Abstract
Serotonin receptor 1A knockout (Htr1aKO) mice show increased anxiety-related behavior in tests measuring innate avoidance. Here we demonstrate that Htr1aKO mice show enhanced fear conditioning to ambiguous conditioned stimuli, a hallmark of human anxiety. To examine the involvement of specific forebrain circuits in this phenotype, we developed a pharmacogenetic technique for the rapid tissue- and cell type–specific silencing of neural activity in vivo. Inhibition of neurons in the central nucleus of the amygdala suppressed conditioned responses to both ambiguous and nonambiguous cues. In contrast, inhibition of hippocampal dentate gyrus granule cells selectively suppressed conditioned responses to ambiguous cues and reversed the knockout phenotype. These data demonstrate that Htr1aKO mice have a bias in the processing of threatening cues that is moderated by hippocampal mossy-fiber circuits, and suggest that the hippocampus is important in the response to ambiguous aversive stimuli.
Htr1aKO mice show enhanced behavioral inhibition and avoidance in several tests of anxiety-related behavior1-4. Although these mice show normal cue- and context-dependent fear conditioning5, it was recently demonstrated that they express enhanced fear responses to contexts containing both conditioned and novel cues6. These findings indicate that Htr1aKO mice might show enhanced anxiety-related behavior specifically under conditions of competing threatening and safety cues, and suggest that these animals have a cognitive defect in the processing of aversive stimuli.
Htr1a is a G protein–coupled receptor (GPCR) expressed both as an autoreceptor on serotonergic neurons in the raphe nuclei, and as a heteroreceptor on nonserotonergic neurons primarily in the mouse forebrain, with its highest expression being in the entorhinal cortex and CA1 region of the hippocampus1,7,8. Activation of both receptor populations results in membrane hyperpolarization and decreased neuronal excitability, an effect that is mediated by its coupling to G protein inward-rectifying K+ channels (GIRKs)9. Expression of Htr1a in the forebrain is sufficient to restore normal anxiety-related behavior to knockout mice1, demonstrating a critical role for this receptor population in the knockout phenotype. Furthermore, a conditional rescue strategy has revealed that expression of the receptor in the forebrain during development is both necessary and sufficient for establishing normal anxiety-related behavior in adulthood1. Moreover, blockade of the receptor in adulthood is not sufficient to reproduce the knockout phenotype1. Thus, structural defects induced by the absence of the receptor during the maturation of forebrain circuits are likely to underlie the anxiety-related phenotype of Htr1aKO mice. Consistent with this hypothesis, electrophysiological and morphological changes have been described at CA3-CA1 synapses in the hippocampus of Htr1aKO mice, including decreased inhibitory neurotransmission, increased excitability and increased dendritic arborization10 (J.E. Monckton, C.T. Gross and R. Hen, Soc. Neurosci. Abstr. 550.1, 2002; J.P. Hornung, personal communication).
In rodents, selective lesions of the hippocampus are associated with reduced avoidance behavior in anxiety-related tests11,12. Moreover, the hippocampus is known to be important in the processing of complex associative stimuli, and the firing rate of pyramidal neurons in the CA1 region has been shown to encode the predictive value of aversively conditioned cues13-15. Thus, it is possible that increased excitability of hippocampal CA3-CA1 synapses as a result of the absence of Htr1a during development contributes to the anxiety-related phenotype of Htr1aKO mice.
Here, we show that Htr1aKO mice express enhanced fear conditioning to partial, but not perfect, conditioned cues, thus demonstrating a bias in their response to ambiguous threatening stimuli. To identify the neural circuits mediating this phenotype, we developed a pharmacogenetic method for the tissue- and cell type–specific inhibition of neural activity in vivo. Using this new pharmacogenetic tool, we show that suppression of neural activity in granule cells of the hippocampal dentate gyrus (DG) selectively suppresses conditioned responses to ambiguous cues and reverses the Htr1aKO phenotype. Our findings implicate hippocampal dentate gyrus circuits in the processing of ambiguous aversive cues and demonstrate that Htr1aKO mice have deficits in hippocampal-dependent anxiety-related behavior.
RESULTS
Htr1aKO mice show enhanced freezing to ambiguous cues
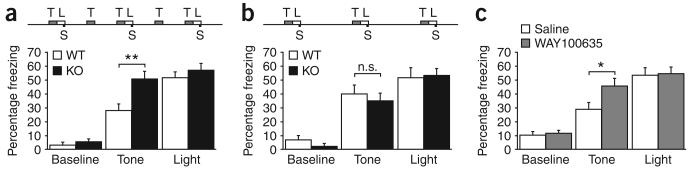
Previously, we demonstrated that Htr1aKO mice showed enhanced contextual fear conditioning only under circumstances in which they were exposed to a context containing both conditioned and novel stimuli6. These findings suggested that Htr1aKO mice might have a bias in their response to aversive cues when those cues are presented in the context of other safety signals. To explicitly test this possibility, we measured the freezing responses of Htr1aKO mice to simultaneously conditioned partial and perfect cues. During training, Htr1aKO mice were exposed to two stimuli, a light and a tone, that were presented together with an unconditioned stimulus, a mild foot shock. The light (perfect cue) was presented three times coterminating with foot shock, whereas the tone (partial cue) was presented three times immediately preceding the light and two times alone. In this way the tone became a partial predictor and the light became a perfect predictor of shock. Htr1aKO and wild-type littermates were sequentially re-exposed to the tone and light 1 d after training and freezing behavior was quantified as a measure of conditioning to these cues. As expected, wild-type mice showed high levels of freezing during the light (perfect cue) and lower levels of freezing during the tone (partial cue). Knockout mice, on the other hand, showed significantly greater freezing to the partial cue when compared with wild-type mice, and similar levels of freezing to partial and perfect cues (Fig. 1a). We observed no differences in baseline freezing before cue presentation, confirming an absence of context-dependent conditioning under these circumstances (P = 0.426). To rule out the possibility that we failed to detect increased freezing to the perfect cue in knockout mice as a result of saturating levels of freezing under our testing conditions (that is, the ‘ceiling’ effect), we repeated the experiments using a protocol that induces lower levels of freezing (0.3 mAversus 0.5 mA shock; ANOVA, main effect of shock/freezing to light: F1,60 = 6.19, P = 0.0156). Regardless of conditioning intensity, increased freezing of knockout mice continued to be restricted to the partial cue (Supplementary Fig. 1 online).
