Abstract
The proper development of digits, in tetrapods, requires the activity of several genes of the HoxA and HoxD homeobox gene complexes. By using a variety of loss-of-function alleles involving the five Hox genes that have been described to affect digit patterning, we report here that the group 11, 12, and 13 genes control both the size and number of murine digits in a dose-dependent fashion, rather than through a Hox code involving differential qualitative functions. A similar dose–response is observed in the morphogenesis of the penian bone, the baculum, which further suggests that digits and external genitalia share this genetic control mechanism. A progressive reduction in the dose of Hox gene products led first to ectrodactyly, then to olygodactyly and adactyly. Interestingly, this transition between the pentadactyl to the adactyl formula went through a step of polydactyly. We propose that in the distal appendage of polydactylous short-digited ancestral tetrapods, such as Acanthostega, the HoxA complex was predominantly active. Subsequent recruitment of the HoxD complex contributed to both reductions in digit number and increase in digit length. Thus, transition through a polydactylous limb before reaching and stabilizing the pentadactyl pattern may have relied, at least in part, on asynchronous and independent changes in the regulation of HoxA and HoxD gene complexes.
Posterior (AbdB-related) Hox genes belonging to both the HoxD and the HoxA complexes are necessary for the proper organization and development of tetrapod digits (for review, see ref. 1). In mice, genetic analyses have revealed that multiple Hox functions cooperate in a rather nonspecific fashion to elaborate the normal sequence of bony elements that compose the appendicular skeleton. For example, the contribution of either Hoxd-13 (2) or Hoxa-13 (3) is required for digit development with double homozygous mutant animals lacking digits entirely (3). Additionally, both the number and the morphogenesis of digits are strongly affected in animals lacking three HoxD functions in cis (Hoxd-11, Hoxd-12, and Hoxd-13; ref. 4), more severely than in the absence of Hoxd-13 alone, indicating that genes belonging to paralogous groups 11 and 12 may participate to these processes as well (see also refs. 5–7). Yet, the combined loss of Hoxd-11 and Hoxa-11 function had only incidental effects on digit morphology. Instead, it drastically truncated the intermediate pieces of both limbs (7), suggesting either that hierarchical relationships exist between Hox functions such that the function of group 11 genes in digits is negligible as long as group 13 proteins are present, or that group 11 and 12 genes can have a function in digits that is not necessarily required in wild-type animals (1, 5).
In Hox mutant strains that show selective distal limb defects, the most commonly observed digit alterations involve reduction in the size of skeletal elements and loss of phalanges. These alterations, sometimes combined with diversions from the pentadactyl formula, were considered to be possible atavisms (2, 3). Recently, similar digit defects also were observed in human patients carrying mutations either in the HOXA13 or in the HOXD13 genes (8, 9). Conversely, the forced ectopic expression of more than the normal amounts of various Hox products in limb buds induced surplus digital material (10–13), which is consistent with a quantitative role of Hox products in the making of a limb.
Although most extant tetrapods have a pentadactyl digit formula, an analysis of the fossil record indicated that ancestral stem tetrapods had polydactylous extremities with seven to eight digits (14). It therefore is assumed that the evolutionary transition between limbs without digits (adactylous), such as in Panderychtis, and limbs with five digits (pentadactylous) involved an intermediate stage of polydactyly, a state that perhaps was linked to the aquatic status of these primitive ancestral tetrapods (15). It is indeed conceivable that the crawling locomotion required by an aquatic environment, favored individuals with multiple and short digits, resulting in paddle-like autopods, rather than a more restricted number of longer digits, which, by contrast, may be advantageous for locomotion in a terrestrial environment. The functional importance of Hox genes in these evolutionary remodelings of the autopods remains speculative. However, three sets of evidence may support a scenario in which at least some of these modifications were driven, or paralleled, by changes in Hox gene regulation and expression. First, phenotypes induced by loss-of-function mutations of posterior Hox genes systematically present a strong atavistic character (2), therefore suggesting that these phenotypes somehow illustrate phylogenetic features or potentialities of the autopod. Second, the expression of Hoxa and Hoxd genes during teleost fin development is compatible with a role for these genes in the fin to limb transition (16, 17). And finally, the observation that the transcriptional regulation of up to five posterior HoxD complex genes active in the embryonic digits act through a shared regulatory mechanism makes it plausible that several Hoxd genes were recruited simultaneously during digit evolution to provide a sufficient dose of Hox proteins (18).
In this work, we used a variety of loss-of-function alleles of murine HoxD and HoxA complex genes expressed in digits to analyze the quantitative and qualitative aspect of this regulation, as well as to investigate the concurrent phenotypic transformations. We show that in compound mutants both the size and number of digits varied in response to quantitative modifications of Hox gene function, and that this response was linear and rather independent of the specific gene involved. A progressive decrease in dosage of functional Hox genes in developing digits induced a graded reduction in the length of the digits and was accompanied by a concomitant transition from pentadactyly to polydactyly, then to oligodactyly, finally reaching the adactyl condition. The relevance of these results is discussed in the context of an evolutionary process involving successive recruitments of the HoxA and HoxD complexes in developing appendages.
