Abstract
Background
The effect of dietary calcium on fecal fat excretion in lactose maldigestion is not known.
Objective
To investigate the effect of dairy and nondairy dietary calcium on fecal fat excretion in lactose digesters and maldigesters during moderate energy restriction.
Design
A randomized cross-over trial comparing the effect of 500 mg vs. 1500 mg dairy and nondairy calcium on fecal fat excretion in 34 healthy adults during moderate (− 30%) energy restriction diet-induced weight loss for 12 weeks. The participants were classified as lactose digester or maldigester on the basis of breath hydrogen test.
Measurements
Anthropometric parameters and body composition, resting energy expenditure, energy and nutrient intake, fecal fat, physical activity, blood pressure, blood and urine sampling for pertinent measurements.
Results
Fecal fat loss expressed as percent of fat intake was significantly higher with 1500 mg (high-Ca) compared to 500 mg (low-Ca) calcium intake per day (mean: 3.0%; the 95% CI: 2.3 to 3.7%; P <0.001) independent of calcium source and lactose digestion status.
Conclusions
During moderate energy restriction induced weight loss a high-Ca diet causes an increase in fecal fat excretion independent of calcium source. Calcium intake related fecal fat loss is also independent of the ability to digest lactose and it is not diminished over time.
Keywords: dietary calcium, dairy, fecal fat, lactose maldigestion, body weight loss
INTRODUCTION
There is an ongoing controversy over the role of calcium intake on body energy regulation. Low levels of dietary calcium and dairy products have been identified as a potential contributing factor to obesity and have also been linked to increased risk of hypertension and insulin resistance1–3. Despite supportive epidemiological reports2, 4–7, interventional and mechanistic studies8–12, the suggested anti-obesity effect of dairy and nondairy calcium supplementation remains far from proven because, not all investigations have confirmed these findings. Several studies indicate that calcium supplementation or dairy products may have no effect13–19 or even an adverse effect20 on body weight. Further, recent reviews and meta-analyses18, 21 of randomized controlled trials with or without concomitant energy restriction indicate that neither calcium supplementation nor dairy products reliably facilitate weight loss. When found, such a relationship relates to decreased rate of weight22 or fat gain, rather than weight or fat loss.
Two recent trials23, 24 have reported over two-fold increase in fecal fat loss with increase in dietary calcium intake for 7 days. The authors concluded that their observation might contribute to an explanation why a high-calcium diet might be inversely related to the body weight. However, the long-term effects of calcium supplementation on fecal fat excretion are unknown. Moreover, there is no clear evidence that dairy- derived calcium would be more effective in causing fecal energy loss than nondairy calcium.
Lactose maldigestion has been linked to low dairy calcium intake and osteoporosis25, although a preponderance of evidence suggests that lactose maldigestion should not limit calcium intake since most of the maldigesters can tolerate several servings of dairy foods daily26. However, the effect of lactose maldigestion on fecal fat loss secondary to dairy and nondairy calcium supplementation has not been systematically studied.
The goal of the present study was to determine the effect of dietary calcium on fecal fat loss. We examined the association between lactose maldigestion, dietary calcium level and source, and weight loss induced by energy restriction and fecal fat loss for 12 weeks. We tested the hypothesis that dietary dairy and nondairy calcium affects energy balance by increasing fecal loss of fat. We also examined a possibility that fecal fat excretion might be different in lactose maldigesters than in lactose digesters.
SUBJECTS and METHODS
Participant Selection
We studied healthy males (10) and females (24), aged 21–50 with BMI 29–35 kg/m2. Volunteers were recruited using flyers, massive emails, and word of mouth. Potential participants with history of medical illness including diabetes, hypertension, renal, liver, or heart disease, pregnant or lactating mothers, those taking medications or dietary supplements that affect body weight, lipid-lowering medications or thyroid hormone substitution, and engaged regularly in heavy or vigorous physical activities were excluded from the study. Volunteers with known or suspected drug or alcohol abuse and tobacco users were also not recruited.
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. The trial was conducted at the Clinical Research Center (CRC) at Vanderbilt University, Nashville, Tennessee, United States, was approved by the Institutional Review Board of Vanderbilt University, and followed the ethical principles of the Helsinki-II Declaration. Each participant provided written consent before the study.
Study Design
Based on breath hydrogen test, lactose digesters and maldigesters were randomly assigned in a single-masked manner to oneof two energy restriction diets: 1) dairy calcium in the form of dairy products and, 2) nondairy calcium in the form of food products excluding dairy. Each diet had two levels of dietary calcium; 500 mg (low-Ca) and 1,500 mg (high-Ca). Nondairy, high-Ca diet was supplemented with calcium citrate (1,000 mg of Ca). A sequence of the two levels of dietary calcium, either low-high calcium (500 – 1,500 mg) or high-low calcium (1,500 – 500 mg) was randomly assigned to each participant for 12 weeks (6 weeks each diet) in cross-over design. Adherence to the protocol was monitored by collecting self-reports of uneaten foods. Urinary nitrogen, sodium, and potassium were measured weekly in 24-h urine as biomarkers of intake.
Diets
Study participants received individualized energy and nutrient controlled diet for the whole study period provided by the CRC metabolic kitchen for consumption at home. The food items in these diets were similar to those regularly used in the U.S. and were adjusted to individual preferences. Energy needs were calculatedas sum of resting energy expenditure (REE) measured using metabolic cart (CPX Optima, MedGraphics, St. Paul, MN), energy expenditure of physical activity measured using accelerometer (RT3, StayHealthy, Monrovia, CA) for one week before the study, and thermic effect of food estimated as 10% of REE. The individualized diet (10-day diet cycle) provided in daily portions contained approximately 70% (± 210 kJ) of daily energy requirements, 52 –54% of energy from carbohydrates, 26–29% of energy from fat, 17–21% of energy from protein, and 17–21 g of fiber. To assure sufficient micronutrient content of the diet, participants received a multivitamin supplement daily (Nature Made, Mission Hills, CA). After six weeks energy content of the diet was lowered (420 to 840 kJ/day) to reflect changes in body weight. No restrictions were posed on amounts of energy-free foodssuch as water, coffee (without sugar or milk), diet soft drinks, salt, and pepper. All food items were marked (breakfast, lunch, dinner, snack), and foods for each day were packed in thermo-insulated bags. The dinner meals were pre-prepared and only needed to be heated. Each participant received a written daily list of foods at every diet pick-up (4 times a week). Any uneaten foods and any additional foods eaten by the participants were reported on daily sheets collected at food pick up. The study dietitian met with each participant weekly to discuss the diet, resolve any barriers and concerns related to food or specimen collection, and to encourage compliance. Each participant was allowed one or two days (i.e., birthday, holiday) on which additional foods could be eaten. Intake data was analyzed for energy and nutrient content using NDS-R database (Nutrition Data System, St. Paul, MN).
