Abstract
We examined the permeabilities of homotypic and heterotypic gap junction (GJ) channels formed of rodent connexins (Cx) 30.2, 40, 43, and 45, which are expressed in the heart and other tissues, using fluorescent dyes differing in net charge and molecular mass. Combining fluorescent imaging and electrophysiological recordings in the same cell pairs, we evaluated the single-channel permeability (Pγ). All homotypic channels were permeable to the anionic monovalent dye Alexa Fluor-350 (AF350), but mCx30.2 channels exhibited a significantly lower Pγ than the others. The anionic divalent dye Lucifer yellow (LY) remained permeant in Cx40, Cx43, and Cx45 channels, but transfer through mCx30.2 channels was not detected. Heterotypic channels generally exhibited Pγ values that were intermediate to the corresponding homotypic channels. Pγ values of mCx30.2/Cx40, mCx30.2/Cx43, or mCx30.2/Cx45 heterotypic channels for AF350 were similar and approximately twofold higher than Pγ values of mCx30.2 homotypic channels. Permeabilities for cationic dyes were assessed only qualitatively because of their binding to nucleic acids. All homotypic and heterotypic channel configurations were permeable to ethidium bromide and 4,6-diamidino-2-phenylindole. Permeability for propidium iodide was limited only for GJ channels that contain at least one mCx30.2 hemichannel. In summary, we have demonstrated that Cx40, Cx43, and Cx45 are permeant to all examined cationic and anionic dyes, whereas mCx30.2 demonstrates permeation restrictions for molecules with molecular mass over ∼400 Da. The ratio of single-channel conductance to permeability for AF350 was ∼40- to 170-fold higher for mCx30.2 than for Cx40, Cx43, and Cx45, suggesting that mCx30.2 GJs are notably more adapted to perform electrical rather than metabolic cell-cell communication.
Keywords: connexin; gap junctions; dye; Lucifer yellow; 4,6-diamidino-2-phenylindole
Connexins (Cx), a family of membrane proteins consisting of 21 distinct isoforms in humans (35), form gap junction (GJ) channels that provide a pathway for electrical signaling and metabolic communication between cells. Cell-cell communication can be organized through homotypic, heterotypic, or/and heteromeric channels with distinct conductance, perm-selectivity, and gating properties (21).
mCx30.2, Cx40, Cx43, and Cx45 are expressed in a variety of tissues but most abundantly in cardiovascular and central nervous systems (reviewed in Ref. 24). mCx30.2, which is expressed most abundantly in the conduction system of the heart (23), is also expressed in neurons of hippocampus and different nuclei of the cerebellum, thalamus, and brain stem of adult mice (46); hCx31.9 is the putative human ortholog of mCx30.2 (26, 44). Until recently, Cx40, Cx43, and Cx45 have been regarded as the three major cardiac connexins with distinct expression patterns that have an effect on the spread of excitation within and between different regions of the heart (36, 34). Blood vessels express Cx40, Cx43, Cx45, and Cx37, with Cx40 most abundantly expressed in endothelial cells and Cx43 in smooth muscle cells (6).
Cell-to-cell dye transfer studies have been used to examine whether dyes of different net charge and size can permeate gap junction channels formed of different Cx isoforms. Techniques used most often to evaluate dye permeability include monitoring dye transfer after scrape-loading in a cell monolayer or injection of dye into a single cell through a microelectrode and monitoring fluorescence recovery after photobleaching (FRAP). These techniques are relatively easy to use, but they do not provide a measure of specific or single-channel permeability without assessment of the number of functional channels mediating dye transfer between cells. There were several attempts to determine specific or single-channel permeability by correlating cell-to-cell transfer of fluorescent dyes or radioactive compounds with GJ channel numbers estimated using electron microscopy (4, 5, 43; reviewed in Ref. 41). More recently, Weber et al. (42) reported single GJ channel permeabilities of six connexin isoforms expressed in Xenopus oocytes for fluorescent Alexa probes that vary in size but possess the same net charge. A similar approach was used to quantify dye flux through single Cx40, Cx43, and Cx45 GJ channels expressed in HeLa cells (38, 39). Recently, Eckert (16) reported single-channel permeabilities for Lucifer yellow (LY) and calcein in mammalian cells expressing Cx43 and Cx46, and Ek-Vitorin et al. (18) showed that permeability of Cx43 channels can be efficiently modulated by phosphorylation.
Previous studies have documented that GJs are permeable to second messengers involved in cell signaling, such as Ca2+ and phosphatidylinositol 1,4,5-trisphosphate (27, 31, 33). Metabolites such as glutamate, glutathione, ADP, and AMP show ∼15-fold higher permeabilities through Cx43 channels, whereas adenosine is ∼10-fold more permeable through Cx32 channels (19, 20). The above-mentioned signaling molecules and metabolites are comparable in molecular mass and net charge to the most often used fluorescent dyes, suggesting that data obtained by studying dye transfer would likely be informative for metabolic communication as well.
In this study, we assessed the single-channel permeability (Pγ) of homotypic and heterotypic GJ channels formed of mCx30.2, Cx40, Cx43, and Cx45. We have demonstrated that values of Pγ for Cx43, Cx40, Cx45, and mCx30.2 homotypic GJ channels to the anionic-monovalent fluorescent dye Alexa Fluor-350 (AF350) are 86, 33, 5.5, and 0.04 × 10−15 cm3/s, respectively. Heterotypic channels containing mCx30.2 paired with Cx43, Cx40, or Cx45 all exhibited low permeabilities, approximately twofold higher than that of the mCx30.2 homotypic GJ channel. The ratio of single-channel conductance to permeability for AF350 (γ/Pγ) was ∼40- to 170-fold higher for mCx30.2 than for Cx40, Cx43, and Cx45, suggesting that mCx30.2 GJs are notably more adapted to perform electrical rather than metabolic cell-cell communication.
Methods
Expanded methods are provided in online data supplements (supplemental data for this article is available online at the American Journal of Physiology-Heart and Circulatory Physiology website).
