Abstract
The molecular and biochemical properties of myosin light chain kinases from chicken skeletal and smooth muscle were investigated by recombinant DNA techniques. Deletion of the amino-terminal region of either the smooth or skeletal muscle myosin light chain kinase resulted in a decrease in Vmax with no significant change in Km values for light chain substrates. Skeletal/smooth muscle chimeric kinases were inactive when a 65-residue region amino-terminal of the catalytic core was exchanged between the two forms. Changing alanine 494 to glutamic acid within this region in the chicken skeletal muscle myosin light chain kinase increased the Km values for light chains 10-fold. These results are consistent with the hypothesis that the region amino-terminal of the catalytic core in myosin light chain kinases is involved in light chain recognition. A skeletal muscle kinase which contained the smooth muscle calmodulin binding domain remained regulated by Ca2+/calmodulin. Thus, the calmodulin binding domains of smooth and skeletal muscle myosin light chain kinases share structural elements necessary for regulation.
Phosphorylation of the regulatory light chain of myosin is required for contraction of smooth muscle and leads to potentiation of twitch tension in skeletal muscle (Kamm and Stull, 1985; Moore and Stull, 1984). The key regulatory enzyme in these processes is Ca2+/calmodulin-dependent MLCK1 (Kemp and Stull, 1990). MLCKs from skeletal and smooth muscles can be distinguished on the basis of molecular mass (Nunnally and Stull, 1984; Edelman et al., 1987), antigenicity (Kamm et al., 1987), and catalytic specificity for synthetic peptide substrates (Michnoff et al., 1986; Kemp and Stull, 1990). Hydrodynamic and CD spectroscopic studies of the native rabbit skeletal muscle MLCK and selected proteolytic fragments have shown that the enzyme exists as an asymmetric amino-terminal tail and globular carboxyl-terminal head (Mayr and Heilmeyer, 1983). The tail fragment, comprising roughly half of the mass of the enzyme, has no known function whereas the globular head region exhibits Ca2+/calmodulin-dependent kinase activity. The smooth muscle MLCK is believed to be structurally similar to the skeletal muscle enzyme (Edelman et al., 1987).
Although all known protein kinases share a homologous catalytic core, this region does not necessarily contain all the determinants for substrate recognition (Hanks et al., 1988; Knighton et al., 1991). For example, a region immediately amino-terminal of the catalytic core of rabbit skeletal muscle myosin light chain kinase apparently binds to myosin light chain (Herring et al., 1990a, 1990b). Similar analyses have not been made with the chicken smooth muscle kinase. Since the rabbit skeletal and chicken smooth muscle enzymes are considered to be skeletal and smooth muscle MLCK prototypes, comparisons of their amino acid sequences have been made in an attempt to associate structure with function (Guerriero et al., 1986). The low sequence identity in the catalytic cores (52%) and calmodulin binding domains (41%) of the rabbit skeletal and chicken smooth muscle MLCKs could account for functional differences between these two kinases (Takio et al., 1985; Guerriero et al., 1986).
Limited proteolysis of both smooth and skeletal muscle MLCKs with trypsin or chymotrypsin results in truncated molecules, lacking both amino- and carboxyl-terminal sequences which exhibit Ca2+/calmodulin-independent kinase activity (Foyt et al., 1985; Ikebe et al., 1987; Kennelly et al., 1987; Mayr and Heilmeyer, 1983). However, the specific activities of these proteolytic fragments are low, and it is unclear whether the decrease in activity is because of partial proteolysis of the amino terminus, carboxyl terminus, or both portions of the MLCKs. Although the calmodulin binding domain has been identified, the mechanism of regulation of MLCK activity continues to be controversial (Edelman et al., 1987; Ikebe et al., 1987; and Pearson et al., 1988; Herring, 1991). Shoemaker et al. (1990) have shown that when the calmodulin binding domain of chicken non-muscle MLCK is altered so that it resembles the calmodulin binding domain of either rabbit skeletal muscle MLCK or Ca2+/calmodulin-dependent kinase II, Ca2+/calmodulin-independent activity is retained. Thus, the specific intramolecular binding of the calmodulin binding domain to the active site may not account for auto-inhibition, and an allosteric mechanism of activation would be favored.
To examine these problems more closely, we have cloned and characterized the chicken skeletal muscle MLCK cDNA. This has allowed a comparison of the skeletal and smooth muscle MLCKs in this animal species. We examined the kinetic properties of truncated forms of skeletal (SK-TR) and smooth (SM-TR) muscle MLCK to determine whether the amino-terminal tail region plays a role in catalysis. We used skeletal/smooth muscle chimeric molecules and site-specific mutants to examine regions inside and outside the catalytic core for involvement in catalysis, substrate recognition, and Ca2+/calmodulin regulation of the enzyme.
MATERIALS AND METHODS
Northern Blot Analysis
Total cellular RNA was isolated from rapidly dissected, quick-frozen tissues, and Northern analysis was performed. All probes were generated from gel-purified cDNA by the random hexamer primer method with [32P]dATP and were hybridized to the Northern blots (Sambrook et al., 1989). Final low stringency wash conditions were in 12.5 mM sodium phosphate, pH 7.4, 150 mM NaCl, 1 mM EDTA (1 × SSPE) (Sambrook et al., 1989), and 1% SDS at 52 °C. Final high stringency wash conditions were in 2.5 mM sodium phosphate, pH 7.4, 30 mM NaCl, 0.2 mM EDTA (0.2 × SSPE), and 0.1% SDS at 68 °C.
Preparation and Screening of cDNA Libraries
RNA was isolated from chicken posterior latissimus dorsi, and the RNA was enriched for poly(A)+ RNA by oligo(dT)-cellulose chromatography (Sambrook et al., 1989). This poly(A)+ RNA was used to make an oligo(dT)-primed cDNA library in λgt10 by the method of Gubler and Hoffman (1983) with modifications as described by McPhaul et al. (1988). Recombinant clones were transferred to nitrocellulose filters and prepared for plaque hybridization by standard procedures (Sambrook et al., 1989). The oligo(dT) library was screened with a probe prepared from the EcoRI-BamHI restriction fragment of the rat skeletal muscle MLCK cDNA (Herring et al., 1989) and washed in low stringency conditions. A 1.9-kb partial clone was isolated from this library.
