to the standard model resulted in upward reclassification of 6.7% of participants who experienced a CVD event as well as upward reclassification of 1.2% of participants who did not experience an event, yielding an overall net reclassification of 5.5% (P=0.15, Table 4). For individuals at intermediate CVD risk (middle two quartiles), addition of aortic PWV resulted in upward reclassification of 14.3% of participants who experienced a CVD event and downward reclassification of 1.4% of participants who did not experience a CVD event, yielding a net reclassification of 15.7% (P=0.03, Table 4). In the full sample, when aortic PWV was added to the standard risk factor model, the discrimination slope increased from 7.8±12.6 to 8.5±13.3%, resulting in an integrated discrimination improvement of 0.7% (95% CI, 0.05 to 1.3%, P<0.05). In participants at intermediate risk, the discrimination slope increased from 1.8±4.5 to 2.7±6.2%, resulting in an integrated discrimination improvement of 0.8% (95% CI, 0.12 to 1.6%, P=0.02). Together these findings indicate that addition of aortic PWV to standard CVD risk factors improved model fit and resulted in a well calibrated model with improved risk discrimination and risk reclassification.
Table 3.
Measures of model fit, discrimination and calibration for various cardiovascular event models with and without carotid-femoral (aortic) pulse wave velocity.
| Model Fit |
Discrimination |
Calibration |
||||
|---|---|---|---|---|---|---|
| Model |
−2 Log Likelihood |
Akaike Information Criterion |
Schwartz’s Bayesian Information Criterion |
C Statistic (95% CI) |
χ2 |
P |
| Age, sex | 2155 | 2159 | 2165 | 0.762 (0.723, 0.801) | 8.7 | 0.46 |
| Add pulse wave velocity | 2136 | 2142 | 2151 | 0.782 (0.746, 0.818)* | 2.9 | 0.97 |
| Age, sex, total cholesterol, HDL cholesterol, systolic pressure, smoking, diabetes and hypertension treatment | 2126 | 2142 | 2166 | 0.796 (0.764, 0.828) | 4.3 | 0.89 |
| Add pulse wave velocity | 2116 | 2134 | 2162 | 0.800 (0.768, 0.832)** | 2.6 | 0.98 |
For a description of the tests displayed in the table, please see statistical methods section.
Comparison of c statistics in rows 1 and 2, P=0.006.
Comparison of c statistics in rows 3 and 4, P=0.3.
Table 4.
Predicted risk for a cardiovascular disease event before and after reclassification with carotid-femoral (aortic) pulse wave velocity in participants who did (A) or did not (B) experience an event within 8 years.
| A. | |||||
|---|---|---|---|---|---|
| Model with PWV |
|||||
| Model without PWV | 0–5% | 5%–11% | 11%–16% | >16% | Total |
| 0–5% | 31 | 5 | 0 | 0 | 36 |
| 5%–11% | 3 | 29 | 3 | 0 | 35 |
| 11%–16% | 0 | 3 | 19 | 13 | 35 |
| >16% | 0 | 0 | 5 | 38 | 43 |
| Total | 34 | 37 | 27 | 51 | 149 |
| B. | |||||
|---|---|---|---|---|---|
| Model with PWV |
|||||
| Model without PWV | 0–5% | 5%–11% | 11%–16% | >16% | Total |
| 0–5% | 1376 | 51 | 0 | 0 | 1427 |
| 5%–11% | 48 | 286 | 40 | 3 | 377 |
| 11%–16% | 1 | 37 | 55 | 36 | 129 |
| >16% | 0 | 0 | 19 | 131 | 150 |
| Total | 1425 | 374 | 114 | 170 | 2083 |
Bold values along the diagonal were similarly classified by both models. Values on each row to the right of the bold value were up classified and those to the left were down classified by the model that included PWV.
