Abstract
Purpose
To examine 1-year changes in insulin dynamics in overweight Hispanic children at high-risk of type 2 diabetes as a function of body composition and pubertal transition.
Experimental Design
Longitudinal changes in insulin dynamics, body composition and maturation were determined in 132 Hispanic children (70 boys/62 girls; aged 10.9±1.8 years).
Methods
Body composition was determined by dual energy x-ray absorptiometry and Tanner stage by physical examination. Insulin sensitivity (SI), the acute insulin response to glucose (AIR) and the disposition index (DI; an index of beta-cell function) were determined using an insulin modified intravenous glucose tolerance test. These measures were conducted at baseline and 1-year later.
Results
Fat mass increased by 13% (3.0 kg) and SI declined by 24%. In repeated measures analysis of variance, the fall in insulin sensitivity over 1 year remained highly significant even after adjusting for baseline fat mass, age, gender and change in fat mass. The fall in SI was not significantly influenced by Tanner stage. However, subjects in earlier maturation showed a compensatory increase in AIR (i.e. appropriate beta-cell compensation), whereas subjects in the latter stages of maturation did not (i.e. poor compensation).
Conclusions
These results indicate that failure to increase AIR in response to the fall in SI may be one factor in the pathogenesis of the progression of pediatric type 2 diabetes in this at risk population.
Keywords: Impaired glucose tolerance, insulin sensitivity, obesity, type 2 diabetes
Introduction
It has been estimated that 50% of Hispanic children born in the year 2000 will develop diabetes during the course of their lifetime (1). The disproportionate burden of obesity and its related co-morbidities in Hispanics is evident early in life. In 2000, 43.8% of Hispanics aged 12–19 were at risk of overweight and 23.4% were overweight, and these values were approximately double that of non-Hispanic whites (2). Hispanic children are also more insulin resistant than Caucasian children independent of body fat content (3), thus placing greater demands on beta-cell function. These factors are likely contributors to the increased risk of developing type 2 diabetes in Hispanic children during adolescent growth.
Pubertal transition may be an additional risk factor for the development of pediatric type 2 diabetes. Rapid and dynamic changes in various metabolic systems occur during this critical period of development. Several studies have demonstrated that insulin sensitivity decreases at the onset of puberty and rebounds toward the end of the maturation process, a normal physiological response that is thought to promote growth (4–7). In longitudinal studies, we have also shown that the decline in insulin sensitivity that occurs in Caucasians recovers by the end of puberty but that African Americans do not show a recovery pattern (8). Based on these findings we hypothesized that the failure to recover from pubertal insulin resistance may ‘trigger’ the development of type 2 diabetes in high-risk, predisposed children. Hispanic children are another high-risk minority group, but longitudinal metabolic changes during puberty have not been previously examined in this group.
In a previous cross-sectional analysis, we failed to identify evidence of a relationship between insulin related variables and Tanner stages in Hispanic children (9). The major objectives of this current analysis were: 1) to determine changes in key variables related to insulin action and secretion and glucose tolerance over a 1-year period in overweight Hispanic children at high-risk of developing type 2 diabetes; and 2) to examine these changes as a function of changes in pubertal status. We hypothesized that: a) insulin sensitivity would decline over time, partly explained by an increase in fat mass; b) the pattern of changes would be influenced by maturation stage (i.e. a fall in insulin sensitivity in the early phases of maturation and a rebound as children approach the end of maturation with appropriate beta-cell compensation), and c) that there would be appropriate beta-cell compensation at all stages of maturation.
Methods
Subjects
The SOLAR (Study Of Latino Adolescents At Risk) is a longitudinal cohort study of ~200 children at high-risk of the development of type 2 diabetes. For the current report we analyzed data from 132 children (70 boys and 62 girls) for whom we currently have a full complement of data from two annual visits, 1 year apart, and who fell into one of the four categories defined below for change in maturation stage. Children were recruited to the SOLAR project through clinics, health fairs, newspaper announcements, and word of mouth. Baseline characteristics of this group have been previously reported (10), and all met the following inclusion criteria: 1) age 8–13 years; 2) BMI ≥ 85th percentile for age and gender (2); 3) Latino ancestry (all four grandparents Latino by self-report), and 4) family history of type 2 diabetes in at least one parent, sibling, or grandparent. Children were of Mexican-American or Central American heritage (~70%). Children were excluded if they had prior major illness including type 1 or type 2 diabetes, or took medications or had a condition known to influence body composition, insulin sensitivity or insulin secretion (e.g. glucocorticoid therapy, hypothyroidism). The Institutional Review Board at the Health Science Campus, University of Southern California approved the SOLAR study. Informed consent and assent were obtained from all parents and children respectively.