A synthetic oligonucleotide-primed library was prepared by standard procedures (Sambrook et al., 1989). The oligonucleotide primer was designed to hybridize to a region located 50 nucleotides 3′ of a unique BglII restriction site near the 5′ end of the initial clone (see Fig. 1A). The primer-extended library was screened with the EcoRI-BglII restriction fragment from the 1.9-kb partial cDNA clone and washed in high stringency conditions. A 2.1-kb clone was isolated from the primer-extended library. The two partial clones were ligated together at the unique BglII restriction site and subcloned into pUC8 and the expression vector pCMV2 (Andersson et al., 1989).
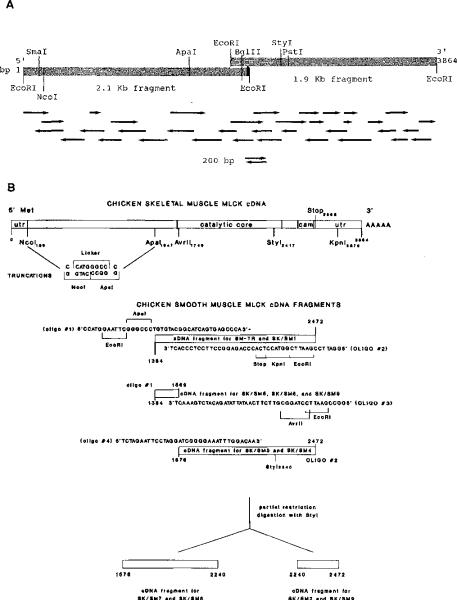
Fig. 1.
Panel A, partial restriction map of the cDNA encoding chicken skeletal muscle MLCK. Restriction endonuclease sites that were used in the construction of the cDNA, truncation mutants, or chimeric MLCKs are indicated at the following positions in the clone: SmaI (141), NcoI (185), ApaI (1546), BglII (2039), StyI (2411), and PstI (2480). EcoRI sites originate from linkers used to prepare the libraries; there are no natural EcoRI sites in the cDNA. The synthetic oligonucleotide primer and its relative position are indicated by the solid black bar at the 3′ end of the 2.1-kb partial clone. Directional arrows indicate individual sequencing reactions. Panel B, construction of truncation and chimeric cDNAs. Oligonucleotide sequences and their relative positions are indicated on the linear schematic of the nucleotide sequence. Numbers correspond to the nucleotides of the chicken skeletal (Fig. 2) or smooth (Olson et al., 1990) muscle cDNAs on the respective cDNAs.
DNA Sequencing
DNA sequencing was performed by subcloning the cDNA into the M13 sequencing vectors mp18 and mp19. Cyclone (International Biotechnologies Inc.) was used to generate a nested set of deletions which were sequenced by the dideoxy method using Sequenase (U. S. Biochemical Corp.).
Data Base Searching and Sequence Analysis
Overlapping clones were assembled using the Assemgel program of Intelligenetics PCGENE. Subsequent analysis was performed using both PCGENE and the University of Wisconsin Genetics Computer Group programs (Devereux et al., 1984). (Wordsearch and Segments use the Wilbur and Lipman search for similarity (Lipman and Pearson, 1985), and Bestfit uses the algorithm of Smith and Waterman (1981).) The data bases GenEMBL and NBRF were searched. Alignments were refined using personal judgment.
Construction of Truncated and Skeletal/Smooth Muscle Chimeric MLCKs
The chicken skeletal muscle MLCK cDNA in pUC8 was digested with NcoI and ApaI restriction enzymes to remove a 1.4-kb NcoI-ApaI restriction fragment from the clone (see Fig. 1B). The remaining portion was recircularized with a synthetic oligonucleotide linker with the sequence CATGGGCC. This construction deletes nucleotides 190–1542 (which encode amino acids 2–452) and utilizes the 5′-untranslated region and initiating M of the wild-type enzyme. The correct reading frame is maintained, beginning with G453. The truncation was confirmed by sequencing, subcloned into pCMV2, and expressed in COS cells by standard methods (Herring et al., 1990b). The initial smooth muscle MLCK fragment used in SM-TR and SK/SM1 was generated from chicken gizzard RNA using PCR. The smaller fragments were generated by utilizing the initial large fragment as a template for subsequent PCR. Fusion of the skeletal and smooth muscle enzymes was accomplished by utilizing convenient restriction sites within the skeletal muscle MLCK cDNA. Oligonucleotides that were used to prime the smooth muscle kinase PCR products contained restriction sites compatible to those in the skeletal muscle enzyme. This construction allowed the fragments to be ligated together and the appropriate reading frame to be maintained. SM-TR was constructed in the same way as SK-TR by joining the NcoI site of the skeletal muscle cDNA to the PCR-generated ApaI site of the smooth muscle cDNA with the synthetic oligonucleotide linker. This construction results in a smooth muscle product except for the two most amino-terminal residues, G and P, from the skeletal muscle enzyme. The chimeric molecules containing smooth muscle cDNA fragments were generated from the following synthetic oligonucleotides as illustrated in Fig. 1 with the resultant protein sequences shown schematically in Fig. 5.
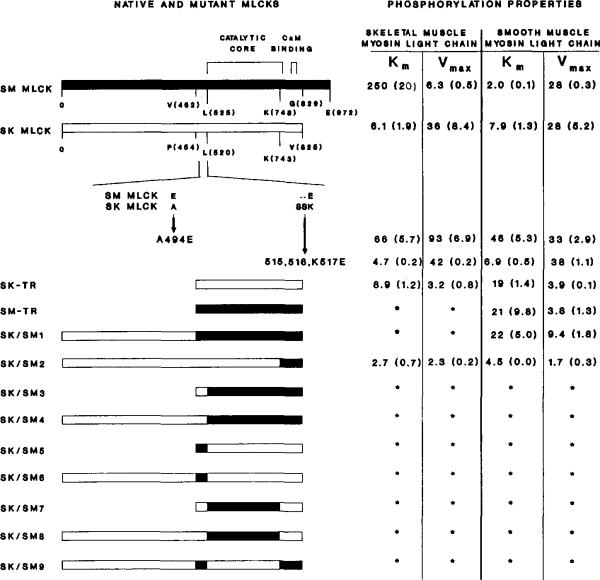
Fig. 5. Linear schematic representation of the primary structures and kinetic properties of chicken skeletal and smooth muscle native, truncated, and chimeric MLCKs.
