Abstract
In photodynamic therapy (PDT), visible light activates a photosensitizing drug added to a tissue, resulting in singlet oxygen formation and cell death. Assessed by confocal microscopy, the photosensitizer phthalocyanine 4 (Pc 4) localizes primarily to mitochondrial membranes in cancer cells, resulting in mitochondria-mediated cell death. A Pc 4 derivative, Pc 181, accumulates into lysosomes. In comparison to Pc 4, Pc 181 was a more effective photosensitizer promoting killing cancer cells after PDT. The mode of cell death after Pc 181-PDT is predominantly apoptosis, and pancaspase and caspase-3 inhibitors prevent onset of the cell death. To assess further how lysosomes contribute to PDT, we monitored cell killing of A431cells after PDT in the presence and absence of bafilomycin, an inhibitor of the acidic vacuolar proton pump that collapses the pH gradient of the lysosomal/endosomal compartment. Bafilomycin by itself did not induce toxicity but greatly enhanced Pc 4-PDT-induced cell killing. In comparison to Pc 4, less enhancement of cell killing by bafilomycin occurred after Pc 181-PDT at photosensitizer doses producing equivalent cell killing in the absence of bafilomycin. These results indicate that lysosomal disruption can augment PDT with Pc 4, which targets predominantly mitochondria, but less so after PDT with Pc 181, since Pc 181 already targets lysosomes.
Keywords: Apoptosis, iron, lysosomes, mitochondria, reactive oxygen species
1. INTRODUCTION
Photodynamic therapy (PDT) involves the administration of a photosensitizing drug, followed by photoirradiation with light of a wavelength absorbed by the photosensitizer. PDT to target cells and tissues results in generation of singlet oxygen (1O2) and/or other reactive oxygen species (ROS) which oxidize tissue lipids and proteins, creating an oxidative stress and killing the cells.
The sub-cellular localization of a photosensitizer determines the site and extent of the initial photodynamic damage during PDT (reviewed in (1)). Photosensitizers usually target three main organelles; mitochondria, endoplasmic reticulum and lysosomes (1–3). PDT mediated by a mitochondrion-targeted photosensitizer dissipates the mitochondrial membrane potential (2), while alternatively, PDT with a lysosome-targeted photosensitizer causes the release of proteolytic lysosomal enzymes (4). Mitochondria-targeted photosensitizers are usually regarded as more efficient inducers of cell killing compared to photosensitizers directed to endoplasmic reticulum or lysosomes. This is thought to stem from the observations that mitochondria-targeted photosensitizers directly induce apoptotic death (1).
For our previous studies, we have used phthalocyanine Pc 4 as a photosensitizer. Pc 4 is a silicon phthalocyanine bearing a dimethylaminopropylsiloxy ligand on the central silicon (5). Pc 4 localizes primarily to mitochondria and endoplasmic reticulum, and in lesser extent to lysosomes, as assessed by confocal microscopy (2,3). Pc 4-PDT induces apoptotic cell death that is mediated by formation of mitochondrial ROS leading to onset of the mitochondrial permeability transition, mitochondrial swelling, and cytochrome c release (Fig. 1) (2). The Kenney group at the Case Western Reserve has recently synthesized a series of analogues of Pc 4 (6,7). These new sensitizers bear two Pc 4-type ligands (Pc 12) or a Pc 4-type ligand and various hydroxylated substituents (Pc 135, Pc 161, and Pc 181 (6). Pc 4 analogues bearing two axial ligands with hydroxylated ligands preferentially localize to lysosomes. Unexpectedly, the lysosome-targeted photosensitizers were more efficient photosensitizers for cells in vitro than those with preferential binding to mitochondria and endoplasmic reticulum. The mechanisms underlying this enhanced PDT-mediated cell killing by the lysosome-directed photosensitizers are not clear. Thus, the aim of this study was to determine how lysosomes contribute to PDT induced by the mitochondrial-targeted photosensitizers such as Pc 4. Our results demonstrate that inhibition of the acidic vacuolar proton pump that collapses the pH gradient of the lysosomal/endosomal compartment greatly enhances mitochondrial-targeted Pc 4-PDT mediated apoptotic death. These results indicate that lysosomal disruption can augment PDT with Pc 4, which targets predominantly mitochondria, but less so after PDT with Pc 181, since Pc 181 already targets lysosomes.
