SUMMARY
Through genetic recombination, the adaptive immune system generates a diverse T cell repertoire allowing recognition of a vast spectrum of foreign antigens. Any given CD8+ T cell specificity is thought to be rare, but none have been directly quantified. Here, major histocompatibility complex tetramer and magnetic-bead technology were coupled to quantitate naive antigen-specific CD8+ T cells and the early response to infection. Among six specificities measured, the number of naive antigen-specific precursors ranged from ~80 to 1200 cells/mouse. After vesicular stomatitis virus infection, the antigen-specific CD8+ T cell response occurred in discrete phases: prolonged activation of a subset of cells over the first 72 hr followed by a rapid proliferative burst. Naive precursor frequency altered response kinetics and regulated immunodominance, as well as the time required for the responding population to shift toward CD62Lhi memory cells. Thus, initial endogenous precursor frequencies were surprisingly diverse and not only regulated initial immune response characteristics but also controlled memory CD8+ T cell lineage decisions.
INTRODUCTION
The ability to generate an immensely diverse T cell repertoire through genetic recombination is a hallmark of the adaptive immune system. Theoretically, this repertoire is made up of ~1015 different receptor combinations (Davis and Bjorkman, 1988). However, a large number of such combinations are selected against because they are unable to bind the appropriate major histocompatibility complex (MHC) molecule or bind it with too high an avidity. This selection process results in ~1013 different possible receptor combinations (Ignatowicz et al., 1996; Bouneaud et al., 2000; Zerrahn et al., 1997). However, only a fraction of the potential repertoire can be utilized given the total number of T cells in a mouse. In addition, the extent to which the size of each clone restricts the overall repertoire is not known, although estimates have been made suggesting that the total mouse T cell repertoire may contain only ~2 × 106 separate clones of ten cells each (Casrouge et al., 2000). Nevertheless, the peripheral repertoire is able to respond to a wide variety of antigens, which is essential to providing protective immunity against infectious diseases.
Technological advances over the past two decades, such as the development of T cell receptor (TCR)-transgenic mice (Berg et al., 1988) and the use of adoptive transfers (Kearney et al., 1994) along with the advent of peptide:MHC (pMHC) tetramers (Altman et al., 1996), have allowed for the dissection of antigen-specific CD4+ and CD8+ T cell responses. In general, T cell responses can be divided into four distinct phases: activation, expansion, contraction, and memory. Upon antigenic stimulation, naive antigen-specific CD8+ T cells become activated and undergo rapid expansion and effector cell differentiation, whereby they increase in numbers by up to 50,000-fold (Blattman et al., 2002; Murali-Krishna et al., 1998; Butz and Bevan, 1998; Busch et al., 1998). After the peak of expansion, ~90%–95% of the effector cells undergo apoptosis, leaving behind the long-lived memory population that develops over the next few weeks (Kaech et al., 2002). This population of renewable memory cells will provide enhanced protection—due in part to the rapid expression of effector functions and localization to nonlymphoid tissues—against secondary antigenic challenge (Sallusto et al., 1999; Masopust et al., 2001b).
Only recently have estimates of naive antigen-specific T cell precursor frequencies been postulated. Using indirect methods, such as titration of TCR-transgenic cells or analysis of the β-chain repertoire, it has been estimated that there are between 10 and 3000 antigen-specific T cells per mouse for a given epitope (Blattman et al., 2002; Kedzierska et al., 2006; Casrouge et al., 2000; Pewe et al., 2004; Bousso et al., 1998; Badovinac et al., 2007). However, a more recent report used an elegant method employing pMHC class II tetramers and magnetic-bead enrichment to directly quantify the size of naive antigen-specific CD4+ T cell populations (Moon et al., 2007). Three specificities were measured and comprise ~20–200 antigen-specific CD4+ T cells per mouse (Moon et al., 2007). With this knowledge, and given the general belief that CD4+ and CD8+ T cells mount very different responses with regard to the extent of proliferation and the overall response magnitude (Foulds et al., 2002), it is therefore important to measure the frequency of endogenous naive antigen-specific CD8+ T cells, which has yet to be reported.
In this paper, we combined magnetic-bead separation and pMHC class I tetramer staining to enable the highly sensitive detection of multiple naive antigen-specific CD8+ T cell populations. In addition, we demonstrate that this technique can be successfully utilized to visualize the early events of CD8+ T cell activation and expansion after viral infection. Therefore, we were able to directly quantify the number of naive antigen-specific CD8+ T cells and track the entire time course of an endogenous immune response from the earliest activation of naive antigen-specific CD8+ T cells through their expansion and subsequent memory cell differentiation.
RESULTS
pMHC Class I Tetramer Enrichment and Enumeration of Multiple Naive CD8+ T Cell Specificities
Thus far, the number of naive antigen-specific CD8+ T cells specific for a given antigen in a mouse is not known. However, pMHC class I tetramer staining and magnetic-bead separation have been coupled to assess infrequent CD8+ T cell epitopes in peripheral blood mononuclear cell (PBMCs) of humans after viral infection (Scriba et al., 2005; Barnes et al., 2004). In addition, this technique has been applied to assess the naive CD4+ T cell population (Hataye et al., 2006; Moon et al., 2007). Therefore, we applied this method to detect naive antigen-specific CD8+ T cells. We initially validated the technique for CD8+ T cells by using naive mice or mice previously infected with a recombinant vesicular stomatitis virus (VSV) expressing an immunodominant epitope of the M45 protein of murine cytomegalovirus (MCMV) (Munks et al., 2006a, 2006b). Cells from the spleen and all macroscopically identifiable lymph nodes (LNs) were pooled from individual mice and were then subjected to enrichment by tetramer reactivity and magnetic-bead enrichment followed by secondary-antibody staining. The enriched lymphocyte population was stained with mAb specific for CD19, CD11b, MHC class II, and CD4 (“dump” gate) and CD8 (Figure 1A). CD8+ T cells were gated, and the tetramer+ population was analyzed by flow cytometry (Figure 1B). One potential pitfall of this approach is that cells that nonspecifically bind to the tetramer may also be enriched. One way to minimize this problem is to stain the samples with both phycoerythrin (PE)- and allophycocyanin (APC)-labeled tetramers of the same specificity, an approach that has been previously used (Stetson et al., 2002). In addition, when ovalbumin (ova)-specific OT-I Rag2−/− TCR-transgenic mice were subjected to the enrichment protocol with the irrelevant M45:Db tetramer, few if any tetramer+ cells were isolated (Figure 1B). This was true for all tetramers used in this study (Figure S1 available online). In contrast,memory cells were greatly enriched, and a population of tetramer+CD8+ T cells from naive C57BL/6 mice was readily detected (Figure 1B). Nearly all tetramer+CD8+ T cells obtained were phenotypically naive CD62LhiCD11aloCD44lo, whereas tetramer+ memory cells were CD62Lhi and loCD11ahiCD44hi (Figure 1C).