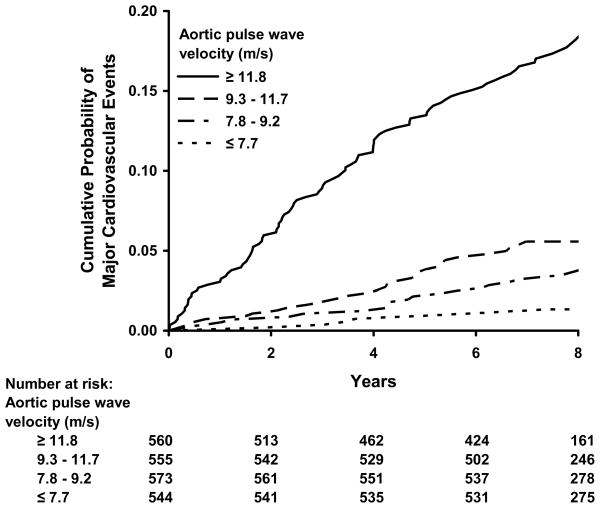
The Figure displays estimated cumulative incidence of major cardiovascular events. When participants were grouped according to quartiles of aortic PWV, the probability of developing a CVD event increased with aortic PWV group. Comparing individuals in the highest (≥11.8 m/s) as compared to the lowest (<7.8 m/s) aortic PWV group after adjusting for age, sex and standard risk factors, individuals in the highest quartile had an adjusted hazards ratio of 3.4 (95% CI, 1.4 to 8.3, P=0.008).
Figure.
Kaplan-Meier plot of cumulative probability of a first major CVD event when participants were grouped according to quartiles of carotid-femoral (aortic) pulse wave velocity.
Discussion
We evaluated relations between carotid-femoral (aortic) PWV, carotid-radial (muscular artery) PWV, central pulse pressure, augmentation index and carotid-brachial pressure amplification and major CVD events in middle-aged and older participants in the community-based Framingham Heart Study. We demonstrated that per standard deviation increase in carotid-femoral PWV, the adjusted risk of a first major CVD event was significantly increased (HR 1.48, 95% CI, 1.16 to 1.91). Measures of model fit and discrimination were improved and calibration was very good when carotid-femoral PWV was added to standard risk factors. The association between carotid-femoral PWV and excess risk persisted after further adjustment for brachial or central pulse pressure or carotid-brachial pulse pressure amplification. In our community-based sample, carotid-radial (muscular artery) PWV, central pulse pressure, augmentation index and carotid-brachial pressure amplification were not associated with risk for a major CVD event in models that included standard risk factors. Our results suggest that the adverse association between CVD outcomes and arterial stiffening, as assessed by increased PWV, may be specific to the aorta as opposed to large muscular arteries, like the brachial and radial arteries. Furthermore, we have shown for the first time that the relation between aortic PWV and events is distinguishable from excessive central pressure pulsatility or abnormal wave reflection.
Prior studies have shown that increased carotid-femoral PWV is associated with excess risk in various high risk 6–9 and community-based samples.10–12 In addition, several cross-sectional and a limited number of longitudinal studies have suggested that central systolic and pulse pressure and measures of wave reflection may provide incremental CVD risk stratification beyond that provided by standard risk factors including conventional blood pressure recorded in the arm.5;13–17 Recently published international guidelines have suggested that PWV and central pressure may be useful as guides to therapy. The 2007 European guidelines for the management of hypertension and guidelines for CVD prevention in clinical practice added aortic PWV as a recommended test for assessment of target organ damage.30;31 The European hypertension guidelines noted that central and peripheral pulse pressure can differ and suggested that central pulse pressure might be a better indicator of risk, although the authors underscored a need for additional prospective data in large-scale studies.31 The present study extends prior work and clarifies the role of central and peripheral pulse pressure by demonstrating that the adverse effects of increased aortic PWV are not captured by assessing potentially related abnormalities in central or peripheral systolic or pulse pressure. Furthermore, we have shown that after accounting for standard risk factors, including conventional systolic blood pressure assessed in the arm, measures of central pulse pressure and wave reflection do not provide incremental CVD risk prediction in this community-based sample of middle-aged and older people.
In contrast to our findings, a number of prior studies have shown that central systolic and pulse pressure may be better predictors of CVD risk than peripheral values.13–17 Differences between our study and prior studies may account for differences in findings. In one study, supine invasive central pulse pressure obtained during clinically indicated cardiac catheterization and compared to seated sphygmomanometric peripheral pulse pressure.17 Thus, the difference in predictive power between central (invasive) and peripheral (noninvasive) pulse pressure in that study may relate to differences in technique. Prior studies have assumed that brachial systolic and diastolic pressures could be applied to the peak and trough of a radial waveform in order to assess mean arterial pressure, which is required to calibrate the carotid pressure waveform.14;15 Subsequent work has shown that the implicit assumption of no amplification between brachial and radial arteries in incorrect and confounds the assessment of central pulse pressure.32 Another study used estimated mean pressure to calibrate a central pressure waveform obtained by using a generalized transfer function16; a calibration procedure that uses estimated mean pressure introduces a variable, mean pressure-related error into the calibration of central pressure. In contrast to the foregoing studies, we measured carotid and brachial pressure waveforms directly, calibrated the brachial waveform to brachial cuff pressure and integrated the calibrated waveform in order to derive an accurate assessment of mean arterial pressure.