Cell lines and culture conditions
Experiments were performed in HeLa cells transfected with wild-type Cx30.2, Cx40, or Cx43 and Cx45 and their fusion forms with color variants of green fluorescent proteins (EGFP or CFP). Vectors used for transfection and cell lines were developed in collaboration with the laboratories of Dr. D. W. Laird (Cx43-CFP and Cx43-EGFP) and Dr. K. Willecke (mCx30.2-EGFP and Cx40-CFP); see Refs. 11, 23, and 30 for more details. To study heterotypic junctions, cells expressing different connexins were seeded on coverslips. Isolated heterotypic cell pairs were selected by identifying those in which the two cells were expressing GFPs differing in color or in which one cell expressed GFP and other was labeled with 4,6-diamidino-2-phenylindole (DAPI) or DiI.
Electrophysiological measurements
For simultaneous electrophysiological and fluorescence recording, cells grown onto coverslips were transferred to an experimental chamber mounted on the stage of inverted microscope equipped with a fluorescence imaging system. Junctional conductance (gj) was measured using the dual whole cell patch clamp. Briefly, each cell of a pair was voltage clamped independently with a separate patch clamp. By stepping the voltage in cell 1 (ΔV1) and keeping the other constant, junctional current (Ij) was measured as the change in current in the unstepped cell 2 (ΔI2): Ij = ΔI2. Thus gj was obtained from the ratio −Ij/ΔV1, where the negative sign indicates that junctional current measured in cell 2 is oppositely oriented to that measured in cell 1. To avoid affects of series resistance on measurements of gj (45), we selected cell pairs exhibiting small junctional plaque(s) (JP), typically exhibiting relatively low levels of coupling, and maintained pipette resistances below 5MΩ.
Fluorescence imaging and dye transfer studies
Fluorescence signals were acquired using UltraVIEW software for image acquisition and analysis (Perkin Elmer Life Sciences, Boston, MA). For dye transfer studies, a given dye was introduced into one cell of a pair (the donor cell) through a patch pipette in whole cell voltage-clamp mode. Typically, this resulted in rapid loading of the cell, followed by dye transfer to the neighboring (recipient) cell. After ∼6–10 min were allowed for dye spread, a whole cell recording was established in the recipient cell to measure gj. Total junctional permeability (Pdye) was evaluated according to Pdye = vol2 × ΔC2/[Δt(C1 − C2)] (22, 40), where C1 and C2 are the dye concentrations in cell 1 (donor) and cell 2 (recipient), respectively; t is time, and vol2 denotes the volume of cell 2. Cell volume was approximated as a hemisphere. The diameter of a hemisphere was determined by averaging the longest and shortest diameters of the cell; on average, the volume of examined HeLa cells was ∼1,800 μm3. Assuming that dye concentration is linearly proportional to fluorescence intensity (FI), which for a number of anionic dyes at concentrations below 1 mM is supported by our studies as well as others (17, 39), then C1 = k·FI1 and C2 = k·FI2, where k is a proportionality constant. Single-channel permeability (Pγ) can be described as follows: Pγ,dye = vol2 × ΔFI2/[Δt(FI1,n − FI2,n)(gj/γ)], where ΔFI2 = (FI2,n + 1 − FI2,n) is the change in FI in cell 2 over the time Δt = (tn + 1 − tn), n is nth time point in the recording, FI1,n − FI2,n is the FI gradient between cells, and gj/γ is the number of open channels. To increase dye detection sensitivity, which is particularly important in cases where coupling is weak and/or channel permeability is low, imaging in time-lapse mode was performed as follows. First, the entire visible field was exposed to excited light that was followed by focused excitation light directed only at the dye recipient cell. The latter allowed us to avoid emission light scattering from the dye donor cell as well as from the dye-filled pipette that can obscure dye transfer to the recipient cell in cases where permeability is low or give the appearance of dye transfer when it is, in fact, absent.
To minimize dye bleaching, imaging was performed in time-lapse mode, i.e., cells were periodically exposed (every 6 s or more) to a low-intensity light for ∼0.4 s. We also used low dye concentrations to load the cells, typically 0.1 mM and below, which minimized photo toxicity but still provided satisfactory fluorescence intensities. We found no difference in fluorescence intensity over time when images were acquired once every 20 s or every 6 s, indicating that dye bleaching was minimal.
Results
Permeability of homotypic GJ channels
Experiments were performed in cell pairs stably expressing mCx30.2, Cx40, Cx43, or Cx45 of wild type and/or fused with color variants of GFP (see methods). Figure 1A shows representative examples of homologous cell pairs containing homotypic junctions in which cell-to-cell transfer of dyes is illustrated. Dyes used include the following (molecular mass of the fluorescent ion, valence): LY (443, −2), AF350 (326, −1), ethidium bromide (EtBr) (314, +1), DAPI (279, +2), and propidium iodide (PrI) (415, +2). Table 1 summarizes the data for cell-to-cell transfer of positively charged dyes, such as EtBr, DAPI, and PrI, which were not quantitatively assessed because of their binding to nucleic acids. The presence or absence of dye transfer was assessed only in cell pairs that were electrically coupled with gj values exceeding 1 nS. Both Table 1 and Fig. 1A show that Cx40, Cx43, and Cx45 are permeable to all dyes we examined. Only mCx30.2 GJs were not permeable to LY and PrI. Both these dyes are larger in molecular mass than the others.
Fig. 1.
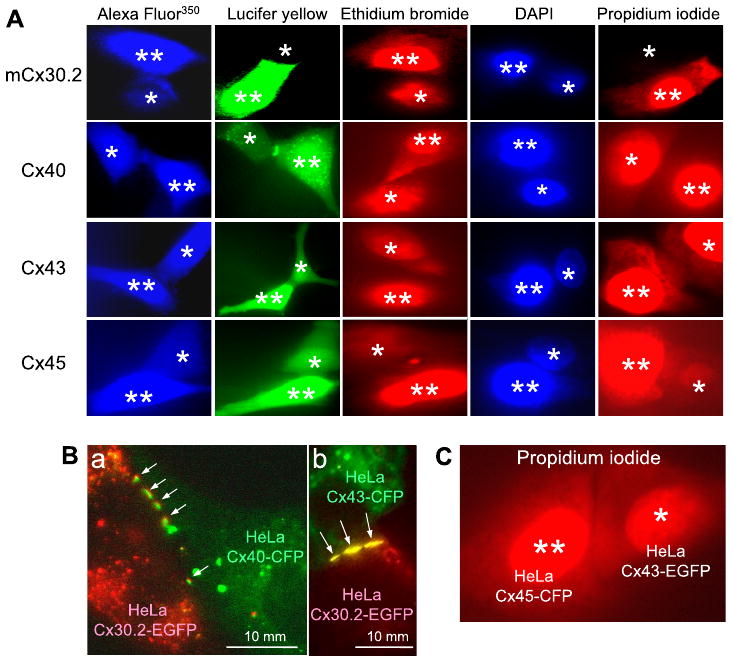
A: representative images demonstrating the presence or absence of dye transfer in homotypic junctions formed of different cardiac connexins (Cxs). Images were recorded ∼10 or more min after opening the patch in cell 1, the dye donor cell (indicated by 2 asterisks). Cell 2 is the dye acceptor cell (indicated by 1 asterisk). DAPI, 4,6-diamidino-2-phenylindole. B: examples of merged images showing that formation of heterotypic junctions is extensive (see arrows) between cells expressing mCx30.2-EGFP (in red) and Cx40-CFP (a; in green) and Cx43-CFP (b; in green). C: a representative image demonstrating propidium iodide transfer in a cell pair forming a Cx43-EGFP/Cx45-CFP heterotypic junction.