Figure 1. Naive Antigen-Specific CD8+ T Cells Are Specifically Enriched with pMHC Class I Tetramers.
(A) Flow-cytometry gates used to detect pMHC class I binding CD8+ T cells from total spleen and lymph node cells. Lymphocytes were gated on the basis of forward scatter (FSC) versus side scatter (SSC) (left plot); this was followed by gating of CD8+ T cells that were negative for the dump stain (CD4, CD11b, CD19, and B220) (right plot).
(B) Representative control plots demonstrating the specificity of the pMHC class I tetramer enrichment protocol. Total spleen and lymph node cells from naive OT-I Rag2−/− mice (left plot), memory mice infected 3 weeks earlier with VSV (center plot), or naive C57BL/6 mice (right plot) were enriched with both PE- and APC-labeled M45:Db tetramers.
(C) Phenotypic analysis of enriched tetramer+ cells. Histograms are gated on the dual tetramer+ cells as described above and are representative of all the specificities analyzed. Filled histograms represent cells enriched from naive C57BL/6 mice, and open histograms are representative of memory cells.
(D) Vβ13 usage by naive and memory CD8+ T cells specific for either the VSV-N:Kb epitope or the Ova:Kb epitope. Histograms are gated on enriched tetramer +CD8+ T cells. These data are representative of two independent experiments, and the value indicates the percentage of antigen-specific CD8+ T cells utilizing the Vβ13 chain.
(E) The majority of Ova:Kb-specific naive CD8+ T cells are deleted in mice constitutively expressing ovalbumin. Total spleen and lymph node cells were pooled from either naive C57BL/6 or naive 232-6 mice. Antigen-specific CD8+ T cells were enriched with either Ova:Kb or VSV-N:Kb tetramers. Contour plots were gated as above and are representative of two independent experiments.
Recently, it was shown that the Vβ repertoire of a CD4+ T cell response correlated with that found in the naive animal (Moon et al., 2007). It is known that after VSV infection the CD8+ T cells responding to the major immunodominant peptide derived from the VSV nucleoprotein (N) (Van Bleek and Nathenson, 1990) express TCRs skewed toward usage of Vβ13 (Kalergis et al., 1999). Thus, to test whether the repertoire of the VSV-N:Kb-specific naive CD8+ T cells predicted the TCR repertoire of responding cells, we compared the usage of Vβ13 within both the naive and memory VSV-N:Kb-specific and, as a control, ovalbumin (Ova)-specific CD8+ T cell populations. Interestingly, the frequency of Vβ13+ cells within both naive and memory VSV-N: Kb-specific CD8+ T cell populations was similar, at ~30%. In contrast, Vβ13 was only rarely used by either naive or memory Ova:Kb-specific CD8+ T cells (Figure 1D). These data indicated that the TCR usage of naive CD8+ T cells, at least in this case, predicted TCR usage during the immune response to infection.
As an additional test of naiveté in our enriched CD8+ T cells, we utilized a transgenic mouse line that expresses ovalbumin constitutively, and as a consequence Ova:Kb-specific CD8+ T cells are not induced by VSV-ova infection (Vezys et al., 2000). Whereas enrichment with the Ova:Kb tetramer resulted in isolation of a population of tetramer+ cells with naive phenotype from normal mice, only a few tetramer+ cells were found in the ova-transgenic mice, which were largely tetramer dull in staining. In contrast, similar populations of VSV-N:Kb-specific CD8+ T cells were present in both preparations (Figure 1E). Thus, expression of the ovalbumin neo-self-antigen resulted in deletion of the majority of tetramer-high, presumably high-avidity, Ova:Kb-specific CD8+ T cells. Together, these findings indicated that the enriched tetramer+ cells represented bona fide antigen-specific naive CD8+ T cells.
We also wished to quantify a number of antigen specificities to begin to establish a range of frequencies for the naive CD8+ T cell repertoire. To this end, enrichment was performed with several tetramers (Figure 2A). The absolute number of naive antigen-specific CD8+ T cells per mouse was quantified (Figure 2B) and was also normalized to the total number of CD8+ T cells in the mouse (Figure 2C). Of the six specificities tested, CD8+ T cells specific for the M45:Db and lymphocytic choriomeningitis virus (LCMV)-derived GP33:Db epitopes had the highest average frequencies, of 1 in 33,000 and 1 in 70,000, respectively. Lower frequencies were observed for CD8+ T cells specific for VSV-N:Kb, Ova:Kb, LCMV NP396:Db, or influenza virus acid polymerase (PA:Db) and ranged from 1 in 120,000 to 1 in 164,000. Among individual mice, the range of antigen-specificCD8+ T cells for all specificities testedwasfrom80–1200cells, indicating the potential for a greater than 10-fold difference in frequencies between given specificities.
Figure 2. Enumeration of the Precursor Frequencies of Multiple Naive Antigen-Specific CD8+ T Cell Populations.
(A) Representative dot plots show the enrichment of tetramer+ cells for all the epitopes tested. Values represent the average precursor frequency (left value) and average cell number (right value) found in an individual C57BL/6 mouse.
(B and C) Graphical depiction of the absolute numbers (B), and reciprocal frequency of the naive antigen-specific CD8+ T cells from total spleen and lymph nodes cells of individual mice (C). Each symbol represents an individual mouse, and the bar indicates mean values.
pMHC Class I Tetramer Enrichment Reveals the Kinetics of Early Expansion of Responding Antigen-Specific CD8+ T Cells
Until now, the only way to examine the very early events in a CD8+ T cell response was to transfer relatively large numbers of single-avidity clonal TCR-transgenic cells (generally 1 × 105–1 × 106). Responses derived from transferred T cells do not mirror endogenous responses (Marzo et al., 2005; Badovinac et al., 2007; Stock et al., 2007). Therefore, we utilized the tetramer enrichment protocol to monitor the antigen-specific CD8+ T cell response to VSV-M45 infection starting from its inception. The tissues were disrupted with collagenase and EDTA in order to circumvent potential issues with the “locking” of responding T cells in lymphoid tissues (Lefrancois et al., 2000; Maxwell et al., 2004; Jabbari et al., 2006).