Lactose Digestion
Lactose digestion was assessedby measuring alveolar breath hydrogen exhalation at 30 minute intervals for three hours following a lactose oral challenge, asdescribed previously26. Participants were classified as lactase deficient (lactose maldigesters) on the basisof a rise in breath hydrogen concentration of greater than 0.90μmol/L (>20 ppm) after ingestion of 25 g of lactosein 250 mL of water.
Body Composition and Anthropometric Data
The National Health and Nutrition Examination Survey (NHANES) protocols27 were followed for all anthropometrical measurements. Body weight was measured at baseline and thrice weekly within 0.1 kg using a calibrated beam platform scale (Detecto-Medic, Detecto Scales, Inc, Northbrook, IL). Height was measured at baseline within 0.5 cm using a calibrated wall-mounted stadiometer (Perspective Enterprises, Portage, MI). Waist circumference was measured in standing position to the nearest 0.1 cm at the midaxillary high point of the iliac crest at minimal respiration at baseline and after 4, 8 and 12 weeks of energy restriction diet. The averages of the two readings were used for analysis. All measurements were performed bythe same investigator. Body composition was determined by DXA at baseline and after 12 weeks using narrow fan-beam technology (GE Lunar Prodigy™ Madison, WI). Fat mass (FM) and fat-free mass (FFM) were determined, and FFM was further divided into lean body mass (LBM) and bone mineral content (BMC). For quality assurance and equilibration, a calibration block was scanned each morning. A spine phantom was scanned on a weekly basis; the coefficient of variation (CV %) was 0.7%. Total body water was measured using the bioelectrical impedance (Quantum-II Desktop, RJL Systems, Clinton Township, MI). The measurement was done promptly after DXA measurement by the same investigator. Data were entered into the software program provided by the manufacturer (Cypress, RLJ Systems, Clinton Township, MI).
Resting energy expenditure (REE)
REE was measured at baseline and after 12 weeks and was defined as the average EE during a 30-min period of lying in a supine position after a 30-min rest following an overnight fast (>10 h) using metabolic cart (CPX Ultima MedGraphics, St Paul, MN).
Physical Activity
Daily physical activity was assessed using an RT3 accelerometer (StayHealthy, Monrovia, CA, US). The participants were instructed to maintain their habitual physical activity throughout the study. They wore theactivity monitor on their right hip while awake for the duration of the study. The results were downloaded weekly and total and physical activity energy expenditure were calculated using energy calculated from the amount of movement measured by the monitor and REE measured at baseline. Physical activity levels (PAL) were calculated by dividing total energy expenditure by REE for each monitored day.
Blood Pressure
Blood pressure was measured 3 times a week in the reclining position after 10 min rest with automatically inflating cuff (Dynamap, General Electric, Milwaukee, WI, USA).
Urine analyses
The complete 24-h urine samples were collected weekly. Urine volume and density was measured and a sample of 10 ml was frozen at −70°C until further analysis in the CRC Core laboratory. Urinary calcium, sodium, and potassium were measured using Vitros 250 Analyzer (Ortho-clinical Diagnostics, Rochester, NY, USA). Urinary nitrogen content was measured using nitrogen analyzer (Antek instrument nitrogen system 9000NS, Antek Instruments, Inc., Houston, TX, USA). The nitrogen excretion in the urine was used as a biological marker for protein intake by multiplying the content of nitrogen in the urine by the factor 7.7228. The sodium content of the urine was used as a biological marker of sodium intake29 and the potassium in the urine divided by 0.77 was used as a biological marker of potassium intake30.
Blood Analyses
Venous blood samples were drawn on the morning after an overnight fast at baseline, at 6 weeks, and on the last day of the study (12 weeks). Basal metabolic panel including glucose and routine hematological indices (hemoglobin concentration, hematocrit, red and white blood cells count) were analyzed in the Vanderbilt University Hospital Laboratory using standard methodologies. Blood for determination of insulin, leptin, parathyroid hormone (PTH), and vitamin D was centrifuged at 2800 × g for 15 min at 4°C. Serum was extracted and the samples were stored at −70°C until later analyses at the Vanderbilt Diabetes Research Center Hormone Assays Laboratory.
Fecal Fat Analyses
All feces excreted during one weekend day (24 h) were collected in plastic containers during all 12 weeks of the study. In addition, the subjects completed a questionnaire on daily defecation frequency during the whole study. The fecal samples were weighed and frozen at −20°C until sent to the analytical laboratory (Arup Laboratories, Salt Lake City, UT, USA) for fecal fat analysis using gravimetric method.
STATISTICAL ANALYSIS
Data are presented as means, standard deviations (s.d.) or ranges. We considered the weekly measurements of fecal fat excretion calculated as percent of fat intake as well as their average over 12 weeks as the major outcomes. Since the fecal fat excretion was consistent over 6 weeks within each calcium intake level and diet source (dairy or nondairy), the average of the fecal fat excretion over 6 weeks was used as the primary outcome in the final analysis. Random-effects models were used with generalized least square estimators and Huber/White/sandwich estimator of variance. As main effects in the model, calcium intake level (500 and. 1500 mg/day), diet source (dairy and nondairy), lactose-digestion status (digester and maldigester), and gender (male and female) were included. Differences between the means for the body fat and weight loss, urinary biomarkers, energy expenditure, and physical activity were analyzed by paired t-test. All tests were two-tailed, and a P-value of < 0.05 was considered significant. Analyses were performed with STATA 9.2 (StataCorp, College Station, TX) and R (www.r-project.org).
RESULTS
A total of 51 volunteers were recruited for the study, 40 were randomized, and 34 completed the study (19 Caucasians, 11 African Americans, and 4 declaring other ethnicities). One participant was dropped due to noncompliance, and five withdrew between week 1 and week 8. Baseline and end of the study characteristics of participants who completed the study are shown in Table 1. There was no difference in initial weight and body composition between lactose maldigesters and digesters and between dairy and nondairy diets (all P >0.05).
