Abstract
Summary
The study assessed whether overweight is associated with better bone densities in healthy youth. It was observed that overweight individuals had better BMDs at the hip but not at other sites after controlling for the bone area. Lean body mass was an important determinant of BMDs in men, but both lean and fat mass were important for BMDs in women.
Introduction
The study assessed the relationship of overweight and obesity to the bone mass in young men and women consuming adequate calcium.
Methods
Bone and body composition parameters were measured using dual energy X-ray absorptiometry in overweight men (n = 74) and women (n = 77) in the age group of 20–35 years and compared with controls having normal body mass index (BMI). Biochemical parameters of bone metabolism were also assessed.
Results
After adjustment for whole body bone area, bone mineral densities (BMDs) at femoral neck and hip were significantly higher in overweight individuals when compared with controls. However, BMD at lumbar spine, forearm, and whole body were not significantly different in the two BMI groups. Overweight women had lower vitamin D and higher parathormone levels than controls. Regression analyses indicated that height was an important determinant of BMD at most of the skeletal sites in both men and women. Lean body mass was an important determinant of BMDs in men, but both lean and fat mass were important for BMDs in women.
Conclusion
Overweight may be associated with better BMDs at the hip but not at other sites after controlling for the bone area. Body composition parameters may have sex-specific associations with BMD.
Keywords: Bone mineral density, Indian youth, Obesity, Lean body mass, Fat mass, Vitamin D
Introduction
Extensive epidemiological data show that high body weight or body mass index (BMI) is associated with high bone mass and reduced risk of fractures [1–3]. Several explanations have been proposed for this relationship. Body weight is thought to affect the bone mass by mechanical loading of the skeleton and by increasing the stress through muscle pull [4, 5]. In addition, higher fat mass may also have positive influence on the bone mass of women as aromatization of androgen to estrogen takes place in fatty tissue [6].
Obesity, however, may be associated with Vitamin D insufficiency and secondary hyperparathyroidism due to reduced availability of Vitamin D3 from cutaneous and dietary sources because of its deposition in body fat compartments [7]. Histomorphometric studies of obese subjects also indicated the possible existence of secondary hyperparathyroidism [8].
Though the positive relationship of body weight to the bone mass is well recognized, results of the studies indicating the relationship of the individual components of body weight (fat and lean tissue) with bone mass are equivocal. Several studies have indicated a positive association between bone mass and lean tissue [9–11]. Whereas a number of other studies have demonstrated that both fat mass and lean mass contribute equally to bone mass especially in women [12–14].
A few epidemiological studies have indicated that adipose tissue may not be beneficial to bones [15, 16]. A study from the USA among adolescents and young adults indicated that fat mass, after accounting for lean mass, had a negative correlation with parameters related to the structure and strength of bone. Only the lean body mass was positively related to bone mass in this study [15].
A number of studies from India have indicated poor bone mass and early onset of osteoporosis and fractures in individuals from the low income group [2, 17, 18]. These studies have shown that body weight is one of the most important determinants of bone density and may be protective against fractures. At the same time, as a result of nutrition transition, over weight and obesity with their associated metabolic consequences are a rapidly escalating problem especially in the high-income group [19, 20]. In addition, Indians and other Asian populations are known to have a higher proportion of body fat for a given BMI than other ethnic groups [21]. As overweight and obesity may exert opposite influence on the risk of metabolic syndrome and osteoporosis, it is necessary to investigate the body composition parameters that mediate the effect of body weight on bone mass.
Since bone mass attained during young adulthood is an important determinant of osteoporosis in later life, this cross-sectional study examined the relationship of overweight and obesity to the bone mass in young men and women. We hypothesized that overweight and obese men and women will have greater bone densities when compared with controls having normal BMI.
Sample size
Assuming 95% CI, 80% power, SD of spine bone mineral density (BMD) of 0.106 g/cm2, and the expected difference of 0.04 g/cm2 in the spine BMD of the two BMI groups, the required sample size was estimated as 71 in each group. Eighty individuals were enrolled in each BMI and sex group. After using the exclusion criteria mentioned below, data on 75 men and 77 women with normal BMI and 74 men and 77 women with BMI > 25 were used for analysis.
Subjects and methods
The study included healthy participants from the high-income group who had adequate calcium intakes and had no apparent constraints to the bone mass development.
Individuals in the age group of 20 to 35 years living a high-income group residential area in Hyderabad were identified by home visits and were requested to participate in the study. Consenting eligible individuals were enrolled in the study until the required sample size in each BMI and sex group was completed. Presence of medical conditions and intake of drugs affecting bone metabolism were regarded as exclusion criteria. Other exclusion criteria included excessive intake of alcohol or caffeine, heavy smoking, residence in fluorotic area, history of lactose intolerance, and breastfeeding within 12 months postpartum in case of women. Elite athletes and other competitive sports persons were also excluded as they have different activity pattern than the general population. Background information regarding socioeconomic status, and information regarding age at menarche, parity, duration of breastfeeding in women was recorded. Weight was measured without footwear to the nearest 0.1 kg on lever type SECA balance (Hamburg, Germany). Height was measured to the nearest 0.1 cm with a stadiometer (SECA, UK). All subjects gave their informed consent to participate in the study. The research protocol was approved by the Institutional Ethics Committee of National Institute of Nutrition (Hyderabad, India).