Twenty-four hours after VSV infection, only a proportion (~35%) of the antigen-specific CD8+ T cells expressed CD69 (Figure 3A), an indication of recent activation (Cebrian et al., 1989). In addition, 12 hr after infection, only ~15% of the antigen-specific CD8+ T cells were CD69+ (data not shown). We also did not observe a global upregulation of CD69 on the bulk CD8+ T cell population, which others have seen in response to viral infection and type I interferons (Bahl et al., 2006), suggesting that this event is the result of TCR engagement. At 24 hr after infection, CD11a expression and cell size were identical to those of naive cells. By 48 hr, CD69 was absent but the cells had begun blasting, and most were CD11ahi (Figure 3A). A small population of CD11alo cells remained, supporting the idea that not all cells had encountered antigen. By 72 hr after infection, the antigen-specific CD8+ T cells remained CD69−, were uniformly CD11ahi, and were at their maximal size. At 96 hr after infection, the cells had decreased in size but retained their phenotype.
Figure 3. Tracking of Early Activation and Expansion of Endogenous Antigen-Specific CD8+ T Cells after VSV Infection.
C57BL/6 mice were infected with 2 × 105 PFU of VSV-M45. M45:Db-specific CD8+ T cells were monitored in the spleen by tetramer enrichment for the first 4 days.
(A) Histograms are gated on enriched M45:Db-specific CD8+ T cells. CD69 expression (left plots), FSC (center plots), and CD11a expression (right plots) were compared. The open histograms represent the antigen-specific CD8+ T cell population and in the top row the shaded histogram depicts “naive” (CD11alo) CD8+ T cells. The same expression pattern was observed for the VSV-N:Kb-specific CD8+ T cell population (data not shown). These data are representative of three independent experiments.
(B) Kinetics of the early expansion of the M45:Db-specific CD8+ T cells. Each dot represents the mean of three or four individual mice with the bar representing the standard deviation of the data. These data are representative of three separate experiments.
(C) Mice were infected with VSV-M45 and given 800 mg of BrdU i.p. daily. Mice were sacrificed 2 or 3 days later, and the enriched M45:Db-specific splenic CD8+ T cells were analyzed for BrdU incorporation by flow cytometry. The contour plots are gated on the CD8+CD11b−CD4−CD19−B220− cells. These data are representative of two independent experiments.
Over the first 48 hr, the cell numbers remained largely unchanged. (Although the values obtained appeared to be less variable than those in Figure 2B, note that only spleen is being analyzed here and that the y axis scales are different between the graphs.) By 72 hr, the cells had increased in number and subsequently rapidly expanded, undergoing approximately five doublings based on day 3 starting numbers (Figure 3B). The activation and early expansion of the antigen-specific CD8+ T cells occurred independently of initial naive precursor frequency, at least for the two specificities tested.
Next we examined when the antigen-specific CD8+ T cells began to synthesize DNA, as a prelude to division. Mice were given BrdU daily after infection, and incorporation was analyzed in the M45:Db-specific T cells. From 0 to 48 hr little, if any, BrdU incorporation was detected. However, from 48 to 72 hr, the majority of the cells had entered the cell cycle and incorporated BrdU (Figure 3C). Thus, BrdU incorporation coincided with blastogenesis (Figure 3A) and the small increase in cell numbers (~1.5 doublings on average) that occurred during this time period (Figure 3B). Taken together with the previous data, the early T cell response comprised a prolonged activation phase lasting approximately 48 hr followed by blastogenesis, with limited division, leading to rapid expansion several days prior to the peak of the response at about day 7 (Masopust et al., 2001a).
Precursor Frequency Dictates the Kinetics and Immunodominance of the Antigen-Specific CD8+ T Cell Response
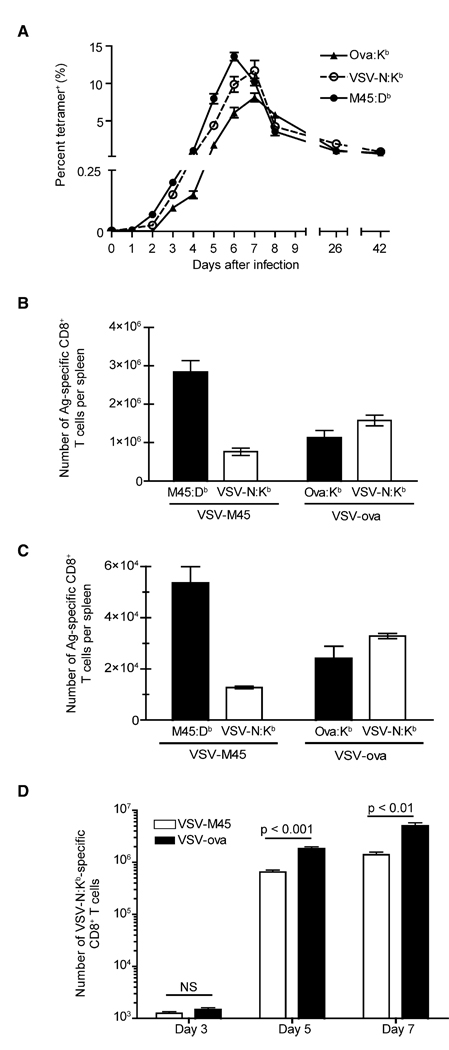
Whether kinetic differences observed in the response are due to differences in endogenous precursor cell numbers has not been tested. In addition, the immunodominance hierarchy of CD8+ T cell responses is a complex phenomenon (Yewdell and Bennink, 1999), but the role that precursor frequency plays in this process has not been explored in detail. For the testing of these ideas, mice were infected with either VSV-M45 or VSV-ova, and the antigen-specific CD8+ T cell responses were continually monitored in the spleen. The response to M45:Db peaked at day 6, ~24 hr prior to the responses to either Ova:Kb or VSV-N:Kb (Figure 4A). Although the M45:Db- and VSV-N:Kb-specific responses differed kinetically, they were of similar overall magnitude, even though their initial precursor frequencies differed on average by 4-fold. Also, whether the infection was initially VSV-ova or VSV-M45, the kinetics of the response to VSV N were similar, although the magnitude differed. These results indicated that the maximum size of the overall response to a particular epitope was limited by additional factors. Interestingly, the magnitude of the VSV-N:Kb-specific CD8+ T cell response was dramatically decreased by the presence of the M45:Db epitope, both at the peak of the response (Figure 4B) and at memory (Figure 4C). Therefore, the size of the memory population correlated with the peak size of the antigen-specific CD8+ T cell population and not necessarily precursor frequency.
Figure 4. The Kinetics and Immunodominance of the CD8+ T Cell Response Is Dictated by Naive Precursor Frequency.
(A) C57BL/6 mice were infected with 2 × 105 PFU of either VSV-M45 or VSV-ova. The frequency of M45:Db-specific (filled circles), VSV-N:Kb-specific (open circles), and Ova:Kb-specific (filled triangles) CD8+ T cells was quantified daily for the first 9 days and then at day 26 and 42 after infection. The N-specific values were obtained from the VSV-ova infection. Each data point is the mean value ± 1 standard deviation from three to five mice. These data are representative of three independent experiments.
