Abstract
Current therapies for Alzheimer disease (AD) such as the acetylcholinesterase inhibitors and the latest NMDA receptor inhibitor, Namenda, provide moderate symptomatic delay at various stages of the disease, but do not arrest the disease progression or bring in meaningful remission. New approaches to the disease management are urgently needed. Although the etiology of AD is largely unknown, oxidative damage mediated by metals is likely a significant contributor since metals such as iron, aluminum, zinc, and copper are dysregulated and/or increased in AD brain tissue and create a pro-oxidative environment. This role of metal ion-induced free radical formation in AD makes chelation therapy an attractive means of dampening the oxidative stress burden in neurons. The chelator desferrioxamine, FDA approved for iron overload, has shown some benefit in AD, but like many chelators, it has a host of adverse effects and substantial obstacles for tissue-specific targeting. Other chelators are under development and have shown various strengths and weaknesses. Here, we propose a novel system of chelation therapy through the use of nanoparticles. Nanoparticles conjugated to chelators show unique ability to cross the blood–brain barrier (BBB), chelate metals, and exit through the BBB with their corresponding complexed metal ions. This method may provide a safer and more effective means of reducing the metal load in neural tissue, thus attenuating the harmful effects of oxidative damage and its sequelae. Experimental procedures are presented in this chapter.
Keywords: Alzheimer disease, chelation therapy, metal dysregulation, nanoparticles
1. Introduction
Alzheimer disease (AD) is a devastating neurodegenerative disease with progressive and irreversible damage to thought, memory, and language. AD is the most common form of dementia among people aged 65 and older, progressing slowly from mild forgetfulness to the need for total care (reviewed in (1)). Unfortunately, an explicative etiology or a viable cure is not available.
Compared with other tissues, the central nervous system may be particularly susceptible to oxidative damage (2, 3). Accumulating evidence supports the hypothesis that oxidative stress generated by various mechanisms may be among the major intermediary risk factors that initiate and promote neurodegeneration, leading to AD (4–12). Oxidation reactions can be catalyzed by transition metals such as iron and copper (13) and, as such, the likelihood that an oxidation reaction will take place is probably increased by the regional concentrations of transition metals (14). Substantial studies show that the metabolism of iron is involved in AD and that the concentration of iron in the brain of AD patients is elevated (8, 15). Aluminum has also received attention in AD, although a role has never been convincingly demonstrated. Nonetheless, aluminum has been found in high concentrations in both senile plaques and intraneuronal neurofibrillary tangles in the brains of subjects with AD, which suggests that this metal may be involved in the etiopathology of AD (8, 15–19). Aluminum, unlike transition metal ions, is unable to participate in redox cycles of electron transfer reactions because of a fixed oxidation state of 3+ in biological systems, but evidence suggests that it can act synergistically with iron to increase free radical damage (2, 20). Strong evidence also shows that other metals implicated in the development of AD include copper (9, 10, 21–25) and zinc (8, 21, 26–28). In the AD brain, the concentration of zinc is significantly elevated in senile plaques and the concentration of copper is elevated in the rim of senile plaques. Overall, these studies indicate that the environmental conditions in AD, due to imbalances of several metals, have the potential for catalyzing and stimulating free radical formation and enhancing neuron degeneration. Moreover, growing studies reveal that all of the aforementioned metals that accumulated in the central nervous system modulate amyloid-β formation and deposition (29). The metals and amyloid-β can form complexes tightly, which also cause neurooxidative damage (11, 20, 24, 30–32).
Simultaneously elevated concentrations of various metals promoting oxidative damage, and hence promoting neurodegeneration, present a complex system of pathophysiology not yet fully understood. Despite this complexity, metal dysregulation may in fact be the Achilles’ heel of AD, opening a door for chelation therapy. An iron chelator, regardless of synthetic or natural origin, can have high affinity for iron, but it may also undesirably chelate other metals in various tissues leading to serious side effects. Affinity for multiple metals such as aluminum, copper, and zinc may pose useful rather than detrimental since various metals are implicated as oxidative instigators. Perhaps this may be the reason why desferrioxamine (DFO), a specific iron chelator with high affinities for aluminum, copper, and zinc has demonstrated some therapeutic benefits for patients with AD.