Their dietary calcium intakes were estimated by a standardized Food Frequency Questionnaire, as described elsewhere [2]. Physical activity was assessed by a pre-tested questionnaire and the overall activity was classified on a scale of 1 to 4 (low to high) based on the bone loading effect of the activities. Sunlight exposure was assessed by documenting average duration of exposure and percentage of the body surface area exposed daily [22].
BMD measurements were carried out using dual-energy X-ray absorptiometry (Hologic QDR 4500 W, Waltham MA, USA) at anteroposterior lumbar spine (L1–L4), hip, forearm as well as whole body including body composition. All scans and analyses were carried out according to the manufacturer’s instructions by a trained technician. The scanner was calibrated daily, and its performance was monitored as per the quality assurance protocol. No sign of scanner drift was observed during the study period. The in vivo precision (coefficient of variation, cv%) was 1% for lumbar spine and hip BMD and <1% for whole body bone mineral content (BMC) measurements. Manufacturer’s normative data were used as a reference range.
A fasting blood sample was drawn in the morning between 0900 to 1000 hours in all the subjects and the estimations of biochemical parameters were carried out using standard procedures. Hemoglobin was estimated by the cyanmethaemoglobin method, serum albumin [23], serum tartrate resistant acid phosphatase [24], and serum bone-specific alkaline phosphatase [25] estimations were carried out on the same day.
The serum was processed, preserved, and estimation of serum calcium was done within a week of sample collection using atomic absorption spectroscopy. Serum 25 (OH) vitamin D was estimated using radioimmunoassay (Dia Sorin Inc., Stillwater, Minnesota, USA). Estimation of intact human parathyroid hormone was done using immunoradiometric assay (Dia Sorin Inc., Stillwater, MN, USA).
Statistical analysis
Mean and SE values were computed for continuous variables and percentages were calculated for categorical variables. Mean values were compared for all the parameters by Student’s t test between groups and also to verify the homogeneity variances between groups. Stepwise regression model was used to study the relationship of BMD at different skeletal sites with age, height, BMI, fat mass, lean mass, dietary calcium intake, serum 25(OH) vitamin D, and serum parathyroid hormone (PTH). Level of significance was considered as 0.05.
Results
A total of 303 men and women participated in the study. Their distribution in the two BMI groups and the characteristics are indicated in Table 1. The mean age, height, and calcium intakes were not significantly different in the two BMI groups among men as well as women. As the two groups were based on BMI, mean (SE) weight was significantly higher in the high BMI (HBMI) when compared to the normal BMI (NBMI) group among both the sexes (85.8 (1.2) vs. 69.5 (0.9) kg in men and 72.5 (1.06) vs. 56.5 (0.72) kg in women). The mean BMI was about six units higher in the HBMI group than NBMI group in both men and women. Both lean and fat mass were significantly higher in the HBMI group than that of NBMI group among men as well as women. Percent fat was higher by about six units in the HBMI group than NBMI group in both the sex groups.
Table 1.
Characteristics of the study participants (Mean ± SE)
| Males | Females | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NBMI (75) | HBMI (74) | P value | NBMI (77) | HBMI (77) | P value | |||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |||
| Age | 26.8 | 0.5 | 27.2 | 0.5 | 0.542 | 28.6 | 0.53 | 28.7 | 0.53 | 0.850 |
| Height | 173.2 | 0.8 | 172.3 | 0.7 | 0.442 | 158.5 | 0.63 | 157.8 | 6.20 | 0.466 |
| Weight | 69.2 | 0.9 | 85.8 | 1.2 | <0.001 | 56.5 | 0.72 | 72.5 | 1.06 | <0.001 |
| BMI | 23.0 | 0.2 | 28.8 | 0.3 | <0.001 | 22.5 | 0.22 | 29.1 | 0.37 | <0.001 |
| Calcium intake | 1054 | 46 | 1055 | 45 | 0.983 | 969 | 34 | 934 | 29 | 0.576 |
| Lean mass | 48.0 | 0.7 | 55.6 | 0.7 | <0.001 | 35.3 | 0.6 | 41.0 | 0.5 | <0.001 |
| Fat mass | 16.4 | 0.5 | 25.2 | 0.7 | <0.001 | 18.8 | 0.5 | 28.0 | 0.6 | <0.001 |
| Fat percent | 23.9 | 0.6 | 30.0 | 0.6 | <0.001 | 33.4 | 0.6 | 39.1 | 0.4 | <0.001 |
| LTM percent | 69.9 | 1.0 | 65.1 | 0.5 | <0.001 | 62.7 | 1.0 | 56.8 | 0.4 | <0.001 |
Numbers enclosed in parentheses are samples. Age in years; height in centimeters; weight, lean mass, and fat mass in kilograms; calcium intake in milligram per day
BMI body mass index, NBMI normal BMI, LTM lean tissue mass, HBMI BMI > 25, NBMI BMI ≤ 25
Mean exposure time to the sunlight and percent of body exposed to the sun were not different between NBMI and HBMI groups. Significantly higher percent of subjects belonging to HBMI, both male and female, were physically active as compared to NBMI group.
