Abstract
Here we report on novel application of scanning electrochemical microscopy (SECM) to enable spatially resolved electrochemical characterization of individual single-walled carbon nanotube (SWNT). The feedback imaging mode of SECM was employed to detect a pristine SWNT (~1.6 nm in diameter and ~2 mm in length) grown horizontally on a SiO2 surface by chemical vapor deposition. The resulting image demonstrates that the individual nanotube under an unbiased condition is highly active for the redox reaction of ferrocenylmethyltrimethylammonium used as a mediator. Micrometer-scale resolution of the image is determined by the diameter of a disk-shaped SECM probe rather than by the nanotube diameter as assessed using 1.5 and 10 μm-diameter probes. Interestingly, the long SWNT is readily detectable using the larger probe although the active SWNT covers only ~0.05 % of the insulating surface just under the tip. This high sensitivity of the SECM feedback method is ascribed to efficient mass transport and facile electron transfer at the individual SWNT.
Electrochemistry of carbon nanotubes is a large and growing field that is both fundamentally and practically important.1, 2 Unique physical and chemical properties of carbon nanotubes render them attractive electrode materials for electroanalysis and electrocatalysis. Most electrochemical studies of carbon nanotubes, however, have been carried out with an ensemble of nanotubes with various structural and electronic properties, thereby limiting our knowledge about fundamental electrochemistry of carbon nanotubes. To overcome this limitation, electrodes based on individual single-walled carbon nanotubes (SWNTs) were fabricated to voltammetrically reveal their high activity for redox reaction.3 Moreover, ex-situ SEM and AFM observation of metallic nanoparticles electrodeposited on a SWNT demonstrated preferential nucleation sites (or defects).4, 5 Single molecule fluorescence microscopy was also employed for in-situ monitoring of adsorption and electrolysis of fluorescent redox molecules at discrete, nanometer-sized reactive sites on a SWNT.6 These non-electrochemical microscopes, however, are applicable only to limited types of redox reactions although their superb spatial resolution is highly attractive.
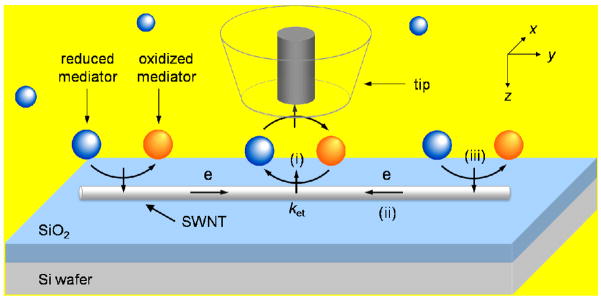
Recently, we proposed a principle of scanning electrochemical microscopy (SECM)7, 8 for spatially resolved electrochemical characterization of individual SWNTs (Figure 1).9 In this SECM feedback method, a disk-shaped ultramicroelectrode is employed as a probe to electrolyze redox mediators at a diffusion-limited rate. When the tip is held less than a tip diameter away from the SWNT, the tip-generated species effectively diffuses to and reacts at the local surface of the SWNT just under the tip. Original mediators thus regenerated by the SWNT are detected at the tip with enhancing its amperometric current response. Consequently, the tip current enhancement based on the feedback effect serves as a measure of the rate of electron transfer at the nanotube surface. Importantly, significant feedback effect is expected even using a tip with the diameter that is much larger than the diameter of an active nanotube, because of the efficient mass transport at the nanotube.9 Moreover, an electronically isolated SWNT is expected to give a quasi-steady-state feedback response when the nanotube is much longer than the tip diameter.10, 11 The unbiased SWNT is not charged by continuous mediator regeneration (step i in Figure 1), which is coupled with electron transport through the nanotube (step ii) and then with electrolysis of original mediators at the exterior sidewall beyond the outer probe tip diameter (step iii) to maintain charge balance in the nanotube. In fact, SECM was successfully applied to the study of externally unbiased carbon nanotube films as grown on insulating surfaces.12, 13 No SECM study of an individual carbon nanotube, however, has been reported.