General protocol
Data were collected over two separate visits; mean time between visits was 1.03 years. The first visit was an out-patient visit and children arrived at the study center at approximately 8.00 am after an overnight fast. Weight and height were measured followed by a physical examination by a pediatrician. Tanner stage was assigned based on breast stage in girls, and pubic hair stage in boys as these clinical features best reflect underlying secretion of gonadal sex steroids. A medical history was conducted including parental interview detailing family history of diabetes and gestational diabetes, and child’s birth weight. Following these procedures an oral glucose tolerance test was conducted. Children who met the screening criteria were invited back for further testing during an in-patient visit. The follow-up visit was an overnight in-patient visit where further measures of body composition and an intravenous glucose tolerance test were administered.
Detailed methodologies
The methods for this ongoing study have been previously reported in detail (11) and are briefly reviewed here. An oral glucose tolerance test was conducted using a dose of 1.75 grams of glucose per kilogram body weight (to maximum 75 grams). Height (by a wall mounted stadiometer) and weight (by a balance beam medical scale) were recorded at each visit to the nearest 0.1 cm and 0.1 kg respectively, and the average of the two measurements was used for analysis. BMI and BMI percentiles for age and gender were determined based upon established CDC normative curves using EpiInfo 2000, Version 1.1. A whole body DXA scan was performed to determine whole body composition using a Hologic QDR 4500W. A frequently sampled intravenous glucose tolerance test was performed with a glucose dose at time zero (25% dextrose, 0.3 g/kg body weight) and sampling at −15, −5, 2, 4, 8, 19, 22, 30, 40, 50, 70, 100, and 180 minutes. Insulin (0.02 U/kg body weight; Humulin® R (REGULAR insulin for human injection; Eli Lilly, Indianapolis, USA) was injected intravenously at 20 min. Values for glucose and insulin were entered into the MINMOD MIL-LENIUM 2002 computer program (Version 5.16, Richard N. Bergman) for determination of insulin sensitivity (SI), and the acute insulin response to glucose (AIR) (12). The disposition index (product of insulin sensitivity and the acute response to glucose) was used as an index of the compensatory adaptation to insulin resistance, a reflection of beta-cell function.
Blood samples during the oral glucose tolerance test were centrifuged and plasma was placed on ice and was analyzed for glucose within 1 hour at the LAC-USC Medical Center Core Laboratory with a Dimension Clinical Chemistry system using the Hexokinase method (Dade Behring, Deerfield, Illinois). Blood samples from the intravenous glucose tolerance test were centrifuged and plasma was stored on ice prior to storage at −80°C. Aliquots were assayed in duplicate for glucose using the glucose oxidase method and a Yellow Springs Instrument 2700 Analyzer (YSI Inc, Yellow Springs, OH, USA). Insulin was assayed in duplicate using an enzyme-linked immunosorbent assay kit from Linco (St Charles, Missouri).
Statistical analysis
Significance of 1-year changes was determined using a paired t-test. The pattern of change in key variables over time was examined as a function of maturation change using repeated measures analysis of variance. To examine the influence of maturation transition, the following four sub-groups were created:
Group 1: Children who remained at either Tanner stage 1 or 2 (i.e. early maturation, non-progressors; n = 49)
Group 2: Children who progressed from Tanner stage 1 or 2 to 3 (i.e. early maturation, progressors; n = 40)
Group 3: Children who progressed from Tanner stage 3 to 4 or 5 (i.e. mid- to late-maturation progressors; n = 22)
Group 4: Children who remained at either Tanner 4 or 5 (i.e. late maturation non-progressors; n = 21)
The pattern of change in insulin sensitivity, acute insulin response to glucose, disposition index, fasting glucose, fasting insulin and 2-hour glucose over time in these groups was examined using repeated measures analysis of variance. In this analysis, gender and tanner change group were included as class variables and baseline body fat mass and age were included as covariates. There were no significant effects or interactions with gender in any of these models. All analyses were performed using SPSS version 11.0 (SPSS Inc, Chicago, IL), with a type I error set at P<0.05.