MATERIALS AND METHODS
Description of the Alleles.
Four loss-of-function alleles, induced by gene inactivation in murine embryonic stem cells through homologous recombination, were used in the course of this work. The Hoxd-13 and Hoxa-13 mutant alleles were homeodomain disruptions, in which the TKneo selection cassette was inserted into the homeobox of either Hoxd-13 (2) or Hoxa-13 (3). For the Hoxa-11 mutant allele, the gene was disrupted by replacing the homeobox containing genomic region with the PGKneo selection cassette (19). The HoxDDel mutant allele was produced as a loxP/cre induced deletion of a genomic region encompassing Hoxd-13 and Hoxd-12, plus lacZ reporter sequences were inserted in-frame in the first exon of the Hoxd-11 gene. This HoxDDel allele thus represents a triple Hoxd-13, Hoxd-12, and Hoxd-11 loss of function, with a Hoxd-11/lacZ fusion product allowing for histochemical detection of β-galactosidase activity (4). All four alleles were generated in 129/Sv-derived embryonic stem cell line (D3 embryonic stem cells; ref. 20, gift of R. Kemler, Max-Planck-Institute of Immunobiology, Freiburg, Germany). Hoxd-13, Hoxa-13, and HoxDDel were maintained in a 129/Sv and C57BL/6 mixed genetic background. The Hoxa-11 allele originally was established in the 129/Sv and CF-1 mixed genetic background, but, in the course of our experiments, it was bred into the 129/Sv and C57BL/6 mixed genetic background for at least four consecutive generations before it was used in the test cross.
Genetic Crosses.
Genetic interactions between the Hoxd-13, Hoxa-13, and HoxDDel null alleles were established by using the following crosses. First, Hoxd-13−/− females were crossed to HoxDDel/+ males to obtain Hoxd-13−/HoxDDel transheterozygous animals. Second, compound heterozygotes for Hoxa-13+/−;Hoxd-13+/− females were crossed to HoxDDel/+ males to obtain Hoxa-13+/−;Hoxd-13−/HoxDDel. Finally, HoxDDel/+;Hoxa-13+/− males and females were intercrossed to obtain all possible genotypes issuing in the F2 generation. Consistent with previous analyses, we found the Hoxa-13−/− constitution to be embryonic lethal (3), whereas the Hoxa-13+/−;Hoxd-13−/−, Hoxa-13+/−;Hoxd-13−/HoxDDel and Hoxa-13+/−;HoxDDel/HoxDDel configurations were semilethal with only occasional animals surviving to adulthood. Genotyping of HoxDDel/+;Hoxa-13+/− F2 progeny was carried out by using yolk sac-derived DNA, according to the Southern blotting protocols reported in the original descriptions of the alleles. Skeletal preparations were performed according to standard procedures (2). To establish genetic interactions with Hoxa-11, we crossed compound heterozygotes of the HoxDDel/+;Hoxa-11+/− genotypes and analyzed the F2 progeny either at birth, at 5 days or at 5 weeks after delivery. The Hoxa-11−/−;HoxDDel/Del constitution was semilethal as well. All genotypes were present at birth following a Mendelian distribution, yet five of six Hoxa-11−/−;HoxDDel/Del animals died by the fifth postnatal day because of kidney agenesis, consistent with the phenotype of Hoxa-11−/−;Hoxd-11−/− compound homozygots (7).
Calculation of the Hox Dose and Digit Length.
Based on previously reported strengths of phenotypes in digits, one wild-type haplotype of Hoxd-11 together with Hoxd-12 was considered to contribute one unit (henceforth Hoxd-11/Hoxd-12) whereas one wild-type allele of both Hoxd-13 and Hoxa-13 were considered to contribute two units of Hox function each. In this way, the wild-type Hox dose is the sum of two units (Hoxd-11/12), plus four units (Hoxd-13) plus four units (Hoxa-13), hence an arbitrary total of 10 units (Fig. 1A). In previous experiments, the most refractory indicator of genetic interactions between Hoxd genes were digits III and IV (see e.g., refs. 3 and 6) and the novel genetic constitutions isolated in the course of this work also confirmed the stability in the size of these digits (Figs. 1 and 2). Five-week-old animals’ forelimb and hindlimb skeletons were observed under a dissecting microscope and the length of digit IV from the proximal end of the metatarsal bone to the tip of the claw was measured with 0.25-mm precision on a millimeter scale. The arithmetic mean of the left and right digits’ length was expressed as a percentage of that observed in age matched controls, siblings whenever possible. The mutant vs. sibling control percentage values proved similar at the 11th day and at 5 weeks of age and were thus entered into the same plot (Fig. 1H). Each entry represented one animal. The entry for a single-dose unit was based on the observation that no digit developed in Hoxd-13−/−;Hoxa-13−/− animals by embryonic day 16.5 (3), the oldest stage at which animals of this genotype can be recovered alive. The entry for two units represented four HoxDDel/Del;Hoxa-13+/− animals; the entry for three units represented one Hoxd-13−/HoxDDel;Hoxa-13+/− animal; the entries for four units represented an Hoxd-13−/−;Hoxa-13+/− and four HoxDDel/Del animals; the entries for five units represented an Hoxd-13−/HoxDDel and three HoxDDel/+;Hoxa-13+/− animals; the entry for six units represented one Hoxd-13−/− animal, and the entries for seven units represented two HoxDDel/+ animals. The linear regression fit of forelimb digit IV length (Fig. 1 H) was calculated by using Cricket Graph software; the regression coefficient was 0.95. The validity of the plot can be controlled by projecting the digit length and Hox doses of other compound genotypes of either Hoxd-11, Hoxd-12, and Hoxd-13 (6) or HoxDDel/Hoxd-11− (21), which were not included in the present curve.