Figure 1.
Increased response to partially conditioned cues in Htr1aKO mice. (a) Training protocol for simultaneous conditioning to partial and perfect cues. Knockout (KO) mice showed greater freezing responses 24 h after training to the partial (T, tone), but not perfect (L, light), conditioned stimulus when compared with wild-type (WT) littermates (S, shock; WT: N = 18, KO: N = 18; ** P < 0.01; n.s., not significant). (b) Same training protocol as in a, but unpaired cue presentations have been omitted. In the absence of partial cue conditioning, wild-type and knockout mice showed indistinguishable freezing responses to both conditioned stimuli during testing (WT: N = 10, KO: N = 10). (c) Mice treated with WAY100635 from P13 to P34 showed greater freezing responses to the partial, but not perfect, conditioned stimulus when compared with saline-treated littermates (saline: N = 10, WAY: N = 12; * P < 0.05). Error bars, s.e.m.
To confirm that the enhanced freezing to the tone that we observed in knockout mice was dependent on the partial nature of conditioning to this cue, we repeated the experiment omitting the unpaired tone presentations. Under these circumstances, both cues were perfect predictors of shock, and knockout and wild-type mice showed indistinguishable freezing behavior (Fig. 1b). These studies confirm that enhanced freezing to the tone in knockout mice reflected a specific defect in their processing of the imperfect contingency of this cue. Moreover, reversal of tone and light cues in the partial conditioning protocol produced similar results, arguing against a contribution of sensory modality to the knockout phenotype (wild type: light −18.8 ± 3.5, tone −60.3 ± 4.7; knockout: light −32.9 ± 5.1, tone −74.7 ± 5.1; knockout versus wild type light, P = 0.035). These findings suggest that Htr1aKO mice have a bias in their assessment of the predictive value of ambiguous aversive cues.
Because the anxiety-related phenotype of Htr1aKO mice is dependent on receptor expression during development, and is associated with structural and physiological changes at CA3-CA1 synapses in the hippocampus1,10 (J.E. Monckton, C.T. Gross & R. Hen, Soc. Neurosci. Abstr. 550.1, 2002; J.P. Hornung, personal communication), we wondered whether the fear conditioning phenotype might also be dependent on Htr1a expression during development. Moreover, because the third and fourth postnatal weeks are a period of maximal dendritic growth and synaptic maturation in the hippocampus16, we asked whether inhibition of Htr1a function specifically during this period might be sufficient to reproduce the knockout phenotype. Wild-type mice were treated with the selective Htr1a antagonist WAY100635 from postnatal day 13 to 34 (P13–34) using subcutaneous osmotic minipumps (1.5 μg h−1, ~0.3 mg per kg of body weight per d). In control experiments, blockade of Htr1a function during WAY100635 treatment was confirmed by the absence of agonist-induced hypothermia at P14 and P28 (data not shown). When treated mice were tested in the ambiguous-cue fear-conditioning protocol in adulthood, WAY100635-treated mice showed significantly greater freezing responses to the partial cue than did vehicle-treated littermates, and like knockout mice, showed similar responses to partial and perfect cues (Fig. 1c). These data demonstrate that blockade of Htr1a function during the third and fourth postnatal weeks is sufficient to reproduce the knockout phenotype and argue that persistent molecular or structural changes induced during the maturation of neural circuits underlie the aberrant processing of ambiguous cues by these mice.
Pharmacogenetic silencing of CeA and DG neurons
To better understand the role of specific neural circuits in the Htr1aKO phenotype, it would be useful to inhibit selected cell populations in the brain transiently during behavioral testing. Such inhibition could perhaps temporarily reverse or mask changes in neural activity induced by the developmental absence of the receptor. To test this approach, we developed a pharmacogenetic strategy for the rapid inhibition of neural activity in selected populations of cells in vivo. We took advantage of the fact that pharmacological activation of inhibitory GPCRs rapidly evokes membrane hyperpolarization by opening GIRK channels9,17. It was recently shown that treatment of rats expressing an inhibitory GPCR could induce rapid inhibition of neural activity in vivo18. Several observations suggest that Htr1a could be used successfully in such a GPCR-mediated pharmacogenetic silencing strategy. First, previous studies have shown that under baseline conditions endogenous serotonin (5-HT) is not sufficient to activate Htr1a in the adult brain1,19, and thus the receptor is not expected to be tonically activated unless pharmacologically challenged by exogenous administration of a receptor agonist. Second, selective Htr1a agonists that efficiently cross the blood-brain barrier make systemic drug delivery possible. Finally, tissue- and cell type–specific expression of Htr1a has been achieved in transgenic mice1. Thus, we set out to determine whether agonist treatment of transgenic mice that expressed Htr1a under the control of a tissue-specific promoter and that lacked endogenous Htr1a (Htr1aTg crossed to Htr1aKO) could be used as a tool for the rapid silencing of neural activity in a cell type–specific manner.
The central nucleus of the amygdala (CeA) is required for the expression of noninstrumental conditioned fear20. Within the CeA, efferent neurons project to mid- and hindbrain structures that mediate the behavioral and physiological responses to conditioned cues21. Thus, inhibition of neuronal activity in CeA should block the expression of conditioned responses to cues regardless of whether they were partially or perfectly conditioned. Granule cells of the hippocampal DG, by contrast, serve as the primary target of entorhinal cortical afferents into the hippocampal DG-CA3-CA1 circuit, and inhibition of these neurons should selectively suppress processing in this circuit. The presence of enhanced excitability and neural transmission at CA3-CA1 synapses in Htr1aKO mice, coupled with the known role of CA1 in encoding cue salience, suggested that suppression of DG could be used to reverse or suppress defects in hippocampal function in the knockout.
