Abstract
A novel, 208-kDa myosin light chain kinase (MLCK) distinct from smooth muscle and non-muscle MLCK has been identified by cross-reaction to two antibodies raised against smooth muscle MLCK. Additional antibodies directed against the amino and carboxyl termini of the smooth muscle MLCK do not react with the 208-kDa MLCK, suggesting these regions are distinct. 208-kDa MLCK phosphorylates 20-kDa myosin light chains in a Ca2+/calmodulin-dependent manner, consistent with it being a member of the MLCK family. Expression of 208-kDa MLCK and smooth muscle MLCK appears to be inversely regulated, with 208-kDa MLCK being most abundant during early development and declining at birth. In contrast, expression of smooth muscle MLCK is relatively low early during development and increases to become the predominant MLCK detected in all adult smooth and non-muscle tissues. The developmental expression pattern of the 208-kDa MLCK suggests this form be named, embryonic MLCK.
Myosin light chain kinases (MLCK)1 are Ca2+/calmodulin-regulated, actin and myosin binding, Ser/Thr protein kinases (Kamm and Stull, 1985; Sellers and Pato, 1984). In skeletal muscle, contraction is regulated by the troponin system and phosphorylation of regulatory light chain by skeletal muscle MLCK has a modulatory role in contraction-induced potentiation of isometric twitch tension (Sweeney et al., 1993). In smooth muscle, phosphorylation of the 20-kDa regulatory light chain of myosin by MLCK is a well characterized event, important for the initiation of contraction (Kamm and Stull, 1985). The role of myosin phosphorylation by MLCK in non-muscle cells is not well characterized but correlates with activities such as cell division, receptor capping, and platelet or endothelial cell contraction (Kerrick and Bourguignon, 1984; Kolodney and Wyslomerski, 1992; Wyslomerski and Lagunoff, 1990; Holzapfel et al., 1983; Ehrlich et al., 1991; Guiliano et al., 1992; Kolega and Taylor, 1993; Adelstein and Conti, 1975; Garcia et al., 1995).
cDNAs representing vertebrate smooth and non-muscle MLCKs have been sequenced and the deduced amino acid sequences of the proteins compared (Gallagher et al., 1991; Kobayashi et al., 1992; Shoemaker et al., 1990; Olson et al., 1990). This comparison reveals a high degree of sequence homology (>97% similarity) that extends beyond the central catalytic core and calmodulin-binding autoinhibitory region (Gallagher et al., 1991; Kobayashi et al., 1992). The regions outside of the catalytic core and autoinhibitory region are comprised of several class I and class II structural motifs that are similar to those in the related family of giant muscle proteins such as twitchin and titin (Olson et al., 1990; Labeit et al., 1992). The amino terminus of the mammalian MLCKs also contains a 175-residue insert not present in the avian smooth and non-muscle MLCKs (Gallagher et al., 1991; Kobayashi et al., 1992). This insert is comprised of 15 tandem copies of a 12-residue repeat of unknown function. Recently, an actin-binding domain has been localized within the NH2 terminus between residues 29 and 80 (Kanoh et al., 1993; Gallagher, 1994). The extreme COOH terminus of smooth muscle MLCK is a highly acidic region of unknown function that is also expressed as an independent protein, telokin (Gallagher and Herring, 1991; Collinge et al., 1992; Yoshikai and Ikebe, 1992). Telokin and by analogy, the COOH terminus of smooth muscle MLCK have been proposed to be involved in myosin binding and myosin filament assembly (Shirinsky et al., 1993).
Determination of molecular masses for smooth muscle MLCKs from several species has shown that these proteins have a similar size between 130 and 155 kDa (Gallagher et al., 1991). However, the masses determined by SDS-PAGE are slightly larger than the masses predicted by the deduced primary sequences (Gallagher et al., 1991; Kobayashi et al., 1993). For example, the rabbit uterine smooth muscle MLCK cDNA encodes a protein of 126 kDa while the recombinant and tissue forms migrate at 152 kDa on SDS-PAGE. The amino terminus of the chicken smooth muscle MLCK has been established by direct sequencing of a CNBr subfragment obtained from the purified protein (Faux et al., 1993). The six NH2-terminal residues (MDFRAN) identified correspond exactly to the first six residues predicted by the 5′ coding region for the chicken, rabbit, and bovine cDNAs (Olson et al., 1990; Gallagher et al., 1991; Kobayashi et al., 1993), suggesting that all of the smooth muscle MLCKs have the same amino termini.
