Abstract
Procedural pain in the neonatal intensive care unit triggers a cascade of physiological, behavioral and hormonal disruptions which may contribute to altered neurodevelopment in infants born very preterm, who undergo prolonged hospitalization at a time of physiological immaturity and rapid brain development. The aim of this study was to examine relationships between cumulative procedural pain (number of skin-breaking procedures from birth to term, adjusted for early illness severity and overall intravenous morphine exposure), and later cognitive, motor abilities and behavior in very preterm infants at 8 and 18 months corrected chronological age (CCA), and further, to evaluate the extent to which parenting factors modulate these relationships over time. Participants were N = 211 infants (n = 137 born preterm ≤32 weeks gestational age [GA] and n = 74 full-term controls) followed prospectively since birth. Infants with significant neonatal brain injury (periventricular leucomalacia, grade 3 or 4 intraventricular hemorrhage) and/or major sensori-neural impairments, were excluded. Poorer cognition and motor function were associated with higher number of skin-breaking procedures, independent of early illness severity, overall intravenous morphine, and exposure to postnatal steroids. The number of skin-breaking procedures as a marker of neonatal pain was closely related to days on mechanical ventilation. In general, greater overall exposure to intravenous morphine was associated with poorer motor development at 8 months, but not at 18 months CCA, however, specific protocols for morphine administration were not evaluated. Lower parenting stress modulated effects of neonatal pain, only on cognitive outcome at 18 months.
Keywords: Pain, Premature infants, Neonatal, Stress, Neurodevelopment, Parent
1. Introduction
Early repeated procedural pain exposure in the neonatal intensive care unit (NICU) has been proposed as one of the factors that may contribute to altered development of cognition, motor function and behavior in infants and children born preterm [2,22,26], although this link has been largely speculative. Neurobiological vulnerability to pain in preterm infants is well established, due to their lower pain threshold, sensitization from repeated pain [16,17], and immature systems for maintaining homeostasis. The physiological perturbations associated with early prolonged exposure to episodic pain appear to contribute to altering the rapidly developing stress systems [24,28]. Nociceptive signals during neonatal blood collection reach the cortex [8,44], and in rat pups, neonatal inflammatory pain may affect the cytoarchitecture of the brain [3].
Due to plasticity of the immature nervous system, long-term effects of early exposure to negative environments may be at least partially ameliorated by positive child-rearing environment [13]. Moreover, effects of neonatal pain on stress systems appear to be prevented by increased maternal behaviors in rodents [45]. In human infants, caregiver interaction and family social context are important modulators of neurodevelopment in infants born preterm, with increasing importance of the socioeconomic and family environment over time [40].
The aims of the present study were to evaluate whether cumulative neonatal procedural pain in very preterm infants is associated with altered cognitive and/or motor neurodevelopment at age 8 and 18 months corrected chronological age (CCA; i.e. adjusted for prematurity), and whether environmental context of parenting stress and parent–infant interaction buffers effects of neonatal pain on neurodevelopment. As a comparison group for neurodevelopment and parent factors, we included a sample of infants born full-term.
Major neurodevelopmental impairments such as cerebral palsy appear to be influenced by other factors, such as intrauterine infection and severe neonatal brain injury [19,41]. Therefore to avoid confounders of effects of neonatal pain, we excluded infants who had major brain injury on neonatal ultrasound or major neurosensory impairments. Therefore this study addressed associations between neonatal procedural pain and neurodevelopmental outcomes in relatively intact infants born very preterm. To our knowledge, this is the first study to examine pain in relation to neurodevelopment in preterm infants past the neonatal period.
2. Methods
2.1. Participants
As part of a larger longitudinal project, N = 211 (137 preterm, 74 full-term) infants completed the Bayley Scales of Infant Development-II ([9] Bayley, 1993) at 8 and/or 18 months CCA, and a parent participated in mother–infant interaction play and completed a questionnaire on parenting stress. Infants with a major congenital anomaly, major neurosensory impairment (legally blind, cerebral palsy, sensori-neural hearing impairment), severe brain injury evident on neonatal ultrasound (periventricular leucomalacia or grade 3 or 4 intraventricular hemorrhage), or maternal report of illicit hard drugs during pregnancy, were excluded. The children seen at the 8 month visit only (116 preterm, 69 full-term) or at the 18 month visit only (102 preterm, 55 full-term) did not differ significantly in birth weight, gestational age, Bayley cognitive or motor scores from the children seen at both 8 and 18 months CCA (82 preterm, 50 full-term).
The preterm infants were born in February 2001–September 2004, and were recruited from the neonatal intensive care unit (NICU) at the Children’s and Women’s (C&W) Health Centre of British Columbia, which is the major tertiary neonatal unit for the province of British Columbia, Canada. Full-term infants were born in May 2001–July 2004 at the same Centre, and were contacted through their pediatricians. Developmental assessments were carried out at age 8 and 18 months CCA blinded to the infant’s pain history and family data. Infant neonatal characteristics and demographic factors with data at one or both ages are shown in Table 1. As expected, the preterm infants had lower gestational age (F[1,203] = 1086.95, p = .0001), and birth weight (F[1,203] = 1009.96, p = .0001). Mothers of the preterm infants had lower number of years of education (F[1,203] = 23.59, p = .0001).
Table 1.
Infant neonatal and demographic characteristics.
| Characteristics | Preterm n = 137 | Full-term n = 74 | p-Value |
|---|---|---|---|
| Gestational age at birth (weeks) mean (SD) | 29.1 (2.6) | 40.0 (1.1) | .0001 |
| Birth weight (grams) mean (SD) | 1263.1 (485.6) | 3534.8 (488.9) | .0001 |
| Illness severity day 1 (SNAP-II) mean (SD) | 13.1 (12.2) | n/a | n/a |
| Skin-breaking procedures (number)* mean (SD) | 121.1 (99.6) | n/a | n/a |
| Mechanical ventilation (days)* mean (SD) | 13.8 (21.4) | n/a | n/a |
| IV morphine exposure (daily average mg/kg × days)* mean (SD) | 2.7 (6.8) | n/a | n/a |
| Postnatal dexamethasone (days)* mean (SD) | 1.5 (6.0) | n/a | n/a |
| Birth weight small-for-gestational-age (%) | 13 | 3 | .06 |
| Sex (% male) | 48 | 48 | 1.00 |
| Number of children in the home mean (SD) | 1.9 (1.0) | 1.7 (0.7) | .18 |
| Mother’s years of education mean (SD) | 14.9 (2.8) | 16.9 (2.9) | .0001 |
| Mother completed high school or above (%) | 92 | 98 | .11 |
| Marital status (% married or common-law) | 77 | 85 | .31 |
| Ethnicity of mother (% Caucasian) | 72 | 78 | .21 |
Recorded daily from birth to term (40 weeks post-conceptional age).