Biochemical parameters
Men and women in the HBMI group had significantly higher hemoglobin levels than men and women in the normal BMI group (P < 0.01). Serum albumin and serum creatinine were not significantly different in the two BMI groups of men and women (Table 2).
Table 2.
Biochemical parameters
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NBMI | HBMI | P value | NBMI | HBMI | P value | |||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |||
| Hemoglobin (g/l) | 143 | 0.9 | 150 | 1.9 | 0.003 | 123 | 2.0 | 133 | 9.7 | 0.001 |
| Serum albumin(g/l) | 47 | 0.5 | 47 | 0.4 | 0.970 | 44 | 0.5 | 43 | 0.3 | 0.924 |
| Serum calcium (mmol/l) | 2.5 | 0.3 | 2.5 | 0.2 | 0.890 | 2.4 | 0.2 | 2.3 | 0.1 | 0.324 |
| Serum phosphorus (mmol/l) | 1.4 | 0.1 | 1.5 | 0.1 | 0.100 | 1.4 | 0.2 | 1.4 | 0.1 | 0.846 |
| Serum alkaline phosphatase (U/l) | 193.8 | 8.4 | 193.9 | 6.0 | 0.992 | 153.6 | 6.5 | 15.8 | 6.4 | 0.895 |
| Serum 25(OH) Vit D (nmol/l) | 58.7 | 5.5 | 51.4 | 3.8 | 0.276 | 50.7 | 6.1 | 24.5 | 3.3 | 0.001 |
| Serum PTH (pmol/l) | 3.9 | 0.4 | 5.2 | 0.6 | 0.085 | 4.7 | 0.5 | 6.8 | 0.6 | 0.009 |
| Serum creatinine (μmol/l) | 123.8 | 34.1 | 132.6 | 32.7 | 0.100 | 114.9 | 4.4 | 106.1 | 9.9 | 0.131 |
| Urinary fluoride (μg/ml) | 0.7 | 0.04 | 0.8 | 0.04 | 0.89 | 0.6 | 0.1 | 0.8 | 0.1 | 0.012 |
BMI body mass index, NBMI normal BMI, HBMI BMI > 25, NBMI BMI ≤ 25
There were no significant differences in bone related biochemical parameters such as serum calcium, phosphorous, and alkaline phosphatase between the BMI groups among both men and women. However, serum 25(OH) vitamin D was significantly lower in HBMI women when compared to NBMI women (mean (SE) 24.5 (3.3) vs. 50.7(6.1) nmol/l, P = 0.001). Similarly, immunoreactive PTH was significantly higher in HBMI women when compared with NBMI women (mean (SE) 6.8(0.6) vs. 4.7(0.5) pmol/l, P = 0.009). Serum 25(OH) Vitamin D and PTH were not significantly different between the two BMI groups of men. Mean urinary fluoride levels, though significantly higher in the HBMI women than NBMI women, were in the normal range in all the groups.
Bone parameters
The BMC and BMD at femoral neck, hip, and lumbar spine were significantly higher in the HBMI group when compared to the NBMI group among both men and women (Figs. 1 and 2).The mean (SE) whole body bone area (WB-BA) was significantly higher among the high BMI group when compared to normal BMI group among both men and women (2,150 (20) vs. 2.086 (24) cm2 in men and 1,809 (15) vs. 1,733 (16) cm2 in women, P = 0.02 and 0.01 for men and women respectively) indicating larger skeletal frame in those having high BMI. As the areal BMD is known to increase with increase in bone area [26], it was thought that the higher BMD values observed in the individuals with high BMI may be because of their higher bone area. To eliminate the effect of different bone areas in the two BMI groups, we compared the BMC and BMD values in the two BMI groups after controlling for the WB-BA. The adjusted values are, therefore, presented in Table 3.
Fig. 1.
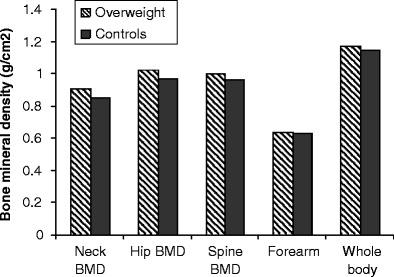
Bone mineral densities at different skeletal sites in the two BMI groups of men. There were significant differences in the BMD in the two BMI groups of men at femoral neck (p = 0.006), hip (p = 0.008), and lumbar spine (p = 0.043)
Fig. 2.