(B and C) The absolute number of antigen-specific CD8+ T cells in the spleen was measured at the peak of the response (day 6 for M45:Db and day 7 for both VSV-N:Kb and Ova:Kb) (B) or 71 days after infection (C) with either VSV-M45 or VSV-ova. Each bar represents the mean for that specificity ± 1 standard deviation from three to five mice. These data are representative of three independent experiments.
(D) Mice were infected with 2 × 105 PFU of either VSV-M45 or VSV-ova. The magnitude of the VSV-N:Kb-specific splenic CD8+ T cell response was measured on days 3, 5, and 7 after infection. Day 3 analysis was performed by tetramer enrichment. Each bar represents the mean of four mice with ± 1 standard deviation. Statistical significance was determined with a two-tailed Student’s t test. These data are representative of two independent experiments.
We also tested when the immunodominance hierarchy was set. On day 3 after infection, the VSV-N:Kb-specific response was similar in magnitude after either infection, although VSV-N: Kb-specific CD8+ T cells were always present in slightly greater numbers during VSV-ova infection. However, by day 5 after infection, the VSV-N:Kb-specific CD8+ T cell response was significantly (p < 0.001) reduced by the presence of the M45:Db epitope (Figure 4D), and this difference was maintained at day 7 (p < 0.01). These data suggested that interclonal competition for resources (i.e., APC interactions, growth factors, or costimulatory molecules) prior to the peak of the response was important in modulating overall clonal expansion.
Transition to a CD62Lhi Central-Memory Phenotype Occurs More Rapidly in the M45:Db-Specific Population
We have previously shown that the number of TCR-transgenic cells transferred can have an impact on the differentiation patterns of memory precursors (Marzo et al., 2005). Because we observed that the M45:Db-specific CD8+ T cells had ~4-fold-greater average precursor frequency than both the Ova:Kb- and VSV-N:Kb-specific CD8+ T cell populations, we asked whether differences in memory-subset differentiation could be detected between different specificities. During the effector stage of the response, the majority of the antigen-specific CD8+ T cells, irrespective of their specificity, had downregulated CD62L expression in keeping with initial proteolytic cleavage of CD62L on activated T cells (Smalley and Ley, 2005). However, at early memory time points (i.e., 30 days after infection), there were significantly (p < 0.01) more CD62Lhi memory cells in the M45:Db-specific population than in either the Ova:Kb- or the VSV-N:Kb-specific populations (Figure 5). This difference was maintained to at least day 75, when the M45:Db-specific memory population contained ~1.5-fold more CD62Lhi cells (~50% of total) than did the populations specific for Ova:Kb or VSV-N:Kb antigens (~25%–30% of total). Interestingly, by 120 days after infection, the percentage of CD62Lhi memory cells among all three specificities had equalized at ~70% of the antigen-specific CD8+ T cells. This phenomenon appeared to be due to the slowing of population conversion of the M45:Db-specific population and continued conversion of the Ova:Kb- and VSV-N:Kb-specific populations.
Figure 5. Initial Precursor Frequency Controls CD62L Expression.
Mice were infected with 2 × 105 PFU of either VSV-M45 or VSV-ova. The expression of CD62L on the cell surface of M45:Db-specific (filled circles), VSV-N:Kb-specific (open circles), and Ova:Kb-specific (filled triangles) splenic CD8+ T cells was analyzed up to ~150 days after infection. Each data point is the mean value from three to five mice ± 1 standard deviation. Statistical significance was determined by a two-tailed Student’s t test (**, p < 0.01). These data are representative of two independent experiments.
Proliferative Capacity or CD62L mRNA Expression Does Not Explain Differences in CD62L Expression
It has long been known that over time the CD62Lhi memory population becomes the predominant population in CD8+ T cell responses (Tripp et al., 1995; Hogan et al., 2001; Roberts et al., 2005). Two main hypotheses, which are not mutually exclusive, have been used to explain this effect (Lefrancois, 2006): conversion of CD62Llo cells to CD62Lhi cells by as-yet-unknown mechanisms or the fact that CD62Lhi memory cells exhibit a higher proliferative rate than do CD62Llo memory cells, thereby resulting in a preferential expansion of CD62Lhi cells over time (Marzo et al., 2005; Wherry et al., 2003). The first explanation appears valid for high-precursor-frequency CD8+ T cells on the basis of adoptive-transfer studies, whereas the latter explains the effect in endogenous CD8+ T cells (Marzo et al., 2005).
However, these studies did not take into account differing endogenous naive CD8+ T cell precursor frequencies. Thus we wished to determine the relative turnover rate of memory CD8+ T cells of the specificities analyzed. To this end, mice were infected with either VSV-M45 or VSV-ova and 30 days later received BrdU in their drinking water for 4 weeks followed by analysis of BrdU incorporation in CD62Lhi and CD62Llo memory CD8+ T cells. If a difference in homeostatic proliferation was the cause of the increased conversion of the M45:Db-specific population, then the ratio of BrdU+CD62Lhi cells to BrdU+CD62Llo cells would be greater than for the VSV-N:Kb- and Ova:Kb-specific memory populations. However, in all three antigen-specific memory CD8+ T cell populations, CD62Lhi cells exhibited a ~1.3-fold-higher proliferative capacity than the CD62Llo population (Figure 6). Thus, increased proliferation based on precursor frequency did not explain the differences noted in CD62L expression.
Figure 6. Naive Precursor Frequency Does Not Alter Homeostatic Proliferation of Endogenous Memory Cells.
Mice infected 30 days previously with either VSV-M45 or VSV-ova were given BrdU in their drinking water for 4 weeks, and BrdU incorporation into splenic antigen-specific CD8+ T cells was determined. The graph shows the BrdU incorporation ratio of the CD62Lhi to CD62Llo antigen-specific memory cells. A value greater than 1.0 indicates that more CD62Lhi memory cells have incorporated BrdU. Each bar represents the mean value of three mice ± 1 standard deviation. These data are representative of two independent experiments.
Because our previous results with TCR-transgenic cells indicate that CD62Llo memory cells derived from a high naive precursor frequency are able to re-express CD62L (Wherry et al., 2003; Marzo et al., 2005), we wished to examine the mechanism by which this occurs and whether a similar process was occurring for endogenous CD8+ T cells. CD62L cell-surface expression is controlled by two mechanisms: proteolytic cleavage and genetic regulation (Smalley and Ley, 2005). First, with real-time PCR we measured CD62L mRNA in adoptively transferred OT-I cells responding to VSV-ova infection. At 1 or 2 weeks after infection, CD62Llo cells derived from a high (5 × 105) or low (5 × 103) initial precursor frequency were purified and CD62L mRNA expression was determined (Figure 7A). Values were standardized to mRNA expression in naive CD62Lhi OT-I cells. At approximately 1 week after infection, CD62Llo OT-I cells from either transfer expressed low amounts of mRNA, although the cells derived from the high-input transfer expressed 2-fold more CD62L mRNA than did those derived from the low-input transfer. However, 2 weeks after infection, CD62Llo cells from the high-input transfer expressed 20-fold-greater amounts of CD62L mRNA than did cells from the low-input transfer. These data indicated that a high precursor frequency resulted in the development of a population of cells that had not silenced CD62L mRNA transcription.
