Abstract
Osteoporosis is a skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture.1 Osteoporosis remains a major health problem worldwide, costing an estimated $13.8 billion in health care each year in the United States. Despite advances in treating osteoporosis in the elderly, no cure exists. Osteoporosis has its roots in childhood. Accrual of bone mass occurs throughout childhood and early adulthood, and peak bone mass is a key determinant of the lifetime risk of osteoporosis. Because the foundation for skeletal health is established so early in life, osteoporosis prevention begins by optimizing gains in bone mineral throughout childhood and adolescence.2,3
Osteoporosis evaluation and prevention is relevant to children with cerebral palsy (CP). CP is the most prevalent childhood condition associated with osteoporosis. Bone density is significantly decreased, and children with CP often sustain painful fractures with minimal trauma that impair their function and quality of life. Preventing or improving osteoporosis and maximizing bone accrual during critical stages of growth will minimize the future lifelong risks of fractures in children with CP. This article addresses the anatomy and structure of bone and bone metabolism, the clinical assessment of bone mass, the causes of osteoporosis and its evaluation and treatment in children with CP.
Keywords: Osteoporosis, Bone density, Bone health, Cerebral palsy, Disabilities
OSTEOPOROSIS
Diagnosis in Adults
The diagnosis of osteoporosis in adults is well defined and based exclusively on the assessment of bone mineral density (BMD). Bone density is reported as a T-score which is the number of standard deviations more than or less than the mean for a healthy 30-year-old Caucasian (nonrace adjusted database) adult of the same sex. The World Health Organization classifies normal bone density as a T-score of −1 or higher. Osteopenia is classified as a T-score between −2.5 and −1, and osteoporosis is a T-score less than or equal to −2.5. If a person has a fracture and a T-score of less than −2.5, then they are considered to have severe osteoporosis. Fracture risk and treatment options have been well investigated and documented in adults. Every 1 standard deviation decrease in BMD is associated with a twofold increase in fracture risk.4 However, comparable information is limited in children.
Osteoporosis in Children
The risk of fracture associated with low BMD, the evaluation of osteoporosis, and treatment options in children are less well defined. However, over the past decade there have been advances in the diagnosis and diagnostic classifications for osteoporosis in children. The International Society of Clinical Densitometry released a position statement defining the parameters for the diagnosis of osteoporosis in children in 2008. Unlike adult osteoporosis, the consensus was that osteoporosis in children should not be determined based on densitometric criteria alone. The diagnosis of osteoporosis requires a clinically significant fracture history and low bone mineral content or bone mineral density (ISCD Pediatric Position Statement, 2008). The current definition for osteoporosis in children includes a BMD Z-score less than −2.0 adjusted for age, gender, and body size plus a clinically significant history of fracture: (1) 2 upper extremity fractures, or (2) vertebral compression fracture, or (3) a single lower extremity fracture. The Z-score is the number of standard deviations the patient’s BMD is more than or less than age-, sex-matched reference values.
BONE EMBRYOLOGY, ANATOMY, AND ARCHITECTURE
To begin to understand osteoporosis a basic understanding of bone embryology, anatomy, and architecture is needed. The musculoskeletal system is derived from embryonic mesoderm at the third week of gestation. Mesenchyme, a subtype of mesoderm, is responsible for bone, cartilage, muscle, tendon, and fibrous connective tissue formation. In the sixth week of gestation, the mesenchymal cells begin the process of ossification of long bones. By the seventh week the cells differentiate into cartilage-forming precursors of long bones. In the eighth week the mesenchymal cells differentiate into osteoblasts, osteoclasts, and chrondroclasts through the process of endochondral ossification. This process transforms cartilage into bone and continues throughout childhood.5
Composition and Structure of Bone
The skeleton of the developing embryo is primarily composed of either fibrous membranes or hyaline cartilage, which provide the medium for ossification. The process of ossification of flat bones such as the skull, ileum, mandible, and scapula occurs through intramembranous ossification, whereas the long bones such as the tibia, femur, and humerus are formed through endochondral ossification. Each long bone is comprised of 2 wider ends (epiphyses), a tubular middle (diaphysis), and the developing zone between the 2 (metaphysis). A layer of cartilage (growth plate) separates the epiphysis and metaphysis in growing bones. This area becomes calcified and remodeled with bone when growth is complete. The outer layer of the bone is comprised of a thick dense layer of calcified tissue known as cortical bone, which provides strength to the bone. Eighty-ninety percent of the volume of cortical bone is calcified. Toward the metaphysis and epiphysis, the cortex becomes thinner and the space is filled with thin calcified trabeculae known as trabecular or cancellous bone. Only 15% to 25% of trabecular bone is calcified. The bone marrow, blood vessels, and connective tissue make up most of the space. There are also 2 surfaces that the bone has with the surrounding soft tissues. The external surface is the periosteal surface and the internal surface is known as the endosteal surface. These are lined with osteogenic cells, which maintain bone formation and absorption.5
Bone Formation and Absorption
The rates of absorption and deposition are equal in nongrowing bones. This delicate balance keeps the total bone mass constant and serves an important role in maintaining the strength of bones. Bones will adjust their strength in proportion to the amount of stress placed on them. Bones thicken with heavy loads and change shape to provide the necessary support. Healthy load-bearing bones and their trabeculae have enough strength to carry a load without breaking suddenly or in fatigue.6 The deposition and absorption of bone aligns with stress patterns. New bone matrix replaces old brittle bone. This balance is maintained through the work of osteoblasts and osteoclasts.