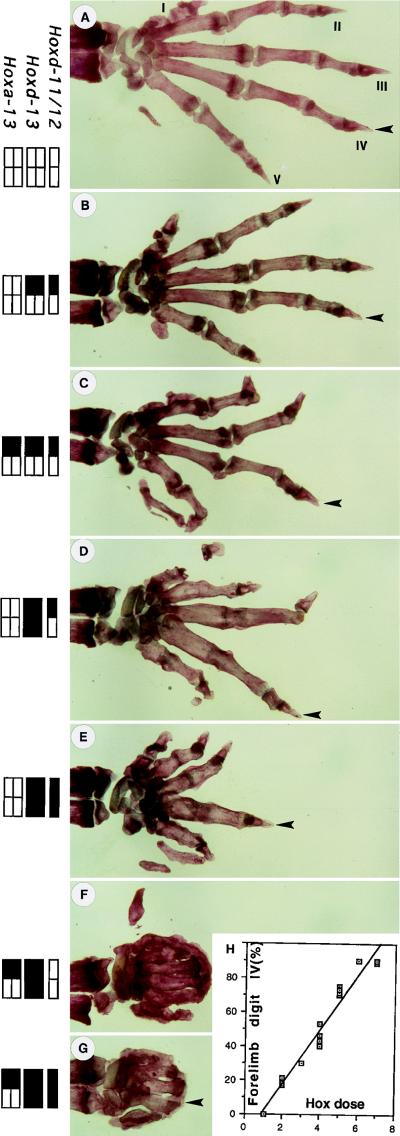
Figure 1.
Digit length is additively determined by the dose of Hoxd-11, Hoxd-12, Hox-13, and Hoxa-13 gene products. The length of forelimb digit IV (arrowheads), including the metacarpal bone, was taken as a reference measure in mice of different genotypes. Compound mutant genotypes are indicated on the left, black rectangles indicating the loss of one dose of the corresponding gene (shown at the top). In this view, the wild-type hand (A) had 10 doses of active products. From B to G doses were progressively removed by using the corresponding genotypes: (B) HoxDDel/+; (C) HoxDDel/+;Hoxa-13+/−; (D) Hoxd-13−/HoxDDel; (E) HoxDDel/Del; (F) Hoxd-13−/−/;Hoxa-13+/−; (G) HoxDDel/Del;Hoxa-13+/−. (H) The length of digit IV was measured, expressed as fraction of wild-type digit length, and plotted against the Hox dose (see Materials and Methods for the calculation of the respective dose per gene). In this way, the length of digit IV varied as a linear function of the dose, regardless of the nature of the combination. (A–G) Anterior is up, posterior is down. I–V indicate digit number with, by convention, digit I being the thumb. In mutants with more or less than five digits, the phalanx pattern makes individual homologization impossible, but the digit found at the position corresponding to wild-type digit IV was always the longest.
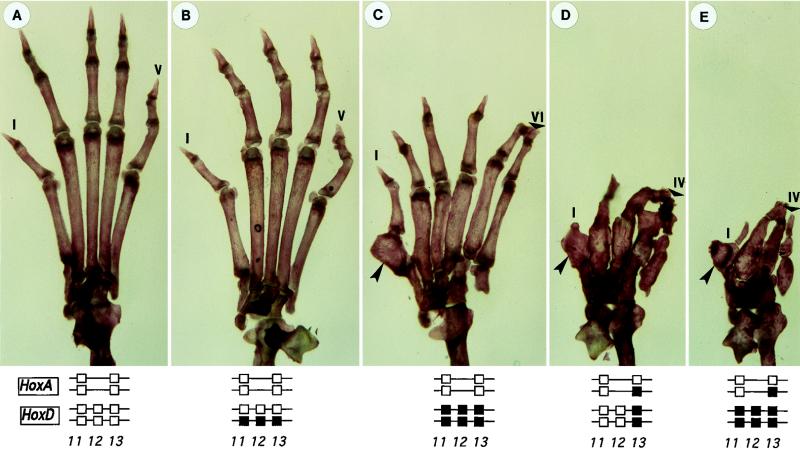
Figure 2.