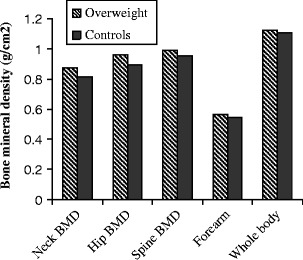
Bone mineral densities at different skeletal sites in the two BMI groups of women. There were significant differences in the BMD of the two BMI groups of women at femoral neck (p < 0.001), hip (p < 0.001), lumbar spine (p = 0.018), and forearm (p = 0.001)
Table 3.
Bone parameters in the two BMI groups men and women (Mean ± SE; adjusted for WB-BA)
| Men | Women | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NBMI | HBMI | P value | NBMI | HBMI | P value | ||||||
| Femoral neck | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |||
| BMC | 4.603 | 0.077 | 4.867 | 0.078 | 0.019 | 3.822 | 0.053 | 3.966 | 0.048 | 0.049 | |
| BMD | 0.861 | 0.013 | 0.899 | 0.013 | 0.048 | 0.827 | 0.012 | 0.866 | 0.011 | 0.023 | |
| Z score | −0.39 | 0.10 | −0.10 | 0.10 | 0.034 | −0.10 | 0.11 | 0.25 | 0.10 | 0.021 | |
| Hip | BMC | 37.529 | 0.617 | 39.380 | 0.621 | 0.038 | 27.611 | 0.393 | 28.158 | 0.3551 | 0.310 |
| BMD | 0.979 | 0.013 | 1.013 | 0.013 | 0.063 | 0.908 | 0.011 | 0.950 | 0.010 | 0.006 | |
| Z score | −0.33 | 0.08 | −0.08 | 0.08 | 0.048 | −0.23 | 0.09 | 0.11 | 0.08 | 0.006 | |
| Spine | BMC | 61.160 | 0.927 | 60.181 | 0.934 | 0.462 | 51.687 | 0.788 | 50.437 | 0.715 | 0.249 |
| BMD | 0.972 | 0.011 | 0.988 | 0.011 | 0.264 | 0.967 | 0.011 | 0.983 | 0.010 | 0.299 | |
| Z score | −1.07 | 0.10 | −0.92 | 0.10 | 0.274 | −0.64 | 0.10 | −0.51 | 0.09 | 0.359 | |
| Forearm | BMC | 16.105 | 0.188 | 16.109 | 0.188 | 0.989 | 10.942 | 0.110 | 10.875 | 0.099 | 0.658 |
| BMD | 0.633 | 0.005 | 0.636 | 0.005 | 0.681 | 0.553 | 0.004 | 0.562 | 0.004 | 0.148 | |
| Z score | −0.80 | 0.09 | −0.74 | 0.09 | 0.644 | −0.08 | 0.09 | 0.13 | 0.08 | 0.072 | |
| Whole Body | BMC | 2437 | 24 | 2487 | 24 | 0.145 | 1998 | 16 | 2000 | 14 | 0.949 |
| BMD | 1.153 | 0.009 | 1.163 | 0.009 | 0.422 | 1.122 | 0.009 | 1.121 | 0.008 | 0.970 | |
| Whole Body | BMC % | 3.5 | 0.1 | 2.9 | 0.1 | <0.001 | 3.5 | 0.1 | 2.8 | 0.1 | <0.001 |
WB-BA whole body bone area, BMI body mass index, NBMI normal BMI, HBMI BMI > 25, NBMI BMI ≤ 25, BMC bone mineral content in g, BMD bone mineral density in g/cm2
Z scores for the whole body BMD were not available
It was observed that BMC, BMD, and Z score at femoral neck were significantly higher in the HBMI group when compared to NBMI group among both men and women (P < 0.05). At the hip, BMC and Z score were significantly higher in the HBMI men when compared to NBMI men (P < 0.05), and there was a trend of higher hip BMD in the HBMI men than NBMI men (P = 0.063). HBMI women had significantly higher hip BMD and Z score when compared with NBMI women (P = 0.006). At the lumbar spine and forearm, all the bone parameters including BMC, BMD, and Z score did not differ among the two BMI groups of men and women. Whole body BMC and BMD were also not significantly different among the two BMI groups of men and women. However, whole body BMC% (BMC as percent of body weight) was significantly higher in the HBMI groups of both men and women.
Regression analysis
Regression models were constructed with BMD at each skeletal site as dependent variable and height, BMI, dietary calcium intake, serum 25(OH) vitamin D, serum PTH, total fat mass, and total lean mass as independent variables (Table 4). Predictors of BMD differed between men and women.
Table 4.