Regions representing skeletal muscle myosin light chain kinase (SK MLCK) are outlined, and regions from smooth muscle kinase (SM MLCK) are darkly shaded. Km (μM) and Vmax (μmol of 32P incorporated per min/mg) values for skeletal and smooth muscle myosin light chains are shown to the right of the native or mutant MLCKs. The numbers represent the means with the S.E. in parentheses. The positions of the catalytic core and calmodulin binding domain are indicated above the linear schematic. The smooth muscle MLCK has 972 residues: residues 519–774 form the catalytic core and residues 797–813 form the calmodulin binding domain. The native skeletal muscle enzyme is composed of 825 amino acids with the catalytic core and calmodulin binding domain spanning residues 512–772 and 795–811, respectively. An amino acid sequence alignment below the native skeletal muscle MLCK illustrates the site-specific mutants A494E and Δ515–516,K517E. The compositions of the truncation mutants and chimeric enzymes are as follows (numbers in parentheses are amino acid numbers): SK-TR = SK (453–825); SM–TR = SM (462–829); SK/SM1 = SK (1–454) and SM (462–829); SK/SM2 = SK (1–742) and SM (748–829); SK/SM3/B> = SK (453–520) and SM (525–829); SK/SM4 = SK (1–520) and SM (526–829); SK/SM5 = SK (453–454), SM (462–525), and SK (521–825); SK/SM6 = SK (1–454), SM (462–525), and SK (521–825); SK/SM7 = SK (453–520), SM (526–748), and SK (744–825); SK/SM8 = SK (1–520), SM (526–748), and SK (744–825); SM (SK/SM9) = SK (1–454), SM (462–525), SK (521–744), and SM (750–829).
Site-specific Mutagenesis
Oligonucleotide mutagenesis was performed as described by Zoller and Smith (1984). A494E was generated using an oligonucleotide with the sequence 5′CAAAGGGTTCGGGCGGAG-3′, and the oligonucleotide 5′CCTAGGATTTCCTCGAGGTTGTACTGGGAGCTG-3′ was used to generate Δ515–516,K517E from M13 single-stranded templates of the skeletal muscle cDNA. All constructions and PCR products were confirmed by sequencing.
Expression
Transfections of COS cells were performed with 2–10 μg of the recombinant or mutant cDNAs in the expression vector pCMV2 as described by Herring et al. (1990b). COS cell lysis buffer was comprised of 10% glycerol; 1% Nonidet P-40 (Tergitol); 100 mM MOPS; 1.25 mM EGTA; 10 mM dithiothreitol; 120 μg/ml N-tosyl-L-phenylalanine chloromethyl ketone and Nα-p-tosyl-L-lysine chloromethyl ketone; 100 μg/ml phenylmethylsulfonyl fluoride; 10 μg/ml each of pepstatin, amidinophenylmethylsulfonyl fluoride, and leupeptin; and 4 μg/ml aprotinin.
Western Immunoblot and Calmodulin Overlay Analysis
COS cell lysates containing wild-type or mutant MLCKs were compared with the native enzyme. Proteins were separated by SDS-PAGE (7.5% acrylamide) and transferred to nitrocellulose. Western immunoblots were performed under the conditions described by Herring et al. (1990b). Conditions for the biotinylated calmodulin overlays (Kameshita and Fujisawa, 1989) were identical to that of the Western immunoblots with the addition of 10 mM CaC12 in all incubation and wash solutions. Biotinylated calmodulin (Billingsley et al., 1985) was used as the first step reactant (25 ng/ml), and horseradish peroxidase-conjugated avidin (1:15,000) (Boehringer Mannheim) was used as the second step. Recombinant MLCK concentrations in COS cell lysates were determined by densitometry of calmodulin overlays or Western immunoblots with purified chicken skeletal muscle MLCK as a standard. In determining the concentration of the truncated kinases by the calmodulin overlay analysis, the lower molecular mass of the enzymes was taken into consideration for the appropriate calculations.
Protein Purification
Chicken skeletal muscle MLCK, skeletal muscle myosin light chains, and smooth muscle myosin light chains were purified as described previously (Nunnally and Stull, 1984; Blumenthal and Stull, 1982; Hathaway and Haeberle, 1983). Chicken smooth muscle MLCK was purified by the method of Adelstein and Klee (1981) and was purified further through a hydroxylapatite column. Calmodulin was purified from bovine testes (Blumenthal and Stull, 1982).
Kinase Activity Measurements
Purified MLCK or COS cell lysates (diluted from 1:20 to 1:2000) were assayed for activity by measuring the amount of 32P incorporated into purified myosin regulatory light chains in the presence of 50 mM MOPS, 10 mM magnesium acetate, 0.3 mM calcium chloride, 1 μM calmodulin, 2 mM dithiothreitol, and 1 mM [γ-32P]ATP (approximately 200 cpm/pmol). The myosin regulatory light chain concentration was calculated from the maximum 32P incorporation after incubation with [γ-32P]ATP and chicken skeletal muscle MLCK. Km, and Vmax values were determined from Lineweaver-Burk double-reciprocal plots.
Protein Sequencing
Approximately 200 pmol of chicken skeletal muscle MLCK was subjected to SDS-PAGE and then transferred to Immobilon membrane (Millipore Corp., Bedford, MA) (Matsudaira, 1987). Automated Edman degradation was performed with an Applied Biosystems Inc. (Foster City, CA) model 470A Sequencer. Although the amino terminus of the enzyme was blocked, sequence data were obtained from unfractionated peptide mixtures derived by cleaving approximately 400 pmol of enzyme either with 70% formic acid or with cyanogen bromide (Landon, 1977; Urdal et al., 1984). Additional peptide mixtures produced by cyanogen bromide cleavage were treated with o-phthalaldehyde (Brauer et al., 1984) at selected cycles of Edman degradation to acquire amino acid sequence information from selected components of the peptide mixture.
RESULTS
A cDNA encoding the full-length chicken skeletal muscle MLCK was constructed from two partial cDNAs (Fig. 1A). Each of the partial clones hybridized to a single chicken skeletal muscle mRNA of 4.0 kb on Northern blots (data not shown). The sequence of the full-length cDNA and the deduced amino acid sequence are shown in Fig. 2. The cDNA expressed in COS cells produces an immunoreactive protein which comigrates with the tissue-purified kinase on SDS-PAGE (see Fig. 4).
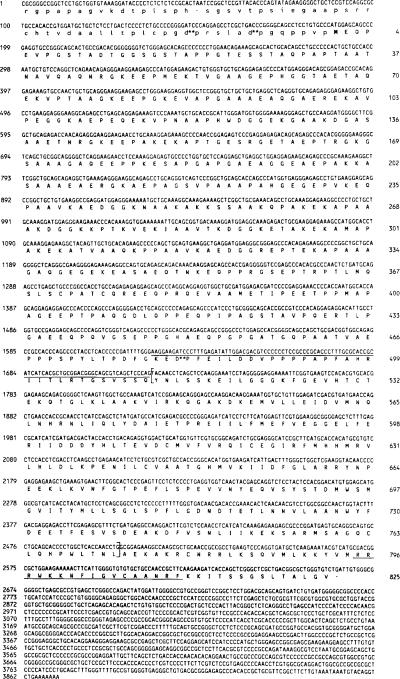
Fig. 2. Nucleotide and deduced amino acid sequence of the cDNA encoding chicken skeletal muscle MLCK.
