Abstract
Few studies have investigated the relationship between breakfast consumption and specific adiposity or insulin dynamics measures in children. The goal of this study is to determine whether breakfast consumption is associated with adiposity, specifically intra-abdominal adipose tissue (IAAT), and insulin dynamics in overweight Latino youth. Participants were a cross-sectional sample of 93 overweight (≥85th percentile BMI) Latino youth (10–17 years) with a positive family history of type 2 diabetes. Dietary intake was assessed by two 24-h recalls, IAAT, and subcutaneous abdominal adipose tissue (SAAT) by magnetic resonance imaging, body composition by dual-energy X-ray absorptiometry, and insulin dynamics by a frequently sampled intravenous glucose tolerance test and minimal modeling. Participants were divided into three breakfast consumption categories: those who reported not eating breakfast on either day (breakfast skippers; n = 20), those who reported eating breakfast on one of two days (occasional breakfast eaters; n = 39) and those who ate breakfast on both days (breakfast eaters; n = 34). Using analyses of covariance, breakfast omission was associated with increased IAAT (P = 0.003) independent of age, Tanner, sex, total body fat, total body lean tissue mass, and daily energy intake. There were no significant differences in any other adiposity measure or in insulin dynamics between breakfast categories. Eating breakfast is associated with lower visceral adiposity in overweight Latino youth. Interventions focused on increasing breakfast consumption are warranted.
INTRODUCTION
As prevalence of overweight among adolescents increases in the United States, more youth are at risk of developing numerous illnesses including diabetes mellitus, coronary heart disease, and hypertension (1). In particular, the prevalence of overweight has increased for Latino adolescents (2), who are more insulin resistant than white adolescents (3). We have previously shown that visceral fat was independently and negatively related to insulin sensitivity (SI) in the same cohort of obese Hispanic children with a family history of type 2 diabetes (4). We have also shown that in this cohort the only dietary variables significantly related to insulin dynamics were total sugar, added sugar, and sugary beverage consumption (5). However, the effect of meal patterns on both adiposity parameters and insulin dynamics in this cohort has not yet been assessed.
Although an imbalance in energy intake and expenditure is partly responsible for childhood obesity, several studies have investigated whether meal patterns, particularly breakfast consumption, play a role in increased adiposity. Daily breakfast intake of adolescents in the United States has decreased steadily between 1965 and 1991; the rates of boys and girls declined from 89.7 and 84.4% in 1965 to 74.9 and 64.7%, respectively, in 1991 (ref. 6). Skipping breakfast was shown to be prevalent in some Hispanic youth (7). Studies investigating the relationship between breakfast consumption behavior and adiposity show mixed results. Several studies reveal that adolescent breakfast skippers are more likely to be overweight (6-10), while others show no association between breakfast omission and overweight status (11,12). Recently, the National Longitudinal Study of Adolescent Health found that skipping breakfast increased with time, and this behavior predicted an increased BMI in young adulthood (13).
To our knowledge, no study has investigated the relationship of breakfast consumption to fat distribution, specifically intra-abdominal adipose tissue (IAAT), and insulin dynamics, i.e., SI, acute insulin response (AIR), and disposition index (DI), in adolescents of any ethnicity, especially those at high risk for diabetes such as Latinos. Thus, the purpose of this study was to examine the association among breakfast consumption, IAAT, and insulin dynamics in overweight Latino adolescents. We hypothesize that skipping breakfast would have a negative impact on fat distribution and insulin dynamics.
METHODS AND PROCEDURES
Participants
This study is a cross-sectional analysis of 110 youth (65 males) who were all part of the University of Southern California Study of Latino Adolescents at Risk for Diabetes (SOLAR Diabetes Project), an ongoing longitudinal study to explore risk factors for the development of type 2 diabetes in overweight Latino youth. The design, data collection methods, and findings of the longitudinal SOLAR cohort have been described in previous publications; however, no analyses of meal patterns using this cohort have been previously published (4,5,14).
Subjects were selected at the beginning of the longitudinal study based on the following inclusion criteria: (i) Hispanic origin (determined by self-report and based on both parents and both sets of grandparents reporting to be Hispanic), (ii) family history for type 2 diabetes (at least one parent, grandparent, or sibling), (iii) age 8–13 years, (iv) BMI ≥85th percentile for age and gender based on the Centers for Disease Control and Prevention guidelines (15) and (v) absence of diabetes, established by an oral glucose tolerance test (OGTT). Subjects were ineligible if they were taking medications known to affect body composition, been diagnosed with syndromes or disease known to affect body composition or fat distribution, or had any major illnesses since birth. This study was restricted to a subset of 110 subjects from the original cohort who had dietary data collected within a week of their clinical visit.