Function of Osteoblasts and Osteoclasts
Osteoblasts are found on the outer surface of bone and in bone cavities. Osteoblast activity occurs in approximately 4% of all living bones. There is continual activity with new bone always being formed.5 At the same time that bone is being formed, bone is also continually being absorbed by osteoclasts. Osteoclasts are large multinucleated cells. They are active on less than 1% of bone surfaces at any one time. Absorption occurs when osteoclasts send out villus-like projections toward bone and secrete proteolytic enzymes, citric acid, and lactic acid, which dissolve the organic matrix of the bone and the bone salts. The fragments of bone salts and collagen are than digested by the osteoclasts. Osteoclasts tunnel out sections of bone. Once the osteoclasts complete the process, osteoblasts invade the tunneled out bone and begin to lay down new bone.5 Normal bones can detect and repair small amounts of microdamage. In some bones this damage can exceed the threshold, escape repair, accumulate, and result in fracture.6
Frost describes a hypothesis of mechanical bone competence that depends on the interactions between a bone’s strength and the magnitude and types of peak voluntary mechanical load on a load-bearing bone during typical activities. Diseased bone or failure to achieve mechanical bone competence can result in nontraumatic fractures in childhood.6 This can be seen in children with CP.
MARKERS OF BONE METABOLISM
Osteogenic Growth Factors
Insulin-like growth factors (IGF) are polypeptides that are synthesized in multiple tissues including bone. These peptides enhance the function of mature osteoblasts, therefore increasing bone matrix synthesis. Insulin-like growth factors inhibit bone collagen degradation and increase collagen synthesis, which help to maintain the bone matrix and bone mass. Alkaline phosphatase is secreted by osteoblasts while actively depositing bone. This activates collagen fibers and causes the deposition of calcium salts. The blood level of alkaline phosphatase is a good indicator of bone formation.7
The Role of Calcium and Vitamin D
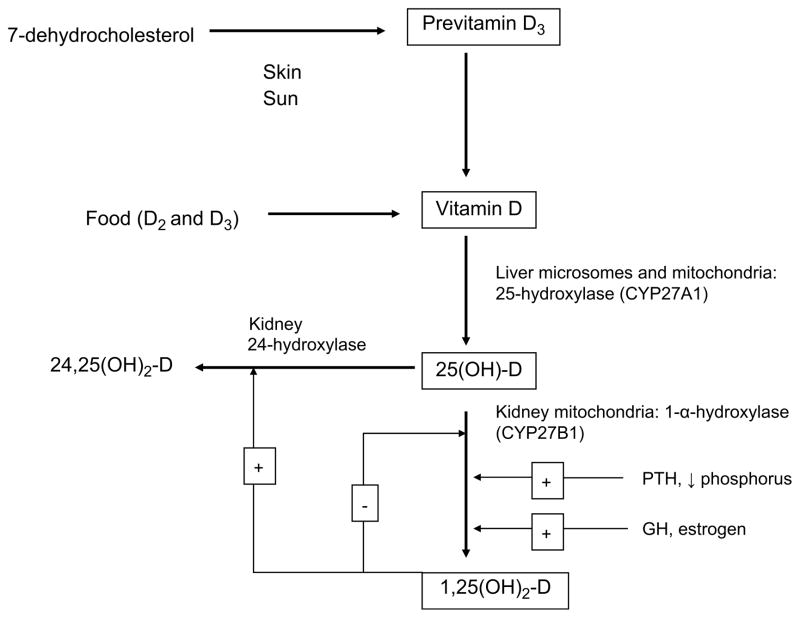
Vitamin D plays a critical role in the mineralization of bone. It is produced in the skin through exposure to sunlight. Vitamin D is biologically inert and must undergo 2 hydroxylations, first in the liver and then the kidneys to become active (Fig. 1). The bio-logically active form is 1,25-dihydroxyvitamin D [1,25(OH)2D]. Its role is to maintain serum calcium in the normal range. It does this by increasing the absorption of calcium in the intestines and signaling stem cells in the bone to become mature osteoclasts. These osteoclasts then mobilize calcium from bone into circulation.5 Vitamin D is found naturally in small amounts in some foods. Oily fish such as salmon, mackerel, and fish liver oils contain vitamin D. Bread products, cereals, milk, and other dairy products are fortified with vitamin D, although the percentage of fortification on the label may not accurately reflect what is found in the food.8
Fig. 1.
Vitamin D pathway.