Results
The mean values over time for key variables, and the change over time is shown in Table I. There was an 8.7 kg increase in body weight, due in part to an increase in fat mass (3.0 kg) and soft lean tissue mass (4.8 kg). There was no significant change in percent body fat. There was a 24% drop in insulin sensitivity, and this was accompanied by an increase in fasting glucose and insulin. In the group as a whole, there was appropriate beta-cell compensation to the fall in insulin sensitivity, since the acute insulin response increased and the disposition index remained constant.
Table I.
Key physical and metabolic variables at baseline and 1-year later in 132 overweight Hispanic children (Means + SD).
| Initial Measure | 1-year Follow-up Measure | Change in Score | |
|---|---|---|---|
| Age, years | 10.9±1.8 | 12.0±1.8 | 1.0±0.2 (p<0.001) |
| Tanner stage | 2.2±1.4 | 2.7±1.4 | 0.5±0.6 (p<0.001) |
| Weight, kg | 61.3±19.1 | 70.0±20.3 | 8.7±4.6 (p<0.001) |
| BMI, kg/m2 | 27.5±5.1 | 29.3±5.4 | 1.8±1.6 (p<0.001) |
| Fat mass, kg | 23.5±9.7 | 26.5±10.1 | 3.0±2.8 (p<0.001) |
| % Fat | 37.8±6.3 | 37.9±6.0 | 0.06±2.5 (ns) |
| Lean tissue mass, kg | 35.7±10.1 | 40.5±11.0 | 4.8±2.8 (p<0.001) |
| Fasting glucose, mg/dL | 91.2±6.8 | 92.5±6.0 | 1.2±6.7 (p=0.04) |
| Fasting insulin | 19.1±11.7 | 25.5±15.2 | 6.4±9.9 (p<0.001) |
| 2-hour Glucose, mg/dL | 126.5±16.8 | 128.4±18.1 | 1.9±18.3 (ns) |
| Insulin sensitivity [×10−4 min−1/(μU/ml)] | 2.31±1.6 | 1.75±1.2 | −0.56±0.96 (p<0.001) |
| Acute insulin response, (μU/ml) | 1650±1220 | 1843±1245 | 193±804 (p=0.007) |
| Disposition index, [×10−4 min−1] | 2583±1144 | 2452±1347 | −130±1126 (ns) |
The fall in insulin sensitivity was significantly correlated with baseline age, body mass index, percent fat, fat mass, and lean tissue mass (positive r values in the range of 0.31 to 0.38; p<0.001). In partial correlation analysis adjusting for baseline fat mass, there were no significant relationships between change in insulin sensitivity and baseline variables or changes in body weight, fat mass or lean mass.
Baseline characteristics of the four sub-groups classified according to 1-year changes in maturation are shown in Table II. As expected, there was a significant and incremental increase in age, body weight, fat mass and fat free mass with increasing maturation status, although fasting and 2-hour glucose were not significantly different across groups. The proportion of children with impaired glucose tolerance (IGT) was not significantly different across the four sub-groups (20.4%, 25.0%, 27.3% and 33.3% of children had IGT in groups 1 to 4 respectively). On an absolute basis, insulin sensitivity was lower with increasing maturation group (probably due to the greater fat mass), but there was no significant group difference for the acute insulin response, resulting in lower disposition index in Groups 3 and 4 compared to 1 and 2.
Table II.