None of the 110 participants in the present analysis had diabetes at the time of clinical and dietary data collection. The Institutional Review Board of the Health Sciences Campus at the University of Southern California approved this study. Written informed consent and youth assent were obtained from all parents and subjects before testing commenced.
Body composition
Anthropometric measures were taken at the University of Southern California General Clinical Research Center. BMI and BMI percentiles for age and gender were determined using EpiInfo 2000, version 1.1 (CDC, Atlanta, GA). Height and weight were measured in duplicate using a beam medical scale and wall-mounted stadiometer, to the nearest 0.1 kg and 0.1 cm, and the average was recorded. A whole-body dual-energy X-ray absorptiometry scan was used to determine body composition (total fat mass and total lean tissue mass) using a Hologic QDR 4500W (Hologic, Bedford, MA). IAAT and subcutaneous abdominal adipose tissue (SAAT) were determined by magnetic resonance imaging, which was performed at the Los Angeles County/University of Southern California Imaging Science Center. A single-slice axial TR 400/16 view of the abdomen at the level of the umbilicus was analyzed for cross-sectional area of adipose tissue. The scan lasted ~2 min and a General Electric 1.5 Sigma LX-Echospeed devise with a General Electric 1.5-Tesla magnet was used (Waukesha, WI). Tanner stage was assessed based on breast stage and pubic hair during a history and physical exam conducted by a licensed pediatric health care provider using established guidelines (16).
Insulin and glucose dynamics
Participants arrived at the University of Southern California General Clinical Research Center at ~0800 hours after an overnight fast. A 2-h OGTT was conducted via the ingestion of 1.75 g oral glucose solution/kg body weight (to a maximum of 75 g). Blood samples were taken via antecubital vein catheter for measurement of blood glucose before (fasting) and 2 h after the glucose load. Those subjects with impaired glucose tolerance were still included in the sample; however, those with a type 2 diabetes status were excluded (as defined as having a fasting glucose ≥126 mg/dl or a 2-h glucose ≥200 mg/dl) (ref. 17).
Within 1 month after the OGTT visit, children were asked to come back to the General Clinical Research Center for an overnight visit where a frequently sampled intravenous glucose tolerance test was performed, which determines insulin dynamics, i.e., SI, DI, and AIR. DI is the product of AIR and SI, which gives a measure of β-cell function. After dinner and an evening snack, participants were only allowed to consume water and noncaloric/noncaffeinated beverages between 2,000 hours and the time of the frequently sampled intravenous glucose tolerance test the following morning. A topical anesthetic (EMLA cream; Aztrozeneca, Wilmington, DE) was applied to the antecubital area of both arms and an hour later a flexible intravenous catheter was inserted into both of the arms. Two fasting blood samples, at −15 and −5 min, were obtained for determination of basal glucose and insulin values. At time zero, glucose (25% dextrose; 0.3 g/kg of body weight) was administered intravenously. Blood samples were then collected at the following time points: 2, 4, 8, 19, 22, 30, 40, 50, 70, 100, and 180 min. Insulin was injected intravenously at 20 min (0.02 units/kg of body weight; Humulin R (regular insulin for human participants; Eli Lilly and Company, Indianapolis, IN)). Plasma was analyzed for glucose and insulin, and values were entered into the Minmod Millennium 2003 computer program (version 5.16; Richard N. Bergman, University of Southern California) for determination of SI, AIR, and DI.
Glucose from the OGTT was analyzed on a Dimension Clinical Chemistry system using an in vitro Hexokinase method (Dade Behring, Deerfield, IL). Blood samples from the frequently sampled intravenous glucose tolerance test were centrifuged immediately to obtain plasma, and aliquots were frozen at −70 °C until assayed. Glucose was assayed in duplicate on a Yellow Spring Instrument 2700 Analyzer (Yellow Spring Instrument, Yellow Springs, OH) using the glucose oxidase method. Insulin was assayed in duplicate using a specific human insulin enzyme-linked immunosorbent assay kit from Linco (St Charles, MO).