Regression models for BMD at different skeletal sites in men and women with age, height, BMI, fat mass, lean mass, dietary calcium intake, physical activity, serum 25(OH) vitamin D, and serum PTH as independent variables
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Dependent variable | Significant variables | R 2 | P value | Significant variables | R 2 | P value |
| Femoral neck BMD | BMI, age (−), height, fat mass (−) | 0.22 | <0.001 | Fat mass, vitamin D (−) | 0.29 | <0.001 |
| Hip BMD | Lean mass | 0.11 | 0.001 | Lean mass, vitamin D (−) | 0.33 | <0.001 |
| Forearm BMD | Height, BMI, fat mass (−) | 0.19 | <0.001 | Lean mass, height | 0.19 | <0.001 |
| Spine BMD | Height, BMI | 0.15 | <0.001 | Fat mass, height, vitamin D (−) | 0.22 | <0.001 |
| WB-BMD | Height, BMI, fat mass (−) | 0.27 | <0.001 | Lean mass, height | 0.15 | <0.001 |
BMI body mass index, BMD bone mineral density, WB-BMD whole body bone mineral density
Men
BMI, height, age, and fat mass were important determinants of femoral neck BMD explaining 22% of variance. BMI and height had positive association whereas age and total fat mass had negative association with femoral neck BMD. Lean mass was the most important determinant of hip BMD whereas height, BMI, and fat mass were important determinants of BMD at forearm. Height and BMI were significant determinants of BMD at spine whereas height, BMI, and fat mass were important for WB-BMD. It can be observed that height, BMI, and lean mass had positive association with BMD at different skeletal sites whereas fat mass consistently showed a negative association with the BMD.
Women
At femoral neck, both fat mass and lean mass were significant, but vitamin D was negatively related to femoral neck BMD. Lean mass and vitamin D were significant determinants of hip BMD also. But again, vitamin D had a negative association with hip BMD. For spine BMD, fat mass and height were positive predictors whereas vitamin D had a negative relationship. Height and lean mass were important determinants of BMD at forearm and whole body explaining 33% and 15% variance, respectively.
Discussion
This study investigates the bone mass in relation to the BMI in young healthy men and women who had adequate calcium intakes. In addition, this study also reveals some interesting relationships between the components of body composition and the BMD at different skeletal sites.
As expected, the mean heights of men and women in both the BMI groups were higher than those reported by the studies in low-income group populations from India [27]. However, the heights were still lower than the young men and women from the developed countries http://www.who.int/growthref/who2007_hight_for_age/en/index.html. Heights were not different among the two BMI groups. The mean calcium intakes were close to 1 g/day in both the BMI and sex groups which can be considered adequate for this age group by western norms [28], though the recommended dietary allowance for calcium in India is much lower [29]. Body composition of women is characteristic of Indian population with high body fat percent of 33.4 even in the NBMI group. As BMI cannot differentiate between fat and fat-free mass, it may not be a reliable measure of obesity. However, we have used this index in the present study because it is the most widely used indicator of obesity. Surprisingly, higher percentage of men and women from the HBMI group were involved in high-impact activities than the NBMI group. However, interviews with the participants revealed that many participants in the HBMI group had started strength training physical exercises within the last 6 months in an attempt to lose weight, and it is unlikely that the physical activity had an influence on BMC and BMD values. Contrary to the speculations that obese men and women may have reduced sunlight exposure due to reduced mobility, sunlight exposure was similar in both the BMI groups (Table 1).
When biochemical parameters were compared between the two BMI groups of men and women, HBMI persons were found to have significantly higher hemoglobin levels than the NBMI persons. It is possible that HBMI persons had higher intake of foods that are rich in iron, folic acid, vitamin B12, etc. However, data on these nutrient intakes are not available. As expected, serum albumin, calcium, phosphorus, alkaline phosphatase, and creatinine values did not differ between the two BMI groups. Though women in the HBMI group had higher urinary excretion of fluoride, the values were in the normal range (Table 2).
It is interesting to note that HBMI women had lower serum vitamin D and higher PTH levels when compared to NBMI women. However, two BMI groups of men did not show differences in the serum vitamin D and PTH levels. A number of studies in men and women have indicated that obesity and increased fat is associated with low serum 25-hydroxy vitamin D concentration [30–32]. The body fat percentage of women in our study was much higher than that of men in this study. This may be responsible for significantly lower circulating vitamin D and higher PTH levels in the HBMI group than NBMI group of women but not in men. Adiposity may be related to low levels of circulating vitamin D through a number of mechanisms. Wortsman et al. demonstrated that there is decreased bioavailability of vitamin D3 from cutaneous and dietary sources in obese individuals because of its deposition in body fat compartments [7]. Bell et al. demonstrated that in obese persons, vitamin D-endocrine system is characterized by secondary hyperparathyroidism and increased serum 1,25 dihydroxy vitamin D [30]. The authors speculated that increased levels of active vitamin D metabolite was responsible for reducing serum 25-hydroxy vitamin D in obese individuals through a negative feedback inhibition of hepatic 25-hydroxy vitamin D synthesis. Other potential reasons may be limited mobility or clothing habits limiting sun exposure in obese individuals. However, estimated sun exposure in HBMI individuals did not differ from NBMI individuals in the present study.
When the bone parameters were analyzed in the two BMI groups of men and women, it was observed that HBMI persons had significantly higher WB-BA when compared to their NBMI counterparts. As skeletal frame size is known to influence the areal BMD measured by DXA [26, 33], we compared the BMC and BMD values between the two BMI groups after controlling for the WB-BA to eliminate the effect of frame size.