Table 1. Summary of data from homotypic GJ channels.
| Dye\Cx | mCx30.2 | Cx40 | Cx43 | Cx45 |
|---|---|---|---|---|
| EtBr | + (4) | + (10) | + (6) | + (4) |
| DAPI | + (5) | + (7) | + (5) | + (5) |
| PrI | − (5) | + (7) | + (4) | + (4) |
Summary data are from homotypic gap junction (GJ) channels in which transfer of positively charged dyes was evaluated as present (+) or absent (−). Numbers in parentheses are the number of experiments (n), which only includes cell pairs in which single-channel conductance (gj) was >1 nS. EtBr, ethidium bromide; DAPI, 4,6-diamidino-2-phenylindole; PrI, propidium bromide.
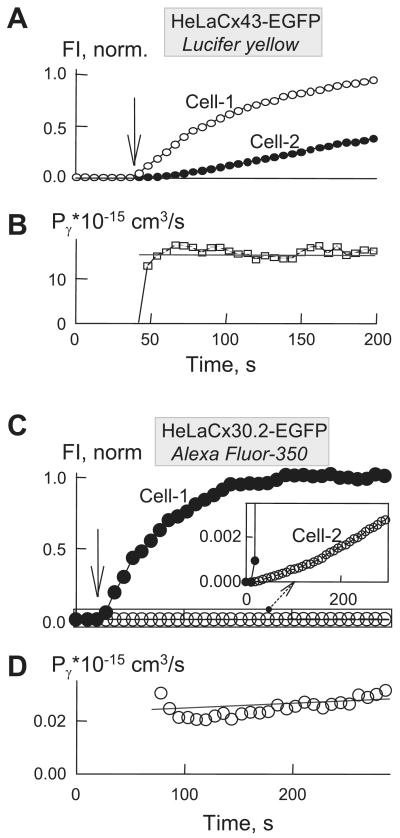
To assess a permeability of the negatively charged dyes AF350 and LY, which do not appreciably change their quantum efficiency in the cytoplasm, we measured changes in fluorescence intensities in both cells (FI1 and FI2) over time (see Fig. 2A for LY transfer in HeLaCx43-EGFP cell pair). From these measurements, we determined flux and then normalized to gj to evaluate Pγ (see methods). In each experiment, a pipette filled with dye was attached to one cell (cell 1) and the pipette without dye was attached to the other cell (cell 2). The patch in cell 1 was opened to fill it with dye and to observe dye transfer to cell 2. Approximately 10 min later, the patch in cell 2 was opened to measure gj in dual whole cell voltage-clamp mode.
Fig. 2.
Permeability of gap junction (GJ) channels. A: time course of the changes in Lucifer yellow (LY) fluorescence in a HeLaCx43-EGFP cell pair. FI, fluorescence intensity. B: plot of single-channel permeability (Pγ) vs. time, calculated from A. C: changes in Alexa Fluor-350 (AF350) fluorescence in cell 1 and cell 2 of a HeLaCx30.2-EGFP cell pair. D: plot of Pγ vs. time, calculated from C. Vertical arrows indicate the time of patch opening in cell 1. Linear regression lines of the first order are shown.
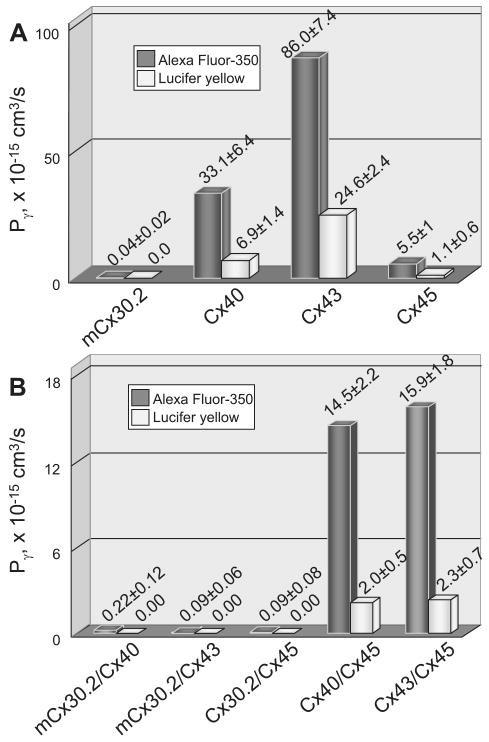
A plot of Pγ vs. time (Fig. 2B) shows that Pγ,LY of a Cx43-EGFP channel was ∼25 × 10−15 cm3/s and remained essentially constant during ∼3 min of recording. Figure 2C shows an example of AF350 transfer in a mCx30.2-EGFP cell pair. Typically, mCx30.2-EGFP channels exhibit very poor dye transfer (see FI measured over time in the inset at an extended scale in Fig. 2C), which can be detected only by using focal excitation of individual cells as described in methods. A plot of Pγ vs. time (Fig. 2D) shows that Pγ,AF-350 of mCx30.2-EGFP channels was ∼4 × 10−17 cm3/s. A bar graph (Fig. 3A) shows the mean Pγ values we obtained for all four homotypic channels for AF350 and LY. The data show that, on average, Pγ values of Cx40, Cx43, and Cx45 GJs for AF350 were ∼4.5-fold higher than for LY. Transfer of LY between mCx30.2-EGFP cells was not detected. Cx43-EGFP and mCx30.2-EGFP exhibited the highest and lowest permeabilities for AF350 with an ∼2,000-fold difference, even though single-channel conductances differ by only ∼12-fold. Cx43 exhibited ∼2.6-fold higher permeability for AF350 and ∼3.6-fold higher permeability for LY than Cx40 despite the fact that the single-channel conductance of a Cx40 channel is higher [150 vs. 115 pS (2, 7, 10)]. Pγ values for AF350 and LY were ∼15-fold lower for Cx45 than for Cx43 despite the fact that conductances of open channels differ by only ∼3.6-fold [32 vs. 115 pS (7, 9)]. We found no difference in Pγ,AF-350 and Pγ,LY for Cx43 and Cx43-EGFP channels, indicating that the GFP tag does not affect dye transfer (data not shown).