The nucleotide sequence and the deduced amino acid sequence of the coding region of the chicken skeletal muscle MLCK are shown in capital letters. The 5′-untranslated region has been translated in lowercase letters. The initiating M is in boldface type. Aspartyl-prolyl bonds are indicated with asterisks as potential sites for cleavage by 70% formic acid. The overlined residues indicate the region amino-terminal of the catalytic core which is homologous to other MLCKs (residues 479–511). The catalytic core of the enzyme is bracketed (residues 512–772), and the calmodulin binding domain is underlined (residues 795–811).
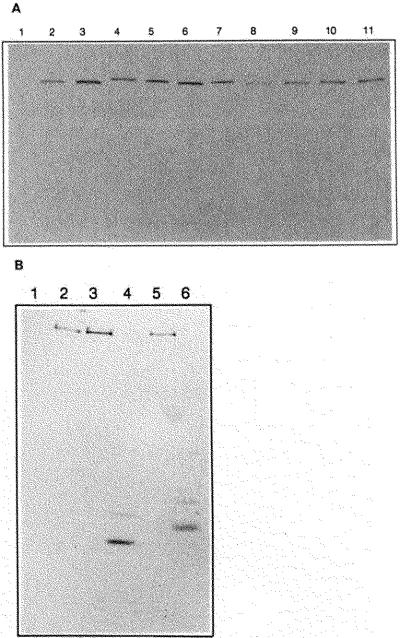
Fig. 4. Western immunoblot and calmodulin overlay analysis of chicken skeletal and smooth muscle MLCKs and mutant enzymes.
Purified MLCKs and COS cell extracts containing recombinant proteins were separated by 7.5% SDS-PAGE and detected with monoclonal antibodies or biotinylated calmodulin as described under “Materials and Methods.” Panel A, Western immunoblot with monoclonal antibody raised to chicken skeletal muscle MLCK. Lane 1, mock transfected COS cell lysate; lane 2, tissue-purified chickn skeletal muscle MLCK (10 ng); lanes 3–11, COS cell lysates transfected with 3, wild-type chicken skeletal muscle MLCK, 4, SK/SM1; 5, SK/SM4; 6, SK/SM6; 7, SK/SM8; 8, SK/SM2; 9, SK/SM9; 10, A494E 11, Δ515–516,K517E. Panel B, biotinylated calmodulin overlay. Lane 1, mock transfected COS cell lysate; lane 2, tissue-purified chicken skeletal myosin light chain kinase; lanes 3–6, COS cell lysates with 3, wild-type chicken skeletal muscle myosin light chain kinase; 4, SK-TR mutant; 5, SK/SM1 mutant; 6, SM-TR mutant.
The translational start site of the chicken skeletal muscle MLCK is encoded by nucleotides 187–189. An in-frame stop codon is present 5′ of this M at nucleotides 49–51. An open reading frame is present from nucleotides 52 to 2661. The amino acid sequence deduced from this cDNA is supported by protein sequence data obtained by Edman degradation after cyanogen bromide digestion of tissue-purified chicken skeletal muscle MLCK bound to Immobilon membrane. All of the amino acids released at each cycle were consistent with the deduced amino acid sequence. To simplify analysis of the data obtained by Edman degradation of the cyanogen bromide peptide mixture, separate samples of the mixture were treated with o-phthalaldehyde (Brauer et al., 1984) prior to the phenylisothiocyanate coupling reaction in cycles 2 and 3, respectively. These cycles were selected because proline residues were determined to be exposed at the amino terminus of at least one component of the cyanogen bromide peptide mixture prior to each of these cycles. Since o-phthalaldehyde couples to all amino acids other than proline and thereby blocks them from further reaction with phenylisothiocyanate, the treatment with o-phthalaldehyde allowed sequence information to be acquired only from those peptides having proline at these positions. Treatment during cycle 2 resulted in the release of amino acids that were consistent with the predicted amino acid sequences carboxyl-terminal of P301 and P400. Treatment during cycle 3 of the Edman degradation resulted in the subsequent release of the homogeneous amino acid sequence PEVPGSTADTGGS, which is identical to the predicted sequence of residues 4–16 (Fig. 2).
To demonstrate that the methionine at nucleotide position 187 was the initiating methionine, the tissue-purified enzyme was treated with a 70% formic acid solution. Cleavage occurs between aspartyl-prolyl bonds under these acidic conditions (Landon et al., 1977). A single aspartyl-prolyl bond is present at D481–P482 in the deduced sequence. However, if protein translation were initiated prior to the ATG at nucleotide 187, two additional aspartyl-prolyl bonds would be present (Fig. 2). The sequence obtained was consistent with initiation at M187.
Comparison of Chicken Skeletal Muscle MLCK with Other Protein Kinases
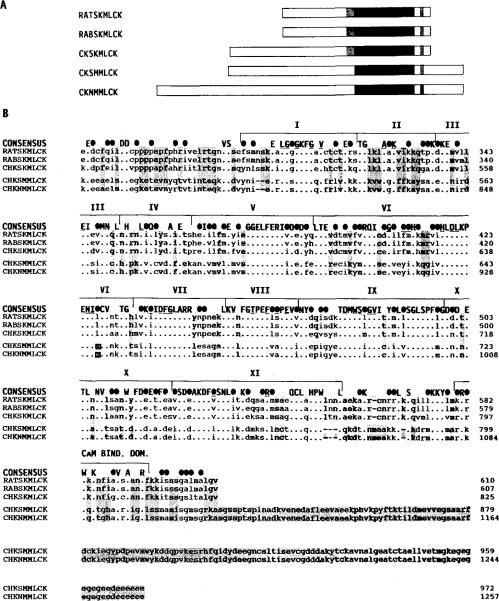
Skeletal muscle MLCKs from different species were found to share more identity than skeletal and smooth muscle MLCKs from the same species. The chicken skeletal muscle enzyme is 84% identical to the rabbit (Takio et al., 1985, 1986) and rat (Herring et al., 1989; Roush et al., 1988) skeletal muscle MLCKs within the catalytic core (amino acid residues 512–772) and 82% identical within the calmodulin binding domain (amino acid residues 795–811, Fig. 3). In contrast, the chicken skeletal muscle MLCK is only 54 and 41% identical to the chicken smooth and non-muscle MLCKs within the catalytic core and calmodulin binding regions, respectively (Guerriero et al., 1986; Shoemaker et al., 1990). A comparison of all MLCK sequences shows 72% conserved (50% identical) residues within the catalytic core and only 52% conserved (29% identical) residues within the calmodulin binding domain. Sequence conservation among the skeletal muscle MLCKs is maintained 33 amino acids amino-terminal of the homologous catalytic core (Fig. 3). Homology among MLCKs is lost amino-terminal of these 33 residues although the amino acid content remains similar. Smooth and non-muscle MLCKs have a carboxyl-terminal extension of conserved amino acids that are not present in skeletal muscle MLCKs (Guerriero et al., 1986; Shoemaker et al., 1990). Not shown in this figure is the amino acid sequence of the rabbit uterine smooth muscle MLCK which has recently been determined (Gallagher et al., 1991). It is 92% similar to the chicken smooth and nonmuscle MLCKs within the region shown in Fig. 3.