Bone mineral density at femoral neck and hip was significantly higher in the HBMI group when compared to NBMI group among men as well as women. Z score values for the femoral neck and hip BMD indicate that the BMD values in the HBMI individuals were close to the western reference standards (Table 3). Considering the bone loading effect of higher weight, it is not surprising to find a higher BMD at these weight-bearing skeletal sites in the HBMI individuals. However, the BMC, BMD, and Z score values at lumbar spine, forearm, and whole body were not significantly different between the two BMI groups in either men or women. In fact, the whole body BMC% which may indicate the mechanical competence of the skeleton was significantly higher in the HBMI groups of both men and women.
A number of previous studies indicated that BMD at lumbar spine, hip, and total body was positively related to body weight and BMI [34] in all the age groups. However, these studies have not controlled for the WB-BA. Therefore, the higher BMD associated with higher weight and BMI in these studies may be a result of larger skeletal frame size of obese individuals. It is known that the bone size independently affects the fracture risk, and one may argue that the higher BMD associated with higher BA may be a better surrogate for fracture risk. However, these findings indicate that high BMI may not be associated with higher BMD at spine, forearm, and whole body when WB-BA is taken into account.
Regression models in Table 4 included the components of body weight, i.e., fat mass and lean mass along with other potential determinants of BMD such as age, height, BMI, serum vitamin D and PTH levels, dietary calcium intake, and physical activity level. It is interesting to note that height was an important determinant of BMD at most of the skeletal sites in men as well as women. Height is known to be closely related to lean body mass; therefore, height may exert its influence on BMD through lean body mass. It was surprising to observe a negative association of femoral neck BMD to age in men, considering the fact the age range of the participants was 20–35 years and BMD is considered to be stable at this age. It may be speculated that age-related loss of BMD at femoral neck started as early as third or fourth decade of life in this group of men. A few other studies have also indicated that femoral neck BMD loss starts in the 20s itself [35].
This study reveals important sex-specific associations between body composition and BMD in these young well-nourished men and women. As reported by a number of previous studies, both lean mass and fat mass were positively related to BMD among women. Studies have indicated that in case of premenopausal women, lean mass is an important determinant of BMD whereas in postmenopausal women, fat mass generally shows greater correlations to BMD than lean mass [36–38]. It is interesting to note that fat mass is an important determinant of BMD in these young premenopausal women as well. Surprisingly, serum vitamin D was a negative predictor of BMD at femoral neck, hip, and spine in women. However, serum vitamin D levels had a negative relationship with the BMI (r = −0.29, P = 0.003) and fat mass (r = −0.24, P = 0.01) and a positive relationship with the height (r = 0.28, P = 0.004). The fact that higher fat mass and height were associated with higher BMDs in women may explain the observed negative relationship of serum vitamin D to the BMD values in women. These findings indicate that mechanical loading effect of weight, lean mass, and fat mass is far more important for BMD despite associated low serum vitamin D level.
In case of men, lean mass was a positive predictor of BMD at hip whereas fat mass had a negative association with BMD at femoral neck, forearm, as well as whole body. This finding concurs with a few other studies which have indicated that fat mass is not beneficial to bone [15, 16]. As increase in fat mass at a given BMI is associated with decrease in lean mass, this finding may simply corroborate importance of lean mass as a determinant of BMD. However, other explanations for this negative relationship are also possible. Adipose tissue is no longer considered a metabolically passive energy reserve, but it secretes multiple adipokines which play role in modulation of various biological functions. Further studies are necessary to investigate the role of adipokines as potential modulators of bone metabolism.
Overweight and obesity with its associated metabolic consequences is a burgeoning problem in India and other countries undergoing nutrition transition. On the other hand, low body weights and BMI are associated with low BMD and increased risk of fracture. This has led to confusion about the desirable body weight that reduces the risk of diabetes, cardiovascular disease, as well as osteoporosis. On this background, this study is important as it demonstrates that a higher lean body mass and not fat mass would be important for better BMDs in men. In case of women, probably the endocrine functions of fat mass may be important for BMD even in young women.
As indicated in Table 1, a higher proportion of HBMI persons were engaged in strength training physical activity. Therefore, physical activity may be a confounding factor for better BMDs. However, physical activity was not a significant predictor of BMD in multiple regression models. Moreover, as mentioned earlier, majority of the HBMI participants had initiated physical exercises within the last 6 months, and it may not be an important factor influencing the BMD.
Limitations of our study include areal BMD measurements by DXA which result in artifactual increases in apparent BMD with body size as a function of increased BMC in bones of greater depth. However, we compared bone parameters in the two BMI groups after controlling for WB-BA to eliminate the effect of different frame sizes.
This study, thus, provides important information regarding the bone mass of young well-nourished Indian men and women having adequate calcium intakes and the contribution of components of body composition to the BMD. Considering the dual burden of increasing obesity in the high-income group and presence of widespread under nutrition in the low-income group in India, these findings help elucidating the importance of lean body mass for better BMDs.