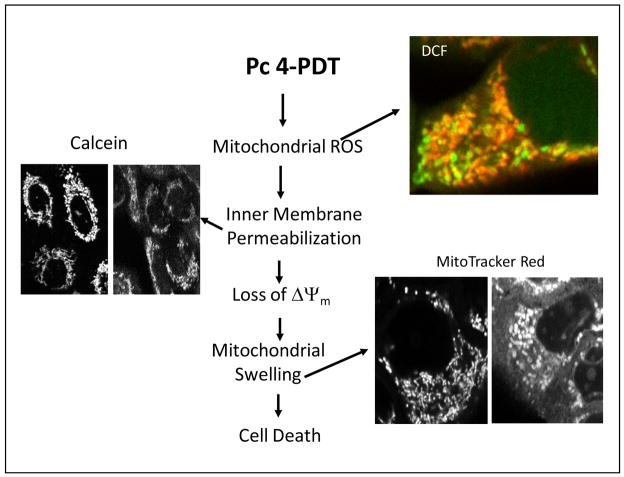
Fig. 1. Pc 4-PDT-mediated mitochondrial events.
Pc 4-PDT induces rapid mitochondrial ROS formation assessed by DCF fluorescence. Green fluorescence is DCF fluorescence, and red fluorescence is TMRM fluorescence. Orange-yellow fluorescence indicates polarized mitochondria, who have increased ROS formation. Green fluorescence indicates depolarized mitochondria with increased ROS production. Inner membrane permeabilization is monitored with calcein fluorescence. Punctate mitochondrial calcein fluorescence changes to diffuse calcein fluorescence as a response of the leakage of calcein from mitochondria due to mitochondrial inner membrane permeabilization. This event is followed by mitochondrial depolarization and swelling, as assessed by MitoTracker Red.
2. METHODS
2.1. Cell Culture
Human A431 epidermoid carcinoma cells were used for the study. Cells were grown in DMEM medium containing 10% fetal bovine serum (FBS) and penicillin/streptomycin (100 μg/ml) in 5% CO2/95% air at 37°C in a humidified incubator. For PDT, cells were loaded with Pc 4 overnight and then exposed to red light (λ = 670 nm) from an Intense-HPD 7404 diode laser (North Brunswick, NJ).
2.2. Caspase-3/7 activity
Caspase-3/7 activity was measured using a Caspase-Glo™ 3/7 kit (Promega) according to the manufacturer’s instructions. At indicated time point, cultured A431 cells were collected into a test-tube followed by centrifugation. The pellet was resuspended and lysed with RIPA buffer. Caspase-Glo™ 3/7 reagent and the lysate were mixed in 1:1 ratio, and luminescence was measured with a luminometer. The resulting luminescence is proportional to caspase activity.
2.3. Subcellular localization of photosensitizers
Localization of Pc 4 and Pc 181 in cells was assessed by confocal microscopy. Briefly, to determine the mitochondrial localization of Pc 4 and Pc 181, cells were preincubated with photosensitizers for 18–20 h followed by incubation with MitoTracker Green, a mitochondria specific probe. Confocal images were collected with a Zeiss LSM 510 NLO laser scanning confocal microscope.
2.4. Mitochondrial membrane potential
Cells were loaded with 150 nM tetramethylrhodamine methylester (TMRM) for 30 minutes in culture medium. After collecting a baseline image, cells were exposed to laser light (198 mJ/cm2) and images were subsequently collected over time using confocal microscopy (543-nm excitation, 560-nm emission).
2.5. Assessment of cell death
Cell death was assessed with propidium iodide (PI) uptake using a multi-well fluorescence plate reader, as previously described (8). Increased PI fluorescence correlates closely with trypan blue uptake (8).
2.6. Clonogenic assay
Cells were exposed to PDT and immediately harvested by trypsinization. Aliquots of cell suspensions were plated onto 60-mm petri dishes in amounts sufficient to yield 50–150 colonies per dish. After 14 days, colonies were stained with 0.1% crystal violet in 20% ethanol and counted by eye.
3. RESULTS
3.1. Pc 4-PDT induces mitochondrial damage
Previously, we showed that Pc 4 preferentially binds to endoplasmic reticulum, Golgi, and mitochondria in A431, L5178Y-R, DU145, and PC-3 cells and induces rapid apoptotic death after exposure to light (2,3,9). However, Pc 4’s binding to mitochondria seems to be the most important factor regarding its efficacy to kill cancer cells. In cancer cells, Pc 4-PDT increases mitochondrial ROS formation within 5 min, as measured by the conversion of non-fluorescent 2′,7′-dichlorofluorescin to fluorescent 2′,7′-dichlorofluorescein (DCF) (2). Mitochondrial ROS is followed by onset of the mitochondrial inner membrane permeabilization, mitochondrial depolarization and swelling, cytochrome c release, and apoptotic death (Fig. 1) (2).