Figure 7. CD62L Gene Expression Is Not Altered by Differences in Endogenous Naive T Cell Precursor Frequencies.
(A) Either 5 × 105 or 5 × 103 OT-I cells (CD45.1+) were transferred to CD45.2+ mice. One day later, the mice were infected with 2 × 105 PFU of VSV-ova. Either 1 or 2 weeks later, splenic CD45.1+CD8+ T cells were flow-sorted on the basis of CD62L expression (purity > 98%), and RNA was analyzed by quantitative RT-PCR. CD62L mRNA expression was normalized to β-actin expression, and its expression relative to naive OT-I cells was determined by the equation 2−ΔΔCt, as previously done (Obar et al., 2004). These data are representative of three independent experiments.
(B) CD62L mRNA expression in the endogenous antigen-specific CD62Llo-CD8+ T cells of different specificities was determined at 2 weeks after infection. As a control, either 2 × 105 or 2 × 103 OT-I cells (CD45.1+) were transferred to naive CD45.2+ mice. Two weeks after VSV-ova infection, CD45.1+CD8+ T cells from the spleen were sorted on the basis of CD62L expression (purity > 98%). For endogenous cells, CD62LloCD8+ T cells were sorted by pMHC class I tetramers (purity > 98%). CD62L mRNA was quantitated as above. These data are representative of two independent experiments.
We then tested whether a similar difference was observed with endogenous CD8+ T cells of high (M45:Db) or low (VSV-N:Kb) frequency. RNA was purified from sorted tetramer+CD62Llo cells 14 days after VSV-M45 infection and subjected to PCR. As a control, responding CD62Llo OT-I cells from high (2 × 105) and low (2 × 103) input numbers were tested. RNA levels were normalized to those of naive CD62Lhi OT-I cells (Figure 7B). CD62L mRNA in CD62Llo OT-I cells from the high-input transfer was readily detected, whereas CD62L mRNA expression was substantially lower in CD62Llo cells from the low-input OT-I transfer. CD62L mRNA expression was also very low in either endogenous CD62Llo population, and there was no difference observed between the mRNA from the two populations. Overall, these findings help explain the ability of CD62Llo cells derived from high precursor transfers to re-express CD62L (Marzo et al., 2005), but did not support the concept that differences in CD62L gene regulation between the two endogenous specificities accounted for the differences in CD62L expression within the population.
DISCUSSION
This work has refined the tetramer enrichment protocol used by others to enumerate rare antigen-specific CD4+ and CD8+ T cell populations in human PBMCs after infection (Scriba et al., 2005; Barnes et al., 2004) and in the naive antigen-specific CD4+ T cell population of mice (Hataye et al., 2006; Moon et al., 2007), enabling the specific detection of endogenous naive antigen-specific CD8+ T cells in mice for the first time. The transfer of graded numbers of TCR-transgenic T cells has been used to estimate naive CD8+ T cell precursor frequency (Blattman et al., 2002; Badovinac et al., 2007). However, only a limited number of TCR specificities are available, these cells are of single avidity, and transfer efficiencies for each are unknown, making it impractical to estimate a broad panel of precursor frequencies via such techniques.
The remarkable finding made with direct quantitation of only six specificities was the broad range of naive CD8+ T cell precursor numbers observed, from 80 to 1200 cells per mouse, with an average of 120–600 cells/mouse, which would not be predictable from previous estimates (Blattman et al., 2002; Casrouge et al., 2000). It should also be noted that our values were probably an underestimate of the absolute total because cells may be lost due to isolation procedures and we did not examine blood or nonlymphoid tissues, both of which contain naive T cells (Cose et al., 2006). Hypothetically, if a large number of specificities comprised ~1000 cells, the overall CD8+ T cell repertoire would appear restricted although, substantial cross-reactivity within the CD8+ T cell repertoire exists and may substantially increase the functional repertoire (Welsh et al., 2000).
Our analysis was based on a recent report that quantitated the number of naive mouse antigen-specific CD4+ T cells (Moon et al., 2007). Three specificities were measured and comprised ~200 cells/mouse specific for the 2W1S:I-Ab epitope, ~20 cells specific for FliC:I-Ab, and ~20 cells specific for Ova:I-Ab. Although it will be necessary to assess a much larger panel of specificities to determine whether such values are representative of the overall CD4+ T cell repertoire, on average these values are substantially smaller than those we obtained for the six CD8+ T cell specificities we measured. However, the data also indicated that overlap in the frequency of particular specificities exists within the CD4+ and CD8+ repertoires (e.g., 200 CD4+ T cells specific for 2W1S:I-Ab and ~120–170 CD8+ T cells specific for VSV-N:Kb, Ova:Kb, NP396:Db, and PA:Db). Therefore, precursor frequency is unlikely to always explain the differences in kinetics and magnitude of certain CD8+ versus CD4+ T cell responses. Thus, our data on CD8+ T cells provide a valuable comparison to what is known at this point regarding the CD4+ T cell repertoire. Our results also indicated that the Vβ repertoire of an antigen-specific CD8+ T cell population was determined by the Vβ usage profile within the naive antigen-specific population. Although the CD8+ T cell response to VSV-N:Kb epitope is skewed toward Vβ13 usage (Kalergis et al., 1999), whether this is due to selection of a small population of responders is unknown. Our findings indicated that rather than selection, skewing of V region usage was a preformed bias of the initial repertoire, similar to what was shown for CD4+ T cells (Moon et al., 2007).