The non-muscle MLCK is reported to be identical to smooth muscle isoforms except for the presence of an additional 286-residues extending the NH2 terminus of the predicted protein (Shoemaker et al., 1990). This additional sequence would increase the mass of the protein by approximately 35 kDa (Shoemaker et al., 1990). Western blotting of smooth and non-muscle tissues demonstrated that the major form of MLCK expressed in these tissues has the same mass as the smooth muscle MLCK. A larger protein corresponding to the non-muscle MLCK and having an appropriate molecular mass (>165 kDa) as predicted by the chicken embryo fibroblast cDNA has not been detected in any tissues (Gallagher et al., 1991). Recently, Gibson and Higgins (1993) suggested that the cDNA encoding the non-muscle MLCK may be incomplete as additional class II structural motifs appear to be present in the 5′-untranslated region of the reported cDNA.
The failure to detect a larger mass MLCK in non-muscle tissues may be because this protein is expressed at low levels or because it is expressed only in specific non-muscle tissues. It is also possible that this cDNA, isolated from primary embryonic fibroblast cells, represents a form of MLCK that is developmentally regulated and its expression may not be detectable in adult tissues. For these reasons, we have examined MLCK expression in embryonic and adult tissues and in cultured cells by Western blotting. These studies reveal the existence of a 208-kDa protein, named embryonic MLCK because its expression can be detected in early embryonic tissues, stem cells, and in proliferating cultured cells. 208-kDa protein is a Ca2+/calmodulin-dependent MLCK that is structurally distinct both from the smooth muscle MLCK and the protein predicted by the chicken non-muscle MLCK cDNA.
Materials and Methods
Western Immunoblotting
Proteins were extracted from tissues and cells using Nonidet P-40 lysis buffer (1% Nonidet P-40, 10 mm MOPS, pH 7.0, 1.25 mm EGTA, 10 mm dithiothreitol, 50 mm MgCl2, 300 mm NaCl, 100 μg/ml phenylmethylsulfonyl fluoride, 4 μg/ml leupeptin, 10 μg/ml aprotinin). Extracts were separated by electrophoresis, transferred to nitrocellulose, and reacted with antibodies to smooth muscle MLCK as described previously (Gallagher et al., 1991) except that proteins were separated on 5% polyacrylamide SDS gels and electrophoretic transfers carried out for 18 h. Immunoreactive proteins were detected using the ECL chemiluminescent detection system according to the manufacturers directions (Amersham). Four antibodies directed against smooth muscle MLCK were used in this study and are described in Table I. Preadsorption of anti-smMLCK or anti-Repeat antibodies was achieved by incubating diluting serum with nitrocellulose saturated with the repeat peptide. The relative intensities of the protein in the bands were quantified by scanning densitometry.
Table I. Antibodies used in this study.
Antibodies recognizing smooth muscle MLCK were previously described as follows: anti-smMLCK (Kamm et al., 1987); anti-Repeat (Gallagher et al., 1991); anti-COOH-terminal (Gallagher and Herring, 1991); anti-NH2-terminal (clone K36, Sigma; Gallagher, 1994). Residues numbered correspond to the rabbit uterine smooth muscle MLCK (Gallagher et al., 1991).
| Antibody | Antigen | Epitope | Type |
|---|---|---|---|
| smMLCK | Purified bovine smMLCK | Full-length protein | Polyclonal |
| Repeat | KPVGNAKPAETL peptide | Residues 102–293 | Polyclonal |
| COOH-terminal | Purified rabbit telokin | Residues 993–1147 | Polyclonal |
| NH2-terminal | Purified chicken gizzard smMLCK | Residues 29–80 | Monoclonal |
Immunoprecipitation
208-kDa embryonic MLCK was immunoprecipitated under nondenaturing conditions from REF cell extracts prepared in Nonidet P-40 lysis buffer. Cells from a 100 mm dish were lysed in 400 μl of Nonidet P-40 lysis buffer and clarified by centrifugation for 5 min. The clarified supernatant was diluted 1:4 in wash buffer (20 mm MOPS, pH 7.0, 10 mm magnesium acetate) prior to immunoprecipitation. 5 μl of anti-Repeat antibody or 5 μl of preimmune serum and 50 μl of protein A-Sepharose (10% suspension) were incubated with the diluted cell extracts for 4 h at 4 °C then centrifuged to collect immune complexes. The immune complexes were washed three times by resuspending the complexes in wash buffer and then subjected to Western immunoblot analysis as described above or resuspended in kinase assay reaction buffer for activity measurements.