Acknowledgements
This work was supported by Indian Council of Medical Research. The authors declare that they have no conflict of interests. The authors would like to thank the directors of the National Institute of Nutrition, Hyderabad, India who provided the facilities and support system to carry out this work. We thank Mr. R. Sambasiva Rao for his help for the data entry and Mr. R. S. Venkateswara Rao for his help for the assessment of dietary calcium intakes and sunlight exposure. We also thank the participants of the study for their cooperation.
Conflicts of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
All the authors were affiliated to the National Institute of Nutrition during the study period.
References
- 1.Bainbridge KE, Sowers M, Lin X, et al. Risk factors for low bone mineral density and the 6-year rate of bone loss among premenopausal and perimenopausal women. Osteoporos Int. 2004;15:439–446. doi: 10.1007/s00198-003-1562-5. [DOI] [PubMed] [Google Scholar]
- 2.Shatrugna V, Kulkarni B, Kumar PA, et al. Bone status of Indian women from a low-income group and its relationship to the nutritional status. Osteoporos Int. 2005;16:1827–1835. doi: 10.1007/s00198-005-1933-1. [DOI] [PubMed] [Google Scholar]
- 3.Margolis KL, Ensrud KE, Schreiner PJ, et al. Body size and risk for clinical fractures in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 2000;133:123–127. doi: 10.7326/0003-4819-133-2-200007180-00011. [DOI] [PubMed] [Google Scholar]
- 4.Beck TJ, Oreskovic TL, Stone KL, et al. Structural adaptation to changing skeletal load in the progression toward hip fragility: the study of osteoporotic fractures. J Bone Miner Res. 2001;16:1108–1119. doi: 10.1359/jbmr.2001.16.6.1108. [DOI] [PubMed] [Google Scholar]
- 5.Vicente-Rodriguez G, Ara I, Perez-Gomez J, et al. Muscular development and physical activity as major determinants of femoral bone mass acquisition during growth. Br J Sports Med. 2005;39:611–666. doi: 10.1136/bjsm.2004.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McTiernan A, Rajan KB, Tworoger SS, et al. Adiposity and sex hormones in postmenopausal breast cancer survivors. J Clin Oncol. 2005;15:1961–1966. doi: 10.1200/JCO.2003.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 8.Compston JE, Vedi S, Ledger JE, et al. Vitamin D status and bone histomorphometry in gross obesity. Am J Clin Nutr. 1981;34:2359–2363. doi: 10.1093/ajcn/34.11.2359. [DOI] [PubMed] [Google Scholar]
- 9.Liu-Ambrose T, Kravetsky L, Bailey D, et al. Change in lean body mass is a major determinant of change in areal bone mineral density of the proximal femur: A 12-year observational study. Calcif Tissue Int. 2006;79:145–151. doi: 10.1007/s00223-006-0098-z. [DOI] [PubMed] [Google Scholar]
- 10.Travison TG, Araujo AB, Esche GR, et al. The relationship between body composition and bone mineral content: threshold effects in a racially and ethnically diverse group of men. Osteoporos Int. 2008;19:29–38. doi: 10.1007/s00198-007-0431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leslie WD, Weiler HA, Lix LM, et al. Body composition and bone density in Canadian White and Aboriginal women: the First Nations Bone Health Study. Bone. 2008;42:990–995. doi: 10.1016/j.bone.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Lim S, Joung H, Shin CS, et al. Body composition changes with age have gender-specific impacts on bone mineral density. Bone. 2004;35:792–798. doi: 10.1016/j.bone.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Makovey J, Naganathan V, Sambrook P. Gender differences in relationships between body composition components, their distribution and bone mineral density: a cross-sectional opposite sex twin study. Osteoporos Int. 2005;16:1495–1505. doi: 10.1007/s00198-005-1841-4. [DOI] [PubMed] [Google Scholar]
- 14.Lee JS, Kawakubo K, Sato H, et al. Relationship between total and regional bone mineral density and menopausal state, body composition and life style factors in overweight Japanese women. Int J Obes Relat Metab Disord. 2001;25:880–886. doi: 10.1038/sj.ijo.0801620. [DOI] [PubMed] [Google Scholar]
- 15.Janicka A, Wren TA, Sanchez MM, et al. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab. 2007;92:143–147. doi: 10.1210/jc.2006-0794. [DOI] [PubMed] [Google Scholar]
- 16.Hsu YH, Venners SA, Terwedow HA, et al. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr. 2006;83:146–154. doi: 10.1093/ajcn/83.1.146. [DOI] [PubMed] [Google Scholar]
- 17.Gupta AK, Samuel KC, Kurian PM, et al. Preliminary study of the incidence of aetiology of femoral neck fractures in Indians. Indian J Med Res. 1967;55:1341–1348. [PubMed] [Google Scholar]