Figure 1.
Scheme of an SECM feedback experiment with a disk ultramicroelectrode probe positioned above an individual SWNT as grown on an insulating substrate.
Here we report on proof-of-concept experiments to demonstrate SECM-based detection of an individual SWNT. To facilitate the experiments, we synthesized very long SWNTs horizontally aligned on a SiO2-covered Si wafer by chemical vapor deposition as reported elsewhere.14 The density of the SWNTs was lowered by employing a lower concentration of Fe nanoparticle catalyst. The long SWNTs are widely separated from the adjacent nanotubes as shown in an SEM image (Figure 2), where the arrow indicates the region of the SWNT investigated by SECM. Since there is a possibility of obtaining bundles with the used synthesis method,15 especially on higher density samples, we confirmed that the investigated nanotube indicated in Figure 2 is an individual SWNT with the diameter of 1.6 ± 0.1 nm as determined by AFM.14 The line in the SEM image appears much thicker than the nanotube diameter because of charging effect on the surrounding SiO2 surface.16
Figure 2.
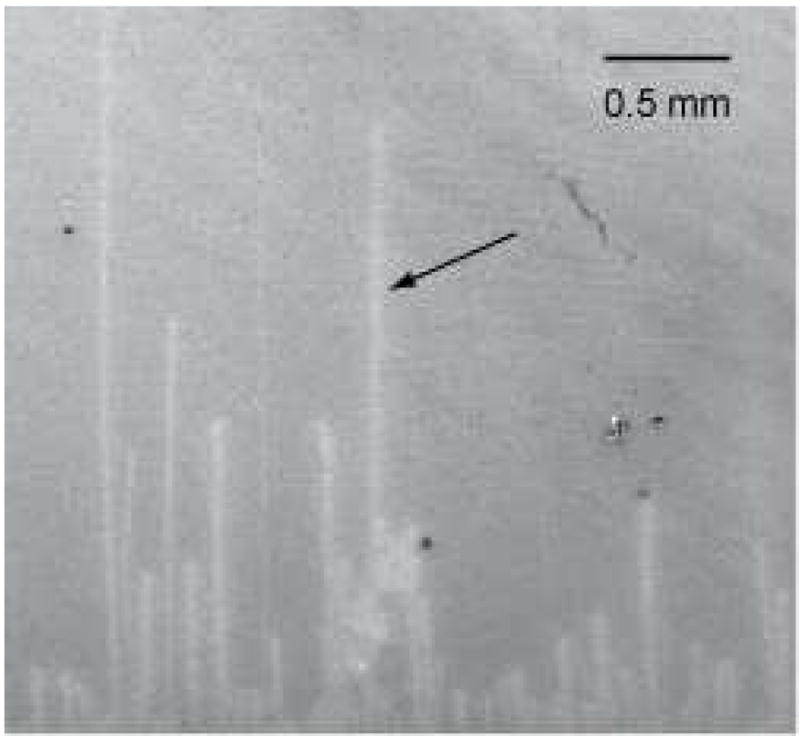
An SEM image of long SWNTs grown on a SiO2/Si wafer at low density. The arrow indicates the region of the SWNT investigated in this work.