Vitamin D plays a role in bone mineralization by maintaining adequate levels of calcium and phosphorus in the blood. This allows the osteoblasts to lay down bone matrix. The production of 1,25(OH)2D is regulated by serum calcium levels through the action of parathyroid hormone (PTH) and phosphorus. As vitamin D stores become depleted due to lack of sunlight exposure or dietary deficiency, intestinal absorption of calcium decreases from 30% to 40% to 10% to 15%. The decrease in calcium levels leads to an increased secretion of PTH. PTH signals the renal conversion of 25(OH)D to 1,25(OH)2D indirectly through renal wasting of phosphorus resulting in decreased intracellular and blood levels. Hypophosphatemia in turn results in the increase in circulating concentrations of 1,25(OH)2D. Multiple other hormones associated with growth and development (growth hormone [GH] and prolactin) also indirectly increase renal production of 1,25(OH)2D.5
The 1,25(OH)2D induces pre-osteoclasts to mature into osteoclasts. The osteoclasts in turn release hydrochloric acid and proteolytic enzymes that dissolve bone and matrix and release calcium into the extracellular space. 1,25(OH)2D also increases the expression of alkaline phosphatase, osteocalcin, osteopontin, and cytokines in osteoblasts.5
FACTORS IMPACTING BONE MASS
Osteoporosis is a disease characterized by a reduction in bone mass accompanied by micro-architectural changes that reduce the bone’s mechanical loading capability and increase its susceptibility to fractures.9 Acquisition of BMD is multifactorial and includes nutritional factors, genetics, hormonal influences, and growth factors.2 Gains in bone size and bone mineral content during childhood and adolescence are achieved only when environmental factors are favorable. Anorexia nervosa, exercise-induced amenorrhea, cystic fibrosis, inflammatory bowel disease, celiac disease, and rheumatologic disorders are associated with early deficits in bone mineral.3
Bone acquisition and remodeling is controlled by mechanical and metabolic factors. Normal skeletal growth, the progression of puberty, and bone mineral accrual all require appropriate hormonal influences, including thyroid hormone, GH, IGF, and sex steroids.3,10 Bone growth is largely dependent on GH before puberty.11 Later, sex steroids become essential for the completion of epiphyseal maturation and mineral accrual in adolescence. The importance of normal endocrine function for bone mineral accrual is highlighted by clinical deficiency states. Reduced bone mineral density is commonly seen in GH-deficient children,12 and has been noted in disorders of estrogen resistance and aromatase deficiency.13 Malnutrition, immobility, sex steroid deficiency, and other factors can interrupt bone mineral accrual and have been found to be a contributing factor to early bone loss in children with CP.14
Overall, appropriate gains in bone size and mineral content are achieved only when environmental conditions are favorable. Frost has discussed the idea that gene expression patterns in utero create baseline bone conditions at birth, including basic bony anatomy and anatomic relationships and neurologic and muscular anatomy and physiology. One also has the “machinery” to increase the strength of a load-bearing bone as needed by adapting to conditions placed on the bone during typical activities. However, factors that decrease a load-bearing bone’s strength could potentiate non-traumatic fractures. According to the “mechanostat hypothesis,” this could be the result of inadequate modeling, excessive disuse mode remodeling, impaired detection or repair of microdamage, degraded properties of bone that potentiate microdamage or a combination of the these.6
Adolescence is typically a period of maximal bone accrual. Recent studies suggest that attainment of peak bone mass occurs at a younger age than was previously believed, with the average age closer to 18 to 25 years than 30 years.15–17 Twenty-five percent of peak bone mass is acquired during the 2-year period surrounding peak height velocity and at least 90% is reached by age 18 years.11 If the process of bone accrual is disrupted during this sensitive period, profound and lifelong osteopenia can result. The label “female athlete triad” refers to a syndrome of disordered eating, amenorrhea, and osteopenia seen in adolescent women who engage in intensive physical training.18–20 Expanding clinical experience with this syndrome confirms that the consequences of early osteopenia can be devastating. Premature fractures can occur, and lost bone mineral density may never be regained.21 The characteristics of affected athletes may be analogous to those of pubertal children with CP, in whom impaired oral intake results in undernutrition and suboptimal body weight, delayed menses, and pubertal progression. This suggests a disruption of the hypothalamic–pituitary–gonadal (HPG) axis and abnormal hormone status.22
ASSESSMENT OF BONE HEALTH
The assessment of bone density is important for 3 reasons: to diagnose osteoporosis, to predict future fracture risk, and to monitor therapy.
Assessment of Bone Density Using Dual Radiograph Absorptiometry
Dual radiograph absorptiometry (DXA) is the most widely used method for assessment of BMD and is considered the “gold standard”. DXA uses 2 different radiographic energies to record attenuation profiles at 2 different photon energies. Attenuation is largely determined by tissue density and thickness. At a low energy, bone attenuation is greater than soft tissue attenuation. At high energy, they are similar. This allows the distinction between bone and soft tissue. The energy absorption of the 2 different energy radiographic beams is used to provide estimates of the amounts of bone mineral. The radiographic photons are collimated into a fan beam that passes through the patients and the photons are selectively attenuated by the bone and soft tissue. After the beam passes through the patient, it is passed to a radiographic detector whereby the intensity of radiation is recorded. This provides a 2-dimensional measurement dependent on the size of the bone and does not separate cortical and trabecular BMD. It can measure central skeletal sites (hip and spine). Extensive epidemiologic data in adults have shown correlations with bone strength in vitro. The DXA scan has been validated in adults and is widely available in the United States (Fig. 2).
Fig. 2.
DXA scanning device.
Bone density measured by DXA is an areal density (g/cm2) rather than a volumetric density (g/cm3). The BMD is the bone mineral content (in grams) per unit area (cm2). The DXA scans are analyzed to generate measures of projected bone area, bone mineral content, and areal bone mineral density. Results are reported as T-scores in adults. This compares the patient’s BMD with the young-normal mean BMD and expresses the difference as a standard deviation score. In children a Z-score is used. This compares BMD with age- and gender-matched references. Typical scan times for cooperative children are roughly 1 minute per scan for lumbar spine or distal femur and 5 to 7 minutes for the whole body.
In normal individuals, much of the pubertal gain in bone density as measured by DXA can be accounted for by increasing bone size. Increases in long bone diameter are matched by proportionate increases in cortical thickness, with no net increase in volumetric density.23 However, bone strength is determined not only by bone density but also by bone geometry (eg, size of bone). Areal BMD may be diminished compared with age-matched normal subjects because of a true decrease in volumetric density or due to differences in the 3-dimenional structure of the bone.24–27 Thinning of the cortex and a smaller outer diameter will both result in diminished areal density as measured by DXA, regardless of whether true volumetric density is decreased. The diameter of a cylindrical bone and the thickness of the cortex are important mechanical parameters. They have a significant impact on the ability of a bone to withstand loads without fracture.27 Assessment of these factors is necessary to understand fracture risk, including in CP.