Fig. 3. Comparison of the primary structures of MLCKs from different tissues and species.
Panel A, a linear schematic shows full-length MLCKs (amino-terminal to carboxyl-terminal) from rat skeletal muscle (RATSKMLCK; Roush et al., 1988), rabbit skeletal muscle (RABSKMLCK; Takio et al., 1986), chicken skeletal muscle, chicken smooth muscle (CHKSMMLCK; Olson et al., 1990), and chicken fibroblasts (CHKNMMLCK; Shoemaker et al., 1990). The solid block indicates the position of the conserved catalytic cores. The shaded region amino-terminal of the catalytic cores of the skeletal muscle enzymes indicates a region of extended homology among these MLCKs. The shqded region carboxyl-terminal of the catalytic core indicates the position of the calmodulin binding domains. Panel B, amino acid sequence alignment of the same MLCKs is shown beginning with the region that is conserved amino-terminal of the catalytic core. A consensus sequence is shown above the sequence alignment. Within the consensus sequence, identical residues are indicated by capital letters and conservative substitutions by solid ovals. Conservation is based on the following groupings of structurally similar amino acids: basic polar R groups (K, R, and H); acidic and uncharged polar R groups (D, E, N, and Q); nonpolar chain R groups (M, L, I, and V); aromatic or ring-containing R groups (F, Y, W, and H); and small R groups with near neutral polarity (A, C, G, S, T, and P). Subdomains I-XI of the catalytic core (Hanks et al., 1988) and the calmodulin binding domain are bracketed above the consensus sequence. Residues that are conserved among all protein kinases are underlined in the consensus sequence. The GXGXXG consensus sequence found among nucleotide-binding proteins is within subdomain I. Subdomain II contains an invariant lysine among all protein kinases. Residues that are identical among all the skeletal muscle enzymes and different from the smooth and non-muscle enzyme are shaded, including the carboxyl-terminal extension of the smooth and non-muscle enzymes which are uniformly absent in the skeletal muscle enzymes.
The amino-terminal 478 amino acids of the chicken skeletal muscle kinase have an unusually high percentage of alanine (21%), proline (14%), and glycine (12%) residues and are highly polar (29% charged residues). Although this amino acid composition is similar to filamentous proteins such as elastin, collagen, and neurofilament proteins, no direct sequence homology has been detected with these proteins (analysis performed with algorithms described by Tokimitsu et al., 1987; Lees et al., 1988). In addition, three short motifs are repeated several times within this same amino-terminal region (Fig. 2). EQP is repeated seven times; , six times; and PAAVKAEDGGK(R), three times. These motifs are not repeated in any other protein in the NBRF data bank.
Analysis of Recombinant Wild-type, Truncated, and Chimeric MLCKs
Although the calculated molecular mass of chicken skeletal muscle MLCK is 87,064 Da, both tissue-purified MLCK and recombinant wild-type kinase expressed in COS cells have an indistinguishable appparent molecular mass of 150,000 Da as determined by Western immunoblots (Fig. 4A). The mobilities of the various chimeric kinases are similar to the wild-type kinase (Fig. 4A). The truncated kinases, identified by calmodulin overlay analysis, migrated as predominant bands at 41,000 and 44,000 Da for the skeletl and smooth muscle forms, respectively (Fig. 4B). However, there were less densely stained bands with apparent masses 3 kDa greater than the predominant bands. Thus, the calmodulin overlay analysis of the truncation mutants showed a slight difference in mobility between the skeletal and smooth muscle forms, but both were close to the molecular mass calculated from their amino acid sequence (SK-TR 42,077 and SM-TR 41,977). Similar results were obtained with Western immunoblots using polyclonal antisera directed against the chicken skeletal and smooth muscle MLCKs (data not shown). Thus, the aberrant migration of the holoenzyme on SDS-PAGE is apparently caused by the amino-terminal portion of the enzyme.
The Km and Vmax values of native and wild-type recombinant chicken skeletal muscle MLCKs with light chain substrates were virtually indistinguishable (Fig. 5). For the native chicken skeletal muscle MLCK, the Km values with skeletal and smooth muscle myosin light chains were 5.8 and 7.4 μM, respectively. The recombinant enzyme had Km values of 6.1 and 8.0 μM with these same substrates. The Vmax values were 37 and 32 μmol of 32P incorporated/min/mg for tissue-purified MLCK and 36 and 28 μmol/min/mg for recombinant MLCK with skeletal and smooth muscle light chain substrates, respectively.
The truncated skeletal muscle MLCK exhibited Ca2+/calmodulin-dependent activity and had Km values for light chains similar to the wild-type enzyme (Fig. 5). However, Vmax values for the truncated kinase were lower than values obtained for the wild-type enzyme with both skeletal and smooth muscle myosin light chains. Truncation of the smooth muscle MLCK at the amino- and carboxyl termini caused a similar decrease in the Vmax value but also increased the Km value for smooth muscle myosin light chain 10-fold. Phosphorylation of the skeletal muscle myosin light chain by SM-TR was not detected. The Vmax values for the truncation mutants were calculated on the predominant form of the kinase (Fig. 4B). If this form is derived from a larger form by partial proteolysis in COS cells, this value will be underestimated assuming that the predominant form has no activity. Additional experiments will be necessary to clarify this point.
To examine structure/function relationships within MLCKs further, segments of SM-TR were selected and exchanged with analogous regions within the native and truncated skeletal muscle enzyme. The resulting chimeric kinases are illustrated in Fig. 5. SK/SM1 represents a chimeric kinase with residues 1–454 of skeletal muscle MLCK fused to SM-TR. The kinetic properties of SK/SM1 were not significantly different from those of SM-TR.