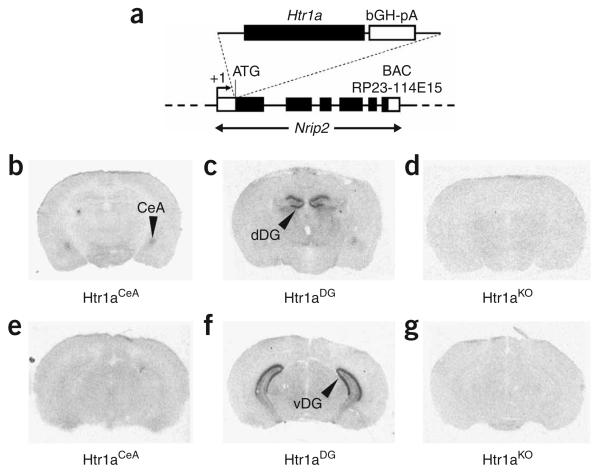
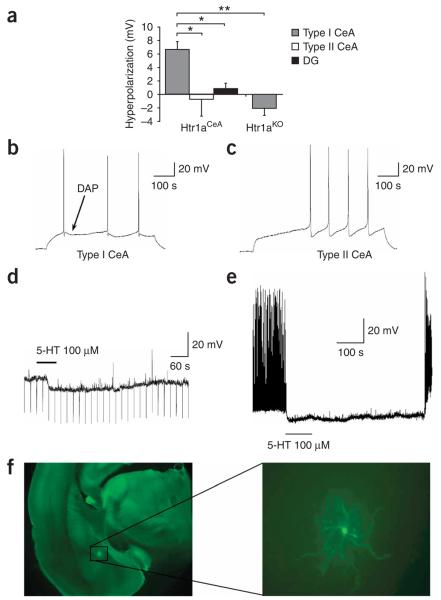
To express Htr1a exclusively in the CeA and DG, we produced transgenic mice in which receptor coding sequences were placed under the control of the Nrip2 promoter (Fig. 2a). Previous studies have shown that Nrip2 mRNA is selectively expressed in the CeA and granule cells of the DG22. Independent transgenic founder mice that were obtained after injection of the Nrip2-Htr1a construct were crossed to Htr1aKO mice and analyzed for tissue- and cell type–specific expression of the receptor. Two founders were selected that showed expression exclusively in the CeA and granule cells of the DG, respectively (Htr1aCeA, heterozygous for the Tg–Nrip2-Htr1aCeA allele and homozygous for the Htr1aKO allele, and Htr1aDG, heterozygous for the Tg–Nrip2-Htr1aDG allele and homozygous for the Htr1aKO allele; Fig. 2b–g and Supplementary Figs. 2 and 3 online). To determine whether pharmacological activation of Htr1a in these lines could be used to specifically inhibit neural activity in these cell populations, we carried out electrophysiological recordings in slices from Htr1aCeA and Htr1aDG mice. Whole-cell recordings in slices from Htr1aCeA mice showed significant hyperpolarization responses to 5-HT exclusively in neurons with high input resistance and depolarizing after-potentials (type I, 7/16 neurons; Fig. 3a–d). No response was seen to 5-HT in those neurons that had lower input resistance and did not have depolarizing after-potentials (type II, 9/16 neurons). Notably, the hyperpolarizing response to 5-HT in type I neurons was completely blocked by administration of WAY100635 (10 μM, N = 2, data not shown), and no response to 5-HT was seen in type I neurons from Htr1aKO mice. To determine whether Htr1a-dependent hyperpolarization was sufficient to suppress neural activity in Htr1aCeA mice, we applied 5-HTwhile recording from slices in which spontaneous neural activity was induced by depolarizing current injection through the recording electrode. Notably, application of 5-HT completely suppressed spontaneous activity in type I CeA neurons from Htr1aCeA mice (Fig. 3e). Finally, histological examination of biocytin-filled type I CeA neurons that showed hyperpolarization responses to 5-HT confirmed that recorded neurons resided in the CeA, but showed no clear correlation between Htr1a response and any particular morphology (spiny versus nonspiny dendrites, and pyramidal versus ovoid soma; Fig. 3f).
Figure 2.
Transgenic lines expressing Htr1a in the CeA and DG. (a) Tissue- and cell type–specific expression of Htr1a was achieved by making transgenic mice with the Htr1a coding sequences under the control of the Nrip2 promoter. (b–g) Crossing of transgenic founder lines to mice lacking the endogenous receptor (Htr1aKO) resulted in receptor expression restricted to cells in the central nucleus of the amygdala (Htr1aCeA; b,e) and granule cells of the dentate gyrus (Htr1aDG; c,f), as detected by autoradiography using the Htr1a ligand 125I-MPPI (dDG, dorsal DG; vDG, ventral DG). No receptor binding was detected in brains from Htr1aKO mice (d,g).
Figure 3.
Electrophysiological characterization of Htr1aCeA line. (a) Magnitude of membrane hyperpolarization by application of serotonin (5-HT, 100 μM) to neurons in brain slices from adult Htr1aCeA mice. Error bars, s.e.m. (b,c) CeA neurons were characterized as type I (b) or type II (c) by the presence or absence of a depolarizing after-potential seen following a depolarizing current–induced action potential, respectively. Significant hyperpolarization was seen in type I, but not type II, CeA neurons (9/16 and 7/16 neurons, respectively; t-test, P = 0.02), and no hyperpolarization response was detected in hippocampal DG neurons (N = 3) from the same mice, or CeA neurons from knockout mice (N = 8). (d) Raw data trace of the membrane potential of a representative type I CeA neuron showing a hyperpolarizing response to 5-HT application. The line depicts the duration of time that 5-HT was in the perfusion buffer. (e) Silencing of depolarizing current–induced spontaneous neural activity in a type I CeA neuron following application of 5-HT. (f) Photomicrograph of a biocytin-labeled type I CeA neuron.