Dose-dependent variation of the dactyly, as illustrated by the feet of mice of various genotypes. The posterior parts of both complexes are schematized below with the genes whose functions were removed in black. (A) Wild type. (B) HoxDDel/+. (C) HoxDDel/Del. (D) Hoxd-13−/−;Hoxa-13+/−. (E) HoxDDel/Del;Hoxa-13+/−. The progressive ectrodactyly goes together with a transition from pentadactyly (A and B) to polydactyly (C) to oligodactyly (E). Digits I (anterior to the left) and V are indicated as well as the most posterior digit IV or VI (small arrowheads in C and D). The large arrowheads in C and D point to a digit I specific alteration produced in absence of Hoxd-13 (2), which identifies this digit. Thus, although the phalanx pattern does not allow homologies with other digits, the extra digit belongs to the II-V domain.
RESULTS AND DISCUSSION
Digit Length.
The most refractory indicator of genetic interactions between Hoxd genes in distal limbs was digit IV. Digit IV is derived from a very posterior position in the developing limb bud, a region where the posterior Hox genes all are expressed early on (36). It is also the first digit to condense as the digital arch forms, which makes it a good indicator of quantitative interactions between gene products, as revealed by its length (Fig. 1). For example, HoxDDel/+ mice, in which the function of one haplotype of Hoxd-11, Hoxd-12, and Hoxd-13 were inactivated simultaneously, showed phalanx 2 defects in digits II and V in the forelimb (Fig. 2B). The size of digit IV nonetheless remained essentially unaltered. However, when one dose of group 13 gene was further removed, either in HoxDDel/+;Hoxa-13+/− (Fig. 1C) or in HoxDDel/Hoxd-13 mice (Fig. 1D), animals had severe defects in every digit, the length of digit IV being also reduced to about 70% of wild-type length (Fig. 2 A, C, and D). Subsequent elimination of the wild-type alleles, for example in specimen of the Hoxd-13−/−;Hoxa-13+/− (Fig. 1F) or HoxDDel/Del;Hoxa-13+/− (Fig. 1G) genotypes, generated an accentuated reduction in digit length, leaving only 30% and 20% of normal size, respectively.
A linear relationship was observed between, on the one hand, the additive dose of Hoxd-11/Hoxd-12 taken together, Hoxd-13 and Hoxa-13, and on the other hand, the adult size of forelimb digit IV (see Materials and Methods and Fig. 1). In this context, Hoxd-13−/HoxDDel mice, for example, had a similar “Hox dose” to HoxDDel/+;Hoxa-13+/− (five doses; Fig. 1 D and C). Correspondingly, they generated very similar sizes for digit IV. In hindlimbs, the same linear relationship was observed between seven and four dose units, although at lower doses involving Hoxd-13−/− and Hoxa-13+/−, further reducing the Hoxd-11/Hoxd-12 dose did not importantly reduce hindlimb digit size, which stayed at about 30 percent of wild type (Fig. 2 D and E).
These results suggested that the function of posterior Hox genes during digit growth is exerted mostly at the quantitative level and does not involve precise qualitative combinatorial information. It is, however, impossible to completely rule out a scenario in which only the group 13 genes would be critical for digits. In such a view, the absence of part or all group 13 function would allow more 3′ genes to exert an “artificial” function in digit development, i.e., a function that they normally would not carry out in the presence of group 13 functions (1, 5). This possibility cannot be assessed by using the available alleles, and the genetic approach also may have to be complemented by biochemical evidence.
Digit Number.
In forelimbs and hindlimbs of mice triple homozygous mutant for Hoxd-11, Hoxd-12, and Hoxd-13 (Fig. 2C; HoxDDel/Del), as well as in forelimbs of animals compound homozygous for the Hoxd-13 mutation and heterozygous for Hoxa-13 (ref. 3 and Fig. 1F), up to seven digit vestiges were found. Therefore, starting from a Hoxd-13 homozygous mutant background, the additional elimination of either the Hoxd-11/Hoxd-12 locus, or one allele of Hoxa-13, led to an increase in digit number. When Hoxd-11/Hoxd-12 and Hoxa-13 levels were reduced simultaneously from the same Hoxd-13-deficient background, novel polydactylous constitutions were found combined with more severe digit size reductions. However, the two extreme genotypes generated by combining these various alleles, i.e., HoxDDel/Del; Hoxa-13+/− (four digits, Figs. 1G and 2E) and HoxDDel/+;Hoxa-13−/− (three digital condensations visible only at embryonic day 14.5, not shown) displayed oligodactyly, with very small digit vestiges.
In Hoxa-13−/− simple mutant animals, i.e., with the normal complement of functional Hoxd genes, oligodactyly also was observed because of the loss of digit I (3). This observation was, however, consistent with the poor expression of Hoxd genes at the anterior edge of the autopod (in digit I primordium), leading to a strong dependence of this digit on the remaining Hoxa-13 function (3). This particular case was thus different from the low Hox dose genotypes reported here, where changes in digit number involved also the domain that normally would give rise to digits II–V. In the HoxDDel/Del;Hoxa-13+/− genotype, oligodactyly was observed in the presence of one Hoxa-13 wild-type allele and thus digit I is presumed to be present (Fig. 2E, arrowhead). Likewise, in HoxDDel/+;Hoxa-13−/− animals, which have only three digit condensations at embryonic day 14 (not shown), more than one digit was missing, indicating that the defect could not be solely ascribed to the absence of digit I.