Three smaller regions were also exchanged in chimeric molecules. The first 65-residue region amino-terminal of the catalytic core was selected because this region may be involved in light chain binding (Herring et al., 1990a, 1990b). The second major region in the skeletal muscle kinase, G521–K743, contains most of the residues highly conserved among protein kinases and is undoubtedly involved in catalysis and substrate binding. The third region exchanged contained the extreme carboxyl terminus of the catalytic core together with the calmodulin binding domain and regulatory region of the enzyme. Whenever the putative light chain binding site was fused to the heterologous catalytic core (either by exchange of the catalytic core or the light chain binding region SK/SM 3–9; Fig. 5), the resultant chimeric kinases were catalytically inactive. However, a chimeric enzyme containing the carboxyl terminus of the catalytic core and the calmodulin binding region of the smooth muscle kinase fused to the remainder of the skeletal muscle kinase (SK/SM2) was catalytically active and Ca2+/calmodulin dependent.
The Km values of SK/SM2 were unchanged from wild-type skeletal muscle kinase for skeletal and smooth muscle myosin light chains. However, the Vmax value decreased 10-fold for both substrates. SK/SMP2 exhibited similar Ca2+/calmodulin-dependent kinase activity with skeletal and smooth muscle myosin light chains (90% ± 1% and 94% ± l%), respectively, as substrates.
Site-specific mutations were made in the region amino-terminal of the catalytic core of the skeletal muscle MLCK (Fig. 5). Mutant Δ515–516,K517E is a deletion of residues S515 and S516, and a substitution of K517 with E, thus making the skeletal muscle MLCK sequence identical to the smooth muscle MLCK sequence at the analogous position predicted by alignment (Fig. 3). Mutant Δ515–516,K517E had catalytic properties similar to the wild-type skeletal muscle enzyme. In contrast, changing A494 to E in mutant A494E increased the Km values of the mutant enzyme 10-fold with both skeletal and smooth muscle myosin light chains without significantly affecting the Vmax values (Fig. 5).
DISCUSSION
Amino-terminal Tail Region
The least conserved region of MLCKs lies amino-terminal of the consensus sequence shown in Fig. 2. The length of the amino-terminal region is variable and accounts for the differences seen in the apparent molecular masses of chicken versus rabbit skeletal muscle MLCKs. Approximately 50% of the molecular mass of MLCKs reside in this amino-terminal region yet no identifiable homology exists between chicken skeletal muscle MLCK versus mammalian skeletal muscle MLCKs. Furthermore, there was no significant homology between chicken skeletal and smooth muscle MLCK. This amino-terminal region is, however, consistently polar and proline rich and exhibits asymmetric hydrodynamic properties. In contrast to the full-length MLCK, the mobility on SDS-PAGE of the chicken skeletal muscle MLCK without the amino terminus (SK-TR) is similar to the calculated molecular mass of 42,000 Da of this molecule. These data are consistent with previous studies of proteolytic fragments of rabbit skeletal muscle MLCK which indicate that it is the amino-terminal tail of the enzyme which is responsible for its aberrant migration on SDS-PAGE (Mayr and Heilmeyer, 1983). The chicken skeletal muscle MLCK amino-terminal tail region also contains repeated motifs that are not found in other proteins and are distinct from the motifs I and II, which have been found in the amino-terminal region of the chicken smooth and non-muscle MLCKs (Olson et al., 1990). These motifs are not found in any of the other skeletal muscle MLCKs. These repeated structures within the amino-terminal tail region of the chicken skeletal muscle MLCK may possess a unique function, but additional experiments will be necessary to support this idea.
Removal of the amino-terminal region of the chicken skeletal muscle enzyme did not significantly affect the ability of the skeletal muscle kinase to recognize light chain substrates but did decrease the maximal rate at which the substrate was phosphorylated. These data indicate that although the primary determinants responsible for recognition of both skeletal and smooth muscle myosin light chain substrates are contained within the globular head region of skeletal muscle MLCKs, the tail region may influence catalysis, perhaps by stabilizing the catalytic domain of the kinase.
A similar truncation of the smooth muscle kinase also caused a decrease in the Vmax value and an increase in the Km value of the enzyme for smooth muscle light chains. The changes in these kinetics properties could not be fully reversed by fusing the amino-terminal region of the skeletal muscle enzyme to the smooth muscle form, suggesting that the amino-terminal region of the kinase is specific to its homologous catalytic core. Bagchi et al. (1989) constructed truncation mutants of the chicken smooth muscle enzyme which also had an apparent decrease in activity although Km and Vmax values were not determined.
Catalytic Domain
The catalytic core is defined as the region that is homologous among all protein kinases (Hanks et al., 1988; Knighton et al., 1991). A comparison of MLCK amino acid sequences with other protein kinases may localize regions of the molecule which are functionally significant. Most of the residues that are conserved among all protein kinases cluster in two general regions around subdomains I and II and around subdomains VI, VII, VIII, and IX. Subdomain I contains the GXGXXG consensus sequence found among nucleotide-binding proteins, and subdomain II contains the invariant K, which is essential for catalytic activity. These domains are spaced by regions of low conservation around subdomains III, IV, V, X, and XI, with subdomains V and X containing no invariable or conserved residues (Hanks et al., 1988). This pattern of conservation is similar among MLCKs except that subdomains V and X are more conserved. The strong conservation of these regions suggests that residues within subdomains V and X may contribute to specific but unidentified functions of MLCKs. A comparison of skeletal and smooth muscle MLCK sequences reveals that a 33-residue region amino-terminal of the catalytic core is highly conserved among the skeletal muscle but not the smooth muscle MLCKs. It has been postulated that acidic residues D269 and D270 in rabbit skeletal muscle MLCK which are located within this homologous region are involved in myosin light chain binding (Herring et al., 1990a). This region was retained in the SK-TR and SM-TR mutants. Seven chimeric enzymes constructed with an exchange of a 65-residue region immediately amino-terminal of the catalytic core (SK/SM3–9) were not active. Several lines of reasoning suggest that the loss of activity was caused by the disruption of the catalytic domain structure rather than by a junction of incompatible regions. The amino-terminal junction of the 65-residue region (SK454/SM462, Fig. 5) is identical to the amino terminus of SM-TR and SK/SM1. Since activity was maintained when the smooth muscle kinase was truncated or fused to the skeletal muscle tail region at this position, a disruption at this junction was probably not the reason for SK/SM3–9 having no activity. The carboxyl-terminal junction of the 65-residue region (SK520/SM526 or SM525/SK521) is very similar to the Δ515–516,K517E mutation which deletes 2 serines and exchanges a lysine for a glutamic acid. This mutation simulated the change that occurs at the chimeric junctions yet the catalytic activity of Δ515–516,K517E was indistinguishable from the wild-type enzyme. Therefore, the change of register which occurs in SK/SM3–9 at this junction would not be expected to cause a loss of activity. In addition, the actual junction between the skeletal and smooth muscle MLCKs at SK520/SM525 or SM524/SK521 was between two identical residues (G) in both skeletal and smooth muscle MLCKs and thus did not alter the sequence at that site. Finally, regions of the catalytic core which are homologous can successfully be interchanged with maintained activity as shown with SK/SM2. Together, these data suggest that the lack of activity in SK/SM 3–9 is caused by an incompatibility between the heterologous amino-terminal and catalytic regions rather than by an artifact of their construction.