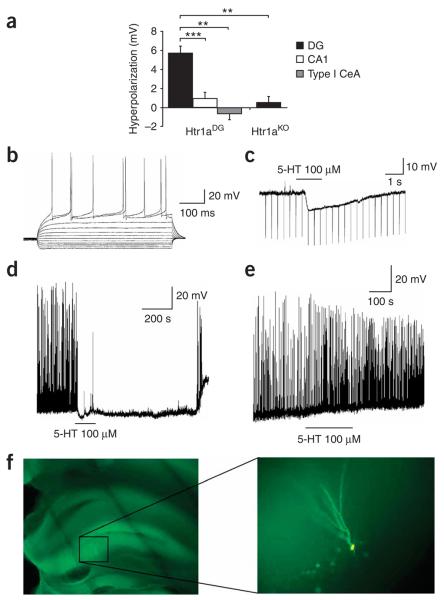
Whole-cell recordings in slices from Htr1aDG mice showed significant hyperpolarization responses to 5-HT exclusively in DG granule cells and not in CA1 pyramidal neurons or CeA neurons from the same animals (Fig. 4a–c). Notably, the hyperpolarizing response to 5-HT in DG granule cells was completely blocked by administration of WAY100635 (10 μM, N = 2, data not shown), and no response to 5-HT was seen in DG granule cells from Htr1aKO mice. Application of 5-HT was able to completely suppress depolarizing current–induced spontaneous activity in granule cells from Htr1aDG mice, but not in similar neurons from Htr1aKO mice (Fig. 4d–e). Histological examination confirmed that 5-HT–responding cells showed a granule cell identity (Fig. 4f). Taken together, these data demonstrate that tissue-specific expression of Htr1a can be used as a pharmacogenetic tool for the rapid cell type–specific silencing of neural activity.
Figure 4.
Electrophysiological characterization of Htr1aDG line. (a) Magnitude of membrane hyperpolarization by application of serotonin (5-HT, 100 μM)) to neurons in brain slices from adult Htr1aDG mice. Significant hyperpolarization was seen in hippocampal DG granule neurons (N = 24), but not in hippocampal CA1 (N = 8; t-test, P = 0.0003) or type I neurons from the CeA (N = 3; t-test, P = 0.003). Similarly, no hyperpolarization response was detected in DG neurons from knockout mice (N = 5). Error bars, s.e.m. (b) Membrane potential response to current injection into a representative DG neuron from a Htr1aDG mouse. (c) Raw data trace of the membrane potential response of a DG neuron showing hyperpolarization response to 5-HT application. The line depicts the duration of time that 5-HT was in the perfusion buffer. (d) Silencing of depolarizing current–induced spontaneous neural activity in a DG neuron after application of 5-HT. (e) No inhibition of current-induced spontaneous neural activity was detected in a DG neuron from a Hrt1aKO mouse. (f) Photomicrograph of a biocytin-labeled DG neuron.
Effects of CeA and DG inhibition on ambiguous-cue response
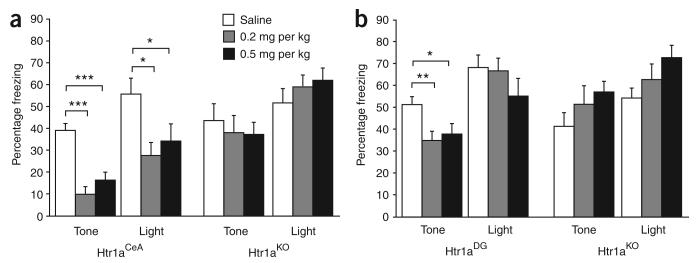
Next, we asked whether suppression of neural activity in type I CeA neurons could block the expression of conditioned responses to aversive cues in our ambiguous-cue fear-conditioning protocol. Htr1aCeA and Htr1aKO littermates were treated subcutaneously with the selective Htr1a agonist 8-hydroxy-2-(dipropylamino)tetralin (8-OH-DPAT) before testing. Htr1aCeA mice treated with either 0.2 or 0.5 mg per kg 8-OH-DPAT showed significantly reduced freezing responses to both the tone (partial cue) and light (perfect cue) when compared with saline-treated animals (Fig. 5a). This effect could be attributed to the activation of Htr1a, as no effect of treatment was observed in Htr1AKO mice. Moreover, saline-treated Htr1aCeA and Htr1aKO mice did not differ significantly in their freezing responses, indicating that basal activation of Htr1a in this neuronal subtype is not sufficient to modulate the expression of conditioned responses. These results are consistent with lesion and pharmacological inhibition studies that demonstrate a critical role for CeA in the expression of noninstrumental fear responses20,21 and show that type I neurons in this structure serve as a common output structure for both partial and perfect conditioned responses.
Figure 5.
Suppression of conditioned responses by inhibition of neurons in the CeA and DG. (a) Administration of 8-OH-DPAT (0.2 or 0.5 mg per kg, subcutaneous) to Htr1aCeA mice before ambiguous-cue fear-conditioning testing suppressed freezing to both partial and perfect cues (saline, N = 12; 0.2 mg per kg, N = 12; 0.5 mg per kg, N = 11; * P < 0.05, *** P < 0.001). Similar treatment of knockout mice was ineffective (saline, N = 11; 0.2, N = 9; 0.5, N = 11). (b) Administration of 8-OH-DPAT (0.2 or 0.5 mg per kg, subcutaneous) to Htr1aDG mice before ambiguous-cue fear-conditioning testing suppressed freezing to the partial, but not perfect, cue (saline, N = 12; 0.2 mg per kg, N = 9; 0.5 mg per kg, N = 10; * P < 0.05, ** P < 0.01). Similar treatment of knockout mice was ineffective (Saline, N = 9; 0.2 mg per kg, N = 10; 0.5 mg per kg, N = 10). Error bars, s.e.m.