1.1. Iron Chelators in the Treatment of AD
DFO is a hexadentate iron chelator and has been found to significantly slow the progression of AD in one clinical trial (33). In this study, the chelation of aluminum was examined, but it is possible that the therapeutic effect may also have been due to removal of iron since DFO preferably chelates iron (34, 35). DFO also has an appreciable affinity for copper and zinc (34, 36). The affinity constants of DFO for Fe(III), Al(III), Cu(II), and Zn(II) are 30.6, 22.0, 14.1, and 11.1 (log K), respectively (37). In this clinical study, copper and zinc were not monitored. Interestingly, 2 years after the initial publication, a verbal report at the International Conference on Alzheimer’s Disease (Padua, Italy, 1992) provides evidence that iron and zinc concentrations are decreased in a postmortem analysis for DFO-treated patients (34, 38).
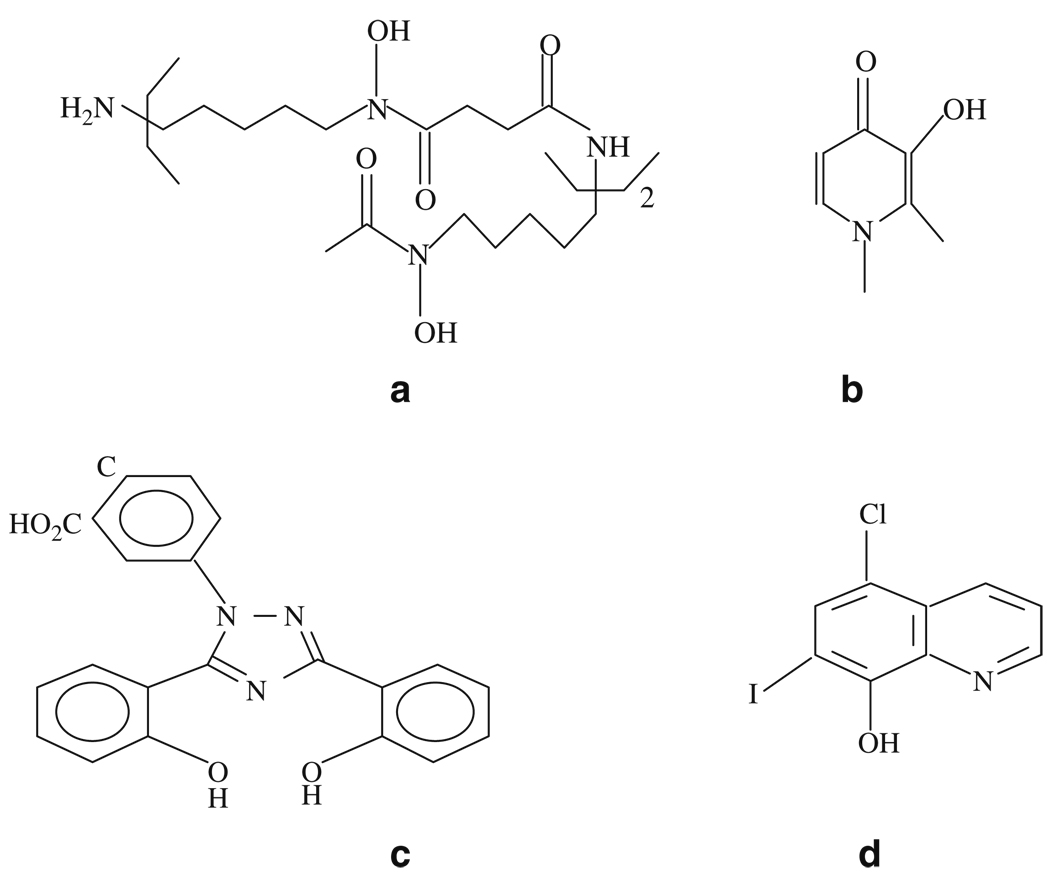
DFO is one of the only two iron chelators approved by the FDA for iron overload disease. DFO is a hexadentate iron chelator (Fig. 8.1a). Its therapy promotes iron excretion and has led to great improvements in the quality and duration of life of patients who suffer from β-thalassemia and other refractory anemias. In addition, DFO also inhibits nigrostriatal degeneration induced by 6-hydroxydopamine (39). Unfortunately, DFO has serious side effects including neurotoxicity and neurological changes (13, 33, 40–45). Furthermore, DFO is poorly absorbed by the gastrointestinal tract and rapidly degrades after administration (46). Therefore, it requires long subcutaneous administration to yield significant iron excretion (35, 47). Moreover, some studies show DFO does not easily penetrate the blood–brain barrier (BBB) due to its hydrophilic nature (48), although this point remains open to debate (49). Some penetration may occur due to a compromised BBB via lesion sites (48). But the neurotoxicity and difficulty of administration and delivery present serious hindrances to the use of DFO for AD treatment.