Myosin Light Chain Phosphorylation
MLCK immunoprecipitated from REF cells or from COS cells transiently expressing recombinant rabbit uterine smooth muscle MLCK was used directly for MLCK activity assays measuring incorporation of 32P into purified regulatory light chains as described previously (Gallagher et al., 1991). Briefly the immunoprecipitate was resuspended in 40 μl of kinase assay reaction buffer (50 mm MOPS, 10 mm magnesium acetate, 0.3 mm calcium chloride, 1.0 μm calmodulin, 2 mm dithiothreitol, 15 μm purified gizzard regulatory light chains). The reaction was initiated by addition of 10 μl of [gamma-32P]ATP to a final concentration of 1 mm. Background rates of incorporation for immunoprecipitated 208-kDa protein using specific antibodies were determined in parallel in the presence of 3 mm EGTA or by protein immunoprecipitated using preimmune serum. The remaining fraction of the assay mixture was diluted with an equal volume of SDS-PAGE sample buffer, and separated on a 15% SDS-polyacrylamide gel. The gel was stained, dried, then exposed to film to detect incorporation of 32P into protein.
Tissues and Cell Lines
Mouse embryos or when discernable, embryonic tissues, were obtained by dissection of embryos at the various times following fertilization. Either whole embryos or specific tissues were dissected and frozen in liquid N2, pulverized, then extracts prepared in Nonidet P-40 lysis buffer. Undifferentiated murine embryonic stem cells (ES-D3 cell line, ATCC CRL1934) were maintained in medium conditioned by leukemia inhibitory factor; differentiated murine embryonic stem cells were obtained by replating embryoid bodies after 4 days in suspension culture in the absence of leukemia inhibitory factor. A10, rat embryonic thoracic aorta cell line (ATCC CRL1476) were cultured in Dulbecco's modified Eagle's medium containing 20% fetal bovine serum. REF, rat embryo fibroblasts (Pavalko and Burridge, 1991); 3T3, mouse fibroblasts (ATCC CCL92); COS fibroblasts (ATCC CRL1650); chicken embryo fibroblasts (ATCC CRL1590), and human umbilical endothelial (ATCC CRL1730) cell lines were cultured in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. AT1 and AT2 cardiac cells were obtained from Dr. Loren Field and are cardiac cells derived from a transplantable transgenic tumor lineage (Kline et al., 1993).
Northern Blot Analysis
RNA was isolated from tissues and cells and a Northern blot prepared as described previously (Gallagher et al., 1991). The blot was probed with 32P-labeled random-primed cDNA corresponding to the 2.9-kb NcoI fragment derived from the cDNA encoding the rabbit smooth muscle MLCK (corresponding to nucleotides 714-3705). Hybridization was performed at 68 °C and final wash was in 2.5 mm sodium phosphate, pH 7.3, 30 mm NaCl, 0.2 mm EDTA, and 0.1% SDS. Poly(A)+ RNA was prepared by magnetic affinity chromatography (Promega).
Results
Expression of MLCKs in Tissues and Cultured Cells
Previous studies have shown that the molecular mass of the smooth muscle MLCK ranges from 130 to 155 kDa depending on the species and this form of MLCK can be detected in both smooth and non-muscle tissues (Gallagher et al., 1991). In the present study, analysis of cellular extracts from the rat A10 smooth muscle cultured cell line demonstrated that in addition to the 136-kDa rat smooth muscle MLCK, a protein of 208 kDa was detected with an anti-smMLCK polyclonal antibody. Examination of several rat tissues and rat embryonic fibroblast (REF) and murine 3T3 cultured cell lines revealed that the 208-kDa protein was apparent only in the cultured cell lines (Fig. 1A). Examination of smooth and non-muscle tissues from three species showed that the predominant immunoreactive protein is the 130–155 kDa smooth muscle MLCK consistent with previous findings (Gallagher et al., 1991) although in some tissues a 208-kDa protein could be detected when the immunoblot is overexposed (Fig. 1B).
Fig. 1. Western analysis of myosin light chain kinase detected in tissues and cells.

A, analysis of rat tissues and rodent cell lines. Amounts of total protein represented in each lane are indicated above figure. B, detection of MLCKs in smooth and non-muscle tissues. Between 50–100 μg (smooth muscle tissues) and 100–175 μg (non-muscle tissues) total protein are represented in each lane. Blot is overexposed to detect expression of 208-kDa protein. C, analysis of muscle-derived and non-muscle cultured cell lines. Cell extracts prepared either from non-muscle (REF, COS, CEF (chicken embryo fibroblast), HUVEC (human umbilical endothelial), or muscle-derived cell lines (A10, AT1, and AT2) were examined. 50 μg of total protein is represented in all lanes except HUVEC, AT1 and AT2 (25 μg each). Positions of molecular weight markers and MLCKs are indicated at the left side of the figure.