Table 1.
Participant Characteristics at the baseline and after 12-week of energy restriction diet.
| Baseline | After 12-week Diet | |||
|---|---|---|---|---|
| Digester (n=18) | Maldigester (n=16) | Digesters (n=18) | Maldigesters (n=16) | |
| Weight (kg) | 105.2 ± 11.9 (80.2 – 124.3) | 105.2 ± 17.1 (74.0 –131.2) | 96.00 ± 10.8a (72.3 –113.2) | 98.65 ± 16.9a (67.9 – 128.7) |
| Body mass index (kg/m2) | 35.4 ± 3.6 (28.9 – 42.1) | 36.1 ± 4.9 (28.9 – 45.6) | 32.3 ± 3.3a (25.6 – 38.2) | 34.2 ± 4.9a (26.5 – 41.3) |
| Body fat mass (kg) | 45.0 ± 7.8 (32.0 – 62.9) | 47.4 ± 11.1 (26.8 – 60.9) | 39.6 ± 7.8a (27.3 – 58.4) | 42.6 ± 12.1a (19.8 – 58.3) |
| Total Body water (l) | 48.1 ± 10.1 (36.6 –68.4) | 47.1 ± 10.5 (36.6 –68.4) | 44.8 ± 9.6a (32.9 –61.7) | 43.8 ± 9.6a (30.4 –65.2) |
| Waist Circumference (cm) | 107.9 ± 10.2 (90 –132) | 106.2 ± 13.3 (87 –134) | 101.2 ± 9.6a (84 –123) | 104.2 ± 14.6a (84 –127) |
| Bone mineral density (g/cm2) | 1.319 ± 0.105 (1.149 –1.542) | 1.355 ± 0.107 (1.212 –1.628) | 1.302 ± 0.096 (1.137 –1.486) | 1.321 ± 0.096 (1.145 –1.526) |
All values are mean ± s.d.. Values in parentheses are ranges.
significantly different from baseline, (p<0.05, paired t-test)
Dietary intake and compliance with the study diets
The participants had an average energy intake of 7.5 ± 1.5 MJ (Table 2). The average intake of energy from carbohydrates, fat, and protein was 54.5 ± 3.0%, 28.9 ± 2.6%, and 18.4 ± 1.85%, respectively. There were no differences between macronutrient and fiber content between the diets (dairy vs. nondairy). Average amount of calcium in low-Ca and high-Ca diets was 503 ± 73 and 1491 ± 131 mg/day, respectively and the level was not different between dairy and nondairy diets. There were no significant differences between the intake of protein, sodium, and potassium and their respective biological markers in weekly urine collections. Ratio of nitrogen in urine and corresponding protein intake were not significantly different between the dairy and nondairy diets (1.13 ± 0.41 and 1.01 ± 0.14, P = 0.105). There were also no differences between the ratios of reported intake and excretion between dairy and nondairy diets for sodium (0.96 ± 0.20 and 0.95 ± 0.19, P = 0.917) and potassium (1.15 ± 0.23 and 1.12 ± 0.16, P = 0.684).
Table 2.
Average daily intake of energy, macronutrients, fiber, vitamin D, and calcium in study participants.
| Dieta | Energy (MJ) | Fats (% energy) | Carbohydrates (% energy) | Proteins (% energy) | Fiber (g) | Vitamin D (mcg) | Calcium (mg) |
|---|---|---|---|---|---|---|---|
| Dairy Diet (n= 17) | |||||||
| Low Calcium (6 weeks) | 7.47±1.4 | 29.3±2.6 | 54.1±3.2 | 18.5±1.6 | 19.0±3.9 | 2.29±1.3 | 515±69 |
| High Calcium (6 weeks) | 7.55±1.3 | 27.8±2.3 | 53.0±2.4 | 21.3±2.2 | 16.7±3.4 | 5.8±2.0 | 1488±131 |
| Total diet (12 weeks) | 7.51 ±1.3 | 28.6±2.5 | 53.5±2.8 | 27.8±2.3 | 19.9±1.9 | 4.0±1.66 | 1002±100 |
| Non-Dairy Diet (n = 17) | |||||||
| Low Calcium (6 weeks) | 7.46±1.5 | 29.1±2.8 | 54.3±3.4 | 18.2±2.1 | 18.4±3.9 | 1.6±0.9 | 491±77 |
| High Calcium (6 weeks) | 7.52±1.5 | 28.7±2.4 | 54.7±2.7 | 18.6±1.6 | 18.8±4.1 | 3.9±1.9 | 1495±136 |
| Total diet (12 weeks) | 7.49 ± 1.5 | 28.9±2.6 | 54.5±3.0 | 18.4±1.8 | 18.6±4.0 | 2.7±1.4 | 992±106 |
All values are mean ± s.d..
Crossover design with high-Ca diet (1500 mg/day) and low-Ca diet (500 mg/day) administered in random order for 6 weeks (total 12 weeks). The nutrient content was estimated using NDS-R dietary assessment software (NDS, St. Paul, Minnesota, US).