Fig. 8.1.
Chemical structures of chelators: (a) DFO; (b) L1; (c) deferasirox; and (d) clioquinol.
Deferiprone or L1 (1,2-dimethyl-3-hydroxyl-4-pyridinone) is a bidentate iron and aluminum chelator (Fig. 8.1b) approved in Europe, but not in the United States (50). Although L1 has high oral activity and BBB penetration ability due to its lipophilicity, its use is limited because of serious side effects (51, 52). In addition, studies have shown that L1 lacks the ability to remove iron from the brain (49) probably due to strong hydrophilicity of the iron–L1 complex. Additionally, there is no carrier-mediated transport system available to remove the complex from the brain. Other L1 derivatives with higher lipophilicity also have the ability to cross the BBB and complex brain iron, but they also possess considerable neurotoxicity (40, 43, 51).
Currently, FDA has approved another iron chelator (Deferasirox) for treatment of transfusional iron overload in thalassemia. Deferasirox is a tridentate iron chelator (Fig. 8.1c) with oral bioavailability (53). However, its long-term profiles are not yet available (54, 55). Furthermore, its lipophilic nature like L1 may raise questions concerning potential toxicity in AD treatment.
Thus, the use of the currently available iron chelators to simultaneously remove several excess metals in the brain of AD is limited because of their toxicity and/or poor transference across the BBB. Most bi- or tridentate iron chelators with small molecular weight and high lipophilicity have the ability to penetrate the BBB, but show toxicity (56). On the other hand, hexadentate iron chelators are considered better candidates for chelation therapy than bi- and tridentate ones because of their lower toxicity before and after chelation (56), but they have difficulty penetrating the BBB (49, 56, 57) due to their hydrophilicity and relative high molecular weight. One strategy to increase the BBB penetration is by enhancing the lipophilicity of the iron chelators (58); however, this is believed to increase toxicity (59). In addition, the increase in lipophilicity of iron chelators will decrease the solubility in aqueous solution with probably a decrease in bioavailability (52). Also, it is possible that some lipophilic chelators, which normally should cross the brain endothelial cells, are rapidly pumped back into the bloodstream by extremely effective efflux pumps. These include multiple organic anion transporter and P-glycoprotein (multidrug resistance protein) (60). Many promising attempts have been made to develop iron chelators with abilities to penetrate BBB and prevent oxidative damage (61–67). However, there is a great need to develop safer and more effective iron chelators for the treatment of AD and other neurodegeneration diseases.
The role of metals in the AD development and the usefulness of chelators for AD treatment have also been demonstrated in the studies with Iodochlorhydroxyquin (clioquinol) (34, 68–70). Clioquinol is a copper and zinc-specific chelator (Fig. 8.1d) with BBB penetrable and is able to dissolve amyloid-β plaques. With clioquinol therapy, the clinical rate of cognitive decline is slowed in a subset of AD patients compared with that in controls (71). However, clioquinol is reported being associated with subacute myelo-optic neuropathy and withdrawn from the market as an antibiotic. In this regard, the second generation of clioquinol has been developed and is under clinical investigations (72).
1.2. Nanoparticle Systems with Iron Chelators: Increased BBB Permeability and Lower Toxicity
Nanoparticles made of natural or artificial polymers ranging in size from about 10–1,000 nm (60, 73) present a possible tool to transport drugs across the BBB (60) and nanoparticles of a size around or less than 300 nm coated with surfactants such as polysorbate 80 have been demonstrated to possess this ability (74–76). Recent studies have also shown the possibility of nanoparticulated drugs for the treatment of AD (77–80). The advantages of nanoparticles include reduced drug toxicity, improved biodistribution, and therapeutic efficacy (81). The mechanism by which the nanoparticles deliver drugs into brain may be involved in preferential absorption of apolipoprotein E (ApoE) and/or B. The particles also appear to mimic LDL and interact with the LDL receptor, resulting in their uptake by brain endothelial cells (60, 74, 82–84). The transferrin transcytosis systems may be also employed by the particulated drug delivery systems to deliver drugs into the brain (60, 82, 85). If an iron chelator can be covalently bonded to a nanoparticle, the particle may serve as a targeting vehicle to deliver the chelator to the brain and cross the BBB. There are three advantages to this approach. First, the chelators need not be lipophilic to cross the BBB. Second, the lipophilic character of the chelator no longer contributes to potential toxicity. And third, hydrophilic hexadentate iron chelators with large molecular weights may be used, as previously demonstrated with nanoparticle technology (75, 76).