3.2. Intracellular localization determines efficacy of the photosensitizer
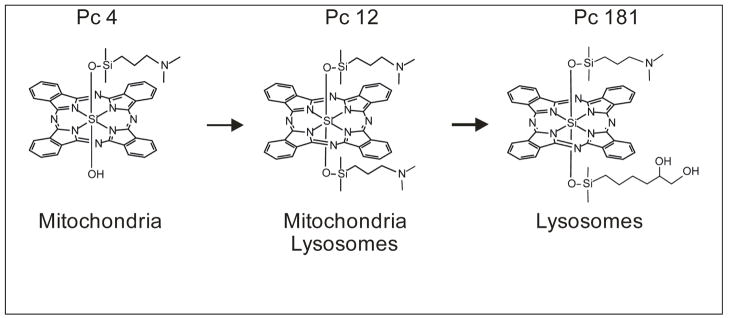
Recently, Pc 4 derivatives with OH groups added to one of the axial ligands were synthesized (Fig. 2) (6). This modification results in change of the photosensitizers’ sub-cellular localization. The parent compound Pc 4 primarily localizes to mitochondria, whereas Pc 181 localizes to lysosomes. A large hydroxylated axial ligand on the Pc 181 structure seems to be responsible for the lysosomal localization. Pc 12 serves as a model compound, which has properties of both mitochondrion- and lysosome-targeted photosensitizer (Fig. 2) (6). Sub-cellular localization is also related to killing efficacy. LD90 dose for Pc 4 and Pc 181 was 100–200 nM and ~20 nM, respectively, as assessed by a clonogenic assay (6).
Fig. 2.
Photosensitizer structure determines sub-cellular localization and killing efficacy
3.3. Contribution of bafilomycin to Pc 4-mediated cell death
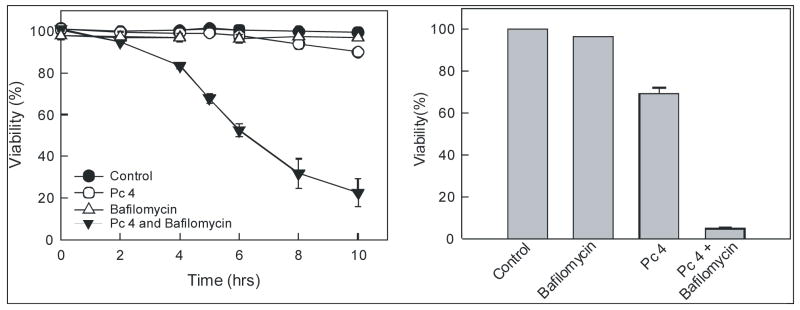
Lysosomes are a primary source for cellular “chelatable iron” that represents free iron and iron bound loosely to a wide variety of anionic intracellular molecules. Chelatable Fe2+ reacts with H2O2 to generate the highly reactive and toxic hydroxyl radical (OH•). The importance of iron-catalyzed OH• formation in cell killing is underscored by the fact that iron chelator, desferrioxamine, suppresses ROS generation and prevents the mitochondrial permeability transition and cell death after oxidative stress to hepatocytes (10). Since lysosome-targeted photosensitizer Pc 181 is more efficient at killing cancer cells after PDT than the mitochondrion-targeted Pc 4, we hypothesized that the possible mechanism underlying this enhanced killing is release of Fe2+ from lysosomes to the cytosol. Fe2+ then induces ROS generation in the cytosol or is taken up by mitochondria to participate in intramitochondrial Fenton-mediated free radical chemistry. Bafilomycin is an inhibitor of the vacuolar proton-pumping ATPase that collapses acidic lysosomal/endosomal pH gradients and increases cytosolic iron concentration (10,11). We determined the contribution of bafilomycin to Pc 4-PDT-mediated cell killing in A431 cells. Cells were loaded with 50 nM Pc 4 for 18 h followed by 1 hour incubation with 50 nM bafilomycin. Subsequently, cells were exposed to light (198 mJ/cm2) at 37°C. Pc 4-PDT alone caused little cell killing as evaluated by PI fluorometry (Fig. 3; left panel). Similarly, bafilomycin alone caused no cell killing over untreated cells. However, the combination of Pc 4-PDT plus bafilomycin caused substantial cytotoxicity over either Pc 4-PDT or bafilomycin alone (Fig. 3; left panel). The results were confirmed with a clonogenic assay (Fig. 3; right panel).