This technology also enabled us to monitor the early phases of the CD8+ T cell response without the aid of adoptive transfer of TCR-transgenic cells, thereby circumventing any issues related to increased precursor frequency or single avidities (Marzo et al., 2005; Badovinac et al., 2007). This type of analysis of endogenous T cells has not been previously reported. One question that remains extant in the field is what proportion of the naive T cell repertoire is recruited into any given response? Despite relatively high virus infection doses, our data suggested that only a subset of antigen-specific CD8+ T cells were activated, as detected by CD69 expression. This result holds important implications for understanding immune-response dynamics in relation to vaccine design because we will now be able to define the parameters necessary for optimal naive T cell recruitment into any given response. Our findings also demonstrated the need for a prolonged activation phase (~72 hr) before the antigen-specific CD8+ T cells were able to undergo rapid proliferative growth. For the first 48 hr, antigen-specific CD8+ T cell numbers in the lymphoid tissues remained relatively stable with only a slight increase, perhaps due to recruitment of cells from the blood and tissues. Between 48 and 72 hr after infection, the antigen-specific CD8+ T cells entered the proliferative phase, and over the next 24 hr the cells began to expand exponentially. These events occurred independently of precursor frequency because the response pattern was nearly identical between the VSV-N:Kb- and M45:Db-specific CD8+ T cells. The initial 48–72 hr may be required for the cells to upregulate molecules important in immunological-synapse formation, such as CD11a (Rothoeft et al., 2006; Dustin, 2004), which are necessary for prolonged TCR engagement. There is evidence for prolonged interactions between CD8+ T cells and DC in the spleen after Listeria monocytogenes infection, which interestingly occurs just prior to the rapid-division phase of the response in the periarterial lymphoid sheath (Khanna et al., 2007). The combination of in situ imaging and analysis of T cells prior to extensive expansion as shown here will provide an exciting opportunity to dissect early events governing the immune response.
Although the kinetics of the early response appeared similar, the peak of the M45:Db-specific CD8+ T cell response occurred 1 day earlier than for either the Ova:Kb- or VSV-N:Kb-specific CD8+ T cells. However, the overall maximum M45:Db-specific response was not substantially greater than the VSV-N:Kb-specific response in the VSV-M45 versus VSV-ova infections, respectively. Therefore, an increased precursor frequency resulted in a more rapid response and additional factors limited the expansion phase. Varying the number of adoptively transferred TCR-transgenic CD8+ T cells also affects the time required to reach the peak response (Badovinac et al., 2007; Stock et al., 2007).
In addition to altering the kinetics of the antigen-specific immune response, endogenous precursor frequency also affected memory cell differentiation in a fashion similar to that of altering precursor frequency through adoptive transfer (Wherry et al., 2003; Marzo et al., 2005). The long-lived memory T cell population consists of at least two subsets based on CD62L expression and functional capacity (Sallusto et al., 1999; Masopust et al., 2001b; Reinhardt et al., 2001; Marzo et al., 2007): CD62Llo, effector-memory cells (Tem) and CD62Lhi, central-memory cells (Tcm). However, upon transfer of high numbers of TCR-transgenic cells, a CD62Llo transitional Tem population develops with high homeostatic proliferative ability and the ability to re-express CD62L over time (Marzo et al., 2005; Wherry et al., 2003). Our present results indicated that at higher endogenous precursor frequencies, the M45:Db-specific population converted toward CD62Lhi more rapidly than did the lower-frequency VSV-N:Kb- or Ova:Kb-specific responses. However, this was not explained by differences in growth potential, given that the ratio of CD62Lhi and CD62Llo cells undergoing homeostatic proliferation was identical among the three specificities. Moreover, we have now defined the mechanism of CD62L re-expression in the transitional Tem population by demonstrating a lack of silencing of CD62L gene expression, thereby allowing CD62L mRNA to be produced in CD62Llo cells. In contrast, little CD62L mRNA was detectable in CD62Llo cells generated from endogenous CD8+ T cells regardless of initial frequency; thus it appears that transitional Tem are not the means of shifting to a predominately CD62Lhi memory population. Although other possibilities exist, we favor the hypothesis that early competitive events result in differential downregulation of CD62L expression and that small changes in the number of CD62Lhi cells between specificities results in more rapid population conversion to CD62L expression as a result of the increased proliferation of the CD62Lhi subset as compared to the CD62Llo subset (Wherry et al., 2003; Marzo et al., 2005; Lefrancois, 2006). The fact that CD62L is proteolytically cleaved early after T cell activation (Jung and Dailey, 1988; Jung et al., 1988; Smalley and Ley, 2005) makes analysis of this possibility problematic. Interestingly, data from a recent report showed that the endogenous LCMV GP33:Db-specific memory CD8+ T cell population became CD62Lhi more rapidly than did the NP396:Db-specific population (Sarkar et al., 2007). This finding was predicted by our demonstration that the endogenous precursor frequency specific for GP33:Db was ~2–3-fold greater than that for NP396:Db. Although that report suggested that endogenous CD62Llo cells could convert to CD62Lhi cells, this result could easily be explained by the level of contamination in their purifications (4%–6%) and the extended time frame over which conversion occurred. Thus, our findings indicated that initial precursor frequency, even within the natural T cell repertoire, regulated the character of the resulting memory population, and we believe that this is a result of events occurring early during T cell activation. Overall, our results provided important insight into understanding the effects of the endogenous CD8+ T cell repertoire on primary and memory responses and supply a framework to analyze the status of the natural T cell repertoire in a variety of normal and pathologic situations.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6 mice were purchased from the National Cancer Institute. TCR-transgenic OT-I Rag−/− (Hogquist et al., 1994) and F5 Rag−/− mice (Mamalaki et al., 1993) were bred in-house. 232-6 mice expressing ovalbumin under control of the intestinal fatty-acid-binding protein promoter were previously described (Vezys et al., 2000). All animal protocols were approved by the University of Connecticut Health Center Animal Care Committee.
Infections
For construction of the VSV expressing the M45:Db epitope (Munks et al., 2006b), the nucleotide sequence encoding the minimal CD8+ T cell epitope was inserted in-frame in the 5′ end of the sequence encoding GFP by PCR, and the virus was generated as previously described (Lawson et al., 1995). The recombinant VSV expressing ovalbumin was previously described (Kim et al., 1998). Mice were infected intravenously with 2 × 105 plaque-forming units (PFU) of VSV.
Tissue Preparation, Flow Cytometry, and pMHC Class I Tetramer Reagents
Single-cell suspensions were prepared by collagenase digestion (Masopust et al., 2001b). Lymphocytes (1 × 107 cells/ml) were stained with peptide:MHC class I tetramers, anti-CD8 (clone 53-6.7), and Fc block for 1 hr at room temperature (RT) and then washed and stained with anti-CD62L, anti-CD69, and anti-CD11a for 30 min at 4°C. H-2Kb tetramers containing either the OVA-derived peptide SIINFEKL or the VSV-N protein-derived peptide RGYVYQGL were generated in our lab as previously described (Altman et al., 1996). H-2Db tetramers containing the MCMV M45-derived peptide HGIRNASFI or the influenza virus PA epitope were obtained from the NIH Tetramer Core Facility.