Sample selection may have further contributed to differences in predictive power of central versus peripheral pulse pressure and pressure amplification in prior studies compared to ours. Prior studies evaluated high risk groups, including patients with known hypertension and other CVD risk factors,15 suspected coronary artery disease resulting in cardiac catheterization17 and end stage kidney disease.14 Another study evaluated a predominantly female sample of American Indians with relatively high prevalences of obesity, diabetes and hypertension and an unusually high event rate (13.3% during 4.7 years of follow-up as compared to with 6.8% during 7.8 years of follow-up in our study).16 In contrast to the foregoing studies, our panel of stiffness measures was routinely ascertained in an unselected, community-based sample.
The inability of central pulse pressure and measures of wave reflection to provide incremental risk stratification beyond that provided by standard CVD risk factors should be interpreted in light of the strong correlation between central and peripheral systolic and pulse pressure in middle-aged and older adults who are at highest risk for CVD events. To minimize the effects of correlation between central and peripheral pressures, we also evaluated carotid-brachial pressure amplification, which assesses only the relative discrepancy between central and peripheral pulse pressure, and again found no independent relation with events. Our findings suggest that when considered in the context of the broad distribution of values for peripheral systolic and pulse pressure found in middle-aged and older people, knowledge of the modest difference between central and peripheral pulse pressure may not contribute substantively to CVD risk prediction in a sample such as ours. Inability of measures of wave reflection, such as augmentation index, to predict CVD outcomes may relate to the observation that augmentation index and wave reflection are confounded by many factors, including several paradoxical associations between lower augmentation and higher CVD risk factor burden. We have previously shown that with advancing age the normally compliant aorta stiffens markedly whereas the relatively stiff muscular arteries remain unchanged. As the properties of the aorta and peripheral muscular arteries become more similar, wave reflection at this interface is reduced and shifted to more distal sites in the arterial system. This “impedance matching” between aorta and large muscular arteries may limit or delay global wave reflection despite the increase in aortic PWV and may thereby facilitate increased transmission of potentially harmful pulsatile energy into the microcirculation.26;33 Thus, the hypothesis that increasing aortic stiffness should result in progressively premature wave reflection and increasing augmentation, rendering augmentation index a suitable marker for aortic stiffening and associated increased risk, was not evident in our community-based sample.
Several limitations of our study should be considered. We acknowledge that an observational study cannot definitively prove that there is a causal link underlying the association between increased carotid femoral PWV and increased CVD events. We cannot rule out residual confounding by duration or severity of associated risk factors, or unknown risk factors. Further, we had only a modest number of events, so we lacked power to examine threshold models or to analyze specific types of CVD events. Additionally, we cannot exclude the possibility that with more follow up or a larger sample other vascular measures might have been related to CVD events. We evaluated a middle-aged and older cohort of predominately white study participants. Therefore, our results may not be generalizable to younger individuals and other ethnicities. Although carotid-femoral PWV is considered the current gold standard measure of aortic stiffness, there are limitations to the measurement. The assessment of transit distance is approximate because of parallel transmission in the aortic arch. In addition, the arterial segment interrogated includes aorta and iliac and femoral arteries, whose properties may change differently with age and risk factor exposure. More specific measures of aortic stiffness may provide superior CVD risk assessment. Strengths of our study include a large, community-based sample with routinely ascertained risk factors and a comprehensive battery of measures of arterial stiffness and wave reflection.
Measurement of PWV is noninvasive, safe and readily implemented in an office setting with relatively inexpensive equipment and modest training, suggesting that attention should be focused on aortic PWV as a potential novel biomarker of cardiovascular risk. Aortic PWV is strongly associated with CVD risk, abnormal in a substantial proportion of middle-aged and older people, and only modestly correlated with standard risk factors, which are all desirable attributes of a potential biomarker.34 Biological correlates and clinical interpretation of aortic PWV values are straightforward in that higher values are directly attributable to excessive aortic wall stiffness and are associated with increasing risk for CVD. In addition, aortic PWV is modifiable, particularly following interventions that target sodium balance (salt restriction, low dose diuretics) or block the renin-angiotensin system. Reductions in PWV of 1 m/s or more are achievable following such interventions even when assessed after a relatively short duration in many studies.35–38 This magnitude of change potentially could translate into a substantial reduction in CVD risk. Importantly, we have shown previously that the prevalence of elevated aortic PWV increases markedly between 50 and 70 years of age, from a few percent prior to 50 years of age to nearly 70% after 70 years of age.20 The combination of aging of the world population, a marked increase in the prevalence of abnormal aortic PWV with age, and a strong relation between aortic PWV and events portends a major increase in the burden of disease potentially attributable to abnormal aortic stiffness. These observations suggest a need to identify and implement effective interventions that limit or reverse arterial stiffening in older people.