Fig. 3. Bafilomycin enhances Pc 4-PDT-mediated cell death.
Left Panel: A431 cells were incubated with Pc 4 (50 nM) for 18 h followed by incubation with bafilomycin (50 nM) for 1 h. Subsequently, cells were exposed to 670-nm light (198 mJ/cm2). Viability was assessed by PI exclusion using fluorometry. Right panel: Cells were treated and irradiated as in the left panel. Subsequently, cells were trypsinized and plated on Petri dishes. After 14 days, colonies were stained with crystal violet. Results are expressed as mean ± S.E.M. from at least 3 independent experiments for each treatment group.
3.4. Contribution of bafilomycin to Pc 4-mediated mitochondrial depolarization
To further characterize cellular responses after Pc 4-PDT with and without bafilomycin, we monitored mitochondrial membrane potential with TMRM using confocal microscopy. After exposure to low dose of Pc 4-PDT (50 nM), small decrease of TMRM fluorescence became evident after 60 minutes (Fig. 4; upper panels). Bafilomycin (50 nM) alone caused no decrease of TMRM fluorescence (Fig. 4; middle panels). By contrast after exposure to Pc 4-PDT plus bafilomycin together, TMRM fluorescence decreased after 5 minutes, and all TMRM fluorescence was lost within 60 minutes (Fig. 4; lower panels). These results indicate that bafilomycin-mediated effect is directed to mitochondria.
Fig. 4. Bafilomycin enhances Pc 4-PDT-mediated mitochondrial depolarization.
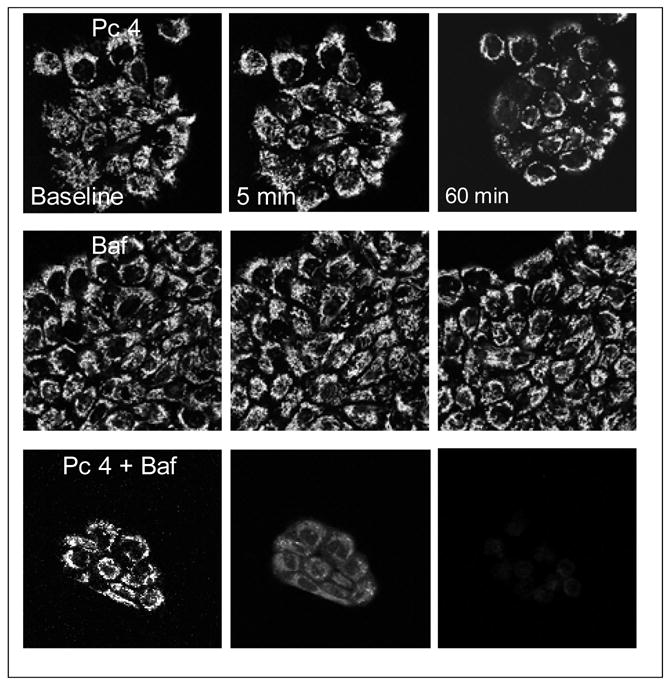
A431 cells were cultured on glass-bottomed Petri dishes, incubated with Pc 4 (50 nM) for 18 h and subsequently incubated with bafilomycin (50 nM) for 1 h before light exposure. Cells were loaded with 100 nM TMRM and Petri dishes were placed on a microscope stage at 37°C. After collecting a baseline image, cells were irradiated at 198 mJ/cm2 and subsequently images were collected over time.