Fecal fat excretion
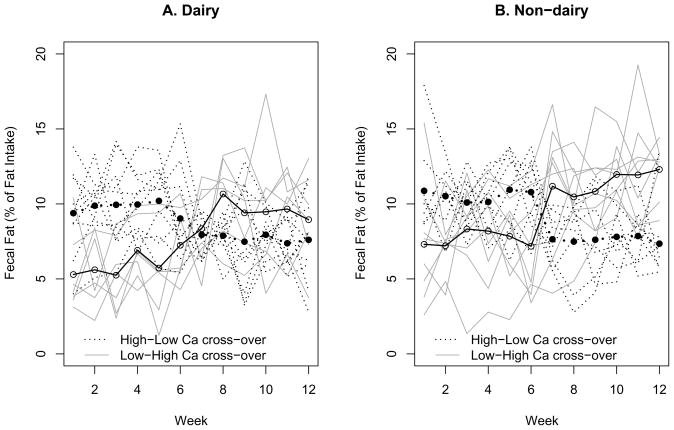
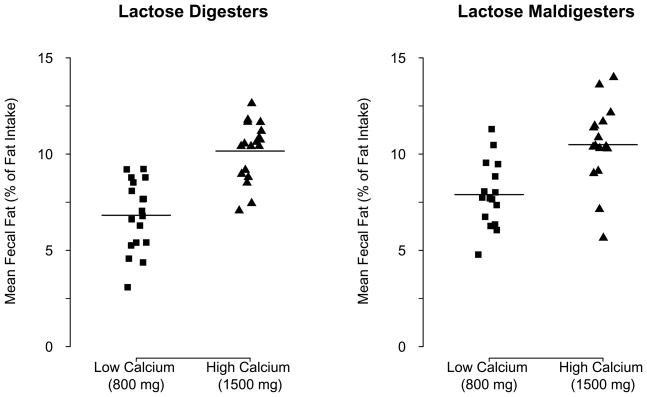
The average fecal fat loss (Table 3) over 6 weeks was significantly higher with high-Ca diet than that with low-Ca diet after adjusting for gender, lactose digestion status, and dairy or nondairy diet source (mean: 3.0%; the 95% confidence interval (CI): 2.3 to 3.7%; P < 0.001). The fecal fat loss increased correspondingly with cross-over to high-Ca diet and vice versa (Figure 1). It was also significantly higher in females than that in males after adjusting for calcium intake level, lactose digestion status, and dairy or nondairy diet source (mean: 2.0%; the 95% CI: 1.2 to 2.9%; P < 0.001). There was no significant difference in fecal fat loss between lactose digesters and maldigesters (Figure 2) after adjusting for gender, calcium intake level, and dairy or nondairy diet source (mean: 0.2%; the 95% CI: −0.5 to 1.0%; P = 0.544). There was a significant positive correlation between calcium intake and fecal fat excretion in dairy (r = 0.460) and nondairy (r = 0.555) diets and in lactose digesters (r = 0.562) and maldigesters (r = 0.463, all P >0.05). Although not statistically significant, the average fecal fat loss over 12 weeks was higher in nondairy than that in dairy diet group (Figure 1) after adjusting for calcium intake level, lactose digestion status and gender (mean: 0.5%; the 95% CI: −0.2 to 1.2%; P = 0.150). In females, the average fecal fat loss over 6 weeks tended to be higher with the non-dairy than that with the dairy diet after adjusting for calcium intake level and lactose digestion status (mean: 0.8%; the 95% CI: −0.1 to 1.6%; P = 0.067).
Table 3.
Comparison of reported average (12 weeks) intake of protein, sodium, and potassium with the corresponding biological markers in urine.
| Intakea | Urinary Excretionb | Ratio of intake to excretion | P-valuec | |
|---|---|---|---|---|
| Protein (g/day) | 85.0 ± 12.0 (64.7 – 114.6) | 87.9 ± 323.6 (43.0 – 132.9) | 1.04 ± 0.32 (0.67 – 2.10) | 0.496 |
| Sodium (g/day) | 3.03 ± 0.44 (2.21 – 4.01) | 3.28 ± 0.83 (1.68–4.97) | 0.96 ± 0.19 (0.57 – 1.46) | 0.246 |
| Potassium (g/day) | 2.83 ± 0.43 (2.01 – 3.81) | 2.53 ± 0.53 (1.74 – 3.58) | 1.13 ± 0.19 (0.94 – 1.68) | 0.432 |
Data are presented as means ± s.d. Values in parentheses are ranges.
Average daily reported intakes of protein, sodium, and potassium assessed by NDSR (Nutrition Diet System, St. Paul, MN).
Nitrogen in 24 h urine (12 weekly collections) multiplied by 7.72 (Bingham and Cummings, 1985), potassium in urine divided by 0.77 (Johansson et al, 1992).
P-values are comparisons of reported intakes of protein, sodium, and potassium with the respective urinary excretion analyzed using the paired t-test.
Figure 1.
Patterns of individual and mean fecal fat excretion with high and low calcium crossover for dairy and nondairy diets
Figure 2.
Mean fecal fat excretion in lactose digesters and maldigesters with high and low calcium diets
Urinary calcium excretion
Urinary calcium excretion (Table 4) was significantly higher in high-Ca than low-Ca (128.3± 57.9 and 146.4 ± 77.9 mg/day−1; the 95% CI for difference: 3.8 to 31.1 mg/day; P = 0.014). The differences in calcium excretion were not different between dairy vs. nondairy diets and in digesters vs. maldigesters (all P > 0.05).
Table 4.
Dietary intake and fecal excretion of total fat
| Low-Ca diet | High-Ca diet | |||||
|---|---|---|---|---|---|---|
| Fat intakea (g/day) | Fecal fat excretionb (g/day) | % of fat intake | Fat intakea (g/day) | Fecal fat excretionb (g/day) | % of fat intake | |
| Digesters (n=18) | 58.2 ± 10.4 (38.7 – 82.8) | 3.8 ± 0.7 (2.5 – 5.2) | 6.8 ± 1.8 (3.1 – 9.2) | 57.9 ± 9.3 (42.9 – 76.3) | 5.8 ± 1.0 (3.6 – 7.1) | 10.2 ± 1.5 (7.1 – 12.6) |
| Maldigesters (n= 16) | 56.6 ± 10.8 (38.2 – 79.4) | 4.4 ± 0.8 (2.8 – 6.1) | 7.9 ± 1.7 (4.8 –11.3) | 56.7± 12.4 (36.1 – 83.1) | 5.9 ± 1.4 (4.1 – 8.7) | 10.5 ± 2.1 (5.6 –14.0) |
Data are presented as means ± s.d. Values in parentheses are ranges.
Average daily reported intake of fat assessed by NDS-R (Nutrition Diet System, St. Paul, MN).
Average daily fat excretion in 24-h stool (12 weekly collections). % of fat intake was calculated as a % ratio of weekly fat excretion to average weekly fat intake.
Body weight and body composition
After following an energy restricted diet for 12 weeks (Table 1), there were no significant differences in weight loss between dairy and nondairy diets (8.5 ± 2.7 and 7.2 ± 2.8 kg, P = 0.116). However, there were significant differences in weight changes in lactose digesters vs. maldigesters (9.2 ± 2.2 and 6.6 ± 2.4, P = 0.003). There was also significant differences between first and second 6-week study periods (5.6± 1.8 and 2.8 ± 1.7 kg, P= 0.001). The differences between dairy and nondairy diets were not significant during the first 6-week (5.7 ± 1.9 and 5.5 ± 1.7 kg) or second 6-week (2.9 ± 1.9 and 2.9 ± 1.8 kg) periods. There were also no significant differences in body fat changes across groups, dairy vs. nondairy (5.4 ± 1.5 and 4.1 ± 1.5; P = 0.393) or lactose digesters vs. maldigesters (5.5 ± 1.3 vs. 4.8 ± 1.7; P = 0.206). There were also no differences in the amount of total body water between the groups.