Fig. 3.
Summary of data from single-channel permeability studies. Bars show averaged Pγ values of homotypic (A) and heterotypic (B) GJ channels for AF350 and LY.
Permeability of heterotypic junctions
Studies were performed in cocultures containing different paired combinations of HeLa cells expressing mCx30.2-EGFP, Cx40-CFP, Cx40, Cx43-CFP, or Cx45. When wild-type (untagged) Cxs were used as one of the cell lines, the cells were identified by prelabeling with DAPI or DiI. Cell pairs forming heterotypic junctions were selected based on GFP fluorescence and/or labeled dye. There are six possible heterotypic junctions. Of these, we found that all but Cx40/Cx43 formed functional channels; Cx40/Cx43 appear to be incompatible to form heterotypic junctions because of their inability to dock (30). Previously (23), our group reported that mCx30.2 forms functional heterotypic junctions with Cx40, Cx43, and Cx45, exhibiting single-channel conductances of 18, 18, and 17 pS, respectively.
All remaining five types of heterotypic junctions, indicated in Fig. 3B, readily showed formation of JPs evident as fluorescing puncta in the junctional region. Figure 1B illustrates two cell pairs forming a heterotypic mCx30.2-EGFP/Cx40-CFP GJ (a) and a heterotypic mCx30.2-EGFP/Cx43-CFP GJ (b); CFP fluorescence is shown in green and EGFP in red. Table 2 summarizes permeability data for all the heterotypic junctions for positively charged dyes. All the junctions were permeable to EtBr and DAPI, whereas PrI permeated only Cx43/Cx45 junctions. PrI did not permeate mCx30.2 homotypic junctions as well as all heterotypic junctions containing mCx30.2 hemichannel.
Table 2. Summary of data from heterotypic GJ channels.
| Dye\Cx | Cx30.2/Cx40 | Cx30.2/Cx43 | Cx30.2/Cx45 | Cx43/Cx45 |
|---|---|---|---|---|
| EtBr | + (3) | + (5) | + (3) | + (5) |
| DAPI | + (7) | + (3) | + (5) | + (5) |
| PrI | − (3) | − (7) | − (4) | + (4) |
Summary data are from heterotypic GJ channels in which transfer of positively charged dyes was assessed as present (+) or absent (−). Numbers in parentheses (n) include only cell pairs in which gj > 1 nS.
Although it is problematic to quantify cell-cell transfer of positively charged dyes, the experiments suggest that permeability of Cx45/Cx43 channels for PrI is higher than that of Cx45 homotypic channels. For example, Fig. 1C shows cell-cell transfer of PrI in a HeLaCx45-CFP/HeLaCx43-EGFP cell pair in which gj was ∼45 nS. Fluorescence intensity measured in the recipient cell (1 asterisk) was ∼0.35 of that in the dye donor cell (2 asterisks). In a Cx45 homotypic cell pair (Fig. 1A, bottom right), in which gj was ∼40 nS and quite similar to that shown in Fig. 1C, the ratio of fluorescence in the injected and recipient cells was ∼0.13, i.e., ∼3-fold smaller. Unitary conductance of a Cx43/Cx45 channel is ∼1.7-fold higher than of a Cx45 channel (9). Thus the Cx45/Cx45 cell pair (Fig. 1A) contains ∼1.5-fold more functional channels than the Cx45/Cx43-EGFP cell pair (Fig. 1C). This example suggests that permeability for PrI is several times lower in Cx45 homotypic GJ channels than in Cx43/Cx45 heterotypic channels.
Figure 3B shows mean values of Pγ for AF350 and LY. We found that heterotypic junctions containing mCx30.2 hemichannels were not permeable to LY. This is in accordance with a lack of LY transfer between HeLa cells expressing mCx30.2-EGFP (23) (see also Fig. 3A). Pγ values of heterotypic channels containing a mCx30.2 hemichannel for AF350 were generally 2-fold higher than those of mCx30.2 homotypic channels and ∼100-fold lower than Pγ values of Cx40-CFP/Cx45 and Cx43-EGFP/Cx45 channels. Cx40-CFP/Cx45 and Cx43-EGFP/Cx45 channels exhibited similar Pγ values for both LY and AF350, ∼2 × 10−15 and 15 × 10−15 cm3/s, respectively. In summary, these dye transfer studies suggest that hemichannels retain their permeability characteristics in both homo- and heterotypic configurations of GJ channels.
Discussion
Cardiomyocytes express several connexins, which can form homotypic, heterotypic, and possibly heteromeric GJ channels. The composition and expression patterns of these channels determines excitation spread as well as metabolic cell-cell communication throughout the heart. Permeability properties of homotypic GJ channels formed of Cx40, Cx43, and Cx45 have been examined quite extensively (16, 20). Recently, mCx30.2 was added to the list of cardiac Cxs, and mCx30.2 GJs were shown to transfer positively and negatively charged dyes such as neurobiotin, EtBr, DAPI, and AF350 but not larger dyes such as LY or PrI (reviewed in Ref. 24). GJs between human embryonic kidney (HEK) cells expressing hCx31.9, the human ortholog of mCx30.2, were reported to lack transfer of LY as well (26). These results suggest that mCx30.2 channels do not appear to be particularly selective on the basis of charge but restrict passage of molecules with molecular mass >400 Da. There are only a few studies examining permeability of heterotypic junctions quantitatively. Cao et al. (14), using cell pairs of Xenopus oocytes and HeLa cells expressing Cx32 and Cx26, showed that Cx32/Cx26 heterotypic channels have a permeability to LY that is intermediate between Cx32 and Cx26 homotypic GJ channels. A more recent study (42) reported single-channel permeabilities of heterotypic junctions formed of Cx26, Cx32, Cx37, and Cx43 and concluded that in heterotypic GJ channels, “permeability characteristics are determined by the more restrictive parental homotypic channel,” For more, see the review in Ref. 20.