Smooth muscle MLCK (130–155 kDa) can be detected in most cultured cell lines, with relatively higher expression levels being detected in muscle-derived cell lines such as A10 smooth muscle, the AT-1 and AT-2 cardiac muscle lines (Fig. 1C). Although not visible in Fig. 1C, 130–155-kDa smooth muscle MLCKs can be detected in chicken embryo fibroblast, COS, and human umbilical endothelial cells, when greater amounts of total protein are analyzed. However, the predominant immunoreactive protein in most cells lines is 208-kDa MLCK. Only two cell lines, REF (Fig. 1C) and undifferentiated murine embryonic stem cells, lack any detectable level of smooth muscle MLCK expression using the available reagents.
Immunoreactivity of 208-kDa Protein
The relationship of the 208-kDa cross-reacting protein detected in A10 and REF cells to the 136-kDa rat smooth muscle MLCK was examined using four anti-smooth muscle MLCK antibodies and biotinylated calmodulin, which binds to high affinity Ca2+/calmodulin binding proteins such as smooth muscle MLCK (Hubbard and Klee, 1987). A 136-kDa rat smooth muscle MLCK was detected in extracts from A10 cells using each of the antibodies but could not be detected in REF cells using any of the antibodies or biotinylated calmodulin, suggesting this cell line does not express smooth muscle MLCK (Fig. 2). The 208-kDa cross-reacting protein was detected in both A10 and REF cells using either anti-smMLCK or anti-Repeat antibodies. Comparison of the signals obtained using these antibodies suggests that the anti-Repeat antibody reacts significantly better with the 208-kDa protein than with the 136-kDa smMLCK, possibly because it has additional sets of the repeated motif “KPVGNAKPAETL” that is found in smMLCK (Gallagher et al., 1991). In contrast, an antibody to the carboxyl terminus and an antibody that binds near the amino terminus do not cross-react with the 208-kDa protein in A10 or REF cell lines, even though both antibodies react with 136-kDa smooth muscle MLCK expressed in A10 cells (Fig. 2A). Fig. 2B shows that a number of Ca2+/calmodulin binding proteins are detected in cell extracts from A10 and REF cells. To unambiguously determine if 208-kDa protein can be detected by Ca2+/calmodulin, anti-Repeat antibody was used to immunoprecipitate 208-kDa protein from cell extracts. After transfer to nitrocellulose, the 208-kDa protein in both A10 and REF cells as well as the 136-kDa smooth muscle MLCK in A10 cells could be detected using biotinylated calmodulin in the presence of calcium (Fig. 2B).
Fig. 2. Immunological relationship of 208-kDa protein and smooth muscle MLCK.
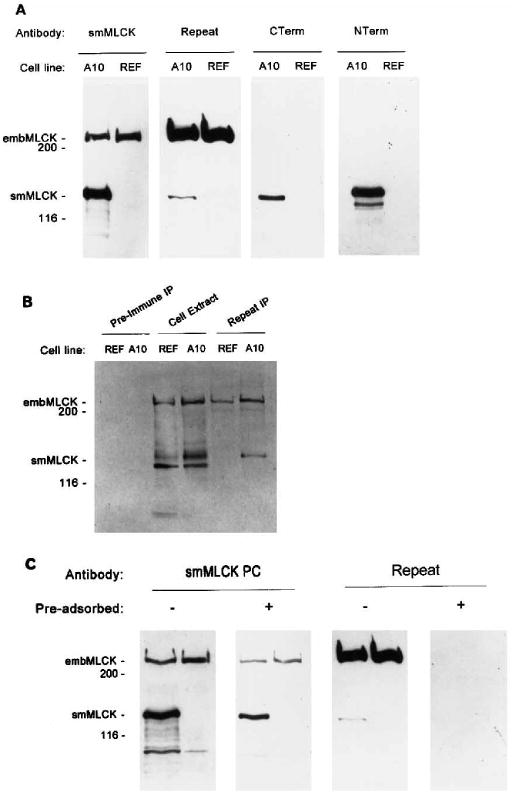
A, Western immunoblotting using four antibodies to detect MLCK in cell extracts from A10 and REF cell lines. Each lane represents 50 μg of total protein. B, ligand blotting to detect immunoprecipitated embryonic MLCK by biotinylated calmodulin in the presence of Ca2+. Embryonic MLCK was immunoprecipitated from REF cells using anti-Repeat antibody (Repeat IP) or preimmune serum (Pre-immune IP) and protein A-Sepharose, separated by SDS-PAGE, transferred to nitrocellulose, and reacted with 20 nm biotinylated calmodulin in the presence of Ca2+. C, Western immunoblotting using unadsorbed (−) or preadsorbed (+) anti-smMLCK or anti-Repeat antibodies to detect MLCK in cell extracts from A10 (left lane) and REF cell lines (right lane). Positions of molecular weight markers and MLCKs are indicated at the left side of the figures.