Resting energy expenditure (REE) and physical activity
There was a difference in REE between the baseline and the end of the study (7.31±1.31 vs. 6.74 ± 1.32 MJ) which became insignificant when REE was adjusted for fat free mass and fat mass (data not shown). There were not REE differences between digesters and maldigesters (Table 6). The differences in the amount of physical activity measured using RT3 accelerometers during the entire study were also not significant. Participants did not change physical activity level (PAL) during the study (Table 6).
Table 6.
Resting energy expenditure, physical activity and physical activity level
| Baseline | After 12-Week Diet | |||
|---|---|---|---|---|
| Digesters (n=18) | Maldigesters (n=16) | Digesters (n=18) | Maldigesters (n=16) | |
| Resting energy expenditure (MJ/day)b | 7.38 ± 1.3 (5.53 – 9.64) | 7.25 ± 1.4 (5.09 – 9.97) | 7.05 ± 1.4a (4.42 – 9.87) | 6.38 ± 1.2a (4.20 – 8.71) |
| Physical activity (MJ/day)b | 3.40 ± 1.5 (1.98 – 6.28) | 3.19 ± 1.2 (1.63–5.58) | 2.81 ± 1.2a (0.99 – 4.71) | 2.85 ± 1.2a (1.32 – 5.38) |
| Physical activity level (PAL) | 1.46 ± 0.25 (1.17 – 2.04) | 1.44 ± 0.14 (1.24 – 1.77) | 1.42 ± 0.17 (1.14 – 1.69) | 1.41 ± 0.19 (1.22 – 1.89) |
Data are presented as means ± s.d. Values in parentheses are ranges.
significantly different from baseline values (P<0.05, paired t-test).
Measured values (see the text for description of the methods). PAL is a ratio of total energy expenditure (resting energy expenditure plus physical activity) and resting energy expenditure.
Blood pressure and blood parameters
Diet had no significant effect on either systolic or diastolic blood pressure (Table 7). Basic hematological parameters were not different between groups (data is not shown). Diet did not have an effect on serum concentrations of insulin and vitamin D. Leptin levels decreased significantly after 12-week on the study diet independent of diet and lactose digestion status (40.6 ± 20.3 vs. 27.3 ± 16.7 ng/ml, P = 0.001, paired t-test).
Table 7.
Changes in blood pressure, hormones, and glucose
| Baseline | After 12-Week Diet | |||
|---|---|---|---|---|
| Digesters (n=18) | Maldigesters (n=16) | Digesters (n=18) | Maldigesters (n=16) | |
| Systolic blood pressure (mmHg) | 127.5 ± 10.5 (111 – 145) | 129.7 ± 16.1 (107 –172) | 124.6 ± 10.3 (95 – 155) | 123.9 ± 13.6 (100 – 146) |
| Diastolic blood pressure (mmHg) | 76.3 ± 11.7 (54 – 97) | 75.1 ± 8.01 (60 – 89) | 73.2 ± 7.3 (63 – 84) | 75.4 ± 10.1 (64 – 95) |
| Vitamin D (ng/mL) | 38.9 ± 14.5 (19 – 65) | 39.4 ± 16.5 (22 – 66) | 40.0 ± 13.41 (21 – 64) | 37.3 ± 14.8 (14 – 64) |
| Parathormone (ng/L) | 40.1 ± 17.9 (19 –81) | 45.5 ± 15.7 (21 – 71) | 34.8 ± 13.4 (16 – 73) | 33.7 ± 10.9a (14 – 42) |
| Insulin (uU/ml) | 19.7 ± 17.7 (6.1 – 74.7) | 26.2 ± 22.1 (6.1 – 80.7) | 21.6 ± 17.6 (6.6 – 70.1) | 31.8 ± 28.6 (9.0 – 110.9) |
| Leptin (ng/ml) | 39.1 ± 22.6 (7.8 – 83.3) | 42.1 ± 18.1 15.1 – 81.2 |
25.2 ± 18.4a (5.5 – 59.8) | 29.7 ± 14.9 a (11.9 – 53.7) |
| Glucose (mg/dL) | 95.0 ± 6.1 (86.1 –109.7) | 99.3 ± 7.5 (88.3 – 100.5) | 95.1 ± 7.5 (87.2 – 118.7) | 91.2 ± 4.3 (85.3 – 100.6) |
Data are presented as means ± s.d. Values in parentheses are ranges.
Significantly different from values after 12 weeks diet (<0.001, paired t-test)
DISCUSSION
The novelty of the present study is that we investigated the random effect of dairy and non-dairy calcium intake on fecal fat excretion in lactose digesters and maldigesters during energy restriction-induced weight loss. The major finding is that high-Ca diet causes an increase in fecal fat loss independent of calcium source (dairy and nondairy) and ability to digest lactose. The effect of dietary calcium on fecal fat loss was not diminished over 12 weeks. The fecal fat loss increased correspondingly with cross over to high-Ca diet and vice versa as illustrated in Figure 1.
Compared to low-Ca diet, high-Ca diet increased the fecal fat excretion by 1.8 g/day, or about 3 % of daily fat intake (6.7 ± 1.9 vs. 4.7 ± 1.8 g/day). This increase in fat excretion could produce 63 –125 kJ (15–30 kcal) per day of fecal energy loss, which could cause approximate 0.4 –0.7 kg body fat loss over the one-year period. This difference might be clinically relevant to long-term body weight regulation.
The results of this 12-week investigation of effect of high calcium diet on fecal fat excretion are in line with prior short-term (1 to 2 weeks) human trials24, 31–33. For example, Denke et al31 have reported a 7% increase in fecal fat by increasing daily calcium intake from 410 mg to 2200 mg for 10 days. Shahkhalili et al32 observed an increase of 4 g/day in fecal fat by supplementing chocolate with 0.9 g calcium/day for 2 weeks. Welberg et al33 demonstrated a graded increase in fecal fat excretion (6.8%, 7.4% and 10.2%) with increasing calcium carbonate supplementation (0, 2 and 4 g per day) for a week. Very recently Bendsen et al23 and Jacobsen et al24 have reported an increase in fecal fat excretion of over 2 and 2.5 fold by increasing daily calcium intake to 2300mg and 1800 mg from 700mg and 500mg respectively. In a 7-day trial with dairy and non-dairy calcium supplementations, Boon et al34 also observed a non-significant trend towards a higher fat excretion on the high-calcium diet, noting a 56% higher fecal fat excretion on 2,500 mg vs. 400 mg calcium diet.