Dietary intake
Dietary intake was determined from two 24-h diet recalls using the multiple pass technique. The initial dietary recall was collected at the outpatient General Clinical Research Center visit by a bilingual dietary technician and the second 24-h dietary recall was obtained via telephone by the same technician ~1 week after the outpatient visit. The majority of the dietary recalls were captured during a weekday. Six participants had a hospital visit on a day that resulted in a dietary recall that captured a weekend day. Parents, if present during the diet recall, were occasionally asked to clarify forgotten meals and/or meal ingredients. For the first recall, portion sizes were clarified by using NASCO three-dimensional food models (Fort Atkinson, WI).
Nutrition data were analyzed using the Nutrition Data System for Research (NDS-R, version 5.0), a windows based software program developed by the Nutrition Coordinating Center at the University of Minnesota. This program includes options to calculate the nutrients, food groups, and meals and incorporates interviewer prompts for conducting 24-h recalls ensuring that all details are collected.
The dietary data were carefully screened for plausibility through a multistep process. Participants’ comments were reviewed and participants with either one or two abnormal diet recalls were eliminated from the sample (i.e., a participant who reported they were sick or ate differently than normal). Of the 110 participants with two dietary recalls, 96 had two viable recalls. Subsequently, the dietary data were examined for plausibility of caloric intake by assessing the residuals of the linear regression of caloric intake and IAAT. Three participants were excluded due to a residual, which was over two standard deviations from the mean, leaving 93 participants in the sample. Thus 93 participants with two complete dietary recalls were used in the present study.
Designation of breakfast categories
Breakfast was defined as any food or beverage consumed between 0500 and 1000 hours with a combined total energy ≥100 kcal (refs. 6,9). Subjects who did not consume breakfast on both days were categorized as breakfast skippers. Subjects who consumed breakfast on one of the two days were categorized as occasional breakfast eaters, while those that consumed breakfast on both days were classified as breakfast eaters.
Statistical analysis
ANOVA and analysis of covariance were performed to assess the relationship of breakfast consumption to various clinical measures (BMI, BMI percentile, SAAT, IAAT, SI, AIR, and DI) before and after controlling for sex, age, Tanner stage, total fat mass, total lean tissue mass, and daily energy intake. When significant differences in clinical measures across breakfast groups were identified, post hoc pairwise comparisons with Bonferroni adjustments were conducted. Analyses were conducted using the Statistical Program for the Social Sciences version 13.0 (SPSS, Chicago, IL). Significance level set at P < 0.05.
RESULTS
Descriptive characteristics for physical parameters of the subjects by breakfast category are presented in Table 1. The mean age of all participants was 14.2 ± 1.7 years of age and 52% of the participants were male. The participants were classified as follows: 21.5% were breakfast skippers, 41.9% were occasional breakfast eaters, and 36.6% were regular breakfast eaters. There were no significant differences in sex, age, or Tanner stage between breakfast categories. There was no significant difference in BMI, BMI percentile, total fat, total lean tissue mass, and SAAT between breakfast categories before or after controlling for sex, age, Tanner, energy, total fat mass, and lean tissue mass. There was no significant difference between SI, AIR, or DI before or after controlling for sex, age, Tanner, total fat mass, and total lean tissue mass.
Table 1.