Key physical and metabolic variables at baseline in the four sub-groups classified according to subsequent 1-year changes in Tanner stage (Means + SD).
| Group 1 (n = 49) | Group 2 (n = 40) | Group 3 (n = 22) | Group 4 (n = 21) | 1-way ANOVA for baseline differences across Groups | ||
|---|---|---|---|---|---|---|
| Age (yr) | Baseline | 9.9±1.5 | 10.5±1.5 | 12.0±1.3 | 12.9±0.7 | P<0.001 |
| 1-Yr later | 10.9±1.5 | 11.6±1.5 | 13.1±1.3 | 13.9±0.8 | ||
| Weight (kg) | Baseline | 51.5±14.6 | 60.0±17.0 | 66.1±17.9 | 82.8±15.4 | P<0.001 |
| 1-Yr later | 59.8±16.3 | 69.7±19.6 | 73.6±16.4 | 90.9±17.2 | ||
| BMI (kg/m2) | Baseline | 25.7±4.1 | 27.6±4.8 | 27.0±5.5 | 32.3±4.5 | P<0.001 |
| 1-Yr later | 27.4±4.5 | 29.3±5.3 | 28.6±5.3 | 34.2±5.0 | ||
| Fat mass (kg) | Baseline | 19.5±7.5 | 24.0±8.7 | 22.5±10.4 | 33.1±9.1 | P<0.001 |
| 1-Yr later | 22.8±8.1 | 26.6±9.3 | 24.9±10.0 | 36.3±9.7 | ||
| % Fat | Baseline | 37.3±5.4 | 39.9±5.4 | 33.6±8.2 | 39.9±5.4 | P = 0.001 |
| 1-Yr later | 38.0±5.1 | 38.7±5.3 | 33.7±7.8 | 40.5±4.8 | ||
| LTM (kg) | Baseline | 30.1±7.1 | 33.8±8.8 | 41.1±9.8 | 46.9±7.3 | P<0.001 |
| 1-Yr later | 34.5±8.3 | 39.9±11.0 | 45.9±9.7 | 50.2±8.1 | ||
| FG (mg/dL) | Baseline | 90.9±5.1 | 92.2±7.7 | 92.1±6.4 | 89.2±8.7 | NS |
| 1-Yr later | 92.6±5.4 | 93.2±5.9 | 91.4±7.0 | 91.7±6.7 | ||
| FI (μU/ml) | Baseline | 15.9±9.6 | 19.9±14.1 | 18.6±10.9 | 25.9±9.4 | P = 0.01 |
| 1-Yr later | 21.7±11.3 | 28.2±20.3 | 23.3±13.2 | 31.6±10.9 | ||
| 2hG (mg/dL) | Baseline | 123.6±15.7 | 126.0±16.9 | 129.9±17.2 | 130.4±18.2 | NS |
| 1-Yr later | 127.0±14.3 | 129.4±17.9 | 128.0±23.1 | 129.9±21.7 | ||
| SI [×10−4 min−1/(μU/ml)] | Baseline | 2.75±1.76 | 2.23±1.54 | 2.41±1.56 | 1.33±1.11 | P = 0.008 |
| 1-Yr later | 2.16±1.32 | 1.55±1.09 | 1.82±0.92 | 1.10±0.65 | ||
| AIR (μU/ml) | Baseline | 1622±1198 | 1686±1323 | 1235±853 | 2078±1324 | NS |
| 1-Yr later | 1985±1311 | 2063±1287 | 1139±653 | 1829±1288 | ||
| DI [×10−4 min−1] | Baseline | 3088±1167 | 2595±1173 | 2056±867 | 1932±681 | P<0.001 |
| 1-Yr later | 3190±1612 | 2402±932 | 1721±693 | 1592±863 |
BMI = body mass index; LTM = lean tissue mass; FG = fasting glucose; FI = fasting insulin; 2hG = 2-hour glucose; SI = insulin sensitivity; AIR = acute insulin response; DI = disposition index
Group 1: Children who remained at either Tanner stage 1 or 2 (i.e. early maturation, non-progressors; n = 49)
Group 2: Children who progressed from Tanner stage 1 or 2 to 3 (i.e. early maturation, progressors; n = 40)
Group 3: Children who progressed from Tanner stage 3 to 4 or 5 (i.e. mid- to late-maturation progressors; n = 22)
Group 4: Children who remained at either Tanner 4 or 5 (i.e. late maturation non-progressors; n = 21)
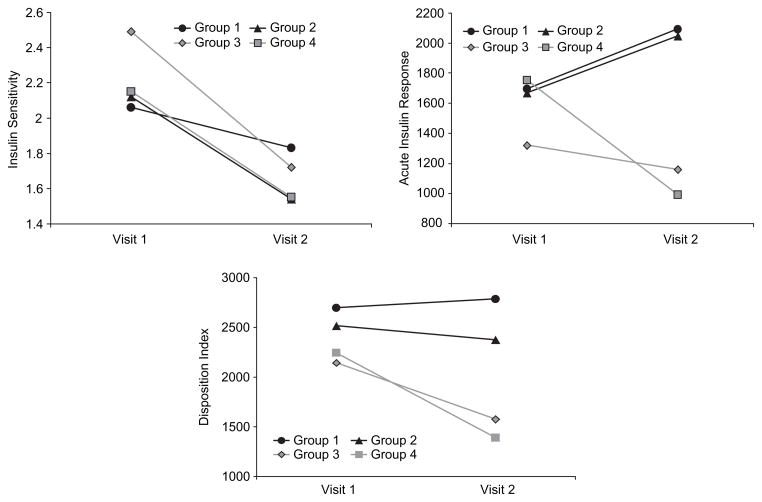