Enrichment of Antigen-Specific CD8+ T Cells
Single-cell suspensions from spleen and lymph nodes (mesenteric, inguinal, cervical, axillary, and brachial) were stained with PE- and APC-labeled pMHC-I tetramers, anti-CD8 (clone 53-6.7), and Fc block for 1 hr at RT in 1 ml of PBS containing 0.1% NaN3, 0.5% BSA, and 2 mM EDTA (MACS buffer). Cells were then washed, resuspended in 500 µl of MACS buffer, and labeled with 50 µl of anti-PE microbeads (Miltenyi Biotec) for 30 min at 4°C, washed, and passed over a magnetized LS column (Miltenyi Biotech). Columns were washed and then removed from the magnet, and bound cells were eluted. Cells were then stained with anti-CD44, anti-CD11a, anti-CD62L, anti-CD4, anti-CD19, anti-IAb, and anti-CD11b for 30 min at 4°C. Anti-CD11b was left out for the enrichment of tetramer+ cells after infection because CD11b may be expressed by some activated T cells. Cells were then washed and fixed with 2% PFA. The entire sample was then analyzed with a LSRII cytometer (Becton Dickinson).
Measurement of BrdU Incorporation
BrdU was administered to infected mice in their drinking water (0.8 mg/ml) for long-term turnover experiments or was injected intraperitoneally (i.p.) daily (800 µg) for short-term studies. The cells were then stained with anti-BrdU according to the BrdU flow kit protocol (BD PharMingen).
Cell Sorting and Quantitative RT-PCR
CD62Lhi and CD62Llo cells were purified on a FACSVantage SE (Becton Dickinson) after magnetic-bead enrichment of CD8+ T cells. Cells were purified to greater than 98% purity. For analysis of CD62L transcripts, RNA was isolated from the FACS sorted cells with the RNeasy Mini Kit (QIAGEN). cDNA was made with SuperScript II RT (Invitrogen) and 0.5 µg of oligo(dT) primer at 42°C for 50 min. Quantitative PCR was performed with 2 µl of the cDNA mixture with Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen) and 400 nM of the appropriate primers. Primer sequences were as follows: CD62L #1, 5′-GGGCTCGAGGAACATCCTGAAG-3′; CD62L #2, 5′-TGCAGAACTTTCTAGCA TTTTCCCA-3′; β-actin #1, 5′-AGAGGGAAATCGTGCGTGAC-3′; and β-actin #2, 5′-CAATAGTGATGACCTGGCCGT-3′. The samples were subjected to 40 cycles of 30 s at 95°C and 30 s at 60°C. qPCR was performed with a Bio-Rad iCycler. CD62L mRNA expression was normalized to β-actin expression, and then relative expression was determined as previously described (Obar et al., 2004).
Statistical Analysis
Statistical significance was determined by the Student’s t test, with a p < 0.01 being significant.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank M. Jenkins (University of Minnesota) for sharing his techniques prior to publication and are grateful to D. Masopust (University of Minnesota) for providing some of the pMHC class I tetramers used in this study. We thank Q.-M. Pham for expert assistance and D. Gran for cell sorting. This work was funded by National Institutes of Health grants AI41576 (L.L.) and F32AI074277 (J.J.O). K.M.K is a Damon Runyon Fellow supported by the Damon Runyon Cancer Research Foundation (DRG-1886-05).
Footnotes
SUPPLEMENTAL DATA
One figure is available at http://www.immunity.com/cgi/content/full/28/6/859/DC1/.
REFERENCES
- Altman JD, Moss PAH, Goulder PJR, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl K, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Selin LK, Welsh RM. IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections. J. Immunol. 2006;176:4284–4295. doi: 10.4049/jimmunol.176.7.4284. [DOI] [PubMed] [Google Scholar]
- Barnes E, Ward SM, Kasprowicz VO, Dusheiko G, Klenerman P, Lucas M. Ultra-sensitive class I tetramer analysis reveals previously undetectable populations of antiviral CD8+ T cells. Eur. J. Immunol. 2004;34:1570–1577. doi: 10.1002/eji.200424898. [DOI] [PubMed] [Google Scholar]
- Berg LJ, Fazekas de St GB, Ivars F, Goodnow CC, Gilfillan S, Garchon HJ, Davis MM. Expression of T-cell receptor alpha-chain genes in transgenic mice. Mol. Cell. Biol. 1988;8:5459–5469. doi: 10.1128/mcb.8.12.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J. Exp. Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: A large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–840. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- Bousso P, Casrouge A, Altman JD, Haury M, Kanellopoulos J, Abastado JP, Kourilsky P. Individual variations in the murine T cell response to a specific peptide reflect variability in naive repertoires. Immunity. 1998;9:169–178. doi: 10.1016/s1074-7613(00)80599-3. [DOI] [PubMed] [Google Scholar]
- Busch DH, Pilip IM, Vijh S, Pamer EG. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 1998;8:353–362. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casrouge A, Beaudoing E, Dalle S, Pannetier C, Kanellopoulos J, Kourilsky P. Size estimate of the alpha beta TCR repertoire of naive mouse splenocytes. J. Immunol. 2000;164:5782–5787. doi: 10.4049/jimmunol.164.11.5782. [DOI] [PubMed] [Google Scholar]
- Cebrian M, Miguel RJ, Lopez-Rivas A, Rodriguez-Tarduchy G, De Landazuri MO, Sanchez-Madrid F. Expression and function of AIM, an activation inducer molecule of human lymphocytes, is dependent on the activation of protein kinase C. Eur. J. Immunol. 1989;19:809–815. doi: 10.1002/eji.1830190505. [DOI] [PubMed] [Google Scholar]
- Cose S, Brammer C, Khanna KM, Masopust D, Lefrancois L. Evidence that a significant number of naive T cells enter non-lymphoid organs as part of a normal migratory pathway. Eur. J. Immunol. 2006;36:1423–1433. doi: 10.1002/eji.200535539. [DOI] [PubMed] [Google Scholar]
- Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Dustin ML. Stop and go traffic to tune T cell responses. Immunity. 2004;21:305–314. doi: 10.1016/j.immuni.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, Woodland DL. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol. 2001;166:1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonistic peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Ignatowicz L, Kappler J, Marrack P. The repertoire of T cells shaped by a single MHC/peptide ligand. Cell. 1996;84:521–529. doi: 10.1016/s0092-8674(00)81028-4. [DOI] [PubMed] [Google Scholar]
- Jabbari A, Legge KL, Harty JT. T cell conditioning explains early disappearance of the memory CD8 T cell response to infection. J. Immunol. 2006;177:3012–3018. doi: 10.4049/jimmunol.177.5.3012. [DOI] [PubMed] [Google Scholar]
- Jung TM, Dailey MO. Reversibility of loss of homing receptor expression following activation. Adv. Exp. Med. Biol. 1988;237:519–524. doi: 10.1007/978-1-4684-5535-9_79. [DOI] [PubMed] [Google Scholar]