The feedback imaging mode of SECM was employed to detect the individual SWNT on the substrate immersed in an aqueous solution of 0.3 mM ferrocenylmethyltrimethylammonium (FcTMA+) hexafluorophosphate and 0.1 M KNO3 (see Supporting Information for details). Initially, a 10 μm-diameter Pt disk probe was biased at 0.35 V (vs Ag/AgCl) and positioned far from the substrate to obtain diffusion-limited tip current of iT,∞ = 430 pA for oxidation of FcTMA+ as given by
| (1) |
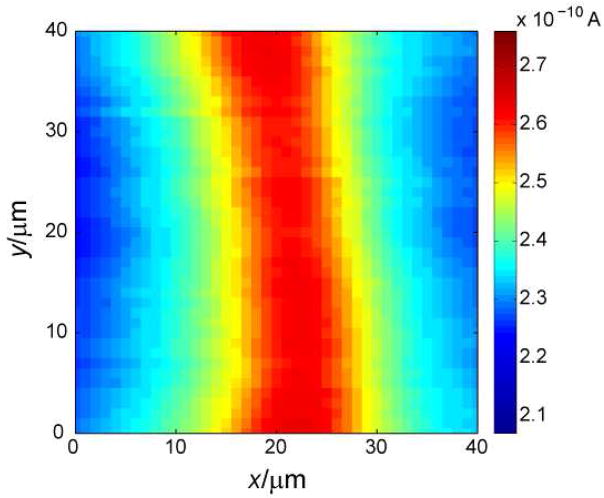
where n is the number of electrons in the tip reaction (n = 1 for FcTMA+/FcTMA2+ couple), F is the Faraday constant, D and c are the diffusion coefficient and concentration of FcTMA+ in the bulk aqueous solution, respectively, and a is the tip radius. Then, the tip was approached toward the SiO2 surface (z-direction) until the tip current decreased to 225 pA (52 % of iT,∞) due to hindered diffusion of FcTMA+ to the tip. This tip current corresponds to the tip–substrate distance, d, of 5.2 μm (d/a = 1.04) according to SECM theory.17 Finally, the tip was held at the constant height and scanned over an individual SWNT in x- and y-directions to obtain a 40 μm × 40 μm image (Figure 3).
Figure 3.
An SECM image of an individual SWNT as obtained using a 10 μm-diameter disk Pt probe at the tip-substrate distance of 5.2 μm. Probe scan rate, 15 μm/s.
The SECM image clearly demonstrates the enhancement of tip current along the length of the SWNT (Figure 3). The enhanced tip current indicates that the tip-generated species, FcTMA2+, was reduced by the SWNT to regenerate FcTMA+, thereby yielding the feedback effect. Importantly, only ~0.05 % of the substrate surface just under the 10 μm-diameter tip is covered by the SWNT with an effective local area of ASWNT = ~4πra (2r = 1.6 nm).9 This extremely high sensitivity of SECM feedback detection is due to fast mass transport9 and facile electron transfer at the SWNT (see below). Moreover, quasi-steady-state feedback responses that are independent of probe scan rate were obtained from the unbiased SWNT, which is much longer than the tip diameter to effectively mediate FcTMA+ oxidation at the exterior sidewall (Figure 1).10 The feedback effect from the SWNT is not limited by electron transport through the nanotube. The tip feedback current above a SWNT varies linearly with the mediator concentration from 0.3 mM (Figure 4a) to 1 mM (data not shown) so that the tip current normalized with respect to iT,∞ (eq 1) is independent of the concentration.
Figure 4.