- 18.Shatrugna V. Osteoporosis in the Asian region: newer questions. In: Shetty P, Gopalan C, editors. Diet, Nutrition and Chronic Diseases: an Asian Perspective. London: London Smith-Gordon; 1998. pp. 81–83. [Google Scholar]
- 19.Misra A, Pandey RM, Devi JR, et al. High prevalence of diabetes, obesity and dyslipidaemia in urban slum population in northern India. Int J Obes Relat Metab Disord. 2001;25:1722–1729. doi: 10.1038/sj.ijo.0801748. [DOI] [PubMed] [Google Scholar]
- 20.Bhardwaj S, Misra A, Khurana L, et al. Childhood obesity in Asian Indians: a burgeoning cause of insulin resistance, diabetes and sub-clinical inflammation. Asia Pac J Clin Nutr. 2008;17:172–175. [PubMed] [Google Scholar]
- 21.Deurenberg-Yap M, Schmidt G, van Staveren WA, et al. The paradox of low body mass index and high body fat percentage among Chinese, Malays and Indians in Singapore. Int J Obes Relat Metab Disord. 2000;24:1011–1017. doi: 10.1038/sj.ijo.0801353. [DOI] [PubMed] [Google Scholar]
- 22.Goswami R, Gupta N, Goswami D, et al. Prevalence and significance of low 25-hydroxyvitamin D concentrations in healthy subjects in Delhi. Am J Clin Nutr. 2000;72:472–475. doi: 10.1093/ajcn/72.2.472. [DOI] [PubMed] [Google Scholar]
- 23.Gustafsson JE. Improved specificity of serum albumin determination and estimation of “acute phase reactants” by use of the broncresol green reaction. Clin Chem. 1976;22:616–622. [PubMed] [Google Scholar]
- 24.Schiele F, Arthur Y, Floc’h AY, et al. Total, tartrate resistant and tartrate-inhibited acid phosphatases in serum biological variations and reference limits. Clin Chem. 1988;34:685–690. [PubMed] [Google Scholar]
- 25.Cadeau BJ, Malkin A. A relative heat stability test for the identification of serum alkaline phosphatase isoenzymes. Clin Chim Acta. 1973;45:235–242. doi: 10.1016/0009-8981(73)90432-4. [DOI] [PubMed] [Google Scholar]
- 26.Lesli WD, Tsang JF, Lix LM. The effect of Total Hip Bone Area on Osteoporosis Diagnosis and Fractures. J Bone Miner Res. 2008;23:1468–1476. doi: 10.1359/jbmr.080416. [DOI] [PubMed] [Google Scholar]
- 27.National Nutrition Monitoring Bureau . Diet and nutritional status of rural population. Hyderabad, India: National Institute of Nutrition; 2002. [Google Scholar]
- 28.Institute of Medicine . Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington: National Academy Press; 1997. [PubMed] [Google Scholar]
- 29.Indian Council of Medical Research . Nutrient requirement and recommended dietary allowances for Indians. NIN: Hyderabad; 1989. [Google Scholar]
- 30.Bell NH, Epstein S, Greem A, et al. Evidence for alteration of vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76:370–373. doi: 10.1172/JCI111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi H, Norman AW, Okamura WH, et al. 1alpha, 25-Dihydroxyvitamin D3 modulates human adipocyte metabolism via nongenomic action. FASEB J. 2001;15:2751–2753. doi: 10.1096/fj.01-0584fje. [DOI] [PubMed] [Google Scholar]
- 32.McKinney K, Breitkopf CR, Berenson AB. Association of race, body fat, and season with vitamin D status among young women: A cross-sectional study. Clin Endocrinol (Oxf) 2008;69(4):535–541. doi: 10.1111/j.1365-2265.2008.03233.x. [DOI] [PubMed] [Google Scholar]
- 33.Wu XP, Liao EY, Liu SP, et al. Relationship of body surface area with bone density and its risk of osteoporosis at various skeletal regions in women of mainland China. Osteoporos Int. 2004;15:751–759. doi: 10.1007/s00198-004-1608-3. [DOI] [PubMed] [Google Scholar]
- 34.Morin S, Tsang JF, Leslie WD. Weight and body mass index predict bone mineral density and fractures in women aged 40 to 59 years. Osteoporos Int. 2008;20(3):363–370. doi: 10.1007/s00198-008-0688-x. [DOI] [PubMed] [Google Scholar]
- 35.Cvijetic S, Colic Baric I, Keser I, et al. Peak bone density in Croatian women: variations at different skeletal sites. J Clin Densitom. 2008;11:260–265. doi: 10.1016/j.jocd.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 36.Douchi T, Yamamoto S, Kuwahata R, et al. Effect of non-weight-bearing body fat on bone mineral density before and after menopause. Obstet Gynecol. 2004;96:13–17. doi: 10.1016/S0029-7844(00)00814-0. [DOI] [PubMed] [Google Scholar]
- 37.Ijuin M, Douchi T, Matsuo T, et al. Difference in the effects of body composition on bone mineral density between pre- and postmenopausal women. Maturitas. 2002;43:239–244. doi: 10.1016/S0378-5122(02)00273-6. [DOI] [PubMed] [Google Scholar]
- 38.Gjesdal CG, Halse JI, Eide GE, et al. Impact of lean mass and fat mass on bone mineral density: The Hordaland Health Study. Maturitas. 2008;59:191–200. doi: 10.1016/j.maturitas.2007.11.002. [DOI] [PubMed] [Google Scholar]