To determine if the anti-smMLCK antibody recognizes epitopes in addition to the repeated sequence present in mammalian MLCK, this serum was preadsorbed using the repeat peptide. A solid phase binding assay was performed to confirm that the antibodies to the repeated region had been completely adsorbed (data not shown). As shown in Fig. 2C, the preadsorbed serum recognizes both 136-kDa smooth muscle MLCK and 208-kDa protein, confirming that at least some antigenic epitopes in addition to the repeated region exist between the smooth muscle MLCK and the 208-kDa protein. A control Western blotting experiment using preadsorbed anti-Repeat antibody showed a loss of detection of both 136-kDa rat smooth muscle MLCK and 208-kDa protein in both A10 and REF cell extracts.
208-kDa Protein Phosphorylates Regulatory Myosin Light Chains in a Ca2+/Calmodulin-dependent Manner
The 208-kDa protein detected in REF cells was immunoprecipitated and tested for its ability to phosphorylate purified myosin regulatory light chains in an in vitro assay. Immunoprecipitation was performed using either anti-Repeat antibody, preimmune serum, or anti-smMLCK antibody. Kinase assays were performed using the immunoprecipitated proteins from 200 μg (100 μl) or 400 μg (200 μl) cell extracts, purified light chains, [32P]ATP, and Ca2+/calmodulin or in the presence of EGTA. Activity was measured as rate of incorporation of 32P into purified regulatory light chains. Fig. 3 shows a representative of four independent assays using different amounts of cell extracts. These assays reveal that the 208-kDa protein immunoprecipitated by anti-Repeat (Fig. 3) or anti-smMLCK antibodies (data not shown) has a linear rate of incorporation of 32P into light chain that is dependent on Ca2+/calmodulin. Only a small amount of incorporation in the absence of Ca2+ (EGTA) or from immunoprecipitates using preimmune serum was detected. Rates of incorporation varied between 2.7 and 5.4 pmol/min/μl cell extract, compared to a rate of 9–15 pmol/min/μl obtained by immunoprecipitating recombinant rabbit uterine smooth muscle MLCK transiently expressed in COS cells (data not shown).
Fig. 3. Myosin light chain phosphorylation by immunoprecipitated 208-kDa embryonic MLCK.

A, representative curves showing Ca2+/calmodulin-dependent incorporation of 32P into myosin light chains following immunoprecipitation of 208-kDa protein using anti-Repeat antibody from 2 volumes of cell extract (200 and 100 μl). Comparable low rates of incorporation were obtained using protein immunoprecipitated by anti-Repeat antibody in the presence of EGTA (200 μl) or using preimmune serum (P.I.). B, autoradiogram showing incorporation of 32P into 20-kDa regulatory light chains. Assays were terminated by addition of SDS-PAGE sample buffer. Proteins were separated by electrophoresis (15% SDS-PAGE) and incorporation of 32P into 20-kDa regulatory light chain detected by autoradiography of the dried gel. Also shown are samples using preimmune serum to immunoprecipitate REF cell extracts.
Examination of proteins labeled by incorporation of 32P during the assay revealed that the only significant radiolabeled protein was the myosin regulatory light chain (Fig. 3B). Autoradiography showed that only those reactions containing Ca2+/CaM and extracts immunoprecipitated by anti-Repeat antibody (Fig. 3B) or anti-smMLCK antibody (data not shown) resulted in significant incorporation of 32P into light chains.