Clinical Summary.
Various measures of arterial stiffness and wave reflection have been proposed as cardiovascular risk markers. However no community-based study has compared prognostic utility of pulse wave velocity (PWV), central pulse pressure and augmentation index. In our study of 2232 Framingham Heart Study participants, after adjusting for risk factors (age, sex, systolic blood pressure, use of antihypertensive therapy, total and HDL cholesterol concentrations, smoking and presence of diabetes), a standard deviation increase in carotid-femoral (aortic) PWV was associated with a 48% increase in risk for a first major cardiovascular disease event. Measures of model fit and discrimination were improved when carotid-femoral PWV was added to standard risk factors. In contrast, carotid-radial (muscular artery) PWV, augmentation index, central pulse pressure and carotid-brachial pulse pressure amplification were not related to events in risk factor-adjusted models. Measurement of PWV is noninvasive, safe and readily implemented in an office setting with inexpensive equipment and modest training. Aortic PWV is strongly associated with risk, abnormal in a substantial proportion of middle-aged and older people, and only modestly correlated with standard risk factors, suggesting that attention should be focused on aortic PWV as a biomarker of cardiovascular risk. The combination of aging of the world population, increasing aortic PWV with age, and increased risk with higher aortic PWV portends a major increase in the burden of disease potentially attributable to abnormal aortic stiffness. These observations suggest a need to identify and implement interventions that limit or reverse arterial stiffening.
Acknowledgments
From the Framingham Heart Study of the National Heart Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine.
Funding Sources
This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. N01-HC-25195) and by HL076784, AG028321, HL070100, HL060040, HL080124, HL071039, HL077447, 2-K24-HL04334 and a grant from the Donald W. Reynolds Foundation.
Footnotes
Disclosures
G.F.M. is owner of Cardiovascular Engineering, Inc, a company that designs and manufactures devices that measure vascular stiffness. The company uses these devices in clinical trials that evaluate the effects of diseases and interventions on vascular stiffness. The remaining authors report no conflicts.
References
- 1.Domanski MJ, Davis BR, Pfeffer MA, Kastantin M, Mitchell GF. Isolated systolic hypertension: prognostic information provided by pulse pressure. Hypertension. 1999;34:375–380. doi: 10.1161/01.hyp.34.3.375. [DOI] [PubMed] [Google Scholar]
- 2.Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation. 1999;100:354–360. doi: 10.1161/01.cir.100.4.354. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell GF, Moye LA, Braunwald E, Rouleau JL, Bernstein V, Geltman EM, Flaker GC, Pfeffer MA. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and Ventricular Enlargement. Circulation. 1997;96:4254–4260. doi: 10.1161/01.cir.96.12.4254. [DOI] [PubMed] [Google Scholar]
- 4.Franklin SS, Lopez VA, Wong ND, Mitchell GF, Larson MG, Vasan RS, Levy D. Single versus combined blood pressure components and risk for cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:243–250. doi: 10.1161/CIRCULATIONAHA.108.797936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 6.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 7.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 8.Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–1206. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 9.Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–2050. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- 10.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 11.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 12.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 13.Agabiti-Rosei E, Mancia G, O’Rourke MF, Roman MJ, Safar ME, Smulyan H, Wang JG, Wilkinson IB, Williams B, Vlachopoulos C. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension. 2007;50:154–160. doi: 10.1161/HYPERTENSIONAHA.107.090068. [DOI] [PubMed] [Google Scholar]
- 14.Safar ME, Blacher J, Pannier B, Guerin AP, Marchais SJ, Guyonvarc’h PM, London GM. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39:735–738. doi: 10.1161/hy0202.098325. [DOI] [PubMed] [Google Scholar]