Clinical characteristics by breakfast category
| Breakfast eater (n = 34) |
Occasional breakfast eater (n = 39) |
Breakfast skipper (n = 20) |
P value | |
|---|---|---|---|---|
| Sex (male, female) | 19/15 | 22/17 | 12/8 | 0.785 |
| Age (years) | 14.3 ± 2.0 | 14.2 ± 1.6 | 13.9 ± 1.1 | 0.675 |
| Tanner stage | 3.41 ± 1.4 | 3.74 ± 1.2 | 3.55 ± 1.4 | 0.585 |
| Height (cm) | 161.0 ± 1.6 | 163.3 ± 1.5 | 162.2 ± 1.6 | 0.563 |
| Weight (kg) | 76.7 ± 3.7 | 83.6 ± 3.0 | 83.6 ± 3.6 | 0.262 |
| BMI (kg/m2) | 29.3 ± 6.9 | 31.2 ± 5.5 | 31.6 ± 5.0 | 0.282 |
| BMI percentile | 94.8 ± 5.3 | 95.6 ± 9.9 | 96.9 ± 4.9 | 0.597 |
| Total fat (kg) | 24.2 ± 9.1 | 29.4 ± 10.7 | 30.1 ± 7.6 | 0.063 |
| Total lean tissue mass (kg) | 46.1 ± 10.0 | 49.5 ± 12.9 | 50.0 ± 9.8 | 0.796 |
| IAAT (cm2) | 29.2 ± 14.8c | 33.5 ± 19.4a | 49.1 ± 22.1b,d | 0.006 |
| SAAT (cm2) | 333.0 ± 134.6 | 369.4 ± 153.5 | 386.0 ± 105.9 | 0.817 |
| n = 31 | n = 35 | n = 17 | ||
| SI [×10−4 min−1/μU/ml)] | 1.8 ± 0.6 | 1.8 ± 0.9 | 1.3 ± 0.6 | 0.077 |
| AIR [μU/ml × 10 min] | 1,340.9 ± 491.7 | 1,404.9 ± 707.6 | 1,648.8 ± 1,223.7 | 0.212 |
| DI (× 10−4/min−1) | 2,330.2 ± 892.4 | 2,092.5 ± 816.2 | 1,719.4 ± 990.0 | 0.060 |
Data are mean ± s.d. Analyses were based on log scores for the following variables: IAAT, SI, AIR, and DI. P values were calculated using χ2-test (age and Tanner) and analysis of covariance. Covariates included sex Tanner, energy, total fat mass, and total lean tissue mass (for IAAT and SAAT). Means with different letters (a and b) across breakfast groups are significantly different from one another using Bonferroni multiple comparisons (P ≤ 0.01). Means with different letters (c and d) show a trend (P < 0.10).
AIR, acute insulin response; DI, disposition index; IAAT, intra-abdominal adipose tissue; SAAT, subcutaneous abdominal adipose tissue; SI, insulin sensitivity.
Twenty-four hour dietary intake by breakfast category is found in Table 2. There were no significant differences in daily energy, macronutrients, total dietary fiber, or total sugar intake between breakfast category groups. In addition, there were no significant differences in any of the other dietary variables between breakfast categories, after controlling for sex, age, Tanner stage, fat mass, or lean tissue mass.
Table 2.
Nutrient intake by breakfast category
| Breakfast eater (n = 34) |
Occasional breakfast eater (n = 39) |
Breakfast skipper (n = 20) |
P value | |
|---|---|---|---|---|
| Energy (kcal/day) | 1,788.4 ± 460.8 | 1,869.5 ± 583.5 | 1,571.3 ± 515.7 | 0.108 |
| Protein | ||||
| (g/day) | 68.9 ± 14.9 | 69.2 ± 21.3 | 64.0 ± 24.8 | 0.622 |
| (% of kcal/day) | 15.7 ± 2.7 | 15.2 ± 3.4 | 17.0 ± 5.6 | 0.223 |
| Fat | ||||
| (g/day) | 63.1 ± 20.6 | 68.8 ± 26.1 | 57.2 ± 22.6 | 0.208 |
| (% of kcal/day) | 31.5 ± 4.4 | 32.0 ± 5.8 | 32.3 ± 6.3 | 0.883 |
| Carbohydrate | ||||
| (g/day) | 242.9 ± 67.6 | 248.5 ± 72.1 | 203.8 ± 76.8 | 0.066 |
| (% of kcal/day) | 54.2 ± 5.7 | 53.9 ± 6.3 | 51.7 ± 8.4 | 0.350 |
| Dietary fiber | ||||
| (g/day) | 14.9 ± 5.0 | 15.0 ± 6.5 | 12.7 ± 5.0 | 0.304 |
| (% of kcal/day) | 3.4 ± 1.1 | 3.2 ± 1.2 | 3.3 ± 1.1 | 0.898 |
| Added sugar | ||||
| (g/day) | 68.4 ± 36.8 | 71.2 ± 41.0 | 53.8 ± 30.6 | 0.246 |
| (% of kcal/day) | 15.0 ± 6.1 | 15.1 ± 7.5 | 14.2 ± 7.3 | 0.890 |
P values were calculated using χ2-test (age and Tanner) and analysis of covariance. Covariates included sex and Tanner.
There was a significant overall effect of breakfast consumption for IAAT (P = 0.01) before (Table 1) and after (Figure 1) controlling for sex, age, Tanner stage, total fat mass, total lean tissue mass, and total daily energy intake. In post hoc analysis, breakfast skippers had significantly higher IAAT than both occasional breakfast eaters (47.2 ± 3.8 vs. 32.1 ± 2.7; P = 0.004) and regular breakfast eaters (32.0 ± 3.0; P = 0.05), after controlling for covariates. There was no significant difference in IAAT between occasional breakfast eaters and breakfast eaters. In addition, this significance remained after adjusting for SI and SAAT (P ≤ 0.05). Even though energy and macronutrient intake did not differ between breakfast categories, we re-ran the analyses including them as covariates and this further adjustment did not affect the findings.