2.2. Measures
2.2.1. Medical chart review
Medical and nursing chart review from birth to term (39 weeks 6 days) was carried out by one neonatal research nurse, including but not limited to birth weight, gestational age, illness severity (SNAP-II) on day 1, days of mechanical ventilation, daily dosage of intravenous (iv) morphine and other medications, and number of skin-breaking procedures (e.g. heel lance, intramuscular injection, chest tube insertion, central line insertion). Procedural pain exposure was operationalized as the sum of every skin-breaking procedure from birth to term, adjusted for early illness severity (SNAP-II on day 1) and iv morphine exposure. Each attempt at a procedure was included, thus the total sum reflected all skin breaks. While it is recognized that procedures differ in pain intensity, in the absence of an empirical basis for assigning weights to every procedure, we count every skin break as a “marker” of cumulative neonatal acute pain exposure in the NICU [e.g. 24,25,27,28]. Total morphine exposure was calculated from birth to term as the average daily dose of iv morphine adjusted for daily weight, multiplied by the number of days on morphine, as we have used previously. For example, if an infant received an average dose of 0.39 mg/kg body weight for 24 treatment days, the morphine score was 9.36 [mg/kg]. All nursing staff in our NICU have been trained to carry out very precise recordings of every skin-breaking procedure, including each attempt, therefore we have highly consistent chart information on every infant.
2.2.2. Neurodevelopment
The Bayley Scales of Infant Development 2nd Edition [9], the most widely used standardized tests of infant and toddler development, were administered at 8 and 18 months CCA. The Mental Development Index (MDI) measures cognitive and language function and includes eye-hand items such as stacking blocks, as well as concrete problem solving tasks, and receptive and expressive vocabulary items; the Psychomotor Development Index (PDI) primarily includes items measuring gross motor development. The MDI and PDI each has a mean of 100 and SD of 15.
2.2.3. Questionnaires
We measured parenting stress using the Parenting Stress Index [1], a 120 item questionnaire, with each item on a 6-point Likert scale from 1 (strongly agree) to 6 (strongly disagree); Cronbach’s alpha was .91 for the Total Stress score. Demographic Information: was obtained by questionnaire. Since maternal education is the single most important socioeconomic status (SES) indicator related to child development [e.g. 12,40], we used mother’s years of education as the SES index in statistical modeling of parent factors.
2.2.4. Parent–child interaction
Interactive parent behaviors during developmentally appropriate semi-structured teaching play at 8 and 18 months were measured using a method validated on preterm and full-term infants and toddlers [15,23]. The parent was asked to play as they would at home. At 8 months a specific set of toys was provided to the parent, ranging from low stimulation (e.g. stuffed toys and books), to high stimulation (with noises and motion that could be activated by the parent). At 18 months CCA, the teaching tasks involved an easier familiar task (stacking or nesting colored cups varying in size), and a novel difficult task (sorting plastic pigs and cows into containers). The parent was rated on each of four measures on a scale from 1 (low) to 5 (high): Gratification, Affect, Sensitivity, and Organization. Coding was carried out from videotapes by two trained experienced blinded raters, a primary coder and a reliability coder. Inter-rater reliability was carried out on 25% of the infants in this study. Weighted kappa using agreement within one scale point was 0.95, 1.0, 0.79 and 0.94 for Gratification, Affect, Sensitivity, and Organization, respectively.
2.3. Procedures
The study was approved by the Clinical Research Ethics Board, University of British Columbia and the Research Review Board of the Children’s and Women’s Health Centre of BC. A parent gave written consent. Infants were tested with the parent who was the primary caregiver, who also completed the questionnaires; all were mothers except one father at 8 months, and five fathers at 18 months.
2.4. Data analysis
Repeated measures ANOVA was used to examine group and age differences. Pearson correlations were used to examine associations among measures. Hierarchical regression analyses were used to examine relationships of neonatal factors to each outcome measure at 8 and 18 months separately. Statistical significance was defined as p < .01 for correlations, and p < .05 for ANOVA and hierarchical regression analyses.
Sample size for the preterm group was based on the standard of 10 subjects per predictor variable for regression analysis [21]. Since full-term control infants were not in the NICU, only the parent variables were used as predictors, and lower sample size was recruited accordingly.
3. Results
3.1. Comparisons of preterm and full-term infants: neurodevelopment, parenting stress, parent–child interaction
Group by Sex Multivariate Analysis of Variance (MANOVA) carried out on the set of outcomes and parenting measures at each age, showed that preterm infants had significantly lower MDI and PDI overall than full-term infants at 18 months only (F[1,152] = 5.17, p = .02), and (F[1,151] = 4.71, p = .03), respectively, as shown in Table 2. The Parenting Stress Index (PSI) total score was significantly higher for the Preterm compared to the Full-term group at both 8 months (F[1,180] = 5.40, p = .004) and 18 months (F[1,151] = 5.08, p = .03) CCA. Parent interaction differed significantly between the groups only for Sensitivity at 8 months (F[1,180] = 3.52, p = .04). There were no statistically significant Sex differences in MDI, PDI, PSI or Parent–Child Interaction at either age (every p > .27).
Table 2.
Developmental (MDI aand PDIb) and parenting characteristics at 8 and 18 months CCAc in preterm compared to full-term infants at 8 and 18 months (mean, SD).
| 8 months CCA | Preterm n = 116 | Full-term n = 69 | p-Value |
|---|---|---|---|
| Bayley Mental Index (MDI) | 94.3 (10.8) | 96.2 (7.7) | .23 |
| Bayley Psychomotor Index (PDI) | 85.0 (16.3) | 88.7 (8.7) | .074 |
| Parenting Stress Index (Total Score) | 210.8 (36.6) | 195.0 (35.4) | .004 |
| Parent–child interaction AFFECT | 4.1 (.7) | 4.0 (.8) | .22 |
| Parent–child interaction GRATIFICATION | 4.0 (1.0) | 3.7 (1.0) | .06 |
| Parent–child interaction SENSITIVITY | 4.2 (.8) | 4.0 (.8) | .04 |
| Parent–child interaction ORGANIZATION | 4.1 (.9) | 4.1 (.8) | .89 |
| 18 months CCA | n = 102 | n = 55 | |
| Bayley Mental Index (MDI) | 88.1 (17.7) | 95.4 (14.0) | .02 |
| Bayley Psychomotor Index (PDI) | 89.4 (13.6) | 94.3 (11.9) | .03 |
| Parenting Stress Index (Total Score) | 210.2 (41.0) | 195.4 (35.4) | .03 |
| Parent–child interaction AFFECT | 3.4 (.5) | 3.3 (.5) | .21 |
| Parent–child interaction GRATIFICATION | 3.1 (.8) | 2.9 (.7) | .17 |
| Parent–child interaction SENSITIVITY | 4.0 (.7) | 4.0 (.7) | .38 |
| Parent–child interaction ORGANIZATION | 3.9 (.6) | 4.0 (.7) | .97 |
3.2. Neonatal factors
Relationships among neonatal characteristics of the preterm infants were examined (see Table 3). The number of neonatal skin-breaking procedures was significantly correlated with illness severity (SNAP-II) on day 1 (r = .52), iv morphine exposure since birth (r = .64), gestational age at birth (r = −.78), and days of mechanical ventilation (r = .90).