For iron chelation to be effective, the chelators must be capable of leaving the brain with the corresponding complexed metal ions. If the nanoparticles are not or controlled biodegradation and can mimic lipoprotein particles by preferentially absorbing Apo-AI, known to facilitate the removal of particles from the brain (60, 86), the same carrier-mediated transport systems will be able to carry the iron complex nanoparticles out of the brain. This is in contrast to lipophilic chelators that can enter the brain, but when complexed, they are unable to cross the BBB due to a change in their lipophilicity. For example, the distribution coefficient (DC) of free L1 determined in n-octanol/Tris–HCl buffer system is 0.24, but when complexed is down to 0.0009 (87). Although L1 can reportedly penetrate the BBB, it fails to remove iron from the brain (49).
Our studies show that nanoparticles have the potential to transfer chelators in and out brain as well, thus effectively preventing metal-associated oxidative damage (80, 88). This novel approach of chelation will provide not only a useful means of AD treatment, but also insights into the mechanisms of AD pathophysiology. It may also show utility in other iron-mediated neurodegenerative diseases such as Friedreich’s ataxia, Parkinson’s disease, and Hallervorden-Spatz Syndrome. More studies are warranted to demonstrate the protective efficacy of the chelator–nanoparticle systems, to evaluate their toxicity and to optimize their capability to cross the BBB.
2. Materials
All chemicals and biochemicals are purchased from Aldrich-Sigma (St. Louis, MO), unless specifically mentioned. The materials obtained are used without further purification. Solutions are prepared following standard protocols.
3. Methods
In order to conjugate nanoparticles covalently, iron chelators must have a functional group to react with an active moiety on the particle surface. The functional group introduced into the iron chelators should not possess adverse effects on the chelator-metal binding. Synthetic methods to produce a series of iron chelators with such functional side chains have been developed (89–91). The metal binding properties of these chelators and some biological properties such as the in vitro ability to remove iron from tissue sections of AD brains and from ferritin (an important protein for iron storage) have been examined (89, 90). Methods for conjugation of various iron chelators to nanoparticles have also been developed. After conjugation, the amounts of chelator that conjugate to the particles and the ability of the chelator–particle systems to bind iron are determined. The human plasma protein absorp tion patterns on iron chelator particle systems and their iron complexes are examined using 2-D PAGE technology to evaluate the ApoE and Apo-AI absorptions (60, 92). These studies indicate that iron chelator–nanoparticle systems have the potential to enter the brain and bring excess metals out of the brain, thus effectively preventing metal-associated oxidative damage. As prototypes, the syntheses of two kinds of iron chelators containing active functional groups have been described (see Note 1). Key experiments are briefly described as follows.
3.1. Synthesis of 2-Methyl-N-(2′-aminoethyl or 3′-aminopropyl)-3-hydroxyl-4-pyridinone (MAEHP and MAPHP) (see Note 2), An Iron Chelator with Functional Groups for Nanoparticle Conjugation
Mix 3-hydroxyl-2-methyl-4-pyranone with benzyl chloride in a molar ration of 1:1.1 in aqueous methanol solution containing NaOH. Reflux for 6 h with the contents being constantly stirred on magnetic stirrer.
Remove methanol under vacuum and add water. Extract the product 3-benzyloxy-2-methyl-4-pyranone into methylene chloride.
Wash the organic (methylene chloride) layer with 5% (w/v) NaOH followed by water and dried it over anhydrous MgSO4.
Evaorate the solvent under vacuum. Add 1,2-diaminoethane or 1,3-diaminopropane in aqueous ethanol solution to the residue containing 3-benzyloxy-2-methyl-4-pyranone reacted and allow the reaction to proceed at the ambient temperature for about 1 week.
Evaporate the solvents and residual diamines under vacuum. Dissolve the residue in chloroform. Wash the chloroform solution with water and dry it over anhydrous Na2SO4.