Patterns of change in key variables in these four sub-groups are shown in Figure 1. Insulin sensitivity was not significantly influenced by maturations status, and the magnitude of the fall in insulin sensitivity was remarkably similar across all four groups, regardless of 1-year changes in maturation. For the acute insulin response, the pattern of change differed by Tanner stage. That is, there was a significant interaction of time and Tanner group change (p = 0.047). Thus, subjects in the earlier stages of maturation (Groups 1 and 2) increased insulin response in response to the decline in insulin sensitivity (appropriate beta-cell response). In contrast, subjects in the later stages of maturation (Groups 3 and 4) had a lower baseline acute insulin response, and actually decreased their acute insulin response after a 1-year follow-up (poor beta-cell response). For the disposition index, there were no overall significant changes over time. However, there was a significant between-subject effect of maturation stage, whereby those children in the more advanced stages (Groups 3 and 4) decreased their disposition index (p = 0.006). The change in disposition index over time was not significant in groups 1 and 2 but fell significantly in groups 3 (−335±708 × 10−4 min−1; p = 0.04 by paired t-test) and 4 (−340±701 × 10−4 min−1; p = 0.04 by paired t-test). There were no significant between-group differences or differences in the pattern of change over time in fasting glucose, 2-hour glucose or fasting insulin.
Figure 1.
Key variables at baseline (visit 1) and 1-year later (visit 2) in sub-groups of children classified according to changes in maturation stage over the 1-year follow-up period. Note that these data are least square means from the repeated measures analysis of variance after adjusting for baseline body fat mass and age:
Group 1 (black line, circles): Children who remained at either Tanner stage 1 or 2 (i.e. early maturation, non-progressors; n = 49)
Group 2 (black line, triangles): Children who progressed from Tanner stage 1 or 2 to 3 (i.e. early maturation, progressors; n = 40)
Group 3 (grey line, rhombus): Children who progressed from Tanner stage 3 to 4 or 5 (i.e. mid- to late-maturation progressors; n = 22)
Group 4 (grey line, square): Children who remained at either Tanner 4 or 5 (i.e. late maturation non-progressors; n = 21)
Discussion
The purpose of this study was to examine 1-year changes in key insulin related variables in a cohort of overweight Hispanic children at high-risk of the development of type 2 diabetes. We noted a 24% decline in insulin sensitivity after a 1-year follow-up that was unrelated to the increase in fat mass and lean body mass. This decline in insulin sensitivity was associated with small but significant increases in fasting (but not 2-hour) glucose and insulin, as well as the acute insulin response to glucose, all factors that would indicate a worsening of insulin resistance. One would predict that the 3.0 kg increase in fat mass (in part due to growth, and continued development of obesity) would lead to a drop in insulin sensitivity. However, the increase in fat mass was not related to the decline in insulin sensitivity (r = 0.03), and percent fat did not change over the 1-year period, suggesting that factors other than changes in body fatness contributed to the decline.