- Jung TM, Gallatin WM, Weissman IL, Dailey MO. Down-regulation of homing receptors after T cell activation. J. Immunol. 1988;141:4110–4117. [PubMed] [Google Scholar]
- Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- Kalergis AM, Ono T, Wang F, DiLorenzo TP, Honda S, Nathenson SG. Single amino acid replacements in an antigenic peptide are sufficient to alter the TCR V beta repertoire of the responding CD8+ cytotoxic lymphocyte population. J. Immunol. 1999;162:7263–7270. [PubMed] [Google Scholar]
- Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Kedzierska K, Day EB, Pi J, Heard SB, Doherty PC, Turner SJ, Perlman S. Quantification of repertoire diversity of influenza-specific epitopes with predominant public or private TCR usage. J. Immunol. 2006;177:6705–6712. doi: 10.4049/jimmunol.177.10.6705. [DOI] [PubMed] [Google Scholar]
- Khanna KM, McNamara JT, Lefrancois L. In situ imaging of the endogenous CD8 T cell response to infection. Science. 2007;318:116–120. doi: 10.1126/science.1146291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Reed DS, Olson S, Schnell MJ, Rose JK, Morton PA, Lefrançois L. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc. Natl. Acad. Sci. USA. 1998;95:10814–10819. doi: 10.1073/pnas.95.18.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Stillman EA, Whitt MA, Rose JK. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. USA. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancois L, Altman JD, Williams K, Olson S. Soluble antigen and CD40 triggering are sufficient to induce primary and memory cytotoxic T cells. J. Immunol. 2000;164:725–732. doi: 10.4049/jimmunol.164.2.725. [DOI] [PubMed] [Google Scholar]
- Lefrancois L. Development, trafficking, and function of memory T-cell subsets. Immunol. Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- Mamalaki C, Elliott J, Norton T, Yannoutsos N, Townsend AR, Chandler P, Simpson E, Kioussis D. Positive and negative selection in transgenic mice expressing a T-cell receptor specific for influenza nucleoprotein and endogenous superantigen. Dev. Immunol. 1993;3:159–174. doi: 10.1155/1993/98015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat. Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo AL, Yagita H, Lefrancois L. Cutting edge: Migration to nonlymphoid tissues results in functional conversion of central to effector memory CD8 T cells. J. Immunol. 2007;179:36–40. doi: 10.4049/jimmunol.179.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Jiang J, Shen H, Lefrançois L. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J. Immunol. 2001a;166:2348–2356. doi: 10.4049/jimmunol.166.4.2348. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001b;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Maxwell JR, Rossi RJ, McSorley SJ, Vella AT. T cell clonal conditioning: A phase occurring early after antigen presentation but before clonal expansion is impacted by Toll-like receptor stimulation. J. Immunol. 2004;172:248–259. doi: 10.4049/jimmunol.172.1.248. [DOI] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J. Immunol. 2006a;177:450–458. doi: 10.4049/jimmunol.177.1.450. [DOI] [PubMed] [Google Scholar]
- Munks MW, Gold MC, Zajac AL, Doom CM, Morello CS, Spector DH, Hill AB. Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J. Immunol. 2006b;176:3760–3766. doi: 10.4049/jimmunol.176.6.3760. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: A reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Obar JJ, Crist SG, Leung EK, Usherwood EJ. IL-15-independent proliferative renewal of memory CD8+ T cells in latent gammaherpesvirus infection. J. Immunol. 2004;173:2705–2714. doi: 10.4049/jimmunol.173.4.2705. [DOI] [PubMed] [Google Scholar]
- Pewe LL, Netland JM, Heard SB, Perlman S. Very diverse CD8 T cell clonotypic responses after virus infections. J. Immunol. 2004;172:3151–3156. doi: 10.4049/jimmunol.172.5.3151. [DOI] [PubMed] [Google Scholar]
- Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J. Exp. Med. 2005;202:123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothoeft T, Balkow S, Krummen M, Beissert S, Varga G, Loser K, Oberbanscheidt P, van den Boom F, Grabbe S. Structure and duration of contact between dendritic cells and T cells are controlled by T cell activation state. Eur. J. Immunol. 2006;36:3105–3117. doi: 10.1002/eji.200636145. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Teichgraber V, Kalia V, Polley A, Masopust D, Harrington LE, Ahmed R, Wherry EJ. Strength of stimulus and clonal competition impact the rate of memory CD8 T cell differentiation. J. Immunol. 2007;179:6704–6714. doi: 10.4049/jimmunol.179.10.6704. [DOI] [PubMed] [Google Scholar]
- Scriba TJ, Purbhoo M, Day CL, Robinson N, Fidler S, Fox J, Weber JN, Klenerman P, Sewell AK, Phillips RE. Ultrasensitive detection and phenotyping of CD4+ T cells with optimized HLA class II tetramer staining. J. Immunol. 2005;175:6334–6343. doi: 10.4049/jimmunol.175.10.6334. [DOI] [PubMed] [Google Scholar]
- Smalley DM, Ley K. L-selectin: Mechanisms and physiological significance of ectodomain cleavage. J. Cell. Mol. Med. 2005;9:255–266. doi: 10.1111/j.1582-4934.2005.tb00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Mohrs M, Mallet-Designe V, Teyton L, Locksley RM. Rapid expansion and IL-4 expression by Leishmania-specific naive helper T cells in vivo. Immunity. 2002;17:191–200. doi: 10.1016/s1074-7613(02)00363-1. [DOI] [PubMed] [Google Scholar]
- Stock AT, Mueller SN, Kleinert LM, Heath WR, Carbone FR, Jones CM. Optimization of TCR transgenic T cells for in vivo tracking of immune responses. Immunol. Cell Biol. 2007;85:394–396. doi: 10.1038/sj.icb.7100076. [DOI] [PubMed] [Google Scholar]
- Tripp RA, Hou S, Doherty PC. Temporal loss of the activated L-selectin-low phenotype for virus-specific CD8+ memory T cells. J. Immunol. 1995;154:5870–5875. [PubMed] [Google Scholar]
- Van Bleek GM, Nathenson SG. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990;348:213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Vezys V, Olson S, Lefrançois L. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity. 2000;12:505–514. doi: 10.1016/s1074-7613(00)80202-2. [DOI] [PubMed] [Google Scholar]
- Welsh RM, McNally JM, Brehm MA, Selin LK. Consequences of cross-reactive and bystander CTL responses during viral infections. Virology. 2000;270:4–8. doi: 10.1006/viro.2000.0278. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- Zerrahn J, Held W, Raulet DH. The MHC reactivity of the T cell repertoire prior to positive and negative selection. Cell. 1997;88:627–636. doi: 10.1016/s0092-8674(00)81905-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.