Several groups have made an attempt to evaluate the permeability of a single GJ channel, Pγ, by using different methodical approaches. Biegon et al. (4) attempted to determine the diffusion coefficient of the Cx43 channel endogenously expressed in Novikoff cells by correlating LY transfer with GJ channel numbers estimated from electron microscopic studies. Eckert (16) used this value of the diffusion coefficient to evaluate Pγ and found it equal to 37 × 10−15 cm3/s. Valiunas et al. (39) calculated the amount of dye flux through single Cx40 and Cx43 GJ channels. Using these data and assuming a constant dye concentration gradient over short time intervals, Pγ for LY is estimated to be ∼2 and 0.4 × 10−15 cm3/s for Cx43 and Cx40, respectively. Similarly, in another study (38), Pγ,LY for Cx45 was estimated to be ∼0.3 × 10−15 cm3/s. Weber et al. (42) determined the permeabilities of Alexa-type dyes in Xenopus oocytes for several connexin isoforms, including Cx43, by using a compartmental model (28) and found Pγ,AF-350 to be 180 × 10−15 cm3/s for Cx43. Recently, Eckert (16) reported a Pγ,LY of ∼17 × 10−15 cm3/s in BICR/M1Rk cells (a rat mammary tumor cell line that endogenously expresses Cx43 channels) and ∼39 × 10−15 cm3/s in HeLa Cx43 transfectants; our Pγ,LY value for HeLaCx43-EGFP cells (24.6 × 10−15 cm3/s) falls in between these two estimates. For more information on the issue of potential sources for differences in Pγ measurements, see the discussions in Refs. 16 and 42.
Our calculations of Pγ assumed that the junctional conductance we measured is defined by the number of channels that are in the open state, n = gj/γopen. All measurements were performed at Vj values close to 0 mV. For Cx40, Cx43, and mCx30.2 GJs, the channels generally reside in the open state at or near Vj = 0 mV (10, 23). For Cx45, it was shown that at Vj = 0 mV, only ∼50% of channels at any given time are open while the other 50% of the channels are fully closed by the slow gating mechanism (9). Thus Cx45 channels at Vj = 0 mV can gate between open and closed states; gating to and from the substate appears at Vj values approximately >20 mV. Therefore, for all Cxs examined in this study, the ratio gj/γopen is a reliable measure of the number of open channels. The situation becomes more complicated when dye transfer is performed at Vj values that can induce gating to the substate by the fast gating mechanism; properties of fast and slow gating mechanisms are reviewed in Ref. 13. As previously shown, the substate or residual state exhibits very different dye permeability characteristics, e.g., in HeLa cells expressing Cx43, GJ channels residing in the residual state are not permeable to AF350, which permeates open Cx43 channels (8), and in Xenopus oocytes expressing Cx43 or Cx46, Vj-gated GJ channels restrict the passage of fluorescent tracer molecules and cAMP (29). Thus a physiological role of the fast voltage gate may be to selectively restrict the passage of large molecules between cells while maintaining electrical coupling. Another critical aspect in determining Pγ is a proper measure of gj, which can be underestimated mainly because of the series resistances of the pipettes (45); typically, resistance of the patch pipette was maintained below 5 MΩ. For a cell pair with gj = ∼1 nS, those underestimates are small, but they can be substantial in cases where gj is larger. Thus, if the conductance of a GJ is underestimated, the calculated Pγ is overestimated, because there are more channels than determined from the ratio gj/γ; see the equation in methods describing Pγ evaluation. This also may explain, to some degree, differences in Pγ,LY estimates for Cx43 in our study and earlier studies (16, 39, 42).
In quantifying permeability of a single GJ channel, there are several potential sources of error in addition to dye bleaching and estimates of cell volume (see methods). These sources include dye binding to intracellular constituents and dye leakage from the cell. To roughly evaluate dye binding to cytoplasmic constituents, we loaded individual cells either with LY or AF350 using a dye-containing patch pipette. We examined loss of dye by time-lapse imaging for ∼10 min, a time period similar to that used to evaluate Pγ in cell pairs, after removing the pipette. Afterward, a second patch pipette lacking dye was used to evaluate the input resistance of the cell and then to injure the cell by fast detachment. Typically, fast (a few seconds) detachment of the pipette leads to a low-resistance seal of the excited patch and leakage of dye from the cell, whereas very slow (∼3–5 min) detachment leads to formation of high-resistance seal of patches and no fast dye leakage from the cell. Fluorescence imaging showed a rapid decay in fluorescence to nearly undetectable levels within 1–2 min of injury. The remaining fluorescence corresponded to, on average, ∼3% for LY and ∼1% for AF350. Thus binding of LY and AF350 within a period of ∼10 min appears to be minimal and would affect measurements of Pγ by underestimating their values by only 3%, at most, for LY and even less for AF350. We found that during the second patching of a cell, the input resistance was similar that measured during the first patching. The cell morphology also showed no changes. Thus loading of the cell with dye and subsequent slow removal of the pipette (typically followed by formation of a high-resistance patch, >1 GΩ) did not compromise cell membrane integrity.
To determine whether there was appreciable dye leakage from the cells through unapposed hemichannels or other pathways, we monitored fluorescence decay over several hours using time-lapse imaging after loading individual cells with LY or AF350. Earlier it was reported that HeLa and RIN cells expressing Cx45 leak ∼18% of LY molecules over a period of 100 min (38); cells were loaded with dye by exposing them for 30 min to 2 mM LY. We expected that dyes could leak through hemichannels formed of mCx30.2 and Cx43, which have been shown to function in HeLa cells (12); Cx40 does not appear to function as a hemichannel under normal perfusion conditions (1). Figure 4 shows three representative records of fluorescence decay over time in a HeLa parental cell and in cells expressing mCx30.2-EGFP and Cx43-EGFP preloaded with AF350; solid lines are fits of the data to a single exponential function. Our data show that, on average, cells expressing mCx30.2-EGFP and Cx43-CFP lose ∼47 ± 3% (n = 6) and 29 ± 4% (n = 6) of AF350 fluorescence, respectively, over a period of 100 min. Cells selected for dye efflux studies exhibited approximately similar levels of GFP fluorescence. Over the same time period, HeLa parental cells lose, on average, ∼21 ± 2% (n = 7) of AF350 fluorescence. Typically, we made calculations of Pγ within 5 min of dye loading so that we could assume that the error due to leakage of AF350 through hemichannels in HeLaCx30.2 could lead to an underestimation of Pγ by ∼3%, at most. For LY, this error is likely smaller because of its higher net charge and molecular mass, and according to the data of Ref. 38, it could be <2% for Cx45. Our data show that there was no statistically significant difference in loss of LY among HeLa parental cells and cells expressing mCx30.2, which suggests that mCx30.2 hemichannels, like their cell-cell channel counterparts, are not permeable to LY. Some degree of a leak for both AF350 and LY in HeLa parental cells can be attributed to endogenous Cx45 hemichannels and/or other pathways. In summary, these data suggest that leakage of dyes may lead to underestimation of Pγ by ∼3% or less for AF350 and ∼2% or less for LY and that these errors are Cx-type dependent.