Based on the expected transient changes in insulin sensitivity across maturation (6,7), we hypothesized a larger fall in insulin sensitivity in children approaching Tanner stage 3 (i.e. Group 2) and a rebound in those approaching Tanner stage 5 (i.e. Group 3), with more stable values in those children who were relatively static in their maturation process (Groups 1 and 4). However, the reduction in insulin sensitivity was consistent across all four groups. These findings suggest that expected rebound of insulin sensitivity after Tanner stage 3 may be blunted, possibly due to the overwhelming effects of obesity and insulin resistance, or other factors unaccounted for. Further cross-sectional and longitudinal studies in other ‘control’ groups (e.g. lean and insulin sensitive, obese with no family history of type 2 diabetes) would be useful to interpret this finding.
Changes in different compartments of body fat may help explain the fall in insulin sensitivity during this period of growth. In the current study we examined changes in intra-abdominal and subcutaneous abdominal fat obtained by single slice MRI in a sub-group of 100 children. Over 1-year there was surprisingly no change in intra-abdominal adipose tissue and a significant increase of 13% in subcutaneous abdominal fat. There were no significant correlations between change in either intra-abdominal or subcutaneous abdominal fat and change in insulin sensitivity. The ‘ectopic fat’ theory (13) suggests that fat deposition outside of adipose tissue (e.g. in muscle or liver) contributes to insulin resistance. Thus, acquisition of fat in muscle and/or liver may be another factor contributing to the decline in insulin sensitivity over and above that explained by the increase in total fat mass. Another factor that was not considered in this study was the role of adipocytokines such as leptin, adiponectin, TNF-alpha, Interleukin-6 or C-reactive protein. These factors are generally associated with body fat and insulin resistance (14) but were not measured in the current study. Thus, individual variation in cytokine level and changes over time/maturation may be another factor contributing to the decline in insulin sensitivity.
Changes in the acute insulin response to glucose, however, showed a different pattern of responses than insulin sensitivity (Figure 1). Subjects in the earlier stages of maturation showed a compensatory increase in the acute insulin response to glucose (i.e. appropriate beta-cell compensation), whereas subjects in the latter stages of maturation showed no compensatory response or even a worsening of the acute insulin response (i.e. poor beta-cell compensation). Evidence from adults shows that insulin resistance alone is insufficient to cause type 2 diabetes. In a variety of studies both insulin resistance and reduced beta-cell function predicted the development of type 2 diabetes (15–20). More recent cross-sectional work in 8th grade Hispanic children by Rosenbaum et al. (21) also support the idea that type 2 diabetes is likely to develop in the face of familial beta-cell dysfunction in response to chronic insulin resistance. Thus the combination of insulin resistance and the inability of beta-cells to compensate by increasing insulin secretion predict the development of type 2 diabetes. This study provides the most direct evidence to date to support the contention that this hypothesis is also likely in children and that late pubertal transition may be a critical period in the pathophysiology.
There are several limitations that should be considered in interpreting these findings. One issue is that it is difficult to tease apart the effects of chronic exposure to insulin resistance from specific effects of puberty as a cause for the failure to increase the acute insulin response. It is possible that the children in the later stages of maturation (Groups 3 and 4) are older and have had a longer exposure to the effects of obesity and insulin resistance and may thus be ‘further along’ in the process of beta-cell failure and development of type 2 diabetes. Further longitudinal studies in this cohort may be useful in this regard. Also, our findings are specific to obese Hispanic children with a positive family history of type 2 diabetes. Other studies in children with a similar degree of obesity but no family history, as well as in lean individuals, are also warranted.