Table 3.
Pearson correlations among neonatal characteristics of the preterm infants (n = 137).
| Birth weight | Illness severity day 1 | Mechanical ventilation (days) | Postnatal dexamethasone (days) | Pain (number of skin-breaking procedures) | IV morphine exposure | |
|---|---|---|---|---|---|---|
| Gestational age at birth | .81** | −.56** | −.73** | −.33** | −.78** | −.41** |
| Birth weight | −.49** | −.64** | −.26* | −.70** | −.39** | |
| Illness severity day 1 (SNAP-II) | .50** | .12 | .52** | .40** | ||
| Mechanical ventilation (days) | .64** | .90** | .78** | |||
| Postnatal dexamethasone (days) | .49** | .62** | ||||
| Pain (number of skin-breaking procedures) | .64** |
p = 0.01.
p = 0.0001.
Correlations between the neonatal factors and outcome measures in the preterm infants are shown in Table 4. At 8 months, lower MDI and PDI were significantly associated with higher number of skin-breaking procedures (r = −.41; r = −.44), higher number of days on mechanical ventilation (r = −.33; r = −.43), lower gestational age at birth (r = .21; r = .26). Higher morphine exposure at 8 months was significantly correlated with lower PDI (r = −.43) but not with MDI. At 18 months, lower MDI and PDI were significantly associated with higher number of skin-breaking procedures (r = −.37; r = −.43), higher number of days on mechanical ventilation (r = −.36; r = −.46), lower gestational age (r = .30; r = .37), and more morphine exposure (r = −.24; r = −.33).
Table 4.
Pearson correlations between neonatal factors (birth to term equivalent) and cognitive (MDIa) and motor (PDIb) outcomes at 8 and 18 months CCAc in preterm infants.
| 8 months CCA |
18 months CCA |
|||
|---|---|---|---|---|
| MDI n = 116 | PDI n = 115 | MDI n = 102 | PDI n = 101 | |
| Gestational age at birth | .21* | .26* | .30** | .37*** |
| Birth weight | .13 | .20* | .36*** | .39*** |
| Pain (number of skin-breaking procedures) | −.41*** | −.44*** | −.37*** | −.43*** |
| Morphine exposure | −.19 | −.43*** | −.24* | −.33*** |
| Mechanical ventilation (days) | −.33*** | −.43*** | −.36*** | −.46*** |
| Illness severity initial (SNAP-II day 1) | −.06 | −.11 | −.25* | −.20 |
| Dexamethasone exposure (days) | −.07 | −.22* | −.24* | −.35*** |
3.3. Parent factors
Correlations between the parent factors and outcome measures in the preterm infants are presented in Table 5. At 8 months CCA, none of the parent factors was significantly correlated with cognitive (MDI) or motor (PDI) development. At 18 months CCA, higher Parent Organization predicted better MDI (r = .24) and PDI (r = .25), and higher maternal education predicted better MDI (r = .29).
Table 5.
Pearson correlations between parent factors and Bayley-II cognitive (MDIa) and motor (PDIb) outcomes at 8 and 18 months CCAc in preterm infants.
| 8 months CCA |
18 months CCA |
|||
|---|---|---|---|---|
| MDI an = 116 | PDIbn = 115 | MDIan = 102 | PDIbn = 101 | |
| Parent interaction: AFFECT | .09 | .01 | .06 | .07 |
| Parent interaction: GRATIFICATION | −.06 | −.11 | .15 | .07 |
| Parent interaction: SENSITIVITY | −.16 | −.21 | .22 | .11 |
| Parent interaction: ORGANIZATION | .05 | .01 | .24* | .25* |
| Parenting Stress Index (Total) | .02 | .03 | −.21 | .06 |
| Mother’s years of education | .12 | .14 | .29* | .15 |
| Children in the home (number) | .03 | .19 | −.04 | −.02 |
3.4. Neonatal and parent predictors for preterm children at 8 and 18 months CCA
To examine the independent contributions of neonatal factors, and the extent that parent factors predict outcome above and beyond neonatal factors, hierarchical regression analysis was carried out in blocks. Prior to hierarchical regression, principal components analysis (PCA) was carried out on the parent-interaction variables at each age, to reduce the number of measures. At 8 months CCA, PCA produced one eigenvalue above 1.0, generating a single parent-interaction behavior measure at that age (labeled PARENT 8 months). At 18 months CCA, two eigenvalues above 1.0 were produced, generating two parent-interaction behavior measures, with AFFECT, GRATIFICATION, and SENSITIVITY predominantly loading on the first factor (labeled PARENT AFFECT 18 months), and ORGANIZATION primarily loading on the second factor (labeled PARENT ORGANIZATION 18 months). Neonatal variables were entered in Block 1: number of skin-breaking procedures, SNAP-II scores at day 1 (to control for early illness severity), number of days on postnatal corticosteroids (dexamethasone), iv morphine exposure from birth to term. Parent factors were entered in Block 2: parent–infant interaction (the factor(s) relevant at each age), parenting stress, number of years of education, and number of children in the home.
Since exposure to skin-breaking procedures was highly correlated with GA and duration of mechanical ventilation (see Table 3), we could not include all these measures in the same regression analysis (due to multicollinearity). Therefore to evaluate whether GA or ventilation rather than procedural pain was the operative factor, we repeated the regression analyses for the neonatal (but not the parent predictors) using GA, and then using days of mechanical ventilation, instead of number of skin-breaking procedures. The conventional cutoff for excluding predictors due to multicollinearlity is r > .80 [21]. To be conservative, we reviewed any correlation r > .70, thus we did not include GA with either mechanical ventilation (r = −.73) or with number of skin breaks (r = −.78) in any regression analysis. However, to maintain the same predictors for each of the regressions, we included iv morphine exposure with mechanical ventilation, despite r = .78 (which is below the standard r = .80 cutoff for multicollinearlity).