Fig. 4.
Representative examples showing the time course of AF350 leakage from individual cells expressing mCx30.2-EGFP and Cx43-EGFP and from HeLa parental cells. Solid lines are a fit of experimental points to a single exponential function.
We examined Pγ of homo- and heterotypic channels that can form among mCx30.2, Cx40, Cx43, and Cx45 for negatively charged dyes, which largely remain unbound in the cytoplasm during the time period examined. Pγ, a measure of single-channel permeability, provides a useful index of dye cell-to-cell transfer that is essentially independent of gj, the intensity of the excited light, and the dye concentration used. Our measurement of Pγ,LY for Cx43 is in relatively good agreement with data from Biegen et al. (4) (24.6 vs. 37 × 10−15 cm3/s) and is inside the range reported by Eckert (16) (6.8 − 150 × 10−15 cm3/s). We found that Cx43 channels have an approximately threefold higher permeability for AF350 and LY than Cx40 channels despite the fact that the single-channel conductance of Cx40 is higher than that of Cx43 (150 vs. 115 pS). Cx40 channels exhibit substantially smaller permeability to anions than cations (2, 3), whereas Cx43 is largely nonselective on the basis of charge (37). This may explain the smaller Pγ value of Cx40 than Cx43 channels for negatively charged dyes. Pγ values of Cx45 for AF350 and LY are ∼15 to 20 times lower than those of Cx43, even though the single-channel conductance of Cx45 is ∼4-fold smaller than that of Cx43. In summary, our data show that Pγ values of Cx43, Cx40, Cx45, and mCx30.2 homotypic GJ channels for AF350 are ∼86, 33, 5.5, and 0.04 × 10−15 cm3/s, respectively (Table 3). Permeabilities of Cx40, Cx43, and Cx45 GJs for LY are ∼4–5-fold lower than for AF350, whereas mCx30.2 GJs are not permeable to LY.
Table 3. Summary of single-channel permeability data from homotypic and heterotypic GJ channels for AF350 and LY.
| Dye\Cx | Cx30.2 | Cx40 | Cx43 | Cx45 | Cx30.2/Cx40 | Cx30.2/Cx43 | Cx30.2/Cx45 | Cx40/Cx45 | Cx43/Cx45 |
|---|---|---|---|---|---|---|---|---|---|
| AF350 | 0.04±0.02 (5) | 33.1±6.4 (8) | 86±7.4 (5) | 5.5±1 (3) | 0.22±0.12 (6) | 0.09±0.06 (9) | 0.09±0.08 (7) | 14.5±2.2 (5) | 15.9±1.8 (6) |
| LY | n.p. (8) | 6.9±1.4 (7) | 24.6±2.4 (6) | 1.1±0.6 (3) | n.p. (3) | n.p. (3) | n.p. (3) | 2±0.5 (6) | 2.3±0.7 (7) |
Values are means ± confidence intervals of single-channel permeability (Pγ; in cm3/s) of homotypic and heterotypic GJ channels for Alexa Fluor-350 (AF350) and Lucifer yellow (LY) dyes. Numbers in parentheses are the number of experiments; n.p., nonpermeable.
Permeabilities of heterotypic mCx30.2/Cx40, mCx30.2/Cx43, or mCx30.2/Cx45 channels for AF350 are all similar and approximately twofold higher than the permeability of a mCx30.2 homotypic GJ channel (0.04 × 10−15 cm3/s). This suggests that the permeability characteristics of these hemichannels are maintained upon docking and that when a mCx30.2 hemichannel docks with a hemichannel exhibiting a much higher permeability, the mCx30.2 hemichannel largely determines the permeability of whole GJ channel. This conclusion is in accordance with studies demonstrating that permeability of heterotypic GJ channel is determined by the more restrictive hemichannel (42). Pγ values of Cx40-CFP/Cx45 and Cx43-EGFP/Cx45 GJ channels to AF350 and LY are similar (∼15 × 10−15 and ∼2 × 10−15 cm3/s, respectively; P < 0.05) despite the fact that permeabilities of Cx40 and Cx43 GJ channels differ approximately fourfold. In summary, our dye permeability studies show that the permeability property of a hemichannel is unchanged when docked homotypically or heterotypically. For any given dye and for any pairing combination we examined, the measured permeability was found to be in between those of the corresponding homotypic junctions, in agreement with earlier reports (20). In heterotypic junctions in which one of the hemichannels is considerably less permeant to a particular dye, the lower permeability hemichannel will largely determine the permeability of the whole channel. In the case of mCx30.2, which exhibits the smallest conductance of any Cx isoform (23) and whose permeability to dyes is generally low, most pairing combinations result in heterotypic channels with very similar permeability characteristics.
Permeabilities of positively charged dyes could only be assessed qualitatively. Our study shows that Cx40, Cx43, and Cx45 channels were permeable to all three of the positively charged dyes we examined. For mCx30.2, we detected transfer of DAPI but did not observe transfer of PrI. Both are divalent, cationic dyes, but DAPI is smaller in size than PrI. This result, together with the lack of LY transfer, indicates that discrimination in mCx30.2 is likely the result of size rather than charge and suggests that there may be a constriction in the pore of mCx30.2 channels that limits the passage of molecules greater than ∼400 Da.