These findings have several important clinical implications. Current assessment of diabetes risk presently involves the assessment of body composition based on body mass index percentile, membership in high-risk ethnic groups, family history, and physical findings suggestive of insulin resistance, while diabetes screening involves the measurement of fasting glucose and sometimes a glucose tolerance test. Our data suggest that this assessment of risk, which essentially reflects factors related to insulin resistance, will be inadequate to actually detect those overweight children most at risk of progression to diabetes. We base this conclusion on the observation that factors such as body fat, fasting and 2-hour glucose, and impaired glucose tolerance at baseline had no relationship to the fall in insulin sensitivity or the deterioration in beta-cell function over the 1-year follow-up. Rather, it appears that clinical assessment of insulin secretion could be needed to more clearly differentiate diabetes risk among the overall population of obese youth. Since our method of assessing insulin secretion using an intravenous glucose tolerance test is not practical in a clinical setting, alternative indicators derived from an oral glucose tolerance test such as the insulinogenic index (change in insulin divided by the change in glucose from 0 to 30 minutes) may be useful in addition to assessment of 2-hour glucose. Further longitudinal follow-up of this cohort may ultimately clarify clinical indicators for distinguishing particular obese adolescents at highest risk for progression to diabetes.
The dramatic decline in insulin sensitivity coupled with the compromised acute insulin response to glucose in advanced Tanner stages suggests that interventions (diet and exercise and potentially pharmacological interventions as well) are warranted during this critical stage of pubertal development. These interventions may need to address multiple ‘targets’ that go beyond weight loss and need to be rigorously tested in randomized trials. Weight loss alone may improve insulin resistance, but may have no effect on the acute insulin response or beta cell function, although one recent study in adults suggests that weight loss does improve beta-cell function at least in older men (22). From a pharmacological perspective, the insulin sensitizer, pioglitazone, has been shown to be effective in preventing the development of type 2 diabetes in women with prior gestational diabetes by promoting beta-cell rest (23). However, pharmacological interventions during pubertal maturation may raise ethical and safety considerations and therefore, needs to be carefully tested for safety and efficacy. One exercise intervention that shows promise and may be particularly suitable in overweight youth is strength training. We have recently completed a randomized trial that shows significant improvements in insulin sensitivity after 16 weeks, but had only a marginal effect on body fat and no effect on the acute insulin response or beta-cell function (24).
In conclusion, we have used a longitudinal study design in high-risk children to identify several findings with important implications for the potential mechanism for the development of pediatric type 2 diabetes in Hispanic youth. First, we observed a significant decline in insulin sensitivity that was unrelated to changes in body fatness or maturation. Second, we found that the acute insulin response to glucose worsens in advanced Tanner stages, thus diminishing the capacity of beta-cells to respond to the decline in insulin sensitivity. Collectively these results indicate our unique finding that the failure to increase the acute insulin response to glucose in response to worsening insulin resistance may be one factor central to the pathogenesis of the progression of pediatric type 2 diabetes in this at risk population. Furthermore, these findings suggest that this compensatory response to continued insulin resistance deteriorates in advanced stages of maturation.
Acknowledgments
We are extremely grateful to Ms Quintilia Avila, project manager, the nursing and bionutrition staff at the General Clinical Research Center at the University of Southern California and the laboratory staff of Dr Bergman for conducting insulin and glucose assays. We are indebted to the children and their families who participated in this study. This study would not have been possible without the committed dedication and effort of these people.
References