Remove the solvent under vacuum and dissolve the residue in methanol. Adjust the pH to approximately 1.0 with HCl. The product 1-(2′-aminoethyl)-3-benzyloxy-2-methyl-4-pyridinone or 1-(3′-aminopropyl)-3-benzyloxy-2-methyl-4-pyridinone separates from methanolic solution as dihydrochloride salt. Collect the dihydrochloride salts by filtration and recrystallize them from a solution of methanol and ether to obtain the pure product(s).
Mix the products with BBr3 (1.0 M CH2Cl2 solution) in CH2Cl2 and stir overnight at room temperature under a nitrogen atmosphere.
Add water and stirring for an additional 4 h at room temperature. The aqueous phase containing MAEHP or MAPHP is separated and evaporated under vacuum.
The MAEHP and MAPHP are purified further through recrystallization from an ethanol/ether solution.
3.2. Synthesis of 2-Methyl (or Ethyl)-N-(2′-hydroxyethoxy)methyl-3-hydroxyl-4-pyridinone (MHEMHP or EHEMHP) (see Note 3), An Iron Chelator with Functional Groups for Nanoparticle Conjucation
Synthesize 3-benzyloxyl-2-alkyl-4-pyridinone as described in Section 3.1, Step 1.
Replace the ring oxygen of 3-benzyloxyl-2-alkyl-4-pyranone by a nitrogen atom via a substitution reaction with aqueous ammonia for 48 h at room temperature.
Silylate the 3-benzyloxyl-2-alkyl-4-pyridinone using hexamethyldisilazane under refluxing and nitrogen gas for 2 h.
Remove the solvent under vacuum. Dissolve the residue in 1,2-dichloroethane and then add benzyloxyethoxymethylchloride (see Note 4) in the presence of a catalytic amount of trimethylsilyl trifluoromethanesulfonate (see Note 5).
Stir the mixture at room temperature for 4 h and then treat with an aqueous solution saturated with sodium bicarbonate.
Discard the aqueous phase. Dry the organic phase over anhydrous Na2SO4 and then evaporate the solvent under vacuum.
Remove the two protection groups simultaneously by hydrogenation with H2/Pt on active carbon in acidic aqueous ethanol at room temperature for 24 h (see Note 6).
Finally, recrystallize the chelators from a 1:1 solution of CH3Cl/MeOH (see Note 7).
3.3. Titration of Chelators with Iron Ions in Buffer Solution
To 2.3 mL of 25 mM Tris–HCl buffer, pH 7.5, containing chelators (0.474 mM), add freshly prepared Fe(NO3) 3 solution (15.1 mM) in Tris buffer gradually in small aliquots of 5 µL each.
Monitor the change in absorbance due to the formation of chelator–iron complexes photometrically at 450 nm or higher. The chelators and iron form purple complexes with typical absorption in the visible range over 450 nm, whereas free chelators absorb maximally 280 nm (see Note 8).
3.4. Iron Removal by Chelators from Ferritin
To study the mobilization of iron from ferritin, incubate horse spleen ferritin (9.2 µL of 100 mg/mL stock solution) with chelators (0.474 mM) in 2.3 mL of Tris buffer solution (25 mM, pH 7.5) at 37°C for 72 h.
Monitor the changes in absorbance due to the formation of iron–chelator complex spectrophotometrically at different time intervals. The kinetics of the iron release was investigated for periods up to 72 h (see Note 9).
3.5. Iron Removal by Chelators from AD Brain Sections In Vitro
The ability of chelators to mobilize iron from brain sections can be examined via histochemical method (80, 93).
Fix the tissue specimens, collected from the hippocampal region of AD patients (see Note 10), in methacarn (see Note 11) overnight at 4°C.
Place the brain tissues in 50% ethanol, dehydrate in ascending concentrations of ethanol and finally embed them in paraffin.
Thin sections (thickness: 6 µm) of the tissue and mounted on silane-coated slides.
Deparaffinize the tissue sections with two changes of xylene (10 min each) and then re-hydrate through graded ethanol/TBS mixture.
Apply 40 µL of PBS containing various concentrations chelator(s) to each section and incubate overnight at 37°C.
At the end of incubation period, rinse the tissue sections thoroughly with TBS.
Incubate at 37°C for 2 h in 7% (w/v) potassium ferrocyanide in 3% (w/v) HCl in water.
Rinse the sections Tris–HCl buffer and subsequently incubate in 0.75 mg/mL 3,3′-diaminobenzidine and 0.015% H2O2 for 5–10 min.