Figure 1.
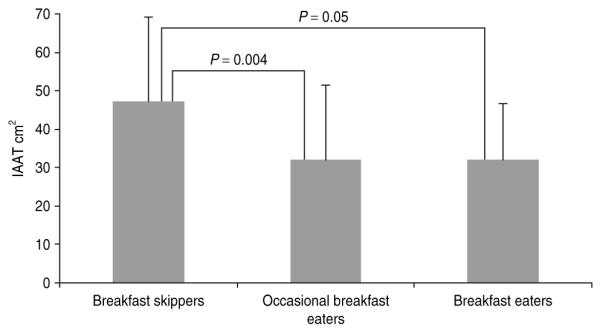
Mean ± s.d. intra-abdominal adipose tissue (IAAT) in 93 overweight adolescent Latinos with a positive family history for type 2 diabetes. There were significant differences between breakfast skippers and breakfast eaters (ANOVA; P = 0.05) and breakfast skippers and occasional breakfast eaters (ANOVA; P = 0.004) when controlling for age, Tanner, sex, total fat, total lean tissue mass, and total energy. There was no significant difference between occasional breakfast eaters and breakfast eaters.
DISCUSSION
Consistent with other reports of breakfast skipping in both adults and adolescents (8,18), 21.5% of our participants regularly skipped breakfast. In this study, individuals who skipped breakfast had higher IAAT compared with both breakfast eaters and occasional breakfast eaters independent of covariates. To our knowledge, this is the first study to show a relationship between breakfast consumption and visceral adiposity. These results suggest that even occasional consumption of breakfast is associated with lower visceral adiposity in overweight Latino adolescents.
The importance of breakfast consumption in youth has recently received a lot of attention. Breakfast has been identified as the most commonly missed meal for adolescents (6). The National Longitudinal Study of Adolescent Health found that 20% of adolescents skipped breakfast the previous day (19). Adolescents who habitually eat breakfast have been shown to have a lower BMI compared with adolescents who frequently skip breakfast (6). Cross-sectional research has shown that adolescents who consume breakfast regularly have a lower BMI compared with those who frequently skip breakfast (20). A recent review of literature also found that adolescents and children who ate breakfast generally consumed more daily calories yet were less likely to be overweight (21). A high BMI was also significantly associated with breakfast skipping in a study of 16-year-old girls and boys in Finland (8). In addition, a study of 6- to 19-year-old children and adolescents found that overweight females were more likely to omit breakfast (22).
Conflicting data exist on adolescent breakfast consumption and ethnicity. One study found that African-Americans and Hispanics, compared with their white peers, were less likely to omit breakfast (9). In contrast, Dwyer et al. showed that Hispanic youth were more likely to skip breakfast than their white counterparts (7). The level of acculturation also seems to play a role in adolescent breakfast consumption. In a study on the behavior of Hispanic immigrants to the United States, the frequency of daily breakfast intake was higher among foreign-born adolescents as compared with US-born adolescents (23). In a cross-sectional survey of 39,000 youth, minorities were more likely to have a BMI above 85th percentile, and the frequency of eating breakfast was inversely associated with being at or above the 85th percentile (24). To our knowledge, there have been no studies that show an association of breakfast skipping with distribution of adiposity in minority populations.
Visceral fat accumulation is an important indicator of disease risk including dyslipidemia and glucose intolerance (25,26). Although BMI provides a general assessment of overweight, it does not account for variations in the distribution of adiposity. The distribution of body fat is known to be important in the development of metabolic disorders associated with obesity (27). Of particular interest is the relationship of visceral fat to the development of insulin resistance in both adults and children. We have previously shown that IAAT was positively associated with higher fasting insulin (4,28), insulin area under the curve after OGTT (29), and insulin resistance (4). However, in the present analysis, the association between breakfast consumption and IAAT remains significant even after adjusting for fasting insulin and insulin sensitivity. These results suggest that breakfast skipping is associated with IAAT independent of insulin action.