Skeletal muscle MLCK phosphorylates smooth and skeletal muscle myosin light chains with low Km values whereas smooth muscle MLCK only phosphorylates smooth muscle myosin light chain with a low Km value. Previous mutagenesis experiments with rabbit skeletal muscle MLCK indicated that two acidic amino acids located 24 and 25 residues amino-terminal of the catalytic core (equivalent to D487 and D488 in chicken skeletal muscle MLCK) were important for low Km values for myosin light chain (Herring et al., 1990a). We therefore constructed two mutant skeletal muscle kinases in which residues conserved among the skeletal muscle enzymes but different from the smooth enzymes were altered to make the mutant enzyme more like the smooth muscle kinase. The aim of these experiments was to change the light chain substrate specificity of the skeletal kinase. Deletion of 515–516 and changing K517 to E had no effect on the kinetic properties of the mutant kinase. Alteration of A494 to E in the chicken skeletal muscle MLCK caused a 10-fold increase in the Km value for both skeletal and smooth muscle myosin light chains. Thus, although these mutations did not affect the specificity of the kinase for light chains, the data are consistent with the proposal that this region is involved in substrate recognition by skeletal muscle MLCKs.
Calmodulin Binding Domain
The calmodulin binding domain is virtually identical in primary structure among skeletal muscle MLCKs or smooth muscle MLCKs but not between the skeletal and smooth muscle enzymes. In fact, the identity between chicken skeletal and smooth muscle MLCKs within the calmodulin binding domain is only about 40%. Calmodulin binding domains in other proteins also share little amino acid identity, but rather they share a similar basic, amphiphilic, α-helical structure (DeGrado and O'Neil, 1990). Indeed, the pattern of basic amino acids is conserved between the two classes of MLCK. A chimeric MLCK was constructed in which the region containing the calmodulin binding domain and part of the catalytic core of the skeletal muscle enzyme was exchanged with the corresponding region of the smooth muscle kinase (SK/SM2). The Km values of SK/SM2 for skeletal and smooth muscle myosin light chains are similar to wild-type values, and the Vmax values are decreased. However, the enzyme is still regulated by Ca2+/calmodulin. Shoemaker et al. (1990) have shown that regulation by Ca2+/calmodulin is also retained when the calmodulin binding domain of the non-muscle MLCK is mutated to sequences similar to the calmodulin binding domains of the skeletal muscle MLCK or Ca2+/calmodulin-dependent protein kinase II. These data indicate that despite significant primary structural differences, the calmodulin binding domains of the skeletal and smooth muscle kinases are interchangeable.
In summary, skeletal and smooth muscle MLCKs within the same animal species have significantly different primary structures whereas enzymes within the same muscle type are highly conserved across species. Despite a 2-fold difference in the apparent molecular mass of chicken skeletal muscle MLCK relative to rabbit skeletal muscle MLCK, the organization of the catalytic and calmodulin binding domains within the enzymes is conserved. The additional mass of the chicken skeletal muscle enzyme relative to other skeletal muscle forms is located entirely within the nonconserved amino-terminal tail region. A 65-residue region amino-terminal of the catalytic core of MLCK has been implicated as being involved in substrate recognition by the enzyme. Further point mutations within this region should suggest a molecular basis for the interaction. The ability of skeletal muscle MLCK to be regulated by either the skeletal or smooth muscle calmodulin binding domain indicates that the mechanism of regulation involves similar structural elements.
Acknowledgments
We wish to thank Clive Slaughter and Carolyn Moomaw for their assistance with protein sequencing, and Phyllis Foley for help with manuscript preparation.
This work was supported by National Institute of Health Grants NIGMS 5T-32 GM08014 and HL26043. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The nucleotide sequence(s) reported in this paper has been submitted to the GenBank™/EMBL Data Bank with accession number(s) M81787.
The abbreviations used MLCK, myosin light chain kinase; SK-TR, truncated skeletal muscle; SM-TR, truncated smooth muscle; SDS, sodium dodecyl sulfate; PAGE, polyacrylamide gel electrophoresis; kb, kilobase(s); PCR, polymerase chain reaction; EGTA, [ethylenebis(oxyethylenenitrilo)]tetraacetic acid; MOPS, 4-morpholinepropanesulfonic acid. Amino acid residues are abbreviated using the single-letter code.