3.5. Contribution of bafilomycin to Pc 4-mediated caspase-3 activation
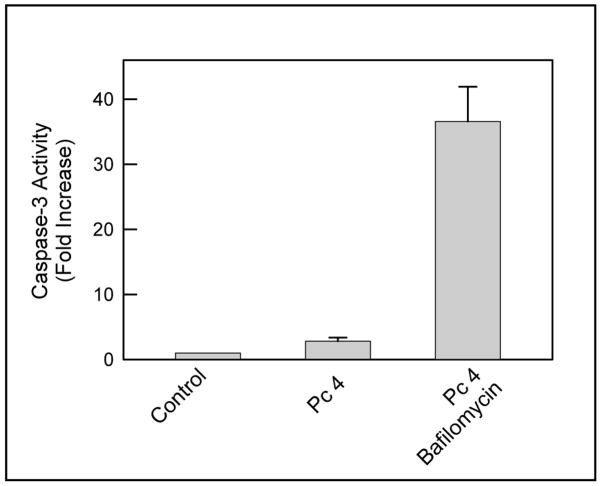
Since bafilomycin had such a remarkable effect on mitochondrial membrane potential, we determined whether bafilomycin also enhances caspase-3 activation after Pc 4-PDT. A431 cells were exposed to light (198 mJ/cm2) and incubated for 2 h. Subsequently, whole cell lysates were prepared for measurement of caspase-3 activity. Pc 4-PDT alone induced a small increase in caspase-3 activity, whereas bafilomycin greatly enhanced Pc 4-PDT-mediated activity (Fig. 5). These results indicate that the damage to mitochondria induced by bafilomycin during PDT is such that it allows cytochrome c to be released from mitochondria to activate caspase-3.
Fig. 5. Bafilomycin enhances Pc 4-PDT-mediated caspase-3 activation.
A431 cells were incubated with Pc 4 (50 nM) for 18 h and subsequently incubated with bafilomycin (50 nM) for 1 h before light exposure. Cells were irradiated at 198 mJ/cm2 at 37°C. After 2 h of post-PDT, whole cell lysates were prepared and caspase-3 activity was measured as described in Methods. Results are expressed as mean ± S.E.M. from three independent experiments.
4. DISCUSSION
Our results show that bafilomycin greatly accelerates mitochondrion-specific Pc 4-PDT-mediated cell killing. Bafilomycin acts on lysosomes/endosomes inhibiting the vacuolar proton-pumping ATPase, collapsing acidic lysosomal/endosomal pH gradients and increasing cytosolic chelatable iron. Although bafilomycin acts on lysosomes its toxic effects were manifested in mitochondria, suggesting a cross talk between lysosomes and mitochondria. Lysosomes are a major source of cellular chelatable Fe2+. Chelatable Fe2+ reacts with H2O2 to generate the highly reactive and toxic OH•. The importance of iron-catalyzed ROS formation in cell killing is underscored by the fact that desferrioxamine suppresses ROS generation and prevents the mitochondrial permeability transition and cell death after oxidative stress to hepatocytes (10). A large portion of mitochondrial ROS formation occurs inside mitochondria, as documented by increased mitochondrial DCF fluorescence (Fig. 1). In order for Fe2+ to effectively participate in mitochondrial ROS formation, Fe2+ needs to enter mitochondria. Mitochondrial Fe2+ uptake by the Ca2+ uniporter can increase intramitochondrial Fe2+ concentration to facilitate ROS generation. Our results suggest that release of lysosomal chelatable Fe2+ to the cytosol, subsequent uptake of Fe2+ by mitochondria and participation in ROS production may be the mechanism by which bafilomycin induces mitochondrial dysfunction and cell death during PDT.
Many lysosomal enzymes, including cathepsins, are overexpressed in cancers (12–14). Enhancing the lysosomal cell death pathway is an alternative strategy to kill cancer cells that have become resistant to caspases. Lysosomes contain a number of proteases, including the aspartic acid protease cathepsin D, the cysteine protease cathepsin B, and a family of papain-like cathepsins related to cathepsin B (15). Several of these enzymes are released to the cytosol during lysosomal permeabilization (15). Although multiple lysosomal proteases may be released to the cytosol during lysosomal permeabilization, the mechanism(s) by which they induce apoptosis are not fully understood. It is unclear what effects bafilomycin has on the lysosomal membranes, whether bafilomycin only collapses pH gradients allowing iron to be released to the cytosol or if more profound physical damage occurs allowing lysosomal enzymes to escape and participate in cell killing. In any case, lysosomal perturbation by bafilomycin effectively enhances mitochondrial-mediated cell killing during PDT. Agents that disturb lysosomal function could potentially be used as an adjuvant treatment with mitochondria-targeted photosensitizers.
Acknowledgments
This research was supported by grants from the US National Cancer Institute, R01 CA119079 and P30 CA138313 (Hollings Cancer Center Cell and Molecular Imaging Shared Facility).