Fecal fat loss in this study (8.2 g/day) was comparable or lower than fat loss reported in other studies. The differences may be largely explained by different calcium and protein contents of the diets used for the studies. It is known that a high protein intake increases calcium bioavailability leaving less calcium available for binding with fat in the intestine35, 36. For example, Jacobsen et al24 have noted modulation of the effect of high-calcium intake on fecal fat excretion by protein content of the energy intake. They have reported a fecal fat loss of 14.2 vs. 6.0 and 5.9 g/day with high calcium (1800 mg)-normal protein (15% of daily energy intake), low calcium (500 mg)-normal protein (15% of daily energy intake), and high calcium (1800 mg)-high protein (23% of daily energy intake) diets, respectively. The authors found no difference in fecal fat excretion between the low calcium-normal protein and the high calcium-high protein diets. We tested the effect of 1500 mg and 500 mg calcium diets with protein content of 17–21% of daily energy intake.
We also observed that fecal fat loss was comparatively less stable in the first 6-week than second 6-week period (Figure 1). We do not have a definite explanation for that. Perhaps it might be related to greater weight loss in the first as compared to the last 6 weeks of energy restriction across all the variables studied. We cannot, however, compare our results to other studies since to the best of our knowledge no trial was reported with similar length (6 or 12 weeks) of controlled calcium intake and fecal fat excretion measurement.
Our results further signify that fecal fat loss with a high calcium diet is not diminished over time, and this effect is not different in lactose digesters and maldigesters and with dairy and non-dairy calcium.
In our study we did not observe any significant differential effect of dairy calcium on fecal fat loss. However, Lorenzen et al37 in a randomized crossover study with four isocaloric meals with differing amount or source of calcium reported a decrease in fat absorption with dairy calcium supplementation. It is not clear why calcium from dairy products but not calcium from supplement was found to inhibit fat absorption. The authors are of the opinion that dairy calcium is largely present as insoluble calcium phosphate, which binds bile acids and impairs micelles formation leading to increased fecal fat loss. However, Bendsen et al23 have recently shown that increasing the daily intake of calcium from low-fat dairy products by 1600 mg for 7 days doubled the total fecal fat excretion (11.5 ± 1.4 g from 5.4 ± 0.5 g), but did not affect the excretion of bile acids. Their results suggest that fecal fat loss is not caused by binding of bile acids but rather it may be due to calcium soap formation in the intestine.
Our results indicate that lactose maldigestion has no significant effect on fecal fat loss or urinary calcium excretion associated with dietary calcium. Approximately 70% of the world’s population loses the ability to digest large amounts of lactose after weaning, and this lactase nonpersistence is inherited as an autosomal recessive trait38. Several investigators have reported increased prevalence of osteoporosis in symptomatic lactose maldigesters39–41. Our findings are in line with the current understanding that inadequate calcium intake rather than lactose maldigestion per se is related to the consequences of calcium deficiency such as osteoporosis42,43.
We noted that mean weight loss but not fat loss tend to be higher in lactose digesters as compared to lactose maldigesters. However, this study was not specifically designed to examine the effect of calcium on weight loss and nor does it have the power to support any such conclusions. All the participants were on isocaloric restriction and we found no significant differences in weight changes with the source of calcium after 12 weeks of energy restriction. Prior studies have reported conflicting effects of dairy or non-dairy calcium supplementation on weight loss, from no effect13–19 to significant effect8–12, 44.
Our study must be interpreted within the context of our experimental design. The sample size was relatively small considering dietary calcium intake level, dairy or nondairy diet source, lactose digestion status, and gender as variables. However, it was a randomized controlled crossover trial with similar numbers of lactose digesters and maldigesters. Secondly, we did not measure fecal calcium but used urinary calcium as a marker of calcium intake. We also did not quantify fecal fat complexes (soaps) as a surrogate to soaponification. Rather, we relied on comparison of fecal fat excretion with low and high dietary calcium intake. Thirdly, 24-h stool samples were collected once per week. However, given constant content and reported intake of energy, macronutrients, and micronutrients including calcium of daily diets prepared by the metabolic kitchen it was reasonable to assume that stool fat content was also not different. Fourthly, the cross-over design was used only for dietary calcium level. Thus, the reason we could not detect the significant difference between non-dairy and dairy diet source could be due to large variability across subjects, constant amount of dietary calcium in respective diets, and possible gender differences. Our results for the effect of diet type or lactose digestion status would require further study.
In summary, we found that during moderate (−30%) energy restriction-induced weight loss a high-Ca diet causes an increase in fecal fat excretion independent of calcium source. The new findings are that calcium intake related fecal fat loss is also independent of the ability to digest lactose and it is not diminished over time.
Table 5.
Comparison of reported daily average calcium intake and urinary calcium excretion during 12 week diet
| Low-Ca diet | High-Ca diet | |||||
|---|---|---|---|---|---|---|
| Ca intakea (mg/day) | Urinary Cab (mg/day) | % of intake | Ca intakea (mg/day) | Urinary Cab mg/day | % of intake | |
| Digesters (n=18) | 504.3 ± 36.8 (429 – 561) | 141.2 ± 47.3 (48.9 – 222.8) | 26.6± 12.1 (13.1 – 46.1) | 1498.9 ± 33.8 (1397 – 1543) | 160.9 ± 69.5 (54.8 – 295.6) | 10.9 ± 6.1 (6.1 – 17.8) |
| Maldigesters (n=16) | 500.1 ± 32.8 (405 –544) | 115.9 ± 54.6 (39.9 – 248.7) | 23.7± 7.0 (7.0 – 40.7) | 1508.8 ± 72.5 (1424 – 1756) | 130.9 ± 85.5 (24.1 – 365.6) | 8.9 ± 7.3 (4.6 – 17.1) |
Data are presented as means ± s.d. Values in parentheses are ranges for weekly averages.
Average daily reported intake of calcium assessed using NDSR software (Nutrition Data System, St. Paul, MN).
Average daily calcium excretion in 24 h urine calculated from 12 weekly collections.