Finally, we asked whether suppression of DG granule cells could modulate conditioned responses in our ambiguous-cue fear-conditioning protocol. Treatment of Htr1aDG mice with either 0.2 or 0.5 mg per kg 8-OH-DPAT before testing significantly reduced freezing responses to the tone (partial cue), but not to the light (perfect cue), when compared with saline-treated mice (ANOVA, genotype × treatment effect for tone: F1,41 = 6.11, P = 0.0177; genotype × treatment effect for light: F1,41 = 0.635, P = 0.430; Fig. 5b). Treatment of Htr1aKO mice with 8-OH-DPAT did not affect freezing behavior, confirming the selectivity of the agonist. Moreover, saline-treated Htr1aDG and Htr1aKO mice showed similar freezing responses, indicating that basal activation of the receptor in this neuronal population is not sufficient to interfere with conditioned cue processing. Taken together, these data show that inhibition of type I CeA neurons blocks the expression of conditioned fear responses, whereas inhibition of DG granule cells selectively attenuates the expression of partially conditioned responses, possibly by modulating the assessment of the predictive value of such cues. Our findings also argue that the enhanced expression of partially conditioned responses seen in Htr1aKO mice can be reversed by the selective suppression of primary inputs to the DG-CA3-CA1 circuit.
DISCUSSION
Our finding that Htr1aKO mice show increased fear conditioning specifically to partially conditioned cues argues that these animals harbor a defect in the cognitive processing of aversive cues. A tendency to interpret ambiguous aversive cues as threatening is a feature of human anxiety, and may serve as a risk factor for pathological anxiety and other mood disorders23-25. Anxiety and depression, for example, show significant comorbidity and share common genetic risk factors26,27. Thus, a better understanding of the neural circuits involved in the processing of ambiguous aversive stimuli could help to further explain the etiology of mood disorders and could suggest neural substrates for therapeutic intervention.
Our observation that suppression of CeA neurons blocks conditioned responses to both partial and perfect cues is consistent with the known role of this structure as a common output circuit for the expression of noninstrumental conditioned fear20,21. Moreover, recent data have indicated that only a single subregion and/or cell type in the CeA serves as the final output circuit that signals to mid- and hindbrain structures for the production of conditioned behavioral and autonomic responses28. Our findings suggest that type I CeA neurons are a critical component of this output circuit. Electrophysiological recordings in rat CeA have described two populations of CeA neurons, type A and type B, with type A neurons showing a significant depolarizing after-potential and a spiny morphology like that seen in a subset of our type I cells29,30. Our study is the first to provide cellular characteristics of mouse CeA neurons (Supplementary Table 1 online) and, although it is difficult to make direct comparisons between our study and previous studies because of differences in species (rat versus mouse) and recording technique (whole cell versus sharp electrode), in at least one other study similar nonpyramidal, spiny neurons have been shown to be GABAergic projection neurons coexpressing peptide neurotransmitters31,32. We speculate that a subset of our type I neurons belongs to this class of projection neuron.
The reversal of the knockout phenotype by the selective inhibition of DG granule cells during the expression of fear conditioning indicates that the bias in processing of ambiguous aversive cues in these mice is modulated by the hippocampal mossy-fiber pathway. The hippocampus has been extensively studied as a critical modulatory circuit for the acquisition, storage and expression of complex associative memories. Post-training lesions of the hippocampus prevent the expression of trace fear conditioning (where presentation of conditioned and unconditioned stimuli are separated in time), while leaving intact simple delay fear conditioning (where presentation of conditioned and unconditioned stimuli coincide)33,34. This requirement has been proposed to depend on the ability of the hippocampus to provide information about the strength of cue association during associative conditioning35,36, a hypothesis that is supported by lesion studies37. Consistent with such a role for the hippocampus, electrophysiological recordings in awake behaving animals revealed that the firing rate of hippocampal CA1 pyramidal neurons encodes the predictive value of the conditioned cue regardless of sensory modality13-15. Notably, CA1 pyramidal neurons receive inputs both directly from the entorhinal cortex and indirectly via the DG-CA3-CA1 pathway, and the integration of information from these pathways has been proposed to have a role in the assessment of the salience of ongoing sensory information38-40. Our finding that inhibition of DG granule cells selectively suppressed freezing to partially conditioned cues suggests that the indirect pathway carries information that is critical for the recall and processing of cue contingency. Alternatively, inhibition of DG granule cells may have disturbed the balance between indirect and direct inputs impinging on CA1 pyramidal neurons, and thus altered the processing of ambiguous cues. Although our data strongly suggest a direct role for DG granule cells in the processing of cue contingency, it remains possible that suppression of this cell population has an indirect effect on neural activity in other brain regions that serve this function.
Finally, although several techniques have been developed for the rapid silencing of neural activity in vivo18,41,42, to our knowledge this is the first example of the use of such a technology to uncover previously unknown neural circuits controlling complex behavior in a mammalian system. Our pharmacogenetic strategy benefits from several advantages, including (i) the efficient coupling of Htr1a to inhibitory GIRK channels, (ii) the use of a receptor that is not tonically activated by its endogenous ligand1,19 and (iii) the use of a selective pharmacological agent that permits systemic administration. However, our system does require that studies be carried out on a Htr1aKO background, a prerequisite that may preclude some experiments that depend on signaling via the endogenous receptor. Furthermore, Htr1a is not exclusively coupled to inhibitory GIRK channels and signaling via other pathways (for example, adenylyl cyclase, Ca+2 channels) could complicate the interpretation of experiments in which long-term or transient inhibition is required.
In summary, we have validated a new pharmacogenetic tool for tissue- and cell type–specific neural silencing in behaving mice and have applied it to show that a subclass of CeA neurons are essential for the expression of conditioned fear and that hippocampal DG circuits modulate the processing of ambiguous conditioned cues. These results indicate a role for hippocampal indirect pathway circuits in the evaluation of conditioned cue contingency and suggest that the hippocampus contributes to the expression of fear behaviors, particularly under conditions of uncertain or ambiguous environmental stimuli. The Htr1aCeA and Htr1aDG lines have the potential to serve as useful tools for dissecting the role of these neurons in the control of complex behavior, and the development of additional Htr1a lines holds promise for the extension of this technique to other circuits and behaviors.