In Cx43, Cx40, Cx45, and mCx30.2 homotypic GJ channels, the ratios of single-channel conductance to permeability for AF350 (γ/Pγ,AF-350) are 1.3, 4.5, 5.8, and 225 × 10−3 S·cm−3·s, respectively. These ratios suggest that mCx30.2 GJ channels are ∼40- to 170-fold more adapted than GJs formed of other cardiac Cxs for electrical cell-to-cell signaling rather than for metabolic communication. Given that the permeability and conductance properties of Cxs appear to be retained in hemichannels and cell-cell channels (see Ref. 39 for Cx45; reviewed in Ref. 32), it is possible that mCx30.2 hemichannels can function as membrane ion channels without introducing leak of metabolites that can be detrimental to the cell. This statement may appear contradictory because of higher dye uptake by HeLa cells expressing mCx30.2 than by cells expressing Cx43 (12). Earlier our group reported that mCx30.2 hemichannels remain largely open at positive and negative membrane potential (Vm) (12), whereas Cx43 hemichannels close at negative Vm and open at large positive Vm (15). Therefore, the open probability of mCx30.2 hemichannels is likely to be much higher than that of Cx43 hemichannels at the resting potential under normal perfusion conditions. We assume that the slow gating mechanism tends to close Cx43 hemichannels at the resting potential because of its substantial sensitivity to voltage. Voltage gating sensitivity of mCx30.2 GJ channels is the lowest among Cx isoforms (23), and this may explain why mCx30.2 hemichannels operate at positive and negative potentials (12). Thus differences in voltage gating sensitivity likely explain the differences in dye uptake among cells expressing Cx43 and mCx30.2. In support of this, our studies show that AF350 leaks faster from cells expressing mCx30.2 than from HeLa parental cells (see Fig. 4 and supporting text) (P < 0.001), whereas there is no statistically significant difference between these two types of cells for the leak of LY (P > 0.4). These data indicate that mCx30.2 hemichannels, like their GJ channel counterparts, are permeable to AF350 but not to LY. The inferred poor selectivity among the monovalent inorganic cations and anions, principally Na+, K+, and Cl−, suggests that open mCx30.2 hemichannels will exert a depolarizing influence on nodal cells that may slow propagation of excitation by inactivating excitatory inward currents (25). Thus mCx30.2 may represent an evolutionary selection, reflecting the need to function in nodal tissues as GJ channels and as membrane channels (hemichannels) without compromising cell survival.
Supplementary Material
Acknowledgments
We thank Dr. Laird and Dr. Willecke for kindly providing the constructs of Cx30.2, Cx40, and Cx43 tagged with GFPs, and Angele Bukauskiene for excellent technical assistance.
Grants: This work was supported by National Institutes of Health Grants R01 NS036706 (to F. F. Bukauskas) and GM54179 (to V. K. Verselis).
References
- 1.Beahm DL, Hall JE. Opening hemichannels in nonjunctional membrane stimulates gap junction formation. Biophys J. 2004;86:781–796. doi: 10.1016/S0006-3495(04)74154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beblo DA, Veenstra RD. Monovalent cation permeation through the connexin40 gap junction channel. Cs, Rb, K, Na, Li, TEA, TMA, TBA, and effects of anions Br, Cl, F, acetate, aspartate, glutamate, and NO3. J Gen Physiol. 1997;109:509–522. doi: 10.1085/jgp.109.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beblo DA, Wang HZ, Beyer EC, Westphale EM, Veenstra RD. Unique conductance, gating, and selective permeability properties of gap junction channels formed by connexin40. Circ Res. 1995;77:813–822. doi: 10.1161/01.res.77.4.813. [DOI] [PubMed] [Google Scholar]
- 4.Biegon RP, Atkinson MM, Liu TF, Kam EY, Sheridan JD. Permeance of Novikoff hepatoma gap junctions: quantitative video analysis of dye transfer. J Membr Biol. 1987;96:225–233. doi: 10.1007/BF01869304. [DOI] [PubMed] [Google Scholar]
- 5.Brink PR, Dewey MM. Nexal membrane permeability to anions. J Gen Physiol. 1978;72:62–86. doi: 10.1085/jgp.72.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruzzone R, Haefliger JA, Gimlich RL, Paul DL. Connexin40, a component of gap junctions in vascular endothelium, is restricted in its ability to interact with other connexins. Mol Biol Cell. 1993;4:7–20. doi: 10.1091/mbc.4.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukauskas FF, Bukauskiene A, Bennett MV, Verselis VK. Gating properties of gap junction channels assembled from connexin43 and connexin43 fused with green fluorescent protein. Biophys J. 2001;81:137–152. doi: 10.1016/S0006-3495(01)75687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukauskas FF, Bukauskiene A, Verselis VK. Conductance and permeability of the residual state of connexin43 gap junction channels. J Gen Physiol. 2002;119:171–186. doi: 10.1085/jgp.119.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukauskas FF, Bukauskiene A, Verselis VK, Bennett MVL. Coupling asymmetry of heterotypic connexin 45/connexin 43-EGFP gap junctions: properties of fast and slow gating mechanisms. Proc Natl Acad Sci USA. 2002;99:7113–7118. doi: 10.1073/pnas.032062099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bukauskas FF, Elfgang C, Willecke K, Weingart R. Biophysical properties of gap junction channels formed by mouse connexin40 in induced pairs of transfected human HeLa cells. Biophys J. 1995;68:2289–2298. doi: 10.1016/S0006-3495(95)80411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bukauskas FF, Jordan K, Bukauskiene A, Bennett MV, Lampe PD, Laird DW, Verselis VK. Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proc Natl Acad Sci USA. 2000;97:2556–2561. doi: 10.1073/pnas.050588497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bukauskas FF, Kreuzberg M, Rackauskas M, Bukauskiene A, Bennett MVL, Verselis VK, Willecke K. Properties of mouse connexin 30.2 and human connexin 31.9 hemichannels: implications for atrioventricular conduction in the heart. Proc Natl Acad Sci USA. 2006;103:9726–9731. doi: 10.1073/pnas.0603372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukauskas FF, Verselis VK. Gap junction channel gating. Biochim Biophys Acta. 2004;1662:42–60. doi: 10.1016/j.bbamem.2004.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao F, Eckert R, Elfgang C, Nitsche JM, Snyder SA, Hulser DF, Willecke K, Nicholson BJ. A quantitative analysis of connexin-specific permeability differences of gap junctions expressed in HeLa transfectants and Xenopus oocytes. J Cell Sci. 1998;111:31–43. doi: 10.1242/jcs.111.1.31. [DOI] [PubMed] [Google Scholar]