Finally, dehydrate tissue sections through graded ethanol, put coverslip, and examine using differential interference microscopy (see Note 12).
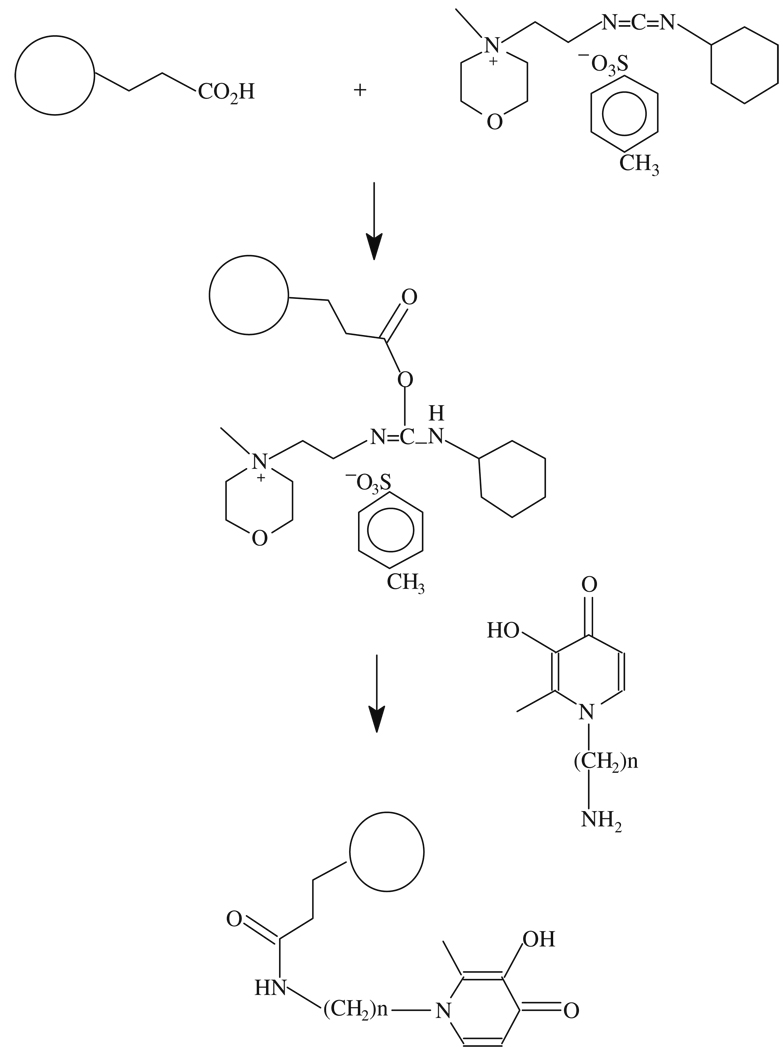
3.6. Conjugation of Iron Chelators with Nano or Microsphere Particles
As a prototypic procedure (see Note 13), a simple method of particle–chelator conjugation by forming an amido bond is presented. Monodispersed polystyrene particles with carboxyl groups on the surface (Bangs Laboratories, Fishers, IN) were used to conjugate MAEHP or MAPHP as prototypic chelators, each of which contained a free primary amino group available for the conjugation (see Note 14).
Prepare a suspension of carboxylated particles by pipetting and vortexing and immediately transfer into a micro-centrifuge tube.
Remove the supernatant by centrifugation.
Re-suspend the particles in 0.01 N NaOH solution, mix well, and repeat the process.
Wash the particles twice with 0.1 M of MES (2-[N-morpholino] ethane sulfonic acid) buffer (pH 5.0) and once with cold Milli-Q water.
Carboxyl groups on the particles are to be activated by adding cold Milli-Q water containing N-cyclohexyl- N′-(2-morpholinoethyl)carbodiimide methyl-p-toluensulfonate (CMC, 0.01M) and incubating for 10 min at 4°C with slow tilt rotation.
After removing the supernatant, add CMC solution again along with MES buffer (0.3 M, pH 5.0).
Vortex the mixture and incubate as described above for 30 min.
Wash the activated carboxyl groups containing particles twice with cold 0.1MMES as quickly as possible and resuspend in MES buffer (0.1 M, pH 5.0) containing excess MAEHP or MAPHP (0.01M) (see Note 15).
Vortex the mixture followed by incubation for 30 min at room temperature with tilt rotation.