- 15.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O’Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 16.Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- 17.Jankowski P, Kawecka-Jaszcz K, Czarnecka D, Brzozowska-Kiszka M, Styczkiewicz K, Loster M, Kloch-Badelek M, Wilinski J, Curylo AM, Dudek D. Pulsatile but not steady component of blood pressure predicts cardiovascular events in coronary patients. Hypertension. 2008;51:848–855. doi: 10.1161/HYPERTENSIONAHA.107.101725. [DOI] [PubMed] [Google Scholar]
- 18.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation. 2007;115:2628–2636. doi: 10.1161/CIRCULATIONAHA.106.667733. [DOI] [PubMed] [Google Scholar]
- 21.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannel WB, Wolf PA, Garrison RJ. Some Risk Factors Related to the Annual Incidence of Cardiovascular Disease and Death in Pooled Repeated Biennial Measurements: Framingham Heart Study, 30 Year Follow-up. Section 34 Bethesda, MD: US Dept of Health and Human Services; 1987. [Google Scholar]
- 23.Frankel DS, Vasan RS, D’Agostino RB, Sr, Benjamin EJ, Levy D, Wang TJ, Meigs JB. Resistin, adiponectin, and risk of heart failure the Framingham offspring study. J Am Coll Cardiol. 2009;53:754–762. doi: 10.1016/j.jacc.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–963. doi: 10.1016/0735-1097(92)90198-v. [DOI] [PubMed] [Google Scholar]
- 25.Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man: relationship to pressure wave forms. Circulation. 1980;62:105–116. doi: 10.1161/01.cir.62.1.105. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 27.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 28.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 29.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 30.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, Dallongeville J, De Backer G, Ebrahim S, Gjelsvik B, Herrmann-Lingen C, Hoes A, Humphries S, Knapton M, Perk J, Priori SG, Pyorala K, Reiner Z, Ruilope L, Sans-Menendez S, Op Reimer WS, Weissberg P, Wood D, Yarnell J, Zamorano JL, Walma E, Fitzgerald T, Cooney MT, Dudina A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Funck-Brentano C, Filippatos G, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Altiner A, Bonora E, Durrington PN, Fagard R, Giampaoli S, Hemingway H, Hakansson J, Kjeldsen SE, Larsen L, Mancia G, Manolis AJ, Orth-Gomer K, Pedersen T, Rayner M, Ryden L, Sammut M, Schneiderman N, Stalenhoef AF, Tokgozoglu L, Wiklund O, Zampelas A. European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Eur J Cardiovasc Prev Rehabil. 2007;14 (Suppl 2):S1–113. doi: 10.1097/01.hjr.0000277983.23934.c9. [DOI] [PubMed] [Google Scholar]
- 31.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A. 2007 ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J Hypertens. 2007;25:1751–1762. doi: 10.1097/HJH.0b013e3282f0580f. [DOI] [PubMed] [Google Scholar]
- 32.Verbeke F, Segers P, Heireman S, Vanholder R, Verdonck P, Van Bortel LM. Noninvasive assessment of local pulse pressure: importance of brachial-to-radial pressure amplification. Hypertension. 2005;46:244–248. doi: 10.1161/01.HYP.0000166723.07809.7e. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 35.Seals DR, Tanaka H, Clevenger CM, Monahan KD, Reiling MJ, Hiatt WR, Davy KP, DeSouza CA. Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: role of arterial stiffness. J Am Coll Cardiol. 2001;38:506–513. doi: 10.1016/s0735-1097(01)01348-1. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell GF, Izzo JL, Jr, Lacourciere Y, Ouellet JP, Neutel J, Qian C, Kerwin LJ, Block AJ, Pfeffer MA. Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the conduit hemodynamics of omapatrilat international research study. Circulation. 2002;105:2955–2961. doi: 10.1161/01.cir.0000020500.77568.3c. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell GF, Dunlap ME, Warnica W, Ducharme A, Arnold JM, Tardif JC, Solomon SD, Domanski MJ, Jablonski KA, Rice MM, Pfeffer MA. Long-term trandolapril treatment is associated with reduced aortic stiffness: the prevention of events with angiotensin-converting enzyme inhibition hemodynamic substudy. Hypertension. 2007;49:1271–1277. doi: 10.1161/HYPERTENSIONAHA.106.085738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karalliedde J, Smith A, DeAngelis L, Mirenda V, Kandra A, Botha J, Ferber P, Viberti G. Valsartan improves arterial stiffness in type 2 diabetes independently of blood pressure lowering. Hypertension. 2008;51:1617–1623. doi: 10.1161/HYPERTENSIONAHA.108.111674. [DOI] [PubMed] [Google Scholar]