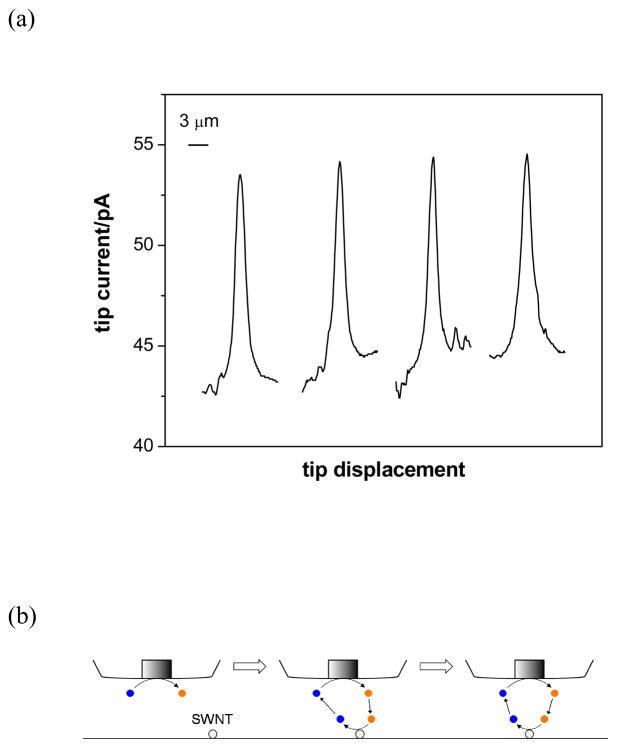
(a) SECM line scans of an individual SWNT at different positions along its length as obtained using a 1.5 μm-diameter disk Pt probe at the tip-substrate distance of 0.525 μm. Probe scan rate, 0.75 μm/s. (b) Scheme of an SECM line scan over an individual SWNT that is positioned (from left) far from, under the edge, and under the center of a disk SECM probe.
The same SWNT was detected with higher spatial resolution using a smaller probe (1.5 μm in diameter). Similar SECM line scans were obtained along the length of the SWNT at four different positions separated every 10 μm (Figure 4a). This result corresponds to uniform reactivity of the nanotube at micrometer resolution determined by the tip diameter.9 The half widths of the peaks are comparable to the tip diameter of 1.5 μm, indicating that a significant feedback effect is obtained from the SNWT when the nanotube is positioned under the edge of the disk probe (Figure 4b). The peak widths are much smaller than the corresponding peak widths as obtained with the 10 μm-diameter probe (Figure 3). Moreover, the line scan experiments allow us to determine the location of the SWNT with high precision, because the tip current reaches the maximum value when the center of the tip is positioned above the SWNT. With the 1.5 μm-diameter probe, the position of a SWNT was able to be determined with precision of ±100 nm (see Figure S1).
The significant feedback effect from a SWNT with a small effective area under the tip implies that mediator regeneration by the nanotube is very fast. In fact, a large rate constant for heterogeneous electron transfer at the SWNT is estimated from the peak currents in the line scan data. In an SECM line scan, the difference in the tip current above the SWNT and the SiO2 surface (~ 10 pA in Figure 4a) corresponds to the feedback current based on mediator regeneration at the SWNT, iSWNT.9, 10 Assuming that (1) the open circuit potential of the long SWNT exposed to a FcTMA+ solution is negative enough to result in electrochemically irreversible reduction of FcTMA2+ at the sidewall under the tip10, 18 and (2) the entire length of the SWNT on the insulating substrate is uniformly active and also uniformly accessible to redox molecules (see Supporting Information for numerical simulations of the accessibility), iSWNT is approximated to
| (2) |
where ket is a rate constant for FcTMA2+ reduction, and cSWNT is the concentration of FcTMA2+ at the nanotube surface under the tip. Since reduction of FcTMA2+ at the nanotube results in cSWNT < c (= 0.3 mM), ket > 4.6 cm/s is estimated from eq 2 with 2r = 1.6 nm and 2a = 1.5 μm. This large rate constant is not unexpected. An extremely large standard rate constant of k0 = 4 ± 2 cm/s was reported for the same redox couple at individual SWNTs, which were lithographically integrated into an electrode format and characterized voltammetrically.3 Also, k0 > 3 cm/s for this redox couple was estimated using a ultramicroelectrode based on a 2D network of SWNTs.19 Moreover, the ket value at the unbiased SWNT is larger than the corresponding k0 value because the open circuit potential of the nanotube is more negative than the standard potential.
In summary, we demonstrated that SECM is a powerful electrochemical method that enables spatially resolved characterization of an individual SWNT as grown on an insulating substrate. High sensitivity of this SECM-based method not only allows for detection of a molecular wide SWNT using a micrometer-sized probe but also indicates that a pristine individual SWNT is highly active for electron transfer.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (GM073439), and the Petersen Institute of NanoScience and Engineering at the University of Pittsburgh. The authors thank the Department of Materials Science and Engineering for the provision of access to the SEM instrumentation.