One possible explanation is that that regularly omitting breakfast may have resulted in a decreased number of total eating episodes by the breakfast skippers, as compared with the other groups. Although we did not examine meal frequency in this study, this is one hypothesis to support our breakfast findings. Several studies have been conducted examining this relationship of meal frequency to adiposity in pediatric populations. Two pediatric studies found an association between eating frequency and prevalence of overweight (30,31), whereas two found no association (32,33). However, these studies used BMI or weight to height proportions to assess adiposity and no assessment of precise measures of adiposity or metabolic factors, i.e., thermic effect of food or glucose or insulin dynamics, was reported. For this particular study, we were interested in examining the effects of breakfast consumption rather than total meal frequency on fat distribution and insulin dynamics. In this study, breakfast consumption was inversely related to visceral adiposity, whereas no association to insulin dynamics was found, and these results were independent of energy intake. Given that there were no differences in daily energy intake and insulin dynamics between the breakfast groups, our findings may be more related to the differences in the thermic effect of food, although not measured in this study. For future analyses and papers, we intend to explore the relationship of meal patterns and frequency with adiposity and metabolic parameters.
Another explanation to consider is the impact of the macro-nutrient content of the breakfast on adiposity and metabolic parameters. Several studies have shown that the type of breakfast consumed, specifically high fiber or whole grain cereals, is associated with reduced BMI (19,34,35) and insulin values (36). In the current analyses, there were no significant differences in daily total macronutrient intake between breakfast groups. In addition, breakfast consumption was inversely related to IAAT independent of macronutrient intake. Thus, the macronutrient intake of the breakfast meal did not appear to influence our findings. However, the purpose of this study was to compare the effects of regular breakfast consumption, regardless of type, to irregular and skipping breakfast on adiposity parameters.
There are several limitations in this study. There may be a bias of dietary reporting and possibly underreporting of overweight subjects. However, because our entire cohort was overweight, we would expect similar underreporting across the whole sample. Also, two dietary recalls may not adequately represent participants’ usual dietary intake patterns. In addition, there is no clear definition of breakfast consumption. A recent study found that the percentage of breakfast skippers varied greatly by how breakfast was categorized (37). However, our definition is similar to one used in past studies (6,9). Our small sample size and lack of adjustment for physical activity may also be limiting factors. Furthermore, because the current study is cross-sectional, no firm conclusions can be drawn about the causative relationship of breakfast consumption and IAAT over time. Finally, for this particular study, we did not look at the food composition of the breakfast consumed, and intend to examine this in later studies.
In summary, we have found that breakfast skipping was related to increased visceral fat independent of age, gender, total fat, total lean tissue, and total energy intake. These findings suggest that interventions promoting breakfast consumption may reduce the risk for visceral adiposity and subsequently diseases associated with visceral adiposity in overweight Latino youth. More pediatric studies should be conducted investigating the role of meal patterns, specifically breakfast consumption, in adiposity, using precise methodology.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant R01 DK 59211 and by General Clinical Research Center, National Center for Research Resources, grant MO1 RR 00043. We thank the project managers Christina Ayala, M.P.H., and Quintilia Avila, M.P.A, the SOLAR team, and the nursing staff. We also appreciate Lourdes Acosta’s assistance in the dietary data collection and entry. We are especially grateful to the participants and their families for their involvement in the study. K.E.A. was responsible for manuscript preparation, statistical analysis, data collection, entry, and evaluation; E.E.V. assisted with evaluation and interpretation of the data and manuscript preparation; D.S.M. assisted with manuscript preparation; M.J.W. oversaw medical procedures and tests in the study and assisted with manuscript preparation; M.I.G. oversaw and managed all aspects of the study, and J.N.D. assisted with manuscript preparation, statistical analysis and data interpretation.
Footnotes
DISCLOSURE The authors declared no conflict of interest.