3.4.1. Cognitive development (Bayley-ll MDI)
3.4.1.1. Number of skin-breaking procedures from birth to term
Results of the hierarchical regression analyses predicting MDI at 8 and 18 months are presented in Table 6. At 8 months (after controlling for early illness severity, iv morphine exposure and days on dexamethasone), higher number of neonatal skin-breaking procedures (β = −.65, p = .0001) predicted lower MDI (Fig. 1). None of the parent variables at 8 months was significant, independent of the neonatal factors. The overall model accounted for 24% of the variance. The neonatal variables accounted for 23% of the variance (p = .0001), and the parent factors for an additional 1% (p = .66), thus the parent factors did not modulate effects of neonatal pain at 8 months.
Table 6.
Results of hierarchical regression analyses predicting cognitive development (Bayley-ll Mental Development Index [MDIa]) at 8 and 18 months CCA for infants born preterm.
| Step | Variables entered | MDI at 8 months CCA n = 116 |
MDI at 18 months CCA n = 102 |
||||
|---|---|---|---|---|---|---|---|
| β | p | Model R2 | β | p | Model R2 | ||
| 1 | Number of skin-breaking procedures | −.65 | .0001 | −.33 | .016 | ||
| Early illness severity (SNAP-II day 1) | .23 | .023 | −.06 | .60 | |||
| iv morphine exposure | .07 | .63 | .07 | .62 | |||
| Postnatal dexamethasone (days) | .17 | .18 | −.12 | .34 | |||
| 23% | 15% | ||||||
| 2 | Number of skin-breaking procedures | −.66 | .0001 | −.23 | .09 | ||
| Early illness severity (SNAP-II day 1) | .25 | .016 | −.08 | .47 | |||
| iv morphine exposure | .04 | .78 | .11 | .46 | |||
| Postnatal dexamethasone (days) | .17 | .17 | −.20 | .11 | |||
| Parenting Stress (PSI Total score) | .08 | .34 | −.19 | .047 | |||
| Maternal education (years) | .10 | .27 | .18 | .06 | |||
| Number of children in the home | .13 | .15 | −.07 | .49 | |||
| Parent behavior during interaction: | |||||||
| PARENT 8 monthsb | .01 | .91 | |||||
| PARENT AFFECT 18 monthsc | .13 | .19 | |||||
| PARENT ORGANIZATION 18 monthsc | .12 | .20 | |||||
| 24% | 27% | ||||||
Mental Development Index (MDI), Bayley Scales of Infant Development [9].
PARENT 8 months was derived from principal components analysis of the parent–interaction behaviors (GRATIFICATION, AFFECT, SENSITIVITY, ORGANIZATION) at 8 months CCA.
PARENT AFFECT 18 months and PARENT ORGANIZATION 18 months were derived from principal components analysis of the parent–interaction behaviors (GRATIFICATION, AFFECT, SENSITIVITY, ORGANIZATION) at 18 months CCA.
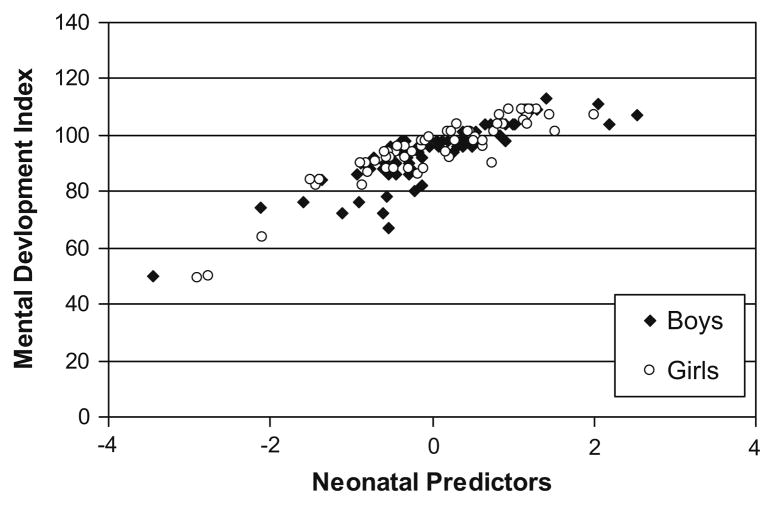
Fig. 1.
Residual regression scores of neonatal predictors (cumulative pain from birth to term, adjusted for early illness severity, intravenous morphine exposure and days on postnatal dexamethasone) in relation to MDI at 8 months CCA.
At 18 months, after controlling for early illness severity, iv morphine exposure and days on dexamethasone, higher number of neonatal skin-breaking procedures (β = −.33, p = .016), predicted lower MDI (Fig. 2). When parent variables were entered, independent of the neonatal factors, lower parenting stress (β = −.19, p = .047) was associated with higher MDI, and higher maternal years of education showed a trend (β = .18, p = .06). The overall model accounted for 27% of the variance. The neonatal variables accounted for 15% of the variance (p = .003), and the parent factors for an additional 12% (p = .02).
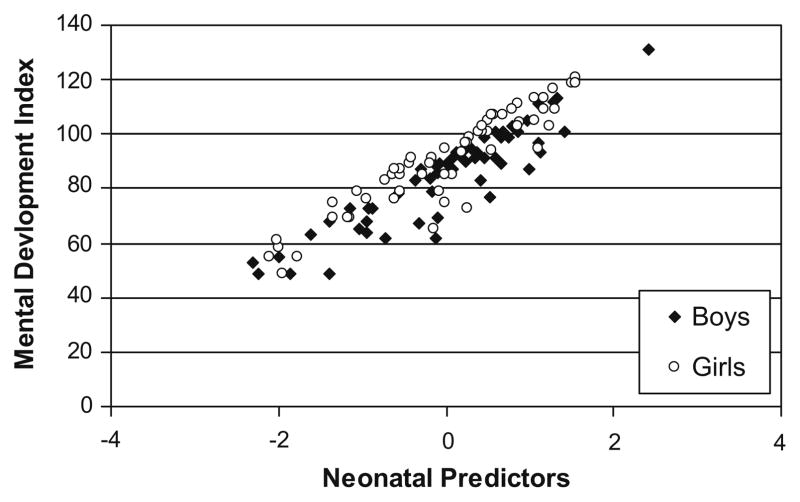
Fig. 2.
Residual regression scores of neonatal predictors (cumulative pain from birth to term, adjusted for early illness severity, intravenous morphine exposure and days on postnatal dexamethasone) in relation to MDI at 18 months CCA.
3.4.1.2. Gestational age at birth
After controlling for early illness severity, cumulative iv morphine and days on dexamethasone, GA was not statistically significantly related to MDI at 8 months (β = .21, p = .08), or 18 months (β = .19, p = .13).