METHODS
Mouse strains
The Htr1a coding sequence followed by a bGH polyadenylation sequence was inserted at the start codon of the Nrip2 gene in a genomic fragment of the RP23-114E15 BAC clone (Chori-BACPAC Resources) containing the full genomic sequences of the target gene using bacterial recombineering43. A FLP-recombinase recognition target dual-promoter neomycin cassette was used to facilitate selection of recombinants and was later excised by transient transfection with a FLP expression vector. Linearized, vector-free BAC insert was prepared for pronuclear injection into (C57BL/6J × CBA/J) × C57BL/6J zygotes by agarose gel purification. Founders carrying the transgene were identified and genotyped by PCR, crossed to Htr1aKO mice4, and maintained on a mixed C57BL/6J;CBA/J;129S6/SvEvTac background. Two out of three founders transmitted the transgene to their offspring that showed detectable expression of Htr1a in brain (Htr1aCeA and Htr1aDG).
Mouse husbandry
Mice were housed in same sex groups of four or five animals per cage with food and water provided ad libitum and under controlled temperature (21 ± 0.5 °C), humidity (55–75%) and lighting (lights on: 7:00–19:00) conditions. Animals were weaned at 3–4 weeks of age and tail biopsies were collected for genotyping. At least 1 week before behavioral testing, mice were transferred to a new room located close to the testing room and switched to a reverse light cycle (lights on: 22:30–10:30) with constant red light illumination (40 W).
Behavioral testing
Procedures were carried out according to EMBL and Italian guidelines for ethical animal treatment following authorized protocols. Training and testing occurred in behavioral chambers (MED Associates) measuring 14 × 15 × 12 cm and equipped to deliver a scrambled foot shock via the grid floor. Freezing was scored manually from videotaped sessions and defined as the complete absence of movement except for respiration. On training and testing days, mice were transported to the behavioral room in their home cages and allowed to acclimate to the room for at least 30 min before testing. Fear conditioning was carried out as described previously35 with the following changes. The training session lasted 18 min, with three 0.5-mA, 1-s shocks delivered at 219, 579 and 939 s. A small exposed light bulb (28V DC, 100 mA) was used as the light cue and was presented three times for 20 s, coterminating with the foot shocks. The tone (85 dB, 3 kHz) was presented five times for 20 s, three times coterminating with light onset and twice alone at 360 and 720 s. On the testing day, freezing was scored during 3-min habituation to the chamber, 6-min tone presentation and 6-min light presentation. To suppress contextual conditioning responses, testing was carried out with different visual, olfactory and tactile cues (a smooth white plastic floor, walls covered with sandpaper, and cinnamon rather than lemon odor).
Osmotic pump implantation
Osmotic minipumps (0.20 μl h−1, Model 1002, Alzet) were implanted subcutaneously to deliver WAY100635 (Sigma) or saline continuously for 21 d. Pumps were filled with 7.1 mg ml−1 (1.5 mg h−1) WAY100635 as estimated to achieve a constant dose of 0.3 mg per kg of body weight. Pumps were soaked overnight in Ringer's solution (Eurospital) at 37 °C to assure steady-state pumping following implantation. Before surgery, all pups were removed from the dam, placed in a beaker containing home cage bedding and kept on a heating pad. Mice were anesthetized with halothane, pumps were subcutaneously implanted in the dorsal thoracic area and wounds were closed with a 9-mm stainless steel clip (Stoelting). After 21 d of treatment mice were anesthetized, pumps were removed and the wounds were closed with a clip. Rectal temperature responses to the Htr1a agonist 8-OH-DPAT (0.3 mg per kg, subcutaneous) were used to verify blockade of endogenous Htr1a 1 d and 14 d after treatment initiation as well as to confirm normal activation of the receptor 1 week after pump removal44 (data not shown).
Statistical analysis
Behavioral data were analyzed by ANOVA followed by post hoc comparisons using the Fisher's test in cases of significance (P < 0.05). Electrophysiological data were analyzed by unpaired Student's t-test. All analyses were carried out using either Excel (Microsoft) or StatView 5.0 (SAS Institute).
Drug administration
8-OH-DPAT was obtained from Sigma-RBI (Natick), dissolved in saline (0.9% NaCl) and administered subcutaneously 15 min before behavioral testing into the scruff of the neck in a volume of 5 ml per kg.
Autoradiography
Animals were killed by carbon dioxide asphyxiation and cervical dislocation, and brains were rapidly removed and frozen in powdered dry ice. We cut 16-μm brain sections in a cryostat, transferred them to slides at 24–25 °C (Superfrost Plus, Menzel-Glaser), and air dried the slides for 30 min before storage at −80 °C. Slides were thawed, preincubated at 24–25 °C in buffer (2 mM MgCl2, 50 mM Tris-HCl, pH 7.4), and incubated in the same buffer containing 0.14 nM 125I-MPPI (gift from H. Kung, University of Pennsylvania) for 1 h. Slides were washed in ice-cold buffer (2 × 7 min), rinsed with distilled water, air-dried overnight and exposed to film (Biomax MR-1, Kodak) for 1–3 d.
Electrophysiology
All procedures were carried out in accordance with the US Public health Service's Policy on Humane Care and Use of Laboratory Animals and approved by the IACUC committee of the Stokes Research Institute of the Children's Hospital of Philadelphia. The slice preparation was similar to what we described previously45. Mice were rapidly decapitated and the head placed in preoxygenated ice-cold artificial cerebrospinal fluid (ACSF: 124 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 2.0 mM MgSO4, 2.5 mM CaCl2, 10 mM dextrose and 26 mM NaHCO3) in which 248 mM sucrose was substituted for NaCl. The brain was blocked by cutting off approximately 2.5 mm from the front of the brain and 1 mm in front of the cerebellum. The blocked brain was affixed to a stage of a Leica microslicer (Leica) with cyanoacrylate glue and submerged with preoxygenated ice-cold sucrose-ACSF. Coronal slices, 200 μm thick, containing the hippocampus and amygdala were cut and placed in a holding vial containing ACSF at 37 °C bubbled with 95% O2/5% CO2 for 1 h. After 1 h, the slices were kept in 24–25 °C ACSF bubbled with 95% O2/5% CO2.