- 15.Contreras JE, Saez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci USA. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckert R. Gap-junctional single-channel permeability for fluorescent tracers in mammalian cell cultures. Biophys J. 2006;91:565–579. doi: 10.1529/biophysj.105.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ek-Vitorin JF, Burt JM. Quantification of gap junction selectivity. Am J Physiol Cell Physiol. 2005;289:C1535–C1546. doi: 10.1152/ajpcell.00182.2005. [DOI] [PubMed] [Google Scholar]
- 18.Ek-Vitorin JF, King TJ, Heyman NS, Lampe PD, Burt JM. Selectivity of connexin 43 channels is regulated through protein kinase C-dependent phosphorylation. Circ Res. 2006;98:1498–1505. doi: 10.1161/01.RES.0000227572.45891.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg GS, Lampe PD, Nicholson BJ. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol. 1999;1:457–459. doi: 10.1038/15693. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg GS, Valiunas V, Brink PR. Selective permeability of gap junction channels. Biochim Biophys Acta. 2004;1662:96–101. doi: 10.1016/j.bbamem.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–427. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 22.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- 23.Kreuzberg MM, Sohl G, Kim J, Verselis VK, Willecke K, Bukauskas FF. Functional properties of mouse connexin30.2 expressed in the conduction system of the heart. Circ Res. 2005;96:1169–1177. doi: 10.1161/01.RES.0000169271.33675.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreuzberg MM, Willecke K, Bukauskas F. Connexin-mediated cardiac impulse propagation: connexin 30.2 slows atrioventricular conduction in mouse heart. Trends Cardiovasc Med. 2006;16:266–272. doi: 10.1016/j.tcm.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meijler FL, Janse MJ. Morphology and electrophysiology of the mammalian atrioventricular node. Physiol Rev. 1988;68:608–647. doi: 10.1152/physrev.1988.68.2.608. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen PA, Beahm DL, Giepmans BN, Baruch A, Hall JE, Kumar NM. Molecular cloning, functional expression, and tissue distribution of a novel human gap junction-forming protein, connexin-31.9. Interaction with zona occludens protein-1. J Biol Chem. 2002;277:38272–38283. doi: 10.1074/jbc.M205348200. [DOI] [PubMed] [Google Scholar]
- 27.Niessen H, Harz H, Bedner P, Kramer K, Willecke K. Selective permeability of different connexin channels to the second messenger inositol 1,4,5-trisphosphate. J Cell Sci. 2000;113:1365–1372. doi: 10.1242/jcs.113.8.1365. [DOI] [PubMed] [Google Scholar]
- 28.Nitsche JM, Chang H, Weber PA, Nicholson BJ. A transient diffusion model yields unitary gap junctional permeabilities from images of cell-to-cell fluorescent dye transfer between Xenopus oocytes. Biophys J. 2004;86:2058–2077. doi: 10.1016/S0006-3495(04)74267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu Y, Dahl G. Function of the voltage gate of gap junction channels: selective exclusion of molecules. Proc Natl Acad Sci USA. 2002;99:697–702. doi: 10.1073/pnas.022324499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rackauskas M, Kreuzberg MM, Pranevicius M, Willecke K, Verselis VK, Bukauskas FF. Gating properties of heterotypic gap junction channels formed of connexins 40, 43 and 45. Biophys J. 2007;92:1952–1965. doi: 10.1529/biophysj.106.099358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saez JC, Connor JA, Spray DC, Bennett MVL. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci USA. 1989;86:2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MVL. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta. 2005;1711:215–224. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanderson MJ. Intercellular calcium waves mediated by inositol trisphosphate. Ciba Found Symp. 1995;188:175–189. [PubMed] [Google Scholar]
- 34.Severs NJ, Dupont E, Coppen SR, Halliday D, Inett E, Baylis D, Rothery S. Remodelling of gap junctions and connexin expression in heart disease. Biochim Biophys Acta. 2004;1662:138–148. doi: 10.1016/j.bbamem.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 35.Sohl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat Rev Neurosci. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- 36.Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–232. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Trexler EB, Bukauskas FF, Kronengold J, Bargiello TA, Verselis VK. The first extracellular loop domain is a major determinant of charge selectivity in connexin46 channels. Biophys J. 2000;79:3036–3051. doi: 10.1016/S0006-3495(00)76539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valiunas V. Biophysical properties of connexin-45 gap junction hemichannels studied in vertebrate cells. J Gen Physiol. 2002;119:147–164. doi: 10.1085/jgp.119.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valiunas V, Beyer EC, Brink PR. Cardiac gap junction channels show quantitative differences in selectivity. Circ Res. 2002;91:104–111. doi: 10.1161/01.res.0000025638.24255.aa. [DOI] [PubMed] [Google Scholar]
- 40.Verselis V, White RL, Spray DC, Bennett MV. Gap junctional conductance and permeability are linearly related. Science. 1986;234:461–464. doi: 10.1126/science.3489990. [DOI] [PubMed] [Google Scholar]
- 41.Verselis VK, Veenstra RD. Gap junction channels. Permeability and voltage gating. In: Hertzberg E, editor. Advances in Molecular and Cell Biology. Greenwich, CT: JAI; 2000. pp. 129–192. [Google Scholar]
- 42.Weber PA, Chang H, Spaeth KE, Nitsche JM, Nicholson BJ. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys J. 2004;87:958–973. doi: 10.1529/biophysj.103.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weingart R. The permeability to tetraethylammonium ions of the surface membrane and intercalated disks of sheep and calf myocardium. J Physiol. 1974;240:741–762. doi: 10.1113/jphysiol.1974.sp010632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White TW, Srinivas M, Ripps H, Trovato-Salinaro A, Condorelli DF, Bruzzone R. Virtual cloning, functional expression, and gating analysis of human connexin31.9. Am J Physiol Cell Physiol. 2002;283:C960–C970. doi: 10.1152/ajpcell.00163.2002. [DOI] [PubMed] [Google Scholar]
- 45.Wilders R, Jongsma HJ. Limitations of the dual voltage clamp method in assaying conductance and kinetics of gap junction channels. Biophys J. 1992;63:942–953. doi: 10.1016/S0006-3495(92)81664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willecke K, Kreuzberg M. Expression pattern of the mouse con-nexin30.2 gene in neurons of hippocampus, thalamus, cerebellum and brain stem. FENS 5th Vienna. 2006:A119.127. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.