The loss of Hoxa-11 function did not significantly modify the dose balance required for proper digit development. Mice of the HoxDDel/Del;Hoxa-11−/− genotype were indeed polydactylous in their hindlimbs, with a reduced incidence of polydactyly in the forelimbs, and their digits were only marginally shorter than those of HoxDDel homozygous mice (not shown). Oligodactyly was not observed in this configuration. Even though both the Hoxa-11 as well as the Evx-2 (located near Hoxd-13) genes were shown to contribute to digit development (22, 23), their combined function was insufficient to rescue digit growth in HoxDDel/Del;Hoxa-13−/− mice, suggesting that the four wild-type alleles of Hoxa-11 and Evx-2 together contribute less than two “Hox dose units,” as measured in the crosses above.
In summary, it appears that both the size and number of digits are dependent on Hoxd-11, Hoxd-12, Hoxd-13, and Hoxa-13, making these four genes the major Hox determinants of digit morphogenesis. Step-wise reduction of Hox dosage induced not only an increasing severity in digit size defects, i.e., ectrodactyly, but also led to a transition from pentadactyly to polydactyly, whereas further reduction generated oligodactyly and finally adactyly, i.e., complete digit loss. This result suggests that a common Hox dose-dependent mechanism may control both the number and the size of digits. Although the effect on digit number may reflect the early role of these genes in the formation of prechondrogenic condensations, the effect on digit growth also might involve the control of chondroblast cell proliferation and maturation (4, 11). Hence, these two important parameters may reflect the two levels at which Hox genes previously were proposed to be required during limb development (2).
Size of Baculum.
As in many rodent and in some other mammalian species, male mice have a bone in their penis. This baculum (os priapi) originates from cells strongly expressing posterior HoxD genes during development, which led to the proposal that limb and genital buds had similar developmental strategies in which posterior Hox genes were essential components (24). Furthermore, this small bone was slightly altered, because of a cellular deficit, in mice lacking Hoxd-13 function (3). We therefore looked for further baculum size reduction in these various compound genotypes.
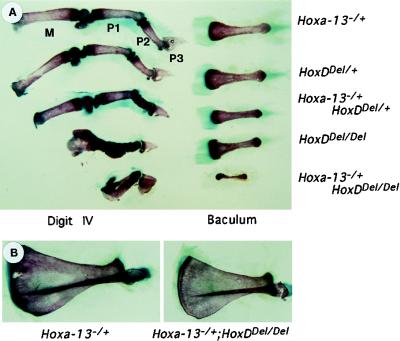
The functional cooperation of the same four genes was clearly observed (Fig. 3). Whereas the inactivation of Hoxd-13 led to a minor localized alteration of the baculum, the simultaneous inactivation of Hoxd-11, Hoxd-12, and Hoxd-13 (HoxDDel/Del) resulted in an overall size reduction of the bone and indeed of the entire organ. The functional input from Hoxa-13 was best evidenced in HoxDDel/+;Hoxa-13+/− males, and an almost complete agenesis of the baculum was seen in HoxDDel/Del;Hoxa-13+/− (Fig. 3A). The remarkably similar functional cooperation of the same four genes during both digit and genital eminence development reflects the coexpression of Hoxd-11, Hoxd-12, Hoxd-13, and Hoxa-13 in overlapping domains of both developing autopods and external genitalia. It suggests that these apparently different structures share important developmental mechanisms, perhaps as a consequence of a common phylogenetic history (18).
Figure 3.
Relationship between the sizes of the penian bone, the baculum, and digit IV in HoxD and HoxA mutant juvenile male mice. (A) Forelimb digit IV (Left) and the penis (Right) of 11-day-old juvenile sibling males were isolated, cleared, and compared. The genotypes are indicated on the right. An extreme reduction of the baculum was observed in mice of the HoxDDel/Del;Hoxa-13+/− genotypes, i.e., mice with the shortest digits. In such specimen, the baculum was ill-formed and barely half of the expected length and thickness. (B) As control for the overall size of the mice, the scapula of the same two genotypes are shown. Although a noticeable reduction of the scapula in HoxDDel/Del;Hoxa-13+/− animals indicate their general shorter statures, this reduction was much less drastic than that observed for the penian bone, which was almost absent.
Autoregulation and Crossregulation.
The existence of autoregulatory and/or crossregulatory interactions between vertebrate Hox genes and their products (e.g., refs. 25 and 26) has introduced an uncertainty in the interpretation of loss-of-function phenotypes, in particular when multiple genes are involved. This uncertainty may be of particular concern when evaluating additive gene doses. For instance, inactivating one gene could in turn double the functional output of another by relieving a repressive effect. Consequently, we looked at HoxD gene activation in selected compound genotypes leading either to the absence of digits, or allowing very limited digit development.