References
- 1.McCreery RL. Chem Rev. 2008;108:2646–2687. doi: 10.1021/cr068076m. [DOI] [PubMed] [Google Scholar]
- 2.Dumitrescu I, Unwin PR, Macpherson JV. Chem Commun. 2009:6886–6901. doi: 10.1039/b909734a. [DOI] [PubMed] [Google Scholar]
- 3.Heller I, Kong J, Heering HA, Williams KA, Lemay SG, Dekker C. Nano Lett. 2005;5:137–142. doi: 10.1021/nl048200m. [DOI] [PubMed] [Google Scholar]
- 4.Fan YW, Goldsmith BR, Collins PG. Nat Mater. 2005;4:906–911. doi: 10.1038/nmat1516. [DOI] [PubMed] [Google Scholar]
- 5.Goldsmith BR, Coroneus JG, Khalap VR, Kane AA, Weiss GA, Collins PG. Science. 2007;315:77–81. doi: 10.1126/science.1135303. [DOI] [PubMed] [Google Scholar]
- 6.Xu W, Shen H, Kim YJ, Zhou X, Liu G, Park J, Chen P. Nano Lett. 2009;9:3968–3973. doi: 10.1021/nl900988f. [DOI] [PubMed] [Google Scholar]
- 7.Bard AJ, Mirkin MV, editors. Scanning Electrochemical Microscopy. Marcel Dekker; New York: 2001. [Google Scholar]
- 8.Amemiya S, Bard AJ, Fan FRF, Mirkin MV, Unwin PR. Ann Rev Anal Chem. 2008;1:95–131. doi: 10.1146/annurev.anchem.1.031207.112938. [DOI] [PubMed] [Google Scholar]
- 9.Xiong H, Gross DA, Guo J, Amemiya S. Anal Chem. 2006;78:1946–1957. doi: 10.1021/ac051731q. [DOI] [PubMed] [Google Scholar]
- 10.Xiong H, Kim J, Kim E, Amemiya S. J Electroanal Chem. 2009;629:78–86. doi: 10.1016/j.jelechem.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim E, Kim J, Amemiya S. Anal Chem. 2009;81:4788–4791. doi: 10.1021/ac900349f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson NR, Guille M, Dumitrescu I, Fernandez VR, Rudd NC, Williams CG, Unwin PR, Macpherson JV. Anal Chem. 2006:7006–7015. doi: 10.1021/ac0610661. [DOI] [PubMed] [Google Scholar]
- 13.Fabre B, Hauquier F, Herrier C, Pastorin G, Wu W, Bianco A, Prato M, Hapiot P, Zigah D, Prasciolu M, Vaccari L. Langmuir. 2008;24:6595–6602. doi: 10.1021/la800358w. [DOI] [PubMed] [Google Scholar]
- 14.Reina A, Hofmann M, Zhu D, Kong J. J Phys Chem C. 2007;111:7292–7297. [Google Scholar]
- 15.Hofmann M, Nezich D, Reina A, Kong J. Nano Lett. 2008;8:4122–4127. doi: 10.1021/nl801461f. [DOI] [PubMed] [Google Scholar]
- 16.Homma Y, Suzuki S, Kobayashi Y, Nagase M, Takagi D. Appl Phys Lett. 2004;84:1750–1752. [Google Scholar]
- 17.Shao Y, Mirkin MV. J Phys Chem B. 1998;102:9915–9921. [Google Scholar]
- 18.Xiong H, Guo J, Amemiya S. Anal Chem. 2007;79:2735–2744. doi: 10.1021/ac062089i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumitrescu I, Unwin PR, Wilson NR, Macpherson JV. Anal Chem. 2008;80:3598–3605. doi: 10.1021/ac702518g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.