RNA Encoding 208-kDa Protein
As antibodies to smooth muscle MLCK detect a 208-kDa protein by Western blotting analysis, Northern blotting was used to determine if additional mRNAs could be detected by probes corresponding to smooth muscle MLCK. Fig. 4 shows that a probe derived from the rabbit smooth muscle MLCK detects the 2.6-kb mRNA encoding telokin, the independently expressed COOH terminus of smooth muscle MLCK and the 5.7-kb mRNA encoding smooth muscle MLCK in both rat uterine tissue and cultured rat A10 cells (Gallagher et al., 1991; Gallagher and Herring 1991). In rat A10 cells, and upon prolonged exposure of the blot in rat uterine tissue, an 8.7-kb mRNA is also detected. As the relative expression of 208 kDa protein appears to be higher in rat A10 cells than in rat uterine tissue, this result would suggest that the 8.7-kb mRNA may encode the 208-kDa protein.
Fig. 4. Northern analysis of mRNAs in rat cells and tissues.
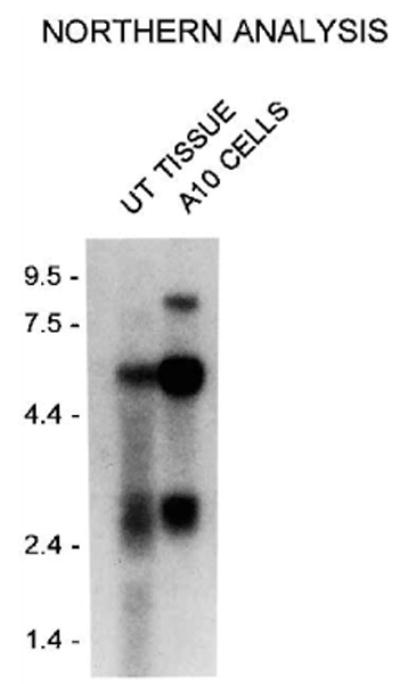
Northern blot analysis of RNA isolated from rat uterine tissue (15 μg of total cellular RNA) and rat A10 cells (5 μg of poly(A)+) following fractionation through a 1.2% agarose gel. The blot was probed with 32P-labeled cDNA probe corresponding to nucleotides 714-3705 (residues 136-1134) of the full-length rabbit uterine smooth muscle MLCK. The positions of the RNA molecular weight standard are shown in kilobases on the left. The blot was exposed for 48 h at −70 °C. Longer exposure of the blot reveals the presence of an 8.7-kb mRNA in rat tissues.
Expression of 208-kDa MLCK and Smooth Muscle MLCK in Embryonic Cells and Tissues
Examination of undifferentiated murine embryonic stem cells by Western blotting revealed that only the 208-kDa protein is expressed (Fig. 5). Following induction of differentiation by replating embryoid bodies expression of 130 kDa murine smooth muscle MLCK can be detected beginning at day 7 (Fig. 5).2 In embryonic tissues the 208-kDa protein and the 130-kDa murine smooth muscle MLCK are both detected, although the relative levels of each change during development. Analysis of extracts from whole mouse embryos shows that 130-kDa murine smooth muscle MLCK and 208-kDa protein are both expressed in E8 and E12 extracts. Fig. 5 shows the relative expression of 208-kDa protein declines 6.3-fold from embryonic day 15 (E15) to neonatal day 5 (N5) to an undetectable level following birth in adult heart. In liver, a less dramatic decline in expression is detected (1.6-fold). In contrast, the relative expression of the smooth muscle MLCK increases during development in both heart (5-fold between E15 and adult) and liver (1.5-fold between E15 and adult). This suggests that expression of smooth muscle MLCK and 208-kDa protein are inversely regulated during development, with expression of the 208-kDa protein declining and expression of smooth muscle MLCK increasing in adult tissues.
Fig. 5. Developmental expression of MLCKs in murine tissues and stem cells.

Immunoblots in panel A were reacted with anti-Repeat antibody which has the highest sensitivity for 208-kDa embryonic MLCK. The blots were stripped and reacted with anti-NH2-terminal antibody which has the highest sensitivity for 130-kDa murine smooth muscle MLCK (panel B). Embryonic stem cells, undifferentiated or differentiated by replating embryoid bodies were extracted as described under “Materials and Methods.” Whole embryos, embryonic heart, or liver tissues were collected at the times (days post-fertilization) indicated above the blots. E, embryonic; Neo, neonatal. Each lane represents 50 μg of total cellular protein. Positions of molecular weight markers (left) and MLCKs (right) are indicated.
Discussion
A 208-kDa MLCK has been identified as a new member of the MLCK family based upon its immunological and biochemical properties. The 208-kDa MLCK is detected in extracts of cultured cells and tissues by two polyclonal antibodies specific for smooth muscle MLCK (anti-smMLCK and anti-Repeat antibody). In activity assays, 208-kDa MLCK phosphorylates purified myosin regulatory light chains in a manner consistent with the Ca2+/calmodulin-dependent properties of all previously characterized eucaryotic MLCKs (Stull et al., 1991; Kamm and Stull, 1985). As the 208-kDa MLCK is expressed in undifferentiated embryonic stem cells and early embryonic tissues, we suggest the name embryonic MLCK.
