Abstract
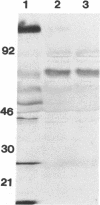
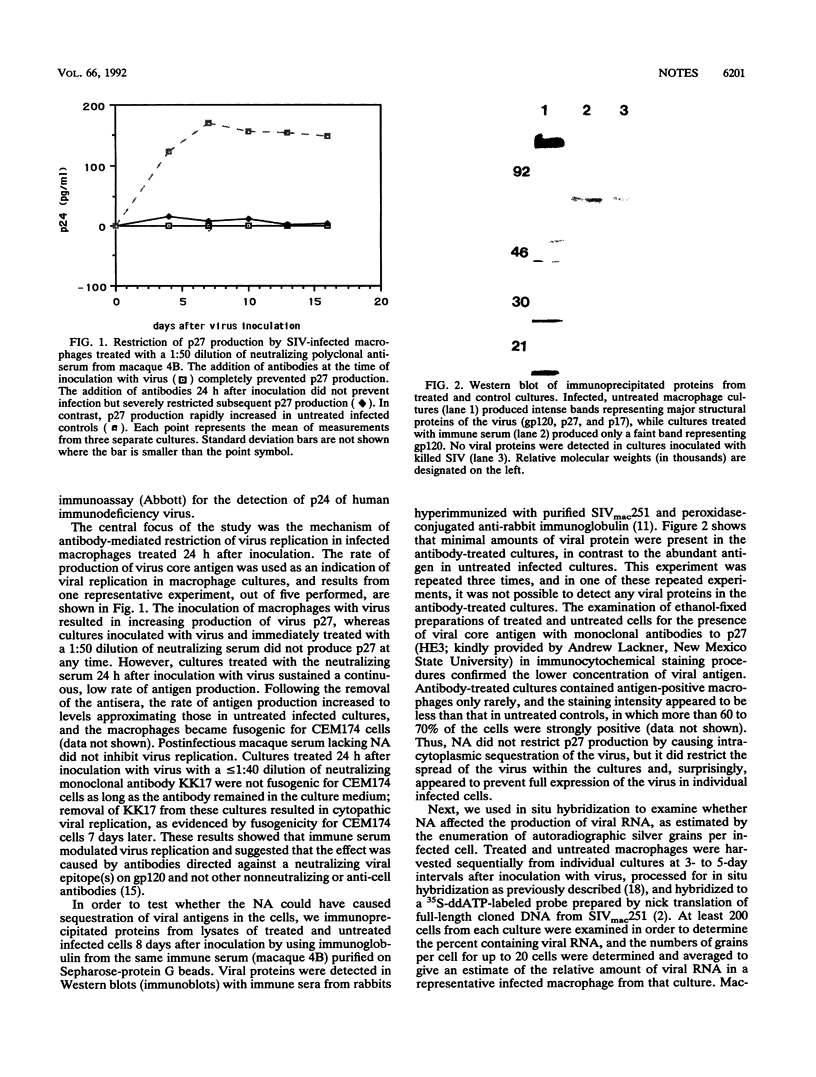
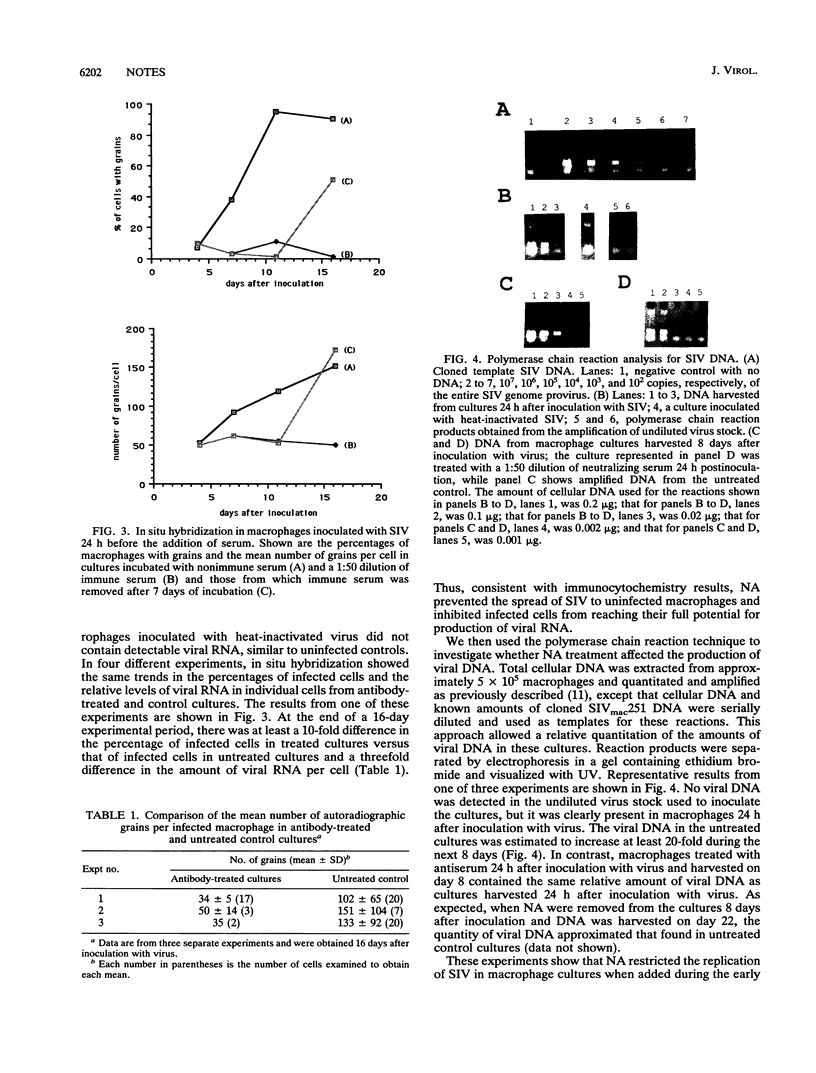
Cultured macaque macrophages are permissive for the replication of SIVmac251, and inoculation with virus is followed by the production of viral p27. Neutralizing macaque polyclonal and murine monoclonal antibodies preincubated with the virus prevented infection but did not prevent cytopathic virus replication when added more than 3 days after inoculation with virus. However, application of the neutralizing antibodies to macrophages 24 h after inoculation with virus resulted in sustained, low-level production of viral antigen. Cell lysates and individual macrophages from treated cultures contained less viral protein by Western blot (immunoblot) and immunocytochemistry than untreated controls. In situ hybridization and polymerase chain reaction procedures for detecting and estimating relative amounts of viral RNA and DNA showed that both viral nucleic acids failed to increase beyond the levels obtained before the addition of neutralizing antibodies. The data suggest that macrophages may need to be infected with a minimum threshold of virus particles in order to reach their full potential for virus replication and that their exposure to neutralizing antibodies prior to reaching this threshold resulted in limited virus replication.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besansky N. J., Butera S. T., Sinha S., Folks T. M. Unintegrated human immunodeficiency virus type 1 DNA in chronically infected cell lines is not correlated with surface CD4 expression. J Virol. 1991 May;65(5):2695–2698. doi: 10.1128/jvi.65.5.2695-2698.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., Sehgal P. K., Hunsmann G., Schmidt D. K., King N. W., Desrosiers R. C. Long-term persistent infection of macaque monkeys with the simian immunodeficiency virus. J Gen Virol. 1987 Dec;68(Pt 12):3183–3189. doi: 10.1099/0022-1317-68-12-3183. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Harris J. D., Traynor B., Ventura P., Peluso R., Brahic M. Visna DNA synthesis and the tempo of infection in vitro. Virology. 1982 Jun;119(2):399–410. doi: 10.1016/0042-6822(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Sarngadharan M. G., Hirsch M. S., Schooley R. T., Rota T. R., Kennedy R. C., Chanh T. C., Sato V. L. Human immunodeficiency virus neutralizing antibodies recognize several conserved domains on the envelope glycoproteins. J Virol. 1987 Jun;61(6):2024–2028. doi: 10.1128/jvi.61.6.2024-2028.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent K. A., Gritz L., Stallard G., Cranage M. P., Collignon C., Thiriart C., Corcoran T., Silvera P., Stott E. J. Production and of monoclonal antibodies to simian immunodeficiency virus envelope glycoproteins. AIDS. 1991 Jul;5(7):829–836. doi: 10.1097/00002030-199107000-00006. [DOI] [PubMed] [Google Scholar]