- 1.Narayan KMV, Boyle JP, Thompson TJ, Sorenson SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–90. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and Trends in Overweight Among US Children and Adolescents, 1999–2000. JAMA. 2002;288:1728–32. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 3.Goran MI, Cruz ML, Bergman RN, Watanabe RM. Insulin resistance and the associated compensatory response in Caucasian, African American and Hispanic children. Diabetes Care. 2002;25:2184–90. doi: 10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- 4.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty: A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. 1986;315:215–9. doi: 10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- 5.Caprio S, Plewe G, Diamond MP, Simonson DC, Boulware SD, Sherwin RS, et al. Increased insulin secretion in puberty: a compensatory response to reductions in insulin sensitivity. J Pediatr. 1989;114:963–7. doi: 10.1016/s0022-3476(89)80438-x. [DOI] [PubMed] [Google Scholar]
- 6.Moran A, Jacobs DR, Jr, Steinberger J, Hong CP, Prineas R, Luepker R. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48:2039–44. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 7.Goran MI, Gower BA. Longitudinal study of pubertal insulin resistance. Diabetes. 2001;50:2444–50. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 8.Ball G, Huang T-T, Gower B, Goran M. Longitudinal changes in insulin sensitivity, insulin secretion and beta-cell function during puberty in Caucasian and African American Youth. J Pediatr. 2006;148:16–22. doi: 10.1016/j.jpeds.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 9.Ball G, Weigensberg M, Cruz M, Shaibi G, Kobasssi H, Goran M. Insulin resistance and beta-cell function across Tanner stages in overweight Hispanic children with a family history of type 2 diabetes. Int J Obes (Lond) 2005;29(12):1471–7. doi: 10.1038/sj.ijo.0803044. [DOI] [PubMed] [Google Scholar]
- 10.Goran MI, Bergman RN, Avila Q, Watkins M, Ball GDC, Shaibi GQ, et al. Impaired glucose tolerance and reduced beta-cell function in overweight Latino children with a positive family history for type 2 diabetes. JCEM. 2004;89:207–12. doi: 10.1210/jc.2003-031402. [DOI] [PubMed] [Google Scholar]
- 11.Arnesen E, Refsum H, Bonaa KH, Ueland PM, Forde OH, Nordrehaug JE. Serum total homocysteine and coronary heart disease. Int J Epidemiol. 1995;24:704–9. doi: 10.1093/ije/24.4.704. [DOI] [PubMed] [Google Scholar]
- 12.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolernce in man. J Clin Invest. 1981;68:1456–67. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann NY Acad Sci. 2002;967:363–78. doi: 10.1111/j.1749-6632.2002.tb04292.x. [DOI] [PubMed] [Google Scholar]
- 14.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 15.Xiang AH, Peters RK, Trigo E, Kjos SL, Lee WP, Buchanan TA. Multiple metabolic defects during late pregnancy in women at high-risk of type 2 diabetes. Diabetes. 1999;48:848–54. doi: 10.2337/diabetes.48.4.848. [DOI] [PubMed] [Google Scholar]
- 16.Buchanan TA. Pancreatic B-cell defects in gestational diabetes: Implications for the pathogenesis and prevention of type 2 diabetes. J Clin Endrocrinol. 2001;86:989–93. doi: 10.1210/jcem.86.3.7339. [DOI] [PubMed] [Google Scholar]
- 17.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–94. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weyer C, Hanson RL, Tataranni PA, CB, Pratley RE. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance. Diabetes. 2000;49:2094–101. doi: 10.2337/diabetes.49.12.2094. [DOI] [PubMed] [Google Scholar]
- 19.Haffner SM, Miettinen H, Gaskill SP, Stern MP. Decreased insulin secretion and increased insulin resistance are independently related to the 7-year risk of NIDDM in Mexican-Americans. Diabetes. 1995;44:1386–91. doi: 10.2337/diab.44.12.1386. [DOI] [PubMed] [Google Scholar]
- 20.Osei K, Rhinesmith S, Gaillard T, Schuster D. Impaired Insulin Sensitivity, Insulin Secretion, and Glucose Effectiveness Predict Future Development of Impaired Glucose Tolerance and Type 2 Diabetes in Pre-Diabetic African Americans: Implications for primary diabetes prevention. Diabetes Care. 2004;27:1439–46. doi: 10.2337/diacare.27.6.1439. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum M, Nonas C, Horlick M, Fennoy I, Vargas I, Schachner H, et al. Beta-Cell function and insulin sensitivity in early adolescence: association with body fatness and family history of type 2 diabetes mellitus. J Clin Endocrinol Metab. 2004;89:5469–76. doi: 10.1210/jc.2004-0971. [DOI] [PubMed] [Google Scholar]
- 22.Utzschneider KM, Carr DB, Barsness SM, Kahn SE, Schwartz RS. Diet-induced weight loss is associated with an improvement in beta-cell function in older men. J Clin Endocrinol Metab. 2004;89:2704–10. doi: 10.1210/jc.2003-031827. [DOI] [PubMed] [Google Scholar]
- 23.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51:2796–803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 24.Shaibi GQ, Cruz ML, Ball GD, Weigensberg MJ, Crespo N, Salem GJ, et al. Effects of resistance training on insulin sensitivity in overweight Hispanic adolescent males. Med Sci Sports Exerc. 2004 doi: 10.1249/01.mss.0000227304.88406.0f. (in press) [DOI] [PubMed] [Google Scholar]