The lack of immunoreactivity of 208-kDa embryonic MLCK with two additional antibodies specific for smooth muscle MLCK (anti-NH2-terminal and anti-COOH-terminal) suggests that the amino and carboxyl termini of this molecule differ from the smooth muscle MLCK, which is detected by both these antibodies. This result also distinguishes 208-kDa embryonic MLCK from the predicted protein encoded by the chicken embryo fibroblast non-muscle MLCK cDNA (Shoemaker et al., 1990).
The developmental expression pattern of 208-kDa embryonic MLCK is of interest, as many actomyosin-dependent events occur in proliferating, migrating cells that are distinct from those occurring in differentiated, non-proliferative tissues. The temporal expression pattern of 208-kDa embryonic MLCK compared to 130-kDa murine smooth muscle MLCK shows that this form is expressed at high levels early during development in embryonic tissues. Expression of 208-kDa embryonic MLCK declines at birth to low or undetectable levels in most adult tissues, although in some tissues such as liver down-regulation of 208-kDa embryonic MLCK is not as dramatic. The decline in expression of embryonic MLCK in cardiac tissues is generally coincident with cessation of cardiomyocyte proliferation and terminal differentiation which occurs shortly following birth in rodents (Rumyantsev, 1991). In liver, a less dramatic decline in expression of embryonic MLCK occurs, possibly reflecting the high regenerative capacity of this tissue. The coincidental expression of 208-kDa embryonic MLCK during early development, in undifferentiated embryonic stem cells and cultured cell lines suggests that the functional role of the 208-kDa embryonic MLCK may be to regulate the activity of early developmental or non-muscle forms of myosin. As non-muscle myosin heavy chain isoforms are expressed in many cultured cell lines and early during development, expression of 208-kDa embryonic MLCK in proliferating cultured cells or in differentiating tissues is consistent with the proposal that this form of MLCK has a unique regulatory function for these myosin isoforms. If this is true, then it is plausible that a family of MLCKs exists, each being specialized to regulate the activities either of specific myosin isoforms or specialized myosin motor activities such as assembly/disassembly, contraction, or cytokinesis.
Collectively, the immunological and biochemical data presented in this report identify the presence of a previously undetected form of MLCK. We suggest this form of MLCK be called embryonic MLCK because of its detection in undifferentiated stem cells and early embryonic tissues. Expression of embryonic MLCK is down-regulated following birth in most adult tissues, with the predominant form of MLCK in adult tissues being smooth muscle MLCK. It is now apparent the expression of smooth muscle MLCK is not tissue-specific and the expression patterns of both forms of MLCK during development suggest that embryonic MLCK and smooth muscle MLCK are inversely regulated. The pattern of immunoreactivity of embryonic MLCK clearly shows that this form of MLCK is related to but clearly distinct from both the smooth muscle MLCK and the predicted non-muscle MLCK.
Acknowledgments
We thank Loren Field for suggestions and for pregnant mice AT1 and AT2 cells, Fred Pavalko for REF cells and assistance with preadsorption data, and Jim Stull for antibodies to smooth muscle MLCK.
Footnotes
This work was supported by American Cancer Society Grant IRG-161-H (to P. J. G.), a Grant-in-Aid from American Heart Association (to P. J. G. and B. P. H.), and a Grant-in-Aid from American Heart Association, Indiana Affiliate (to B. P. H.).
The abbreviations used are: MLCK, myosin light chain kinases; MOPS, 4-morpholinepropanesulfonic acid; SDS-PAGE, SDS-polyacrylamide gel electrophoresis; kb, kilobase pair(s); REF, rat embryonic fibroblast; sm, smooth muscle.