3.4.1.3. Days on mechanical ventilation
Controlling for initial illness severity, days on dexamethasone from birth to term, and iv morphine exposure, higher number of days on mechanical ventilation predicted lower MDI at 8 (β = −.65, p = .0001) and 18 months (β = −.39, p = .029). Together these neonatal variables accounted for 16% of the variance at 8 months and 14% at 18 months.
3.4.2. Motor development (Bayley-II PDI)
3.4.2.1. Number of skin-breaking procedures from birth to term
Results of the hierarchical regression analyses predicting PDI at 8 and 18 months are presented in Table 7. At 8 months, controlling for early illness severity and days on dexamethasone, higher number of neonatal skin-breaking procedures (β = −.36, p = .005) and greater iv morphine exposure (β = −.37, p = .01) independently predicted lower PDI motor scores (Fig. 3). Independent of the neonatal factors, more children in the home (β = .23, p = .009) predicted better PDI scores, but no other parent factor was statistically significant. The overall model accounted for 30% of the variance. The neonatal variables accounted for 25% of the variance (p = .0001), and the parent factors for an additional 5% (p = .11).
Table 7.
Results of hierarchical regression analyses predicting motor development (Bayley-ll Psychomotor Index [PDIa]) at 8 and 18 months CCA for infants born preterm.
| Step | Variables entered | PDI at 8 months CCA n = 115a |
PDI at 18 months CCA n = 101a |
||||
|---|---|---|---|---|---|---|---|
| β | p | Model R2 | β | p | Model R2 | ||
| 1 | Number of skin-breaking procedures | −.36 | .005 | −.34 | .012 | ||
| Early illness severity (SNAP-II day 1) | .17 | .08 | −.02 | .87 | |||
| iv morphine exposure | −.37 | .01 | .10 | .49 | |||
| Postnatal dexamethasone (days) | .20 | .10 | −.27 | .032 | |||
| 25% | 20% | ||||||
| 2 | Number of skin-breaking procedures | −.38 | .003 | −.26 | .068 | ||
| Early illness severity (SNAP-II day 1) | .21 | .036 | −.01 | .91 | |||
| iv morphine exposure | −.34 | .016 | .14 | .35 | |||
| Postnatal dexamethasone (days) | .21 | .08 | −.32 | .016 | |||
| Parenting Stress (PSI Total score) | .07 | .81 | .04 | .67 | |||
| Maternal education (years) | .08 | .85 | .09 | .36 | |||
| Number of children in the home | .23 | .009 | −.01 | .96 | |||
| Parent behavior during interaction: | |||||||
| PARENT 8 monthsb | |||||||
| PARENT AFFECT 18 monthsc | −.04 | .60 | |||||
| PARENT ORGANIZATION 18 monthsc | .09 | .37 | |||||
| .15 | .14 | ||||||
| 30% | 23% | ||||||
One preterm child was missing the motor Bayley-ll (PDI) at 8 months, and another at 18 months. The PDIa was the last test administered, and due to fatigue they did not complete all the items.
Psychomotor Development Index (PDI), Bayley Scales of Infant Development [9].
PARENT 8 months was derived from principal components analysis of the parent-interaction behaviors (GRATIFICATION, AFFECT, SENSITIVITY, ORGANIZATION) at 8 months CCA.
PARENT AFFECT 18 months and PARENT ORGANIZATION 18 months were derived from principal components analysis of the parent-interaction behaviors (GRATIFICATION, AFFECT, SENSITIVITY, ORGANIZATION) at 18 months CCA.
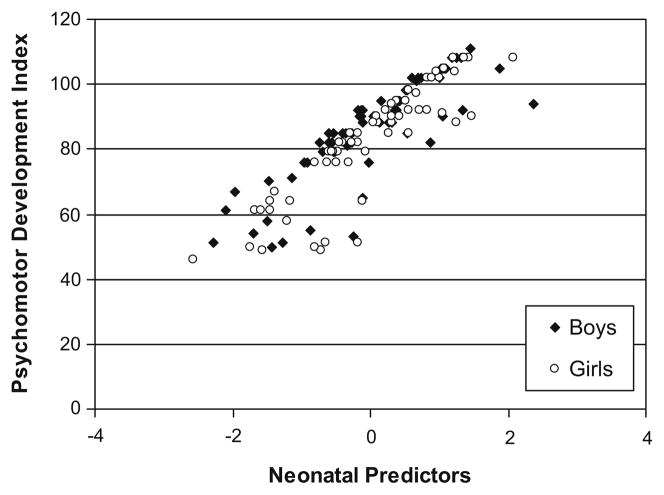
Fig. 3.
Residual regression scores of neonatal predictors (cumulative pain from birth to term, adjusted for early illness severity, intravenous morphine exposure and days on postnatal dexamethasone) in relation to PDI at 8 months CCA.
At 18 months, independent of early illness severity, higher number of neonatal skin-breaking procedures (β = −.34, p = .012), higher days on dexamethasone (β = −.27, p = .032), but not iv morphine exposure (β = .10, p = .49) predicted lower PDI motor scores (Fig. 4). Independent of neonatal variables, no parent factors predicted PDI scores. The overall model accounted for 23% of the variance. The neonatal variables accounted for 20% of the variance (p = .0001), and the parent factors for an additional 3% (p = .58), thus parent factors did not modulate effects of neonatal pain on motor development at 18 months.
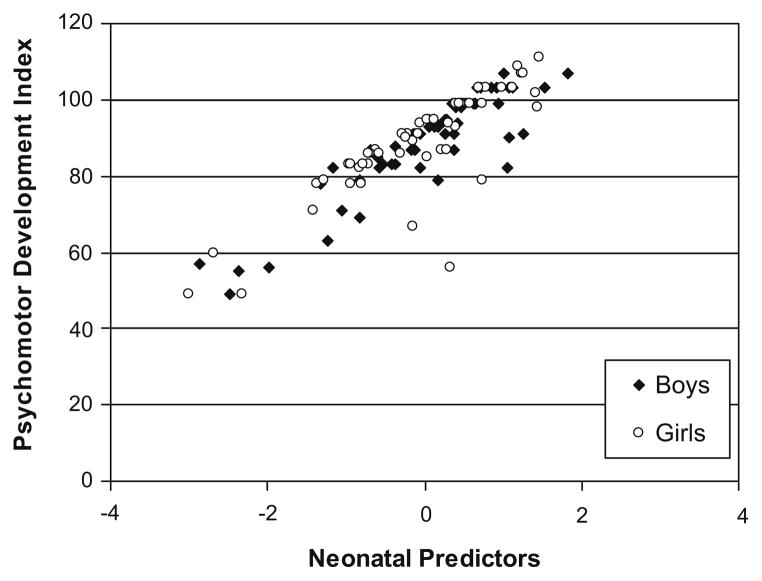
Fig. 4.