A single slice was placed in a recording chamber (Warner Instruments) and continuously perfused with oxygenated ACSF (1.5–2.0 ml min−1) at 32 °C, maintained by an in-line solution heater (TC-324, Warner Instruments). Neurons were visualized using an upright microscope (Zeiss Axioskop) fitted with a 63 × water-immersion objective, differential interference contrast and infrared filter. The image from the microscope was enhanced using a CCD camera and displayed on a monitor. Recording pipettes were fashioned on a P-97 micropipette puller (Sutter Instruments) using borosilicate glass capillary tubing (1.2-mm outer diameter, 0.69-mm inner diameter; Warner Instruments). The resistance of the electrodes was 5–10 MΩ when filled with an intracellular solution of 130 mM potassium gluconate, 5 mM NaCl, 10 mM sodium phosphocreatine, 1 mM MgCl2, 0.02 mM EGTA, 10 mM HEPES, 2 mM MgATP, 0.5 mM Na2GTP, 0.1% biocytin, pH 7.3.
A visualized cell in the CeA, DG or CA1 hippocampus was approached with the electrode, a GΩ seal established and the cell membrane ruptured to obtain a whole-cell recording using a HEKA EPC 10 amplifier (HEKA Electronik). Signals were filtered at 1 kHz, digitized at 10 Hz and stored on-line. Using current-clamp recording, the following cellular characteristics were measured as previously described45: resting membrane potential, input resistance, and action potential threshold, amplitude and width. To examine the effect of Hrt1a receptor activation, we added 5-HT (100 μM) to the perfusion buffer. All chemicals for making the ACSF, electrolyte solution and 5-HT hydrochloride were purchased from Sigma-Aldrich. Standard immunofluorescence procedures were used to visualize the recorded cell. After recording, the slice was fixed by submersion in 4% paraformaldehyde prepared in 0.1 M phosphate buffer, pH 7.4, and then stored in phosphate buffer. Biocytin was visualized using streptavidin-conjugated Alexa Fluor 633 (1:100, Molecular Probe) for 60 min at 24–25 °C. Immunofluorescence label was visualized using a Leica DMR fluorescence microscope (Leica). Images were captured using a digital camera and Openlab 3.09 software (Improvision).
Supplementary Material
ACKNOWLEDGMENTS
We thank V. Carola, J. Rientjes, T. Ferreira, S. Santanelli, F. Zonfrillo and the members of the EMBL Transgenic Facility for expert help. We are grateful to F. Crestani for helpful suggestions on the ambiguous-cue fear-conditioning protocol, A. Akanwa for doing the immunohistochemistry on brain slices, and K. Ploessl and H. Kung for the gift of 125I-MPPI. This work was supported by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (C.G.), a grant from the Fritz Thyssen Stiftung (C.G.), funds from the EMBL Ph.D. Programme (T.T. and L.L.I.), and grants from the US National Institutes of Health MH048125 and MH07540407 (S.G.B.).
Footnotes
Supplementary information is available on the Nature Neuroscience website.
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
References
- 1.Gross C, et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- 2.Heisler LK, et al. Elevated anxiety and antidepressant-like responses in serotonin 5–HT1A receptor mutant mice. Proc. Natl. Acad. Sci. USA. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc. Natl. Acad. Sci. USA. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramboz S, et al. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc. Natl. Acad. Sci. USA. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groenink L, et al. 5-HT1A receptor knockout mice and mice overexpressing corticotropin-releasing hormone in models of anxiety. Eur. J. Pharmacol. 2003;463:185–197. doi: 10.1016/s0014-2999(03)01281-0. [DOI] [PubMed] [Google Scholar]
- 6.Klemenhagen KC, Gordon JA, David DJ, Hen R, Gross CT. Increased fear response to contextual cues in mice lacking the 5-HT1A receptor. Neuropsychopharmacology. 2006;31:101–111. doi: 10.1038/sj.npp.1300774. [DOI] [PubMed] [Google Scholar]
- 7.Chalmers DT, Watson SJ. Comparative anatomical distribution of 5-HT1A receptor mRNA and 5-HT1A binding in rat brain—a combined in situ hybridisation/in vitro receptor autoradiographic study. Brain Res. 1991;561:51–60. doi: 10.1016/0006-8993(91)90748-k. [DOI] [PubMed] [Google Scholar]
- 8.Kia HK, et al. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J. Comp. Neurol. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein–coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic, but not presynaptic, transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- 10.Sibille E, Pavlides C, Benke D, Toth M. Genetic inactivation of the serotonin(1A) receptor in mice results in downregulation of major GABA(A) receptor α subunits, reduction of GABA(A) receptor binding, and benzodiazepine-resistant anxiety. J. Neurosci. 2000;20:2758–2765. doi: 10.1523/JNEUROSCI.20-08-02758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bannerman DM, et al. Regional dissociations within the hippocampus—memory and anxiety. Neurosci. Biobehav. Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Kjelstrup KG, et al. Reduced fear expression after lesions of the ventral hippocampus. Proc. Natl. Acad. Sci. USA. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Best MR, Best PJ. The effects of state of consciousness and latent inhibition on hippocampal unit activity in the rat during conditioning. Exp. Neurol. 1976;51:564–573. doi: 10.1016/0014-4886(76)90180-1. [DOI] [PubMed] [Google Scholar]
- 14.Delgado-Garcia JM, Gruart A. Building new motor responses: eyelid conditioning revisited. Trends Neurosci. 2006;29:330–338. doi: 10.1016/j.tins.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Munera A, Gruart A, Munoz MD, Fernandez-Mas R, Delgado-Garcia JM. Hippocampal pyramidal cell activity encodes conditioned stimulus predictive value during classical conditioning in alert cats. J. Neurophysiol. 2001;86:2571–2582. doi: 10.1152/jn.2001.86.5.2571. [DOI] [PubMed] [Google Scholar]