REFERENCES
- Adelstein RS, Klee CB. J. Biol. Chem. 1981;256:7501–7509. [PubMed] [Google Scholar]
- Andersson S, Davis DL, Dahlbäch H, Jörnvall H, Russell DW. J. Biol. Chem. 1989;264:8222–8229. [PubMed] [Google Scholar]
- Bagchi IC, Kemp BE, Means AR. J. Biol. Chem. 1989;264:15843–15849. [PubMed] [Google Scholar]
- Billingsley ML, Pennypacker KR, Hoover CG, Brigati DJ, Kincaid RL. Proc. Natl. Acad. Sci. U. S. A. 1985;82:7585–7589. doi: 10.1073/pnas.82.22.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal DK, Stull JT. Biochemistry. 1982;21:2386–2391. doi: 10.1021/bi00539a017. [DOI] [PubMed] [Google Scholar]
- Brauer AW, Oman CL, Margolies MN. Anal. Biochem. 1984;137:134–142. doi: 10.1016/0003-2697(84)90359-2. [DOI] [PubMed] [Google Scholar]
- DeGrado WF, O'Neil KT. Trends Biochem. Sci. 1990;15:59–63. doi: 10.1016/0968-0004(90)90177-d. [DOI] [PubMed] [Google Scholar]
- Devereux J, Haeberli P, Smithies O. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman AM, Blumenthal DK, Krebs EG. Annu. Rev. Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Foyt HL, Guerriero V, Jr., Means AR. J. Biol. Chem. 1985;260:7765–7774. [PubMed] [Google Scholar]
- Gallagher PJ, Herring BP, Griffin S, Stull JT. J. Biol. Chem. 1991;266:23936–23944. [PMC free article] [PubMed] [Google Scholar]
- Gubler U, Hoffman BJ. Gene (Amst.) 1983;25:263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Guerriero V, Jr., Russo MA, Olson NJ, Putkey JA, Means AR. Biochemistry. 1986;25:8372–8381. doi: 10.1021/bi00374a007. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Quinn AM, Hunter T. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hathaway DR, Haeberle JR. Anal. Biochem. 1983;135:37–43. doi: 10.1016/0003-2697(83)90726-1. [DOI] [PubMed] [Google Scholar]
- Herring BP. J. Biol. Chem. 1991;266:11838–11841. [PubMed] [Google Scholar]
- Herring BP, Nunnally MH, Gallagher PJ, Stull JT. Am. J. Physiol. 1989;256:C399–C404. doi: 10.1152/ajpcell.1989.256.2.C399. [DOI] [PubMed] [Google Scholar]
- Herring BP, Fitzsimons DP, Stull JT, Gallagher PJ. J. Biol. Chem. 1990a;265:16588–16591. [PMC free article] [PubMed] [Google Scholar]
- Herring BP, Stull JT, Gallagher PJ. J. Biol. Chem. 1990b;265:1724–1730. [PMC free article] [PubMed] [Google Scholar]
- Ikebe M, Stepinska M, Kemp BE, Means AR, Hartshorne DJ. J. Biol. Chem. 1987;260:13828–13834. [PubMed] [Google Scholar]
- Kameshita I, Fujisawa H. Anal. Biochem. 1989;183:139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Kamm KE, Stull JT. Annu. Reu. Phurmacol. Toricol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Kamm KE, Leachman SA, Michnoff CH, Nunnally MH, Persechini A, Richardson AL, Stull JT. Prog. Clin. Biol. Res. 1987;245:183–193. [PubMed] [Google Scholar]
- Kemp BE, Stull JT. In: Peptides and Protein Phosphorylation. Kemp BE, editor. CRC Press; Boca Raton, FL: 1990. pp. 115–133. [Google Scholar]
- Kemp BE, Pearson RB, Guerriero V, Jr., Bagchi IC, Means AR. J. Biol. Chem. 1987;262:2542–2548. [PubMed] [Google Scholar]
- Kennelly PJ, Edelman AM, Blumenthal DK, Krebs EG. J. Biol. Chem. 1987;262:11958–11963. [PubMed] [Google Scholar]
- Knighton DR, Zheng J, Ten Eyck LF, Ashford VA, Xuong N-H, Taylor SS, Sowadski JM. Science. 1991;253:407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- Landon M. Methods Enzymol. 1977;47:145–149. doi: 10.1016/0076-6879(77)47017-4. [DOI] [PubMed] [Google Scholar]
- Lees JF, Shneidman PS, Skuntz SF, Carden MJ, Lazzarini RA. EMBO J. 1988;7:1947–1955. doi: 10.1002/j.1460-2075.1988.tb03032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman DJ, Pearson WR. Science. 1985;227:1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. J. Biol. Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- Mayr GW, Heilmeyer LMG., Jr. Biochemistry. 1983;22:4316–4326. doi: 10.1021/bi00287a024. [DOI] [PubMed] [Google Scholar]
- McPhaul MJ, Noble JF, Simpson ER, Mendelson CR, Wilson JD. J. Biol. Chem. 1988;263:16358–16363. [PubMed] [Google Scholar]
- Michnoff CH, Kemp BE, Stull JT. J. Biol. Chem. 1986;261:8320–8326. [PubMed] [Google Scholar]
- Moore RL, Stull JT. Am. J. Physiol. 1984;247:C462–C471. doi: 10.1152/ajpcell.1984.247.5.C462. [DOI] [PubMed] [Google Scholar]
- Moore RL, Houston ME, Iwamoto GA, Stull JT. J. Cell. Physiol. 1985;125:301–305. doi: 10.1002/jcp.1041250219. [DOI] [PubMed] [Google Scholar]
- Nunnally MH, Stull JT. J. Biol. Chem. 1984;259:1776–1780. [PubMed] [Google Scholar]
- Olson NJ, Pearson RB, Needleman DS, Hurwitz MY, Kemp BE, Means AR. Proc. Natl. Acad. Sci. U. S. A. 1990;87:2284–2288. doi: 10.1073/pnas.87.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RB, Wettenhall REH, Means AR, Hartshorne DJ, Kemp BE. Science. 1988;241:970–973. doi: 10.1126/science.3406746. [DOI] [PubMed] [Google Scholar]
- Roush CL, Kennelly PJ, Glaccum MB, Helfman DM, Scott JD, Krebs EG. J. Biol. Chem. 1988;263:10510–10516. [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratow Manual. 2nd ed. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Shoemaker MO, Lau W, Shattuck RL, Kwiatkowski AP, Matrisian PE, Guerra-Santos L, Wilson E, Lukas TJ, Van Eldik LJ, Watterson DM. J. Cell Biol. 1990;111:1107–1125. doi: 10.1083/jcb.111.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TF, Waterman MS. Adv. Appl. Math. 1981;2:482–489. [Google Scholar]
- Takio K, Blumenthal DK, Edelman AM, Walsh KA, Krebs EG, Titani K. Biochemistry. 1985;24:6028–6037. doi: 10.1021/bi00343a002. [DOI] [PubMed] [Google Scholar]
- Takio K, Blumenthal DK, Walsh KA, Titani K, Krebs EG. Biochemistry. 1986;25:8049–8057. doi: 10.1021/bi00372a038. [DOI] [PubMed] [Google Scholar]
- Tokimitsu I, Tajima S, Nishikawa T, Tajima M, Fukasawa T. Arch. Biochem. Biophys. 1987;256:455–461. doi: 10.1016/0003-9861(87)90602-3. [DOI] [PubMed] [Google Scholar]
- Urdal DL, Mochizuki D, Conlon PJ, March CJ, Remerowski ML, Eisenman J, Ramthun C, Gillis S. J. Chromatogr. 1984;296:171–179. doi: 10.1016/s0021-9673(01)96410-6. [DOI] [PubMed] [Google Scholar]
- Zoller MJ, Smith M. DNA (N. Y.) 1984;3:479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]