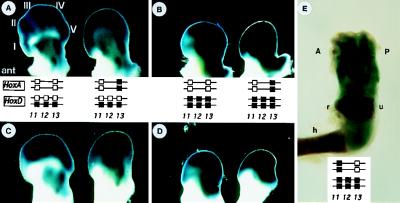
We isolated HoxDDel/+ and HoxDDel/Del fetuses which, in addition, lacked Hoxa-13 function (Fig. 4). In the latter case, the extent of skeletal condensations was expected to be even less developed than in Hoxd-13−/−;Hoxa-13−/− mice, which did not show any genuine digit (3). These animals were used to asses the activity of the Hoxd-11/lacZ maker gene present at the 5′ end of the HoxDDel locus (4). HoxDDel/Del and HoxDDel/Del;Hoxa-13−/− forelimbs and hindlimbs showed comparable levels of Hoxd marker gene expression (Fig. 4 B and D), similarly to HoxDDel/+ and HoxDDel/+;Hoxa-13−/− (Fig. 4 A and C). Activation of the Hoxd marker in HoxDDel/Del;Hoxa-11−/− mice also was unaltered as shown by the blue staining in both forearm and digits (Fig. 4E). These results illustrated that neither the Hoxd-11, Hoxd-12, Hoxd-13, Hoxa-13 combination, nor the Hoxd-11, Hoxd-12, Hoxd-13, Hoxa-11 combination was required for Hoxd gene activity. These data strongly suggested that activation of Hoxd genes in digit primordia was independent of both Hoxa-13 and Hoxa-11, as well as of autoregulatory mechanisms. Activation of the Hoxd reporter in the genital bud and genital eminence mirrored the situation in digits, supporting a previous suggestion that a common regulatory mechanism is responsible for posterior Hoxd gene expression in the primordia of these two structures (18).
Figure 4.
Absence of crossregulatory and autoregulatory interferences as judged by a HoxD reporter gene in HoxA and HoxD compound mutant mice. Hoxd-11/lacZ reporter gene expression (4) in pairs of forelimbs (A and B) and hindlimbs (C and D) derived from mice of four different genotypes schematized in between as in Fig. 2. The presumptive digits are labeled from I (anterior to the left) to V. Although the morphology of these developing limbs vary with the genotypes, they all strongly expressed the HoxD reporter gene in both the distal and proximal domains. This expression indicated that neither the HOXD, nor the HOXA13 proteins are necessary for the function of the Hoxd transgene in limbs. (E) Hoxd-11/lacZ reporter gene expression in a forelimb of HoxDDel/Del;Hoxa-11−/− mouse 5 days after birth. Expression of the Hoxd-11/lacZ reporter gene was detected in both the autopod (digits) and around the zeugopod (radius and ulna), indicating that HOXA11 is not required for the activation of HoxD genes in the developing limbs. The forearm of the limb shown under E is truncated because of the absence of both Hoxd-11 and Hoxa-11 functions (7). A, anterior; P, posterior; r, radius; u, ulna; h, humerus.
CONCLUSIONS
From these and previous results (6), it appears that, similar to vertebral specification (e.g., refs. 27 and 28), digit size and number are fixed as a quantitative function of Hox protein dose, rather than by a qualitative Hox code. However, various Hox complexes seem to have particular roles in digit patterning, as in these mutant stocks we were not able to generate a polydactylous mouse without affecting the HoxD complex, whereas oligodactylous mice could be obtained only by perturbing HoxA. This observation may be relevant in an evolutionary context. It has been established, that at least four Hox complexes were present at the time of the emergence of digits, including all the loci described in this work (29, 30). We therefore may speculate that, in the course of this important remodeling of distal limb structures, the two complexes became involved independently and at different times through the design of complex-specific regulatory mechanisms (Fig. 5). In this model, incipient limiting doses of Hox proteins, initially provided by Hoxa genes (16, 17) could have been involved in distal appendages of the polydactylous short-digited stem tetrapod forms (14, 15), or even before, as suggested by distal Hoxa-13 expression in developing teleost paired fins (30). The occurrence of polydactyly in the Hoxa-11/HoxDDel compound homozygous mice, a situation where digit development almost exclusively relied on the function of Hoxa-13, is consistent with this view. It therefore is reasonable to propose that the emergence of a novel distal expression domain for posterior Hoxd genes may have contributed to abbreviation of serial iterative generation of digits and provided for an intensive and elongated growth phase of the endoskeleton necessary for long-digit formation (17), possibly through the elaboration of a shared-digit activation unit involving at least Hoxd-11, Hoxd-12, Hoxd-13, and Evx-2 (18). Whenever a threshold Hox dose was surpassed, digits could have become longer and the pentadactyl pattern stabilized. The dual role of the Hox dose in digit number and size determination was deduced from mouse mutants and may not directly apply to anamniotes, which, according to fossil evidence, reached pentadactyly independently (15). It is also likely that in different species, the output of the HoxD complex may have varied independently in the early limb bud, thereby influencing digit number and, subsequently, in digital condensations, contributing to the final phalanx length. In this context, important parameters could be determined by the specific times of recruitment of the HoxA and HoxD complexes in distal appendages, followed by gene-specific expression patterns, to build up locally effective Hox protein doses to determine cellular growth rates.