REFERENCES
- 1.Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics. 1998;101:518–525. [PubMed] [Google Scholar]
- 2.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 3.Tortolero SR, Goff DC, Jr, Nichaman MZ, et al. Cardiovascular risk factors in Mexican-American and non-Hispanic white children: the Corpus Christi Child Heart Study. Circulation. 1997;96:418–423. doi: 10.1161/01.cir.96.2.418. [DOI] [PubMed] [Google Scholar]
- 4.Cruz ML, Bergman RN, Goran MI. Unique effect of visceral fat on insulin sensitivity in obese Hispanic children with a family history of type 2 diabetes. Diabetes Care. 2002;25:1631–1636. doi: 10.2337/diacare.25.9.1631. [DOI] [PubMed] [Google Scholar]
- 5.Davis JN, Ventura EE, Weigensberg MJ, et al. The relation of sugar intake to β cell function in overweight Latino children. Am J Clin Nutr. 2005;82:1004–1010. doi: 10.1093/ajcn/82.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siega-Riz AM, Popkin BM, Carson T. Trends in breakfast consumption for children in the United States from 1965–1991. Am J Clin Nutr. 1998;67:748S–756S. doi: 10.1093/ajcn/67.4.748S. [DOI] [PubMed] [Google Scholar]
- 7.Dwyer JT, Evans M, Stone EJ, et al. Adolescents’ eating patterns influence their nutrient intakes. J Am Diet Assoc. 2001;101:798–802. doi: 10.1016/s0002-8223(01)00198-5. [DOI] [PubMed] [Google Scholar]
- 8.Keski-Rahkonen A, Kaprio J, Rissanen A, Virkkunen M, Rose RJ. Breakfast skipping and health-compromising behaviors in adolescents and adults. Eur J Clin Nutr. 2003;57:842–853. doi: 10.1038/sj.ejcn.1601618. [DOI] [PubMed] [Google Scholar]
- 9.Affenito SG, Thompson DR, Barton BA, et al. Breakfast consumption by African-American and white adolescent girls correlates positively with calcium and fiber intake and negatively with body mass index. J Am Diet Assoc. 2005;105:938–945. doi: 10.1016/j.jada.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Fiore H, Travis S, Whalen A, Auinger P, Ryan S. Potentially protective factors associated with healthful body mass index in adolescents with obese and nonobese parents: a secondary data analysis of the third national health and nutrition examination survey, 1988–1994. J Am Diet Assoc. 2006;106:55–64. doi: 10.1016/j.jada.2005.09.046. quiz 76–79. [DOI] [PubMed] [Google Scholar]
- 11.Resnicow K. The relationship between breakfast habits and plasma cholesterol levels in schoolchildren. J Sch Health. 1991;61:81–85. doi: 10.1111/j.1746-1561.1991.tb03242.x. [DOI] [PubMed] [Google Scholar]
- 12.Sampson AE, Dixit S, Meyers AF, Houser R., Jr The nutritional impact of breakfast consumption on the diets of inner-city African-American elementary school children. J Natl Med Assoc. 1995;87:195–202. [PMC free article] [PubMed] [Google Scholar]
- 13.Niemeier HM, Raynor HA, Lloyd-Richardson EE, Rogers ML, Wing RR. Fast food consumption and breakfast skipping: predictors of weight gain from adolescence to adulthood in a nationally representative sample. J Adolesc Health. 2006;39:842–849. doi: 10.1016/j.jadohealth.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Goran MI, Bergman RN, Avila Q, et al. Impaired glucose tolerance and reduced β-cell function in overweight Latino children with a positive family history for type 2 diabetes. J Clin Endocrinol Metab. 2004;89:207–212. doi: 10.1210/jc.2003-031402. [DOI] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 16.Tanner JM. Growth and maturation during adolescence. Nutr Rev. 1981;39:43–55. doi: 10.1111/j.1753-4887.1981.tb06734.x. [DOI] [PubMed] [Google Scholar]
- 17.Type 2 diabetes in children and adolescents. American Diabetes Association. Pediatrics. 2000;105:671–680. doi: 10.1542/peds.105.3.671. [DOI] [PubMed] [Google Scholar]
- 18.Vanelli M, Iovane B, Bernardini A, et al. Breakfast habits of 1,202 northern Italian children admitted to a summer sport school. Breakfast skipping is associated with overweight and obesity. Acta Biomed. 2005;76:79–85. [PubMed] [Google Scholar]
- 19.Cho S, Dietrich M, Brown CJ, Clark CA, Block G. The effect of breakfast type on total daily energy intake and body mass index: results from the Third National Health and Nutrition Examination Survey (NHANES III) J Am Coll Nutr. 2003;22:296–302. doi: 10.1080/07315724.2003.10719307. [DOI] [PubMed] [Google Scholar]