- 16.Pokorny J, Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. I. Development of dendritic arborisation in pyramidal neurons. Brain Res. Bull. 1981;7:113–120. doi: 10.1016/0361-9230(81)90075-7. [DOI] [PubMed] [Google Scholar]
- 17.Okuhara DY, Beck SG. 5-HT1A receptor linked to inward-rectifying potassium current in hippocampal CA3 pyramidal cells. J. Neurophysiol. 1994;71:2161–2167. doi: 10.1152/jn.1994.71.6.2161. [DOI] [PubMed] [Google Scholar]
- 18.Tan EM, et al. Selective and quickly reversible inactivation of mammalian neurons in vivo using the Drosophila allatostatin receptor. Neuron. 2006;51:157–170. doi: 10.1016/j.neuron.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Haddjeri N, Blier P, de Montigny C. Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J. Neurosci. 1998;18:10150–10156. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 21.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation and expression of pavlovian fear conditioning. J. Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greiner EF, et al. Differential ligand-dependent protein-protein interactions between nuclear receptors and a neuronal-specific cofactor. Proc. Natl. Acad. Sci. USA. 2000;97:7160–7165. doi: 10.1073/pnas.97.13.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck AT, Clark DA. Anxiety and depression: an information processing perspective. Anxiety Research. 1988;1:23–36. [Google Scholar]
- 24.Hazlett-Stevens H, Borkovec TD. Interpretive cues and ambiguity in generalized anxiety disorder. Behav. Res. Ther. 2004;42:881–892. doi: 10.1016/S0005-7967(03)00204-3. [DOI] [PubMed] [Google Scholar]
- 25.Mogg K, May J, Eysenck M. Implicit and explicit memory bias in anxiety. J. Abnorm. Psychol. 1989;98:236–240. doi: 10.1037//0021-843x.98.3.236. [DOI] [PubMed] [Google Scholar]
- 26.Gorman JM. Comorbid depression and anxiety spectrum disorders. Depress. Anxiety. 1996;4:160–168. doi: 10.1002/(SICI)1520-6394(1996)4:4<160::AID-DA2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 27.Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am. J. Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 28.Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J. Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 29.Lopez de Armentia M, Sah P. Firing properties and connectivity of neurons in the rat lateral central nucleus of the amygdala. J. Neurophysiol. 2004;92:1285–1294. doi: 10.1152/jn.00211.2004. [DOI] [PubMed] [Google Scholar]
- 30.Schiess MC, Callahan PM, Zheng H. Characterization of the electrophysiological and morphological properties of rat central amygdala neurons in vitro. J. Neurosci. Res. 1999;58:663–673. [PubMed] [Google Scholar]
- 31.Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological and immunocytochemical study. J. Comp. Neurol. 1986;246:478–499. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- 32.Veinante P, Stoeckel ME, Freund-Mercier MJ. GABA- and peptide-immunoreactivities colocalize in the rat central extended amygdala. Neuroreport. 1997;8:2985–2989. doi: 10.1097/00001756-199709080-00035. [DOI] [PubMed] [Google Scholar]
- 33.Chowdhury N, Quinn JJ, Fanselow MS. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav. Neurosci. 2005;119:1396–1402. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- 34.Quinn JJ, Oommen SS, Morrison GE, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12:495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- 35.Crestani F, et al. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat. Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- 36.Holland PC. Brain mechanisms for changes in processing of conditional stimuli in Pavlovian conditioning: implications for behavior theory. Anim. Learn. Behav. 1997;25:373–399. [Google Scholar]
- 37.Han JS, Gallagher M, Holland P. Hippocampal lesions disrupt decrements, but not increments, in conditioned stimulus processing. J. Neurosci. 1995;15:7323–7329. doi: 10.1523/JNEUROSCI.15-11-07323.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarsky T, Roxin A, Kath WL, Spruston N. Conditional dendritic-spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat. Neurosci. 2005;8:1667–1676. doi: 10.1038/nn1599. [DOI] [PubMed] [Google Scholar]
- 39.McNaughton N. Cognitive dysfunction resulting from hippocampal hyperactivity—a possible cause of anxiety disorder? Pharmacol. Biochem. Behav. 1997;56:603–611. doi: 10.1016/s0091-3057(96)00419-4. [DOI] [PubMed] [Google Scholar]
- 40.Papez JW. A proposed mechanism of emotion. 1937. J. Neuropsychiatry Clin. Neurosci. 1995;7:103–112. doi: 10.1176/jnp.7.1.103. [DOI] [PubMed] [Google Scholar]
- 41.Karpova AY, Tervo DG, Gray NW, Svoboda K. Rapid and reversible chemical inactivation of synaptic transmission in genetically targeted neurons. Neuron. 2005;48:727–735. doi: 10.1016/j.neuron.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 42.Hazen M, Ordway RW. Fast synaptic fatigue in shibire mutants reveals a rapid requirement for dynamin in synaptic vesicle membrane trafficking. Nat. Neurosci. 2000;3:859–860. doi: 10.1038/78753. [DOI] [PubMed] [Google Scholar]
- 43.Muyrers JP, Zhang Y, Testa G, Stewart AF. Rapid modification of bacterial artificial chromosomes by ET-recombination. Nucleic Acids Res. 1999;27:1555–1557. doi: 10.1093/nar/27.6.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin KF, Phillips I, Hearson M, Prow MR, Heal DJ. Characterization of 8-OH-DPAT–induced hypothermia in mice as a 5-HT1A autoreceptor response and its evaluation as a model to selectively identify antidepressants. Br. J. Pharmacol. 1992;107:15–21. doi: 10.1111/j.1476-5381.1992.tb14457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beck SG, Pan YZ, Akanwa AC, Kirby LG. Median and dorsal raphe neurons are not electrophysiologically identical. J. Neurophysiol. 2004;91:994–1005. doi: 10.1152/jn.00744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.