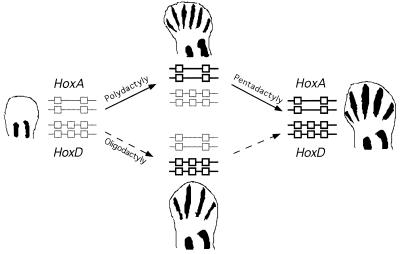
Figure 5.
Scheme showing the relationships between the function of the HoxA and HoxD complexes in distal limbs and the dactyly. Hox complexes are shown either in light gray, when not functional, or bold boxes when functional. In the absence of HoxA and HoxD expression, a complete adactyly is observed. This situation may reflect an ancestral step in which neither complexes had been recruited in distal limbs. Activation of the HoxA complex genes in distal limb, in absence of HoxD function (pathway on the top) coincides with the appearance of a series of truncated digit-like bony elements with a clear polydactyly. The subsequent recruitment of Hoxd genes in the digit domain could lengthen all the digits while reducing their number to the pentadactyl formula. Alternatively, activation of the Hoxd genes first (pathway in the bottom) would have generated an oligodactyl limb, with potentially long digits, and the subsequent activation of the Hoxa genes would have shortened digits and extended their number to five. Paleonthological and developmental evidence support the first pathway (see the text).
The potential successive activation of these two Hox complexes in the course of limb evolution is supported by both developmental and phylogenetic arguments (17, 31–33). During limb development, distal expression of Hoxa-13 is observed before that of Hoxd-13 and appears to be generated through a different dynamic, which does not necessarily involve the apparent anterior expansion of Hoxd expression domains (32, 34–36). And, Hoxa-13 is expressed during teleost fin development in a way similar to what is seen in developing limbs (30), in contrast to Hoxd genes that show fundamental differences (16), suggesting that Hoxa-13 was already functional in distal appendages at the time of the origin of teleosts whereas Hoxd genes became involved secondarily.
Acknowledgments
We thank S. S. Potter and S. Small for the gift of mice lacking Hoxa-11 function and P. Dollé for his early involvement in this work. We also thank M. Kumar for her suggestions and all of the members of the laboratory for discussions and sharing reagents. This work was supported by funds from the Canton de Genève, the Swiss National Research Fund, the Human Frontier Programme, and the Claraz and Latsis foundations.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Rijli F, Chambon P. Curr Opin Gen Dev. 1997;7:481–487. doi: 10.1016/s0959-437x(97)80074-3. [DOI] [PubMed] [Google Scholar]
- 2.Dollé P, Dierich A, LeMeur M, Schimmang T, Schuhbaur B, Chambon P, Duboule D. Cell. 1993;75:431–441. doi: 10.1016/0092-8674(93)90378-4. [DOI] [PubMed] [Google Scholar]
- 3.Fromental-Ramain C, Warot X, Messadeq N, LeMeur M, Dollé P, Chambon P. Development (Cambridge, UK) 1996;122:2997–3011. doi: 10.1242/dev.122.10.2997. [DOI] [PubMed] [Google Scholar]
- 4.Zákány J, Duboule D. Nature (London) 1996;384:69–71. doi: 10.1038/384069a0. [DOI] [PubMed] [Google Scholar]
- 5.Kondo T, Dollé P, Zákány J, Duboule D. Development (Cambridge, UK) 1996;122:2651–2659. doi: 10.1242/dev.122.9.2651. [DOI] [PubMed] [Google Scholar]
- 6.Davis A P, Capecchi M R. Development (Cambridge, UK) 1996;122:1175–1185. doi: 10.1242/dev.122.4.1175. [DOI] [PubMed] [Google Scholar]
- 7.Davis A P, Witte D P, Hsieh-Li H M, Potter S S, Capecchi M R. Nature (London) 1995;375:791–795. doi: 10.1038/375791a0. [DOI] [PubMed] [Google Scholar]
- 8.Mortlock D P, Innis J W. Nat Genet. 1997;15:179–180. doi: 10.1038/ng0297-179. [DOI] [PubMed] [Google Scholar]
- 9.Muragaki Y, Mundlos S, Upton J, Olsen B R. Science. 1996;272:548–551. doi: 10.1126/science.272.5261.548. [DOI] [PubMed] [Google Scholar]
- 10.Morgan B A, Izpisúa-Belmonte J-C, Duboule D, Tabin C J. Nature (London) 1992;358:236–239. doi: 10.1038/358236a0. [DOI] [PubMed] [Google Scholar]
- 11.Goff D, Tabin C J. Development (Cambridge, UK) 1997;124:627–636. doi: 10.1242/dev.124.3.627. [DOI] [PubMed] [Google Scholar]
- 12.Yokouchi Y, Nakazato S, Yamamoto M, Goto Y, Kameda T, Iba H, Kuroiwa A. Genes Dev. 1995;9:2509–2522. doi: 10.1101/gad.9.20.2509. [DOI] [PubMed] [Google Scholar]
- 13.Gérard M, Zákány J, Duboule D. Dev Biol. 1997;190:32–40. doi: 10.1006/dbio.1997.8679. [DOI] [PubMed] [Google Scholar]
- 14.Coates M I, Clack J A. Nature (London) 1990;347:66–68. [Google Scholar]
- 15.Coates, M. I. (1994) Development (Cambridge, UK) Suppl., 169–180.