Assessment of Bone Density Using Peripheral Quantitative Computed Tomography
Peripheral quantitative computed tomography (pQCT) (Fig. 3) provides a 3-dimensional assessment of volumetric BMD. This differs from a DXA scan, which measures a 2-dimensional areal BMD. The limitations of DXA are relevant to growing children, as a DXA scan may not accurately capture changes in bone size that relate to bone strength. DXA can underestimate true volumetric BMD in growing children with small bone size. The advantages of pQCT are that it requires less radiation exposure and has good precision. The pQCT provides measures of bone size and geometry that are not attainable with DXA. The pQCT technology allows a 3-dimensional approach to measure bone density and bone geometry. This provides a more accurate assessment of change during growth. The pQCT is able to estimate cortical width and bone endosteal and periosteal circumference, allowing for better characterization of bone strength. Peripheral QCT is independent of size. Children with CP typically have smaller than normal bones with thin cortex. These are important parameters that impact on the bone’s ability to withstand load and resistance to bending without fracture.28 The use of pQCT is not yet widely used or validated in children with CP.
Fig. 3.

pQCT device.
In addition, pQCT can distinguish between the 2 main types or compartments of bone: trabecular (eg, spine or distal radius) and cortical bone (eg, radial shaft). Trabecular and cortical bone differ in their rates of bone turnover and pattern of bone accrual during normal growth. Trabecular bone in particular is often more rapidly affected by disease or therapies. Peripheral QCT imaging obtains trabecular bone measurements at an ultradistal site, whereas cortical bone measurements are acquired from the shaft of the bone. The separate analysis of cortical and trabecular bone is also advantageous when studying the response to therapeutic interventions.29 Measurements can include a potential weight-bearing site (tibia) and a non–weight-bearing site (radius). The trabecular site is evaluated at 4% of the length of the tibia or forearm. In addition, a second site at 20% of the length of the tibia or forearm is measured to assess a purely cortical bone. Bone mineral content, volumetric BMD, and area of the trabecular and cortical compartments can be calculated at both sites. Periosteal and endosteal circumferences and measurements of bone strength, the polar strength–strain index (pSSI), are measured at the 20% site. The pSSI is calculated considering the geometric properties (bone size) and material properties (bone density) of the bone. Settings to obtain the scans and analysis modes, including pSSI, in children with CP have been previously reported.28 The scan time is approximately 90 seconds per slice (approximately 10 minutes total time).
Risks
Bone density scans (DXA and pQCT) expose the patient to a small amount of radiation. The total amount of radiation in performing DXA and pQCT (5 tests in total) is less than 4.0 mrem. The total radiation dose is similar to a round-trip cross-country plane flight, which is from 2 to 5 mrem per flight. The average background radiation to the general public is approximately 360 mrem per year. The total radiation exposure to complete these studies is therefore equivalent to a round-trip cross-country plane flight and is a small fraction (<2%) of the average background radiation that the general public receives per year. The risk from such a diagnostic procedure is not precisely known, but is believed to be small.
Challenges in Bone Density Assessment in CP
Assessment of bone density in children with CP has presented some challenges. Henderson and colleagues have been studying bone density and related factors in children with developmental disabilities including CP since 1993.27,30–33 Henderson and colleagues30 have demonstrated that reliable DXA measurements of bone density in children with CP may be obtained at the distal femur.34 Assessment of bone mineral density in this region is clinically useful because this is the most common site of fractures. This innovative technique allows use of DXA technology in children whose spasticity or contractures preclude measurement at the traditional proximal femur site. Henderson and colleagues26,27,30 have also compiled a database of DXA measurements (including distal femur values) in normal children, allowing standardization and comparison of DXA studies. Values for the reliability and coefficient of variation of the techniques are reported in these studies.
Peripheral QCT is not distorted by bone size or body weight, which is important when evaluating children with CP who often have smaller height and weight compared with age-matched peers. However, the assessment of bone density and strength in children with CP by pQCT also presents technical challenges. Binkley and colleagues28 attempted pQCT scans in 15 children with moderate to severe CP. They were unable to obtain scans in 2 children due to issues with positioning in the scanner. They report on how to provide support for the extremities in children with CP, including splints to support legs, rolled towels, allowing the child to remain in their wheelchair, and help of staff to hold the necessary position.28
CP AND BONE HEALTH
Cerebral palsy is the most common physical disability of childhood.35 Cerebral palsy describes a group of permanent disorders of the development of movement and posture, causing activity limitations, which are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain. The motor disorders of cerebral palsy are often accompanied by disturbances of sensation, cognition, communication and behavior, epilepsy, and secondary musculoskeletal problems.36 The average cumulative incidence rate of CP is 2.7 per 1000 live births. In recent years, the incidence rate of CP has been increasing internationally due to increased survival of low birth weight infants.37–39 It has been estimated that more than 100,000 children in the United States today have some degree of neurologic disability attributed to CP.40 Children with CP frequently grow slowly. The impact of this altered growth on skeletal development and bone density is a significant health problem. In typically growing children, the accrual of peak bone mass follows peak height velocity. However, in children with CP, differences in linear growth become more accentuated over time compared with their typically growing peers. In addition, as growth slows, the bone mineral density also falls further outside the normal range.
Growth in CP: Risk Factors for the Development of Osteoporosis
Bone growth, as assessed by BMD, is an important aspect of growth in children with CP. In addition to diminished linear growth, children with CP often sustain painful pathologic fractures due to poor mineralization of bone, often with minimal trauma.41,42 Thus, bone growth and bone density are highly relevant to overall linear growth, nutritional health, and health-related quality of life. Henderson and colleagues30 initially investigated nutritional status and BMD in 139 children with CP in a cross-sectional study. They found that BMD was variable, but averaged −1SD. Functional severity (increasing severity) and lower nutritional status correlated with lower BMD. Low calcium intake and immobilization were also contributors to low BMD. Vitamin D levels and anticonvulsants did not correlate with BMD when the severity of CP and nutritional status were controlled. Serum calcium, alkaline phosphatase, and osteocalcin were also found not to correlate with BMD.