Residual regression scores of neonatal predictors (cumulative pain from birth to term, adjusted for early illness severity, intravenous morphine exposure and days on postnatal dexamethasone) in relation to PDI at 18 months CCA.
3.4.2.2. Gestational age at birth
After controlling for early illness severity, iv morphine and days on dexamethasone, GA was not significantly related to PDI at 8 months (β = .11, p = .32), or 18 months (β = .21, p = .08).
3.4.2.3. Days on mechanical ventilation
After controlling for initial illness severity, days on dexamethasone from birth to term, and iv morphine exposure, higher days on mechanical ventilation predicted lower PDI scores at 8 (β = −.38, p = .014) and 18 (β = −.46, p = .008) months. Together these neonatal variables accounted for 24% and 20% of the variance at 8 and 18 months, respectively.
4. Discussion
While compelling arguments have been advanced to link neonatal pain to neurodevelopment [2,22], to our knowledge this is the first study to empirically address this question in preterm infants, past NICU discharge. We confirmed our hypothesis that (after controlling for early illness severity, overall intravenous morphine exposure, and days on postnatal dexamethasone), higher number of skin-breaking procedures from birth to term predicted lower cognitive and motor development at 8 and 18 months CCA. While cumulative neonatal pain exposure was associated with cognitive and motor outcomes, we considered whether this relationship may be driven by other neonatal factors. Importantly, number of skin-breaking procedures was highly correlated with duration of mechanical ventilation. Both these variables predicted neurodevelopment to the same extent. In contrast, although lower GA was correlated with poorer cognitive and motor outcomes, after controlling for early illness severity, days on iv morphine and days on postnatal dexamethasone, GA at birth was not significantly associated with cognitive or motor outcome at 8 or 18 months. Major impairment is known to be associated with lower GA [e.g. 5,34], and most studies examining effects of neonatal factors include infants who had severe brain injury [e.g. 5,11,34], whereas we excluded infants with grade 3 or 4 intraventricular hemorrhage or any periventricular leucomalacia, in order to reduce confounding factors that impact outcomes. Extent of exposure to neonatal pain and the number of days on mechanical ventilation, therefore, may be more specific indicators of later development than factors considered traditionally in the neonatal medical course such as birth weight and gestational age, when infants with major risk factors or severe neurosensory impairment are excluded.
We did not have a specific morphine protocol in this study. Morphine was administered according to clinical judgement, therefore we were unable to directly evaluate effects of morphine on later cognitive and motor development. In this exploratory study, we controlled for iv morphine exposure statistically, in order to elucidate relationships between number of skin-breaking procedures and neurodevelopmental outcomes. Recognizing the limitations of this study, however, we did find that poorer motor development at 8, but not at 18, months was associated with greater overall exposure to iv morphine (independent of the number of skin-breaking procedures), consistent with recent findings that higher morphine exposure [39] and repeated sucrose [32] were linked to poorer motor outcome at term. Our finding that morphine exposure was not related to cognitive development, is consistent with results from a major controlled trial at age 5 years [42]. While efficacy of morphine remains unclear [10,14,43] reanalysis of the original NEOPAIN trial [4] indicated that cautious use of morphine may be safe for micropreemies [30]. Our exploratory results in the present study suggest that further research is needed on long-term effects of morphine on the full spectrum of motor development, including subtle motor dysfunction.
In general, preterm boys show more adverse neurodevelopmental outcomes than girls [48]. Surprisingly, we found no sex differences in cognitive or motor development, parenting stress or parent–child interaction at either 8 or 18 months CCA in the present study, which may be due to our excluding infants with major brain injury or neurosensory impairments. One important issue that we were not able to consider in the present study, and has not been addressed to our knowledge in any previous studies of human preterm infants, is the possibility that long-term effects of neonatal pain (and neonatal morphine) may differ for male and female infants. In rats, learning deficits following prenatal stress have been found to be more evident in male than female rats [46], and sex differences in effects of neonatal pain [33,37] and in opioid pharmacology [47], have been reported. Sex differences in long-term effects should be examined in future studies of large cohorts of human preterm infants.
Exposure to exogenous glucocorticoids can impair brain development [7,36], therefore we controlled for this variable in all predictive analyses. Higher number of days on postnatal dexamethasone was related to poorer motor function at 8 and 18 months, and lower cognitive ability at 18 months. However, after adjusting for other neonatal variables, greater postnatal steroid exposure predicted poorer motor but not cognitive performance, at 18 months only.
To our knowledge, this is the first study to address parent factors as modulators of neonatal pain exposure. Consistent with other studies showing the increasing importance of the environment for cognitive development, we found (after controlling for neonatal factors), that lower parenting stress buffered effects of cumulative neonatal pain on infant cognition, but only at 18 months CCA [35]. While greater organization in parent interaction was significantly correlated with better motor and cognitive development at 18 months, consistent with previous work [18], after controlling for neonatal factors, parent interaction was not significant. Maternal education is known to promote development of “at risk” infants [40], and similarly we found that mother education was positively correlated with cognition at 18 months, but only marginally after adjusting for neonatal factors. Importantly, more children in the home buffered motor development at 8 (but not at 18) months, but was not related to motor function in full-term infants. Preterm infants with older siblings have more experienced parents, who may provide more opportunities for motor exploration than first-borns, however, by 18 months no parent factors were linked to motor outcome (after controlling for neonatal factors).
Surprisingly, for the full-term infants, we found none of the parent factors in this study were related to motor or cognitive development. Both the preterm and full-term groups had relatively high maternal education (mean years of education 14.9 and 16.9, respectively), and full access to prenatal and postnatal care. Our findings confirm previous observations that preterm infants may be more vulnerable to differences in parent behavior than full-term infants [18,35], possibly because of poorer ability to self-regulate physiological and behavioral stress systems [23,24,28,29]. Studies emphasizing the importance of SES on development generally comprise a wide range of SES, including socially disadvantaged families. The relatively high SES of the participants in our study is an advantage in addressing long-term effects of pain on neurodevelopment, without the confounding detrimental effects of social deprivation.