Acknowledgments
We thank Nobuko Hongu, PhD, RD, LeMonica Adkerson, BS, Damaris Santana, BS, and Lauren Whitaker, BS for their help with conducting the study. We acknowledge our participants for their enthusiasm and commitment to this study. We also thank staff of the Clinical Research Center at Vanderbilt University for their help with this project.
Supported in part by NIH Vanderbilt CTSA grant 1 UL1 RR024975, Vanderbilt Diabetes Research and Training Center grant DK20593, and Vanderbilt Digestive Disease Research Center grant P30DK058404.
Footnotes
U.S. Clinical Trial Registration: Clinicaltrials.gov NCT00808275
References
- 1.Griffith LE, Guyatt GH, Cook RJ, Bucher HC, Cook DJ. The influence of dietary and nondietary calcium supplementation on blood pressure : An updated metaanalysis of randomized controlled trials. American Journal of Hypertension. 1999;12:84–92. doi: 10.1016/s0895-7061(98)00224-6. [DOI] [PubMed] [Google Scholar]
- 2.Pereira MA, Jacobs DR, Jr, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: The CARDIA study. JAMA. 2002;287:2081–9. doi: 10.1001/jama.287.16.2081. [DOI] [PubMed] [Google Scholar]
- 3.Zemel MB. Calcium and dairy modulation of obesity risk. Obesity. 2005;13:192–3. doi: 10.1038/oby.2005.26. [DOI] [PubMed] [Google Scholar]
- 4.Davies K, Heaney R, Recker R, et al. Calcium intake and body weight. J Clin Endocrinol Metab. 2000;85:4635–38. doi: 10.1210/jcem.85.12.7063. [DOI] [PubMed] [Google Scholar]
- 5.Jacqmain M, Doucet E, Despres J, Bouchard C, Tremblay A. Calcium intake, body composition, and lipoprotein-lipid concentrations in adults. Am J Clin Nutr. 2003;77:1448–52. doi: 10.1093/ajcn/77.6.1448. [DOI] [PubMed] [Google Scholar]
- 6.McCarron D, Morris C, Henry H, Stanton J. Blood pressure and nutrient intake in the United States. Science. 1984;29:1392–8. doi: 10.1126/science.6729459. [DOI] [PubMed] [Google Scholar]
- 7.Shapses S, Heshka S, Heymsfield S. Effect of calcium supplementation on weight and fat loss in women. J Clin Endocrinol Metab. 2004;89:632–7. doi: 10.1210/jc.2002-021136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metz J, Karanja N, Torok J, McCarron D. Modification of total body fat in spontaneously hypertensive rats and Wistar-Kyoto rats by dietary calcium and sodium. Am J Hypertens. 1988;1:58–60. doi: 10.1093/ajh/1.1.58. [DOI] [PubMed] [Google Scholar]
- 9.Shi H, Dirienzo D, Zemel M. Effects of dietary calcium on adipocyte lipid metabolism and body weight regulation in energy-restricted aP2-agouti transgenic mice. FASEB J. 2001;15:291–3. doi: 10.1096/fj.00-0584fje. [DOI] [PubMed] [Google Scholar]
- 10.Zemel M, Richards J, Mathis S, Milstead A, Gebhardt L, Silva E. Dairy augmentation of total and central fat loss in obese subjects. Int J Obes Relat Metab Disord. 2005;29:391–7. doi: 10.1038/sj.ijo.0802880. [DOI] [PubMed] [Google Scholar]
- 11.Zemel MB. The role of dairy foods in weight management. J Am Coll Nutr. 2005;24:537S–46. doi: 10.1080/07315724.2005.10719502. [DOI] [PubMed] [Google Scholar]
- 12.Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC. Regulation of adiposity by dietary calcium. FASEB J. 2000;14:1132–8. [PubMed] [Google Scholar]
- 13.Bowen J, Noakes M, Clifton P. Effect of calcium and dairy foods in high protein, energy-restricted diets on weight loss and metabolic parameters in overweight adults. Int J Obes (Lond) 2005;29:957–65. doi: 10.1038/sj.ijo.0802895. [DOI] [PubMed] [Google Scholar]
- 14.Cleghorn D, O’Loughlin P, Schroeder B, Nordin B. An open, crossover trial of calcium-fortified milk in prevention of early postmenopausal bone loss. Med J Aust. 2001;175:242–5. doi: 10.5694/j.1326-5377.2001.tb143554.x. [DOI] [PubMed] [Google Scholar]
- 15.Gunther CW, Legowski PA, Lyle RM, et al. Dairy products do not lead to alterations in body weight or fat mass in young women in a 1-y intervention. 2005;81:751–6. doi: 10.1093/ajcn/81.4.754. [DOI] [PubMed] [Google Scholar]
- 16.Jensen L, Kollerup G, Quaade F, Sorensen O. Bone minerals changes in obese women during a moderate weight loss with and without calcium supplementation. J Bone Miner Res. 2001;16:141–7. doi: 10.1359/jbmr.2001.16.1.141. [DOI] [PubMed] [Google Scholar]
- 17.Rajpathak SN, Rimm EB, Rosner B, Willett WC, Hu FB. Calcium and dairy intakes in relation to long-term weight gain in US men. Am J Clin Nutr. 2006;83:559–66. doi: 10.1093/ajcn.83.3.559. [DOI] [PubMed] [Google Scholar]
- 18.Trowman R, Dumville J, Hahn S, Torgerson D. A systematic review of the effects of calcium supplementation on body weight. Br J Nutr. 2006;95:1033–8. doi: 10.1079/bjn20051727. [DOI] [PubMed] [Google Scholar]
- 19.Yanovski J, Parikh S, Yanoff L, et al. Effects of calcium supplementation on body weight and adiposity in overweight and obese adults: a randomized trial. Ann Intern Med. 2009 Jun 16;150:821–9. W145–6. doi: 10.7326/0003-4819-150-12-200906160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barr SI, McCarron DA, Heaney RP, et al. Effects of increased consumption of fluid milk on energy and nutrient intake, body weight, and cardiovascular risk factors in healthy older adults. Journal of the American Dietetic Association. 2000;100:810–7. doi: 10.1016/S0002-8223(00)00236-4. [DOI] [PubMed] [Google Scholar]
- 21.Lanou A, Barnard N. Dairy and weight loss hypothesis: an evaluation of the clinical trials. Nutr Rev. 2008;66:272–9. doi: 10.1111/j.1753-4887.2008.00032.x. [DOI] [PubMed] [Google Scholar]
- 22.Christensen R, Lorenzen J, Svith C, et al. Effect of calcium from dairy and dietary supplements on faecal fat excretion: a meta-analysis of randomized controlled trials. Obes Rev. 2009 Jul;10:475–86. doi: 10.1111/j.1467-789X.2009.00599.x. [DOI] [PubMed] [Google Scholar]
- 23.Bendsen N, Hother A, Jensen S, Lorenzen J, Astrup A. Effect of dairy calcium on fecal fat excretion: a randomized crossover trial. Int J Obes. 2008;32:1816–24. doi: 10.1038/ijo.2008.173. [DOI] [PubMed] [Google Scholar]
- 24.Jacobsen R, Lorenzen JK, Toubro S, Krog-Mikkelsen I, Astrup A. Effect of short-term high dietary calcium intake on 24-h energy expenditure, fat oxidation, and fecal fat excretion. Int J Obes Relat Metab Disord. 2005;29:292–301. doi: 10.1038/sj.ijo.0802785. [DOI] [PubMed] [Google Scholar]
- 25.Callegari C, Lami F, Levantesi F, et al. Post-menopausal bone density, lactase deficiency and milk consumption. J Hum Nutr Diet. 1990;3:159–64. [Google Scholar]
- 26.Johnson A, Semenya J, Buchowski M, Enwonwu C, Scrimshaw N. Adaptation of lactose maldigesters to continued milk intakes. Am J Clin Nutr. 1993;58:879–81. doi: 10.1093/ajcn/58.6.879. [DOI] [PubMed] [Google Scholar]
- 27.Center fDC. NHANES: Anthropometry Procedures Manual. In; 2000 December:3:20–3 & 3:30–21.