Henderson31 then evaluated whether BMD can predict fractures in an observational cohort study of 43 children with quadriplegic CP followed for a mean of 3.8 years. During the follow-up, 9 fractures occurred. The predictive variables were history of a previous fracture and spica casting, but not lumbar spine BMD. Fractures in this population often occurred in the extremities or in the spine. Spine BMD did not correlate well with BMD in the extremities, specifically the femur. However, in this population of children, who frequently have orthopedic surgeries, hardware, or contractures, assessment of BMD of the proximal femur could not be determined consistently. Subsequently, a new technique has been proposed for measuring BMD in the distal femur in children with CP in the lateral position, as this position can be more easily obtained in most children with CP and is more relevant to the site where fractures frequently occur. Scanning the hip was instituted in adults as this is the location at which fractures occur, but the distal femur is the most common location of fractures in individuals with CP.34
Further investigation into bone density in children with CP focused on those with moderate to severe motor impairment27 (Gross Motor Function Classification System, GMFCS, III to V43). Significantly decreased bone density is virtually universal in non-ambulatory children with moderate to severe CP after the age of 10 years; however, predicting which children will fracture is a challenge.27 Studies have found that the percentage of children with CP with a history of fractures ranges from 12% to 26%.27,44 Multiple predisposing factors for bone fragility in individuals with disabilities have been investigated, including weight-bearing activity, muscle mass, calcium and phosphate homeostasis, nutrition, and medication use, especially glucocorticoids and anticonvulsants (Table 1).14 In children with CP, these risk factors seem to disrupt bone homeostasis and result in microdamage that in turn predisposes them to non-traumatic fractures. Henderson and colleagues45,46 have studied longitudinal assessments over 2 years of bone density in children and adolescents with moderate to severe CP (GMFCS III to V), finding that lower BMD Z-scores at initial evaluation were associated with greater severity of CP (GMFCS level), feeding difficulty, and poorer growth and nutrition as judged by weight Z-scores. Large variability in changes in bone density from 42% per year to −31% was seen in the distal femur and lumbar spine. Despite increases in BMD, distal femur BMD Z-scores decrease with age in this population.
Table 1.
Risk factors for osteoporosis in CP
| Poor growth and nutritional status | Low calcium Intake |
| poor sun light | Immobility |
| Low vitamin D | Medications that interfere with vitamin D metabolism |
| Lack of weight bearing | Growth hormone insufficiency |
Fracture rate was investigated by Stevenson and colleagues in a longitudinal cohort study of 245 patients with moderate to severe CP. At baseline, 15.7% reported a history of fractures. Children with fractures were older and had higher body fat content than those who did not fracture. Level of severity (GMFCS) and gender were not significant. Twenty children reported 24 fractures during 604 person-years of follow-up, with 4 fractures per 100 person years (4% per year). With a history of prior fracture at baseline, the rate increased to 7% per year. Having a gastrostomy tube (6.8% per year) and high body fat at baseline (9.7% per year) were also associated with increased risk of fracture.47
Binkley and colleagues28 investigated bone density and strength assessment using pQCT in a cross-sectional study of 13 children with moderate to severe CP. Bone strength was compromised in children with CP secondary to smaller and thinner bones, not lower cortical bone density.
TREATMENT OPTIONS
Minimize Known Risk Factors
The first step in the management of osteoporosis in children with CP is to reduce the known risk factors. When possible, medications such as anticonvulsants that have the least impact on BMD should be chosen. Children need exposure to sunshine to maximize their absorption of vitamin D. Because sunscreen can reduce the ability to absorb vitamin D from the sun, 10 to 15 minutes of exposure 3 times a week before applying sunscreen are recommended.5 The time needed can vary by location and time of year.
General Nutrition, Vitamin D and Calcium
Optimizing nutritional status, especially vitamin D and calcium levels, are important in the prevention and treatment of osteoporosis. Melanin reduces the production of vitamin D3. Individuals with darker skin color require longer exposure (up to five- to tenfold) to sunlight to make the necessary vitamin D3. Latitude, time of day, and season of the year affect the production of vitamin D3 in the skin. Casual exposure to the sun provides most of the vitamin D needed. Excess is stored in fat to be used during winter months when exposure may be limited. However, topical use of sunscreen dramatically reduces the amount of vitamin D absorbed. A sun protection factor of 8 (SPF 8) reduces absorption by greater than 97%. Chronic sunscreen use can result in vitamin D deficiency.5
Vitamin D deficiency is a concern for children with CP who may not be exposed to ample amounts of sunshine and who may have insufficient dietary intake. Jekovec-Vrhovsěk and colleagues evaluated BMD before and after supplementation with vitamin D and calcium. They followed 20 children with CP living in residential care. These children had severe motor impairment and used multiple and chronic anticonvulsant therapy. Thirteen children received vitamin D and 500 mg of calcium supplementation for 9 months. All children had increases in BMD. Of the 7 not treated and monitored, BMD remained the same or decreased.48
In 2008 the American Academy of Pediatrics (AAP) increased its recommendation for vitamin D supplementation for children. Exclusively and partially breastfed infants should receive supplements of 400 IU/d of vitamin D shortly after birth and continue supplementation until the child is weaned and consumes 1000 mL/d or more of vitamin D-fortified formula or whole milk. Nonbreastfed infants ingesting less than 1000 mL/d of vitamin D-fortified formula or milk should receive vitamin D supplementation of 400 IU/d. The AAP also recommends that children and adolescents who do not obtain 400 IU/d through vitamin D-fortified milk and foods should take a 400 IU vitamin D supplement daily.49 The recommended daily intake of calcium varies based on age (Table 2).50
Table 2.