- Kestler H., Kodama T., Ringler D., Marthas M., Pedersen N., Lackner A., Regier D., Sehgal P., Daniel M., King N. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990 Jun 1;248(4959):1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., Daniel M. D., Sehgal P. K., Desrosiers R. C., Hunt R. D., Waldron L. M., MacKey J. J., Schmidt D. K., Chalifoux L. V., King N. W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985 Oct 4;230(4721):71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- McEntee M. F., Sharma D. P., Zink M. C., Adams R. J., Flexner C., Clements J. E., Narayan O. Rhesus monkey macrophages infected with simian immunodeficiency virus cause rapid lysis of CD4-bearing lymphocytes. J Gen Virol. 1991 Feb;72(Pt 2):317–324. doi: 10.1099/0022-1317-72-2-317. [DOI] [PubMed] [Google Scholar]
- Pauza C. D., Galindo J. E., Richman D. D. Reinfection results in accumulation of unintegrated viral DNA in cytopathic and persistent human immunodeficiency virus type 1 infection of CEM cells. J Exp Med. 1990 Oct 1;172(4):1035–1042. doi: 10.1084/jem.172.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Zinkus D. M. Accumulation of human immunodeficiency virus type 1 DNA in T cells: results of multiple infection events. J Virol. 1990 Oct;64(10):4836–4841. doi: 10.1128/jvi.64.10.4836-4841.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott E. J. Anti-cell antibody in macaques. Nature. 1991 Oct 3;353(6343):393–393. doi: 10.1038/353393a0. [DOI] [PubMed] [Google Scholar]
- Tersmette M., Gruters R. A., de Wolf F., de Goede R. E., Lange J. M., Schellekens P. T., Goudsmit J., Huisman H. G., Miedema F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol. 1989 May;63(5):2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Joy A. E., Temin H. M. Correlation between cell killing and massive second-round superinfection by members of some subgroups of avian leukosis virus. J Virol. 1980 Jan;33(1):494–506. doi: 10.1128/jvi.33.1.494-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink M. C., Yager J. A., Myers J. D. Pathogenesis of caprine arthritis encephalitis virus. Cellular localization of viral transcripts in tissues of infected goats. Am J Pathol. 1990 Apr;136(4):843–854. [PMC free article] [PubMed] [Google Scholar]