Wash twice the chelator–particle systems with 0.1 M MES buffer and PBS, store in PBS at 4°C.
Determine the yields of chelator conjugation by measurements of the free chelator concentrations in the solutions before and after conjugation using UV-visible spectrometer (or HPLC) at the wavelength of maximum absorption (94).
Concentrations and size distributions of the chelator–particle systems could be determined using a Beckman Coulter Multisizer II in a counting cuvette containing Isoton II diluent or using a Coulter N4 Plus Sub-micron Particle Sizer.
3.7. Reaction of Chelator–Particle Systems with Ferric Iron
Add an aliquot of freshly prepared ferric iron solution (Fe(NO3)3, 0.002 M in MES buffer 0.01 M, pH 5.0) to MES (0.01 M, pH 5.0) solution containing suspended MAPHP–particle systems as prototype, or plain particles as a control.
Allow the mixture to rotate at room temperature for 4 h. The iron–chelator–particle systems and supernatant are separated by centrifugation.
Wash the systems thoroughly with MES buffer 5 times to remove non-complexed iron ions.
After combination of the supernatants, add excess MAPHP in MES buffer (0.01 M) to complex the iron ions that does not react with the chelator–particle systems. The visible absorbance of the iron–MAPHP complex is measured using UV-visible spectrophotometry at a maximum wavelength of 455 nm (ε 3.02 × 103) after the chelating reaction reached equilibrium.
Obtain a standard curve for iron concentration by measuring several solutions of iron–MAPHP complex with known iron concentrations to estimate the amount of non–complexed iron with the chelator–particle systems (see Note 16).
3.8. Protein Absorptions of Chelator–Particle Systems and Chelator–Particle Systems with Iron
The absorbed proteins on chelator–particle systems and chelator–particle systems with iron, which are obtained by reaction of ferric iron with chelator–particle systems, were evaluated using 2-D PAGE analyses.
Incubate separately the chelator–particle systems that are overcoated with polysorbate 80 at room temperature and the chelator–particle systems with iron (100 µL of each system, 2.5% w/v in PBS buffer) in 1 mL of citrated human plasma for 5 min at 37°C (92).
After separating by centrifugation, wash the systems four times with Milli-Q water.
Elute the adsorbed proteins from the particle surface with a protein solubilizing solution (5% SDS), 5% dithioerythritol, 10% glycerol, and 60 mM Tris, pH 6.8) (92).
In the first dimension of the 2D-PAGE analysis, isoelectric focusing (IEF), the proteins are separated according to their isoelectric points (pI). Carry out the IEF in glass tubes of inner diameter 2.0 mm using 2.0% pH 3.5–10 ampholines for 9,600 V-h.
In the second dimension of SDS-PAGE, the separation is based on molecular weight (MW). Equilibrate each tube for 10 min in 62.5 mM Tris, pH 6.8, buffer containing 2.3% SDS, 50 mM dithioerythritol, and 10% glycerol.
Seal to the top of a stacking gel that is on the top of a 10% acrylamide slab gel (145 × 145 × 0.75 mm).
Perform SDS slab gel electrophoresis for about 4 h at 12.5 mA/gel.
After SDS-PAGE, dry the gels between sheets of cellophane and silver-stained (92) (see Note 17).