Recommended daily allowance of calcium intake
| Age | Calcium Intake (mg/d) |
|---|---|
| 0–6 mo | 210 |
| 7–12 mo | 270 |
| 1–3 y | 500 |
| 4–8 y | 800 |
| 9–18 y | 1300 |
Vitamin D status can be determined by assessing levels of 25(OH)D. A level of less than 12.5 ng/mL is severe deficiency. Deficiency is defined as a level less than 37.5 ng/mL, and insufficiency as a level between 37.5 and 50 ng/mL. Sufficient levels of vitamin D are between 50 and 250 ng/mL. Aggressive therapy is needed for significant depletion. Pharmacologic doses of vitamin D should be used orally at 50,000 IU of vitamin D once weekly for 8 weeks.5
Activity and Weight Bearing
Caulton and colleagues51 evaluated the impact of standing/weight bearing on BMD in a randomized clinical trial of 26 prepubertal children with severe CP, comparing children receiving 50% increase in regular standing versus no increase in standing for a 9-month period. Range of standing was between 180 and 675 minutes per week. Improvement in lumbar spine BMD of 6% was reported in the standing group over the control group. No change was seen in tibial BMD. These investigators concluded that, whereas increased standing may decrease the risk of vertebral fractures, it is unlikely to impact lower extremity fractures. The magnitude of an increase in BMD sufficient to decrease the risk of fracture has not been defined for children with CP.
Low Frequency Oscillation
Ward and colleagues52 evaluated the influence of low-level mechanical stimulation on BMD in ambulatory children with disabilities in a double-blinded randomized control trial. Twenty children aged 4 to 19 years were randomized to standing on active or placebo devices for 10 minutes per day. Treatment was 5 days per week for 6 months. Volumetric trabecular BMD of the proximal tibia and spine (L2) was assessed using 3-dimensional QCT. The children receiving low-level mechanical stimulation had improved BMD in the tibia after 6 months, compared with the children receiving sham treatment. This noninvasive, nonpharmacologic treatment option warrants further investigation in children with CP.
Growth Hormone
Administration of growth hormone (GH) has been shown to improve BMD in children with CP. Ali and colleagues53 investigated GH treatment in a pilot randomized control study of 10 children with CP. Five children received GH daily for 18 doses. The remaining 5 children received no treatment. Linear growth improved significantly in the GH treatment group. Spinal BMD Z-scores, adjusted for height, also increased by 1.17 in the GH-treated group, in comparison to an increase of 0.24 (P = .03) in the control group.
Pharmacologic Bisphosphonates
Bisphosphonates are used to inhibit osteoclast-mediated bone resorption. In the United States, several bisphosphonates are available for use, including etidronate (Didronel), pamidronate (Aredia), alendronate (Fosamax), ibandronate (Boniva), and residronate (Actonel). Currently, none of the bisphosphonates are approved by the US Food and Drug Administration for use in children, and their use for osteoporosis in CP would be considered off-label in children.
Henderson and colleagues54 investigated the use of pamidronate in a group of children with quadriplegic CP. Six pairs of children were matched within pairs for age, sex, and race. All the children had a BMD Z-score less than −2.0 and 11/12 had previous fractures. The treatment protocol involved a daily intravenous infusion for 3 days, with 3-day dosing repeated every 3 months for 1 year. The children were also followed for 6 months for observation after treatment ended. All children received calcium and vitamin D supplementation. Intravenous bisphosphonates safely and effectively increased BMD for the duration of the study. Although a promising treatment, for whom, when, and for how long bisphosphonate treatment should be considered remains uncertain. Although oral bisphosphonates are available, they have yet to be sufficiently studied in children, including those with CP. The impact on future fracture rates is unclear.
TAKE-HOME MESSAGE AND PLANS FOR THE FUTURE
Children with severe CP develop clinically significant osteopenia. Unlike elderly adults, this is not primarily from true losses in bone minerals, but from a rate of growth in bone mineral that is diminished relative to healthy children, a failure to accrue bone mass. The efficacy of interventions to increase BMD can only be assessed once the magnitude and natural course of bone maturation is understood in children with CP before intervention. There continues to be a need for research in the area of bone accrual, and prevention and treatment options for osteoporosis in children with CP.
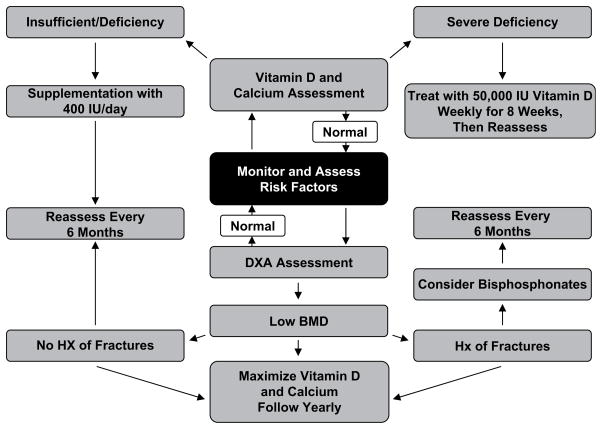
Children with CP should have their risk of osteoporosis assessed at each visit. Calcium and vitamin D intake should be evaluated by the medical team. When necessary, supplementation should be started and levels followed closely. Available software for reference Z-scores for DXA scans for the lumbar spine begin at the age of 6 years. Reference Z-scores for the distal lateral femur are also available for children at the age of 6 years. If a child is considered at risk, DXA scans should be performed for a baseline at the age of 6 years with follow-up every 1 to 2 years depending on individual risk factors. If a child with CP meets the criteria for osteoporosis, the clinician also needs to consider the use of a bisphosphonate to improve BMD and possibly prevent future fractures (Fig. 4).
Fig. 4.