- 16.Sordino P, van der Hoeven F, Duboule D. Nature (London) 1995;375:678–681. doi: 10.1038/375678a0. [DOI] [PubMed] [Google Scholar]
- 17.Sordino P F, Duboule D. Trends Ecol Evol. 1996;11:114–119. doi: 10.1016/0169-5347(96)81089-5. [DOI] [PubMed] [Google Scholar]
- 18.van der Hoeven F, Zákány J, Duboule D. Cell. 1996;85:1025–1035. doi: 10.1016/s0092-8674(00)81303-3. [DOI] [PubMed] [Google Scholar]
- 19.Small K, Potter S S. Genes Dev. 1993;7:2318–2328. doi: 10.1101/gad.7.12a.2318. [DOI] [PubMed] [Google Scholar]
- 20.Doetschman T C, Eistetter H, Katz M, Schmidt W, Kemler R. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 21.Zákány J, Gérard M, Favier B, Duboule D. EMBO J. 1997;16:4393–4402. doi: 10.1093/emboj/16.14.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis A P, Capecchi M R. Development (Cambridge, UK) 1994;120:2187–2198. doi: 10.1242/dev.120.8.2187. [DOI] [PubMed] [Google Scholar]
- 23.Hérault Y, Hraba-Renevey S, van der Hoeven F, Duboule D. EMBO J. 1996;15:6727–6738. [PMC free article] [PubMed] [Google Scholar]
- 24.Dollé P, Izpisúa-Belmonte J-C, Brown J M, Tickle C, Duboule D. Genes Dev. 1991;5:1767–1776. doi: 10.1101/gad.5.10.1767. [DOI] [PubMed] [Google Scholar]
- 25.Zappavigna V, Renucci A, Izpisúa-Belmonte J-C, Urier G, Peschle C, Duboule D. EMBO J. 1991;10:4177–4187. doi: 10.1002/j.1460-2075.1991.tb04996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Studer M, Lumsden A, Arivza-Mc Naughton L, Bradley A, Krumlauf R. Nature (London) 1996;384:630–634. doi: 10.1038/384630a0. [DOI] [PubMed] [Google Scholar]
- 27.Horan G S B, Ramirez-Solis R, Featherstone M S, Wolgemuth D J, Bradley A, Behringer R R. Genes Dev. 1995;9:1667–1677. doi: 10.1101/gad.9.13.1667. [DOI] [PubMed] [Google Scholar]
- 28.Zákány J, Gérard M, Favier B, Potter S S, Duboule D. Dev Biol. 1996;176:325–328. doi: 10.1006/dbio.1996.0137. [DOI] [PubMed] [Google Scholar]
- 29.van der Hoeven F, Sordino P, Fraudeau N, Izpisúa-Belmonte J-C, Duboule D. Mech Dev. 1996;54:9–21. doi: 10.1016/0925-4773(95)00455-6. [DOI] [PubMed] [Google Scholar]
- 30.Sordino P, Duboule D, Kondo T. Mech Dev. 1996;59:165–175. doi: 10.1016/0925-4773(96)00587-4. [DOI] [PubMed] [Google Scholar]
- 31.Coates M I. Curr Biol. 1995;5:844–848. doi: 10.1016/s0960-9822(95)00169-2. [DOI] [PubMed] [Google Scholar]
- 32.Duboule D. Science. 1994;266:575–576. doi: 10.1126/science.7939709. [DOI] [PubMed] [Google Scholar]
- 33.Duboule D. Curr Opin Genes Dev. 1995;5:525–528. doi: 10.1016/0959-437x(95)90058-o. [DOI] [PubMed] [Google Scholar]
- 34.Haack H, Gruss P. Dev Biol. 1993;157:410–422. doi: 10.1006/dbio.1993.1145. [DOI] [PubMed] [Google Scholar]
- 35.Nelson C E, Morgan B A, Burke A C, Laufer E, DiMambro E, Murtagh L C, Gonzales E, Tessarollo L, Parada L F, Tabin C. Development (Cambridge, UK) 1996;122:1449–1466. doi: 10.1242/dev.122.5.1449. [DOI] [PubMed] [Google Scholar]
- 36.Vargesson N, Clarke J D, Vincent K, Coles C, Wolpert L, Tickle C. Development (Cambridge, UK) 1997;124:1909–1918. doi: 10.1242/dev.124.10.1909. [DOI] [PubMed] [Google Scholar]