B. P. Herring, unpublished observations.
References
- Adelstein RS, Conti MA. Nature. 1975;256:597–598. doi: 10.1038/256597a0. [DOI] [PubMed] [Google Scholar]
- Collinge M, Matrisian PE, Zimmer WE, Shattuck RL, Lukas TJ, Van Eldik LJ, Watterson DM. Mol Cell Biol. 1992;12:2359–2371. doi: 10.1128/mcb.12.5.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faux M, Mitchelhill K, Katsis F, Wettenhall R, Kemp B. Mol Cell Biochem. 1993;127/128:81–91. doi: 10.1007/BF01076759. [DOI] [PubMed] [Google Scholar]
- Gallagher PJ. Mol Biol Cell. 1994;5:151a. [Google Scholar]
- Gallagher PJ, Herring BP. J Biol Chem. 1991;266:23945–23952. [PMC free article] [PubMed] [Google Scholar]
- Gallagher PJ, Herring BP, Griffin SA, Stull JT. J Biol Chem. 1991;266:23936–23944. [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Davis H, Patterson C. J Cell Physiol. 1995;163:510–522. doi: 10.1002/jcp.1041630311. [DOI] [PubMed] [Google Scholar]
- Gibson T, Higgins D. DNA Sequence. 1993;3:333–335. doi: 10.3109/10425179309020833. [DOI] [PubMed] [Google Scholar]
- Guiliano KA, Kolega J, DeBiasio RL, Taylor DL. Mol Biol Cell. 1992;3:1037–1048. doi: 10.1091/mbc.3.9.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel G, Wehland J, Weber K. Exp Cell Res. 1983;148:117–126. doi: 10.1016/0014-4827(83)90192-1. [DOI] [PubMed] [Google Scholar]
- Hubbard M, Klee C. J Biol Chem. 1987;262:15062–15070. [PubMed] [Google Scholar]
- Kanoh S, Ito M, Niwa E, Kawano Y, Hartshorne D. Biochemistry. 1993;32:8902–8907. doi: 10.1021/bi00085a023. [DOI] [PubMed] [Google Scholar]
- Kamm KE, Stull JT. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Kerrick WG, Bourguignon LY. Proc Natl Acad Sci U S A. 1984;81:165–169. doi: 10.1073/pnas.81.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RP, Sorota S, Dresdner KP, Steinhelper ME, Lanson NA, Wit AL, Claycomb WC, Field LJ. J Cardio Electrophys. 1993;4:642–660. doi: 10.1111/j.1540-8167.1993.tb01251.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Inoue A, Mikawa T, Kuwayama H, Hotta Y, Masaki T, Ebashi S. J Biochem. 1992;112:786–791. doi: 10.1093/oxfordjournals.jbchem.a123976. [DOI] [PubMed] [Google Scholar]
- Kolega J, Taylor DL. Mol Biol Cell. 1993;4:819–836. doi: 10.1091/mbc.4.8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodney MS, Wyslomerski RB. J Cell Biol. 1992;117:73–82. doi: 10.1083/jcb.117.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeit S, Gautel M, Lakey A, Trinick J. EMBO J. 1992;11:1711–1716. doi: 10.1002/j.1460-2075.1992.tb05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson NJ, Pearson RB, Needleman D, Hurwitz MY, Kemp B, Means AR. Proc Natl Acad Sci U S A. 1990;87:2284–2288. doi: 10.1073/pnas.87.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko F, Burridge K. J Cell Biol. 1991;114:481–491. doi: 10.1083/jcb.114.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumyantsev PP. In: Growth and Hyperplasia of Cardiac Muscle Cells. Carlson BM, editor. Harwood Academic Press; New York: 1991. pp. 70–157. [Google Scholar]
- Sellers JR, Pato MD. J Biol Chem. 1984;259:7740–7746. [PubMed] [Google Scholar]
- Shirinsky VP, Vorotnikov A, Birukov KG, Nanaev AK, Collinge M, Lukas T, Sellers J, Watterson DM. J Biol Chem. 1993;268:16578–16583. [PubMed] [Google Scholar]
- Shoemaker MO, Lau W, Shattuck R, Kwiatkowski A, Matrisian PE, Guerra-Santos L, Wilson E, Lukas T, VanEldik L, Watterson D. J Cell Biol. 1990;111:1107–1125. doi: 10.1083/jcb.111.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull JT, Gallagher PJ, Herring BP, Kamm KE. Hypertension. 1991;17:723–732. doi: 10.1161/01.hyp.17.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney HL, Bowman BF, Stull JT. Am J Physiol. 1993;264:C1085–1095. doi: 10.1152/ajpcell.1993.264.5.C1085. [DOI] [PubMed] [Google Scholar]
- Wyslomerski RB, Lagunoff D. Proc Natl Acad Sci U S A. 1990;87:16–20. doi: 10.1073/pnas.87.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikai S, Ikebe M. Arch Biochem Biophys. 1992;299:242–247. doi: 10.1016/0003-9861(92)90270-7. [DOI] [PubMed] [Google Scholar]