Treatment algorithm.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit:
References
- 1.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy [see comment] JAMA. 2001;285:785–95. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 2.Gelfand IM, DiMeglio LA. Bone mineral accrual and low bone mass: a pediatric perspective. Rev Endocr Metab Disord. 2005;6(4):281–9. doi: 10.1007/s11154-005-6186-y. [DOI] [PubMed] [Google Scholar]
- 3.Bachrach LK. Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol Metab. 2001;12(1):22–8. doi: 10.1016/s1043-2760(00)00336-2. [DOI] [PubMed] [Google Scholar]
- 4.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures [see comment] BMJ. 1996;312(7041):1254–9. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Favus M, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 5. Washington, DC: The American Society for Bone and Mineral Research; 2003. pp. 1–12.pp. 129–37. [Google Scholar]
- 6.Frost HM. Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:1081–101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- 7.Guyton A. Parathyroid hormone, calcitonin, calcium and phosphate metabolism, vitamin D, bone and teeth. In: Dreibelbis D, editor. Textbook of medical physiology. 2. Philadelphia: Saunders Company; 1986. pp. 937–53. [Google Scholar]
- 8.Holick MF, Glorieux JH. Rickets. New York: Raven Press; 1991. Photosynthesis, metabolism, and biologic actions of vitamin D; pp. 1–22. [Google Scholar]
- 9.Formica CA, Nieves JW, Cosman F, et al. Comparative assessment of bone mineral measurements using dual X-ray absorptiometry and peripheral quantitative computed tomography. Osteoporos Int. 1998;8(5):460–7. doi: 10.1007/s001980050092. [DOI] [PubMed] [Google Scholar]
- 10.Bachrach LK. Bone mineralization in childhood and adolescence. Curr Opin Pediatr. 1993;5(4):467–73. doi: 10.1097/00008480-199308000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Bailey DA, Martin AD, McKay HA, et al. Calcium accretion in girls and boys during puberty: a longitudinal analysis. J Bone Miner Res. 2000;15(11):2245–50. doi: 10.1359/jbmr.2000.15.11.2245. [DOI] [PubMed] [Google Scholar]
- 12.Boot AM, Engels MA, Boerma GJ, et al. Changes in bone mineral density, body composition, and lipid metabolism during growth hormone (GH) treatment in children with GH deficiency. J Clin Endocrinol Metab. 1997;82:2423–8. doi: 10.1210/jcem.82.8.4149. [DOI] [PubMed] [Google Scholar]
- 13.Carani C, Qin K, Simoni M, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997;337(2):91–5. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd ME, Spector TD, Howard R. Osteoporosis in neurological disorders. J Neurol Neurosurg Psychiatr. 2000;68(5):543–7. doi: 10.1136/jnnp.68.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu PW, Briody JN, Ogle GD, et al. Bone mineral density of total body, spine, and femoral neck in children and young adults: a cross-sectional and longitudinal study. J Bone Miner Res. 1994;9(9):1451–8. doi: 10.1002/jbmr.5650090918. [DOI] [PubMed] [Google Scholar]
- 16.Matkovic V, Jelic T, Wardlaw GM, et al. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J Clin Invest. 1994;93(2):799–808. doi: 10.1172/JCI117034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vuori I. Peak bone mass and physical activity: a short review. Nutr Rev. 1996;54(4 Pt 2):S11–4. doi: 10.1111/j.1753-4887.1996.tb03892.x. [DOI] [PubMed] [Google Scholar]
- 18.Hobart JA, Smucker DR. The female athlete triad. Am Fam Physician. 2000;61(11):3357–64. 3367. [PubMed] [Google Scholar]
- 19.Sabatini S. The female athlete triad. Am J Med Sci. 2001;322(4):193–5. doi: 10.1097/00000441-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Skolnick AA. ‘Female athlete triad’ risk for women. JAMA. 1993;270(8):921–3. [PubMed] [Google Scholar]
- 21.Drinkwater BL, Bruemner B, Chesnut CH., III Menstrual history as a determinant of current bone density in young athletes. JAMA. 1990;263(4):545–8. [PubMed] [Google Scholar]
- 22.Hemingway C, McGrogan J, Freeman JM. Energy requirements of spasticity [see comment] Dev Med Child Neurol. 2001;43(4):277–8. doi: 10.1017/s0012162201000524. [DOI] [PubMed] [Google Scholar]
- 23.Seeman E. From density to structure: growing up and growing old on the surfaces of bone. J Bone Miner Res. 1997;12(4):509–21. doi: 10.1359/jbmr.1997.12.4.509. [DOI] [PubMed] [Google Scholar]
- 24.Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7(2):137–45. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 25.Cowell CT, Lu PW, Lloyd-Jones SA, et al. Volumetric bone mineral density – a potential role in paediatrics. Acta Paediatr Suppl. 1995;411:12–6. doi: 10.1111/j.1651-2227.1995.tb13852.x. [discussion: 17] [DOI] [PubMed] [Google Scholar]
- 26.Henderson RC, Lark RK, Newman JE, et al. Pediatric reference data for dual X-ray absorptiometric measures of normal bone density in the distal femur. AJR Am J Roentgenol. 2002;178(2):439–43. doi: 10.2214/ajr.178.2.1780439. [DOI] [PubMed] [Google Scholar]
- 27.Henderson RC, Lark RK, Gurka MJ, et al. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics. 2002;110:e5. doi: 10.1542/peds.110.1.e5. [DOI] [PubMed] [Google Scholar]
- 28.Binkley T, Johnson J, Vogel L, et al. Bone measurements by peripheral quantitative computed tomography (pQCT) in children with cerebral palsy. J Pediatr. 2005;147:791–6. doi: 10.1016/j.jpeds.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Ward KA, Adams JE, Hangartner TN. Recommendations for thresholds for cortical bone geometry and density measurement by peripheral quantitative computed tomography. Calcif Tissue Int. 2005;77(5):275–80. doi: 10.1007/s00223-005-0031-x. [DOI] [PubMed] [Google Scholar]