- 28.Bingham S, Cummings J. Urine nitrogen as an independent validatory measure of dietary intake: a study of nitrogen balance in individuals consuming their normal diet. Am J Clin Nutr. 1985;42:1276–89. doi: 10.1093/ajcn/42.6.1276. [DOI] [PubMed] [Google Scholar]
- 29.Bingham S. Biomarkers in nutritional epidemiology. Public Health Nutrition. 2002;5:821–7. doi: 10.1079/phn2002368. [DOI] [PubMed] [Google Scholar]
- 30.Johansson G, Callmer E, Gustafsson J. Validity of repeated dietary measurements in a dietary intervention study. Eur J Clin Nutr. 1992;46:717–28. [PubMed] [Google Scholar]
- 31.Denke M, Fox M, Schulte M. Short-term dietary calcium fortification increases fecal saturated fat content and reduces serum lipids in men. Am J Clin Nutr. 1993;123:1047–53. doi: 10.1093/jn/123.6.1047. [DOI] [PubMed] [Google Scholar]
- 32.Shahkhalili Y, Murset C, Meirim I, et al. Calcium supplementation of chocolate: effect on cocoa butter digestibility and blood lipids in humans. Am J Clin Nutr. 2001;73:246–52. doi: 10.1093/ajcn/73.2.246. [DOI] [PubMed] [Google Scholar]
- 33.Welberg J, Monkelbaan J, de Vries E, et al. Effects of supplemental dietary calcium on quantitative and qualitative fecal fat excretion in man. Ann Nutr Metab. 1994;38:185–91. doi: 10.1159/000177810. [DOI] [PubMed] [Google Scholar]
- 34.Boon N, Hul G, Stegen J, et al. An intervention study of the effects of calcium intake on faecal fat excretion, energy metabolism and adipose tissue mRNA expression of lipid-metabolism related proteins. Int J Obes. 2007;31:1704–12. doi: 10.1038/sj.ijo.0803660. [DOI] [PubMed] [Google Scholar]
- 35.Kerstetter J, O’Brien K, Insogna K. Dietary protein, calcium metabolism, and skeletal homeostasis revisited. Am J Clin Nutr. 2003;78:584S–92S. doi: 10.1093/ajcn/78.3.584S. [DOI] [PubMed] [Google Scholar]
- 36.Kerstetter J, O’Brien K, Insogna K. Low protein intake: the impact on calcium and bone homeostasis in humans. J Nutr. 2003;133:855S–61S. doi: 10.1093/jn/133.3.855S. [DOI] [PubMed] [Google Scholar]
- 37.Lorenzen J, Nielsen S, Holst J, Tetens I, Rehfeld J, Astrup A. Effect of dairy calcium or supplementary calcium intake on postprandial fat metabolism, appetite, and subsequent energy intake. Int J Obesity. 2007;85:678–87. doi: 10.1093/ajcn/85.3.678. [DOI] [PubMed] [Google Scholar]
- 38.Sahi T, Isokoski M, Jussila J, Launiala K, Pyorala K. Recessive inheritance of adult-type lactose malabsorption. Lancet. 1973;2:823–6. doi: 10.1016/s0140-6736(73)90862-3. [DOI] [PubMed] [Google Scholar]
- 39.Corazza G, Benati G, Di Sario A, et al. Lactose intolerance and bone mass in postmenopausal Italian women. Br J Nutr. 1995 Mar;73:479–87. doi: 10.1079/bjn19950050. [DOI] [PubMed] [Google Scholar]
- 40.Elbon S, Johnson M, Fischer J, et al. J Nutr Elder. 1999;19:25–39. [Google Scholar]
- 41.Jackson K, Savaiano D. Lactose maldigestion, calcium intake and osteoporosis in African-, Asian-, and Hispanic-Americans. J Am Coll Nutr. 2001;20:198S–207S. doi: 10.1080/07315724.2001.10719032. [DOI] [PubMed] [Google Scholar]
- 42.Savaiano D. Lactose intolerance: a self-fulfilling prophecy leading to osteoporosis. Nutr Rev. 2003;61:221–3. doi: 10.1301/nr.2003.jun.221-223. [DOI] [PubMed] [Google Scholar]
- 43.Bone health and osteoporosis: a report of the Surgeon General. [Accessed December 27, 2006]. www.surgeongeneral.gov/library/bonehealthDoHaHS. [PubMed]
- 44.Thompson WG, Rostad HN, Janzow DJ, Slezak JM, Morris KL, Zemel MB. Effect of energy-reduced diets high in dairy products and fiber on weight loss in obese adults. Obesity. 2005;13:1344–53. doi: 10.1038/oby.2005.163. [DOI] [PubMed] [Google Scholar]