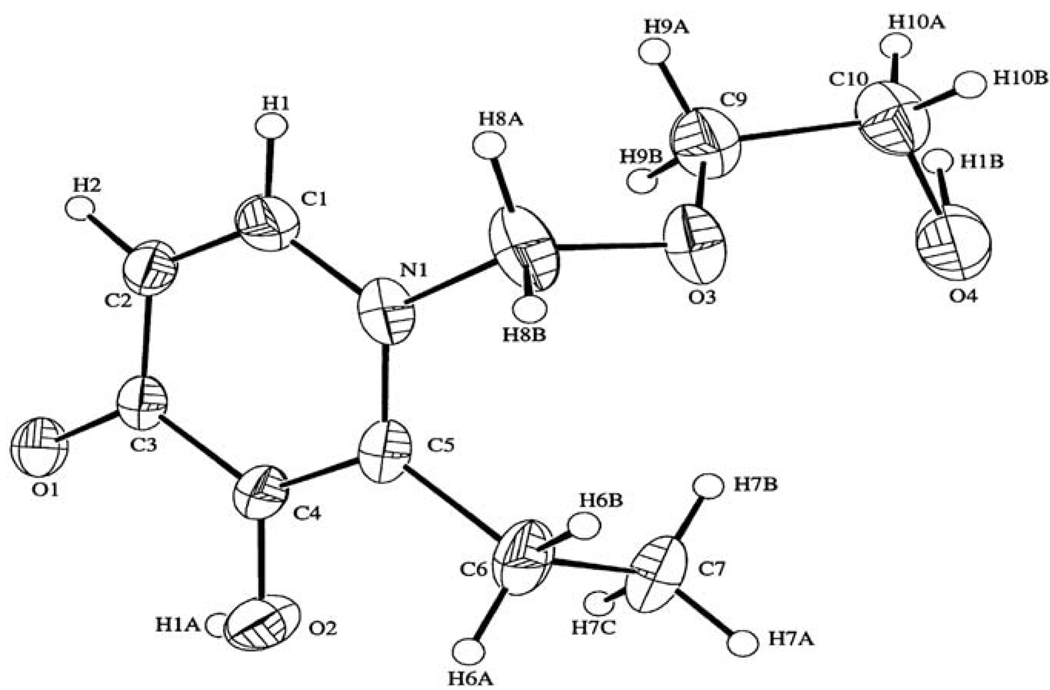
Fig. 8.2.
ORTEP stereoview of chelator EHEMHP.
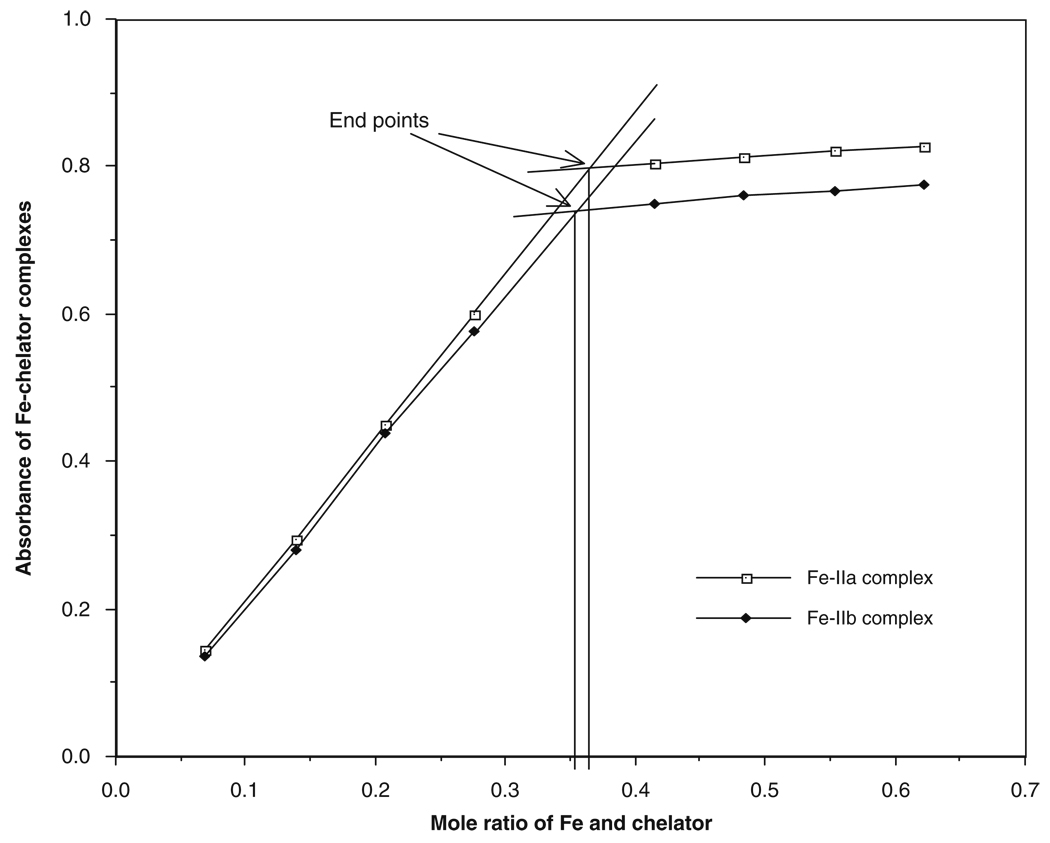
Fig. 8.3.
Titration of MHEMHP (IIa) and EHEMHP