References
- 1.Oleinick NL, Morris RL, Belichenko I. The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochem Photobiol Sci. 2002;1:1–21. doi: 10.1039/b108586g. [DOI] [PubMed] [Google Scholar]
- 2.Lam M, Oleinick NL, Nieminen AL. Photodynamic therapy-induced apoptosis in epidermoid carcinoma cells. Reactive oxygen species and mitochondrial inner membrane permeabilization. J Biol Chem. 2001;276:47379–47386. doi: 10.1074/jbc.M107678200. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi NS, Wang HW, Nieminen AL, Oleinick NL, Izatt JA. Quantitative analysis of Pc 4 localization in mouse lymphoma (LY-R) cells via double-label confocal fluorescence microscopy. Photochem Photobiol. 2000;71:634–639. doi: 10.1562/0031-8655(2000)071<0634:qaopli>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Reiners JJ, Jr, Caruso JA, Mathieu P, Chelladurai B, Yin XM, Kessel D. Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves Bid cleavage. Cell Death Differ. 2002;9:934–944. doi: 10.1038/sj.cdd.4401048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oleinick NL, Antunez AR, Clay ME, Rihter BD, Kenney ME. New phthalocyanine photosensitizers for photodynamic therapy. Photochem Photobiol. 1993;57:242–247. doi: 10.1111/j.1751-1097.1993.tb02282.x. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez ME, Zhang P, Azizuddin K, Delos Santos GB, Chiu SM, Xue LY, Berlin JC, Peng X, Wu H, Lam M, Nieminen AL, Kenney ME, Oleinick NL. Structural Factors and Mechanisms Underlying the Improved Photodynamic Cell Killing with Silicon Phthalocyanine Photosensitizers Directed to Lysosomes Versus Mitochondria. Photochem Photobiol. 2009 doi: 10.1111/j.1751-1097.2009.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anula HM, Berlin JC, Wu H, Li YS, Peng X, Kenney ME, Rodgers MA. Synthesis and photophysical properties of silicon phthalocyanines with axial siloxy ligands bearing alkylamine termini. J Phys Chem A. 2006;110:5215–5223. doi: 10.1021/jp056279t. [DOI] [PubMed] [Google Scholar]
- 8.Nieminen AL, Gores GJ, Bond JM, Imberti R, Herman B, Lemasters JJ. A novel cytotoxicity screening assay using a multiwell fluorescence scanner. Toxicol Appl Pharmacol. 1992;115:147–155. doi: 10.1016/0041-008x(92)90317-l. [DOI] [PubMed] [Google Scholar]
- 9.Usuda J, Chiu SM, Murphy ES, Lam M, Nieminen AL, Oleinick NL. Domain-dependent photodamage to Bcl-2. A membrane anchorage region is needed to form the target of phthalocyanine photosensitization. J Biol Chem. 2003;278:2021–2029. doi: 10.1074/jbc.M205219200. [DOI] [PubMed] [Google Scholar]
- 10.Uchiyama A, Kim JS, Kon K, Jaeschke H, Ikejima K, Watanabe S, Lemasters JJ. Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology. 2008;48:1644–1654. doi: 10.1002/hep.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagliardi S, Rees M, Farina C. Chemistry and structure activity relationships of bafilomycin A1, a potent and selective inhibitor of the vacuolar H+-ATPase. Curr Med Chem. 1999;6:1197–1212. [PubMed] [Google Scholar]
- 12.Campo E, Munoz J, Miquel R, Palacin A, Cardesa A, Sloane BF, Emmert-Buck MR. Cathepsin B expression in colorectal carcinomas correlates with tumor progression and shortened patient survival. Am J Pathol. 1994;145:301–309. [PMC free article] [PubMed] [Google Scholar]
- 13.Castiglioni T, Merino MJ, Elsner B, Lah TT, Sloane BF, Emmert-Buck MR. Immunohistochemical analysis of cathepsins D, B, and L in human breast cancer. Hum Pathol. 1994;25:857–862. doi: 10.1016/0046-8177(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 14.Sinha AA, Gleason DF, Staley NA, Wilson MJ, Sameni M, Sloane BF. Cathepsin B in angiogenesis of human prostate: an immunohistochemical and immunoelectron microscopic analysis. Anat Rec. 1995;241:353–362. doi: 10.1002/ar.1092410309. [DOI] [PubMed] [Google Scholar]
- 15.Turk B, Turk D, Turk V. Lysosomal cysteine proteases: more than scavengers. Biochim Biophys Acta. 2000;1477:98–111. doi: 10.1016/s0167-4838(99)00263-0. [DOI] [PubMed] [Google Scholar]