- 30.Henderson RC, Lin PP, Greene WB. Bone-mineral density in children and adolescents who have spastic cerebral palsy. J Bone Joint Surg Am. 1995;77:1671–81. doi: 10.2106/00004623-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Henderson RC. Bone density and possible predictors of fracture risk in children and adolescents with spastic quadriplegia. Dev Med Child Neurol. 1997;39:224–7. doi: 10.1111/j.1469-8749.1997.tb07415.x. [DOI] [PubMed] [Google Scholar]
- 32.Henderson RC. The correlation between dual-energy X-ray absorptiometry measures of bone density in the proximal femur and lumbar spine of children. Skeletal Radiol. 1997;26:544–7. doi: 10.1007/s002560050283. [DOI] [PubMed] [Google Scholar]
- 33.Lin PP, Henderson RC. Bone mineralization in the affected extremities of children with spastic hemiplegia. Dev Med Child Neurol. 1996;38:782–6. doi: 10.1111/j.1469-8749.1996.tb15112.x. [DOI] [PubMed] [Google Scholar]
- 34.Harcke HT, Taylor A, Bachrach S, et al. Lateral femoral scan: an alternative method for assessing bone mineral density in children with cerebral palsy. Pediatr Radiol. 1998;28:241–6. doi: 10.1007/s002470050341. [DOI] [PubMed] [Google Scholar]
- 35.Back S. Cerebral palsy. Philadelphia: WB Saunders; 1999. [Google Scholar]
- 36.Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- 37.Rosen MG, Dickinson JC. The incidence of cerebral palsy. Am J Obstet Gynecol. 1992;167(2):417–23. doi: 10.1016/s0002-9378(11)91422-7. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki J, Ito M. Incidence patterns of cerebral palsy in Shiga Prefecture, Japan, 1977–1991. Brain Dev. 2002;24(1):39–48. doi: 10.1016/s0387-7604(01)00395-3. [DOI] [PubMed] [Google Scholar]
- 39.Colver AF, Gibson M, Hey EN, et al. Increasing rates of cerebral palsy across the severity spectrum in north-east England 1964–1993. The North of England Collaborative Cerebral Palsy Survey. Arch Dis Child Fetal Neonatal Ed. 2000;83(1):F7–12. doi: 10.1136/fn.83.1.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuban KC, Leviton A. Cerebral palsy. N Engl J Med. 1994;330:188–95. doi: 10.1056/NEJM199401203300308. [DOI] [PubMed] [Google Scholar]
- 41.Bischof F, Basu D, Pettifor JM. Pathological long-bone fractures in residents with cerebral palsy in a long-term care facility in South Africa. Dev Med Child Neurol. 2002;44:119–22. doi: 10.1017/s0012162201001773. [DOI] [PubMed] [Google Scholar]
- 42.Lohiya GS, Crinella FM, Tan-Figueroa L, et al. Fracture epidemiology and control in a developmental center. West J Med. 1999;170(4):203–9. [PMC free article] [PubMed] [Google Scholar]
- 43.Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 44.Leet AI, Mesfin A, Pichard C, et al. Fractures in children with cerebral palsy. J Pediatr Orthop. 2006;26(5):624–7. doi: 10.1097/01.bpo.0000235228.45539.c7. [DOI] [PubMed] [Google Scholar]
- 45.Henderson RC, Gilbert SR, Clement ME, et al. Altered skeletal maturation in moderate to severe cerebral palsy. Dev Med Child Neurol. 2005;47:229–36. doi: 10.1017/s0012162205000459. [DOI] [PubMed] [Google Scholar]
- 46.Henderson RC, Kairalla JA, Barrington JW, et al. Longitudinal changes in bone density in children and adolescents with moderate to severe cerebral palsy. J Pediatr. 2005;146:769–75. doi: 10.1016/j.jpeds.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 47.Stevenson RD, Conaway M, Barrington JW, et al. Fracture rate in children with cerebral palsy. Pediatr Rehabil. 2006;9:396–403. doi: 10.1080/13638490600668061. [DOI] [PubMed] [Google Scholar]
- 48.Jekovec-Vrhovsek M, Kocijancic A, Prezelj J. Effect of vitamin D and calcium on bone mineral density in children with CP and epilepsy in full-time care. Dev Med Child Neurol. 2000;42:403–5. [PubMed] [Google Scholar]
- 49.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children and adolescents. American Academy of Pediatrics Section on Breast-feeding, American Academy of Pediatrics Committee on Nutrition. Pediatrics. 2008;122:1142–52. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 50.Greer FR, Krebs NF American Academy of Pediatrics Committee on Nutrition. Optimizing bone health and calcium intakes of infants, children, and adolescents. Pediatrics. 2006;117:578–85. doi: 10.1542/peds.2005-2822. [DOI] [PubMed] [Google Scholar]
- 51.Caulton JM, Ward KA, Alsop CW, et al. A randomized controlled trial of standing program on bone mineral density in non-ambulant children with cerebral palsy. Arch Dis Child. 2004;89:131–5. doi: 10.1136/adc.2002.009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward K, Alsop C, Caulton J, et al. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360–9. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 53.Ali O, Shim M, Fowler E, et al. Growth hormone therapy improves bone mineral density in children with cerebral palsy: a preliminary pilot study. J Clin Endocrinol Metab. 2007;92:932–7. doi: 10.1210/jc.2006-0385. [DOI] [PubMed] [Google Scholar]
- 54.Henderson RC, Lark RK, Kecskemethy HH, et al. Bisphosphonates to treat osteopenia in children with quadriplegic cerebral palsy: a randomized, placebo-controlled clinical trial. J Pediatr. 2002;141:644–51. doi: 10.1067/mpd.2002.128207. [DOI] [PubMed] [Google Scholar]