- 20.Wolfe WS, Campbell CC, Frongillo EA, Jr, Haas JD, Melnik TA. Overweight schoolchildren in New York State: prevalence and characteristics. Am J Public Health. 1994;84:807–813. doi: 10.2105/ajph.84.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rampersaud GC, Pereira MA, Girard BL, Adams J, Metzl JD. Breakfast habits, nutritional status, body weight, and academic performance in children and adolescents. J Am Diet Assoc. 2005;105:743–760. doi: 10.1016/j.jada.2005.02.007. quiz 761–762. [DOI] [PubMed] [Google Scholar]
- 22.O’Dea JA, Caputi P. Association between socioeconomic status, weight, age and gender, and the body image and weight control practices of 6- to 19-year-old children and adolescents. Health Educ Res. 2001;16:521–532. doi: 10.1093/her/16.5.521. [DOI] [PubMed] [Google Scholar]
- 23.Gordon-Larsen P, Harris KM, Ward DS, Popkin BM. Acculturation and overweight-related behaviors among Hispanic immigrants to the US: the National Longitudinal Study of Adolescent Health. Soc Sci Med. 2003;57:2023–2034. doi: 10.1016/s0277-9536(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 24.Delva J, Johnston LD, O’Malley PM. The epidemiology of overweight and related lifestyle behaviors: racial/ethnic and socioeconomic status differences among American youth. Am J Prev Med. 2007;33:S178–S186. doi: 10.1016/j.amepre.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Goran MI, Gower BA. Relation between visceral fat and disease risk in children and adolescents. Am J Clin Nutr. 1999;70:149S–156S. doi: 10.1093/ajcn/70.1.149s. [DOI] [PubMed] [Google Scholar]
- 26.Bjorntorp P. Abdominal fat distribution and disease: an overview of epidemiological data. Ann Med. 1992;24:15–18. doi: 10.3109/07853899209164140. [DOI] [PubMed] [Google Scholar]
- 27.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 28.Huang TT, Johnson MS, Gower BA, Goran MI. Effect of changes in fat distribution on the rates of change of insulin response in children. Obes Res. 2002;10:978–984. doi: 10.1038/oby.2002.133. [DOI] [PubMed] [Google Scholar]
- 29.Gower BA, Nagy TR, Trowbridge CA, Dezenberg C, Goran MI. Fat distribution and insulin response in prepubertal African American and white children. Am J Clin Nutr. 1998;67:821–827. doi: 10.1093/ajcn/67.5.821. [DOI] [PubMed] [Google Scholar]
- 30.Toschke AM, Kuchenhoff H, Koletzko B, von Kries R. Meal frequency and childhood obesity. Obes Res. 2005;13:1932–1938. doi: 10.1038/oby.2005.238. [DOI] [PubMed] [Google Scholar]
- 31.Fabry P, Hejda S, Cerny K, Osancova K, Pechar J. Effect of meal frequency in schoolchildren. Changes in weight-height proportion and skinfold thickness. Am J Clin Nutr. 1966;18:358–361. doi: 10.1093/ajcn/18.5.358. [DOI] [PubMed] [Google Scholar]
- 32.Nicklas TA, Yang SJ, Baranowski T, Zakeri I, Berenson G. Eating patterns and obesity in children. The Bogalusa Heart Study. Am J Prev Med. 2003;25:9–16. doi: 10.1016/s0749-3797(03)00098-9. [DOI] [PubMed] [Google Scholar]
- 33.Nicklas TA, Morales M, Linares A, et al. Children’s meal patterns have changed over a 21-year period: the Bogalusa Heart Study. J Am Diet Assoc. 2004;104:753–761. doi: 10.1016/j.jada.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 34.Kosti RI, Panagiotakos DB, Mihas CC, et al. Dietary habits, physical activity and prevalence of overweight/obesity among adolescents in Greece: the Vyronas study. Med Sci Monit. 2007;13:CR437–CR444. [PubMed] [Google Scholar]
- 35.Barton BA, Eldridge AL, Thompson D, et al. The relationship of breakfast and cereal consumption to nutrient intake and body mass index: the National Heart, Lung, and Blood Institute Growth and Health Study. J Am Diet Assoc. 2005;105:1383–1389. doi: 10.1016/j.jada.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Farshchi HR, Taylor MA, Macdonald IA. Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am J Clin Nutr. 2005;81:388–396. doi: 10.1093/ajcn.81.2.388. [DOI] [PubMed] [Google Scholar]
- 37.Dialektakou KD, Vranas PB. Breakfast skipping and body mass index among adolescents in Greece: whether an association exists depends on how breakfast skipping is defined. J Am Diet Assoc. 2008;108:1517–1525. doi: 10.1016/j.jada.2008.06.435. [DOI] [PubMed] [Google Scholar]