We have operationalized cumulative neonatal pain-related stress exposure as number of skin-breaking procedures from birth to term, controlling for early illness severity and cumulative intravenous morphine exposure (daily dosage adjusted for weight). Due to sensitization to tactile stimulation, activation of nociceptors in infants born very preterm cannot be functionally isolated from “wind-up” effects whereby even intrinsically non-invasive procedures such as diaper change can trigger as much behavioral and cardiac reactivity as blood collection under certain conditions; specifically, even routine non-invasive clustered nursing caregiving is more stressful for ELGA infants than for preterm infants born more physiologically mature [31]. Therefore we consider our index to reflect cumulative pain-related stress, rather than pain per se. Other studies that measure infant pain in the NICU have included a range of procedures such as suctioning [14]. Our method [24,25,27,28] has been to only include skin-breaking procedures, in order to operationalize exposure to procedural pain without using clinical judgment to decide which other procedures and/or handling might induce pain. Our system likely underestimates cumulative pain exposure, and is therefore conservative.
Limitations
While cumulative pain-related stress in the NICU may contribute to adverse neurodevelopment, there are multiple factors that impact developmental trajectories. For example, preterm infants may have been exposed to maternal stress and/or infection in utero as potential precipitating causes of preterm delivery [20]; these factors may affect both brain development and the stress axis prior to delivery. Multiple interacting pre and postnatal factors likely impact neurodevelopment in the vulnerable population of infants born ≤32 weeks GA [6,20,30,38]. As a first study (to our knowledge) to evaluate the relationship of neonatal procedural pain to neurodevelopment beyond NICU discharge, it was not designed to address the effects of specific morphine protocols, thus each individual skin-breaking event was not specifically linked to the reason that infant was or was not receiving morphine. In our NICU during this study, each infant received morphine for clinical indications at the discretion of the medical staff, for a wide variety of reasons and in varying regimens.
In conclusion, our findings suggest that early repetitive procedural pain-related stress in very preterm neonates is associated with poorer neurodevelopment in the first 2 years of life. However, it was not possible to disentangle duration of mechanical ventilation from prolonged repetitive pain exposure in the NICU. Whether neonatal morphine negatively impacts early motor development could not be concluded, from this study. However, further work is needed to address this important question.
Acknowledgments
We thank the families who participated in this study and the staff of the Early Experience Unit, Centre for Community Child Health Research, Child and Family Research Institute, and the Neonatal Follow-up Programme at the Children’s and Women’s Health Centre of BC. This study was supported by grants to REG from the National Institute for Child Health and Human Development (HD39783), Canadian Institutes for Health Research grant (MOP42469), Human Early Learning Partnership (HELP) and Michael Smith Foundation for Health Research. Dr. Grunau is supported by a Distinguished Scholar Award from the Child and Family Research Institute, and a Senior Scholar Award from HELP.
Footnotes
Conflict of interest
None of the authors had any financial or other relationship that might lead to a conflict of interest.
References
- 1.Abidin RR. Parenting stress index. 3. Odessa (FL): Psychological Assessment Resources, Inc; 1995. [Google Scholar]
- 2.Anand KJ. Effects of perinatal pain and stress. Prog Brain Res. 2000;122:117–29. doi: 10.1016/s0079-6123(08)62134-2. [DOI] [PubMed] [Google Scholar]
- 3.Anand KJS, Garg S, Rovnaghi CR, Narsinghani U, Bhutta AT, Hall RW. Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr Res. 2007;62:283–90. doi: 10.1203/PDR.0b013e3180986d2f. [DOI] [PubMed] [Google Scholar]
- 4.Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, Boyle EM, Carbajal R, Bhutani VK, Moore MB, Kronsberg SS, Barton BA NEOPAIN Trial Investigators Group. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363:1673–82. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- 5.Anderson PJ, Doyle LW. Cognitive and educational deficits in children born extremely preterm. Semin Perinatol. 2008;32:51–8. doi: 10.1053/j.semperi.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Aylward GP. Neurodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr. 2005;26:427–40. doi: 10.1097/00004703-200512000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Barrington KJ. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr. 2001;1:1. doi: 10.1186/1471-2431-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartocci M, Bergqvist LL, Lagercrantz H, Anand KJ. Pain activates cortical areas in the preterm newborn brain. Pain. 2006;122:109–17. doi: 10.1016/j.pain.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Bayley N. Manual for the Bayley Scales of Infant Development. San Antonio (TX): Psychological Corporation; 1993. [Google Scholar]
- 10.Bellù R, de Waal KA, Zanini R. Opioids for neonates receiving mechanical ventilation. Cochrane Database Syst Rev. 2008:CD004212. doi: 10.1002/14651858.CD004212.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhutta AT, Anand KJ. Vulnerability of the developing brain. Neuronal mechanisms Clin Perinatol. 2002;29:357–72. doi: 10.1016/s0095-5108(02)00011-8. [DOI] [PubMed] [Google Scholar]
- 12.Bohm B, Katz-Salamon M, Institute K, Smedler AC, Lagercrantz H, Forssberg H. Developmental risks and protective factors for influencing cognitive outcome at 5 1/2 years of age in very-low-birthweight children. Dev Med Child Neurol. 2002;44:508–16. doi: 10.1017/s001216220100247x. [DOI] [PubMed] [Google Scholar]
- 13.Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–74. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- 14.Carbajal R, Lenclen R, Jugie M, Paupe A, Barton BA, Anand KJ. Morphine does not provide adequate analgesia for acute procedural pain among preterm neonates. Pediatrics. 2005;115:1494–500. doi: 10.1542/peds.2004-1425. [DOI] [PubMed] [Google Scholar]
- 15.Crnic KA, Greenberg MT, Ragozin AS, Robinson NM, Basham RB. Effects of stress and social support on mothers and premature and full-term infants. Child Dev. 1983;54:209–17. [PubMed] [Google Scholar]
- 16.Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–20. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald M, Millard C, McIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anaesthesia. Pain. 1989;39:31–6. doi: 10.1016/0304-3959(89)90172-3. [DOI] [PubMed] [Google Scholar]
- 18.Forcada-Guex M, Pierrehumbert B, Borghini A, Moessinger A, Muller-Nix C. Early dyadic patterns of mother–infant interactions and outcomes of prematurity at 18 months. Pediatrics. 2006;118:e107–14. doi: 10.1542/peds.2005-1145. [DOI] [PubMed] [Google Scholar]
- 19.Gabriel R, Grolier F, Graesslin O. Can obstetric care provide further improvement in the outcome of preterm infants? Eur J Obstet Gynecol Reprod Biol. 2004;117:S25–8. doi: 10.1016/j.ejogrb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimm LG, Yarnold PR. Reading and understanding multivariate statistics. Washington (DC): American Psychological Association; 1995. [Google Scholar]
- 22.Grunau RE. Early pain in preterm infants: a model of long-term effects. Clin Perinatol. 2002;29:373–94. doi: 10.1016/s0095-5108(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 23.Grunau RE. Self-regulation and behavior in preterm children: effects of early pain. In: McGrath PJ, Finley GA, editors. Pediatric pain: biological and social context, progress in pain research and management. Seattle: IASP Press; 2003. pp. 23–55. [Google Scholar]
- 24.Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007;150:151–6. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grunau RE, Holsti L, Haley DW, Oberlander T, Weinberg J, Solimano A, Whitfield MF, Fitzgerald C, Yu W. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113:293–300. doi: 10.1016/j.pain.2004.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grunau RE, Holsti L, Peters JW. Long-term consequences of pain in human neonates. Semin Fetal Neonatal Med. 2006;11:268–75. doi: 10.1016/j.siny.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Grunau RE, Oberlander TF, Whitfield MF, Fitzgerald C, Lee SK. Demographic and therapeutic determinants of pain reactivity in very low birth weight neonates at 32 weeks’ postconceptional age. Pediatrics. 2001;107:105–12. doi: 10.1542/peds.107.1.105. [DOI] [PubMed] [Google Scholar]
- 28.Grunau RE, Weinberg J, Whitfield MF. Neonatal procedural pain and preterm infant cortisol response to novelty at 8 months. Pediatrics. 2004;114:e77–84. doi: 10.1542/peds.114.1.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haley DW, Grunau RE, Oberlander TF, Weinberg J. Contingency learning and reactivity in preterm and full-term infants at 3 months. Infancy. 2008;13:570–95. doi: 10.1080/15250000802458682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall RW, Kronsberg SS, Barton BA, Kaiser JR, Anand KJS. Morphine, hypotension, and adverse outcomes among preterm neonates: who’s to blame? Secondary results from the NEOPAIN trial. Pediatrics. 2005;115:1351–9. doi: 10.1542/peds.2004-1398. [DOI] [PubMed] [Google Scholar]
- 31.Holsti L, Grunau RE, Oberlander TF, Whitfield MF. Prior pain induces heightened motor responses during clustered care in preterm infants in the NICU. Early Hum Dev. 2005;81:293–302. doi: 10.1016/j.earlhumdev.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Johnston CC, Filion F, Snider L, Majnemer A, Limperopoulos C, Walker CD, Veilleux A, Pelausa E, Cake H, Stone S, Sherrard A, Boyer K. Routine sucrose analgesia during the first week of life in neonates younger than 31 weeks’ postconceptional age. Pediatrics. 2002;110:523–8. doi: 10.1542/peds.110.3.523. [DOI] [PubMed] [Google Scholar]
- 33.LaPrairie JL, Murphy AZ. Female rats are more vulnerable to the long-term consequences of neonatal inflammatory injury. Pain. 2007;132:S124–33. doi: 10.1016/j.pain.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larroque B, Ancel PY, Marret S, Marchand L, André M, Arnaud C, Pierrat V, Rozé JC, Messer J, Thiriez G, Burguet A, Picaud JC, Bréart G, Kaminski M EPIPAGE Study Group. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet. 2008;371:813–20. doi: 10.1016/S0140-6736(08)60380-3. [DOI] [PubMed] [Google Scholar]
- 35.Muller-Nix C, Forcada-Guex M, Pierrehumbert B, Jaunin L, Borghini A, Ansermet F. Prematurity, maternal stress and mother–child interactions. Early Hum Dev. 2004;79:145–58. doi: 10.1016/j.earlhumdev.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Murphy BP, Inder TE, Huppi PS, Warfield S, Zientara GP, Kikinis R, Jolesz FA, Volpe JJ. Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics. 2001;107:217–21. doi: 10.1542/peds.107.2.217. [DOI] [PubMed] [Google Scholar]
- 37.Page GG, Blakely WP, Kim M. The impact of early repeated pain experiences on stress responsiveness and emotionality at maturity in rats. Brain Behav Immun. 2005;19:78–87. doi: 10.1016/j.bbi.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Perlman JM. Cognitive and behavioral deficits in premature graduates of intensive care. Clin Perinatol. 2002;29:779–97. doi: 10.1016/s0095-5108(02)00051-9. [DOI] [PubMed] [Google Scholar]
- 39.Rao R, Sampers JS, Kronsberg MS, Brown J, Desai NS, Anand KJS. Neurobehavior of preterm infants at 36 weeks postconception as a function of morphine analgesia. Am J Perinatol. 2007;24:511–7. doi: 10.1055/s-2007-986675. [DOI] [PubMed] [Google Scholar]
- 40.Resnick MB, Stralka K, Carter RL, Ariet M, Bucciarelli RL, Furlough RR, Evans JH, Curran JS, Ausbon WW. Effects of birth weight and sociodemographic variables on mental development of neonatal intensive care unit survivors. Am J Obstet Gynecol. 1990;162:374–8. doi: 10.1016/0002-9378(90)90389-o. [DOI] [PubMed] [Google Scholar]
- 41.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 42.Rozé JC, Denizot S, Carbajal R, Ancel PY, Kaminski M, Arnaud C, Truffert P, Marret S, Matis J, Thiriez G, Cambonie G, André M, Larroque B, Bréart G. Prolonged sedation and/or analgesia and 5-year neurodevelopment outcome in very preterm infants: results from the EPIPAGE cohort. Arch Pediatr Adolesc Med. 2008;162:728–33. doi: 10.1001/archpedi.162.8.728. [DOI] [PubMed] [Google Scholar]
- 43.Simons SH, van Dijk M, van Lingen RA, Roofthooft D, Duivenvoorden HJ, Jongeneel N, Bunkers C, Smink E, Anand KJ, van den Anker JN, Tibboel D. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA. 2003;290:2419–47. doi: 10.1001/jama.290.18.2419. [DOI] [PubMed] [Google Scholar]
- 44.Slater R, Cantarella A, Gallella S, Worley A, Boyd S, Meek J, Fitzgerald M. Cortical pain responses in human infants. J Neurosci. 2006;26:3662–6. doi: 10.1523/JNEUROSCI.0348-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker CD, Kudreikis K, Sherrard A, Johnston CC. Repeated neonatal pain influences maternal behavior, but not stress responsiveness in rat offspring. Brain Res Dev Brain Res. 2003;140:253–61. doi: 10.1016/s0165-3806(02)00611-9. [DOI] [PubMed] [Google Scholar]
- 46.Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem Res. 2007;32:1730–40. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- 47.White DA, Michaels CC, Holtzman SG. Periadolescent male but not female rats have higher motor activity in response to morphine than do adult rats. Pharmacol Biochem Behav. 2008;89:188–99. doi: 10.1016/j.pbb.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitfield MF, Grunau RV, Holsti L. Extremely premature (<800 g) schoolchildren: multiple areas of hidden disability. Arch Dis Child. 1997;77:F85–90. doi: 10.1136/fn.77.2.f85. [DOI] [PMC free article] [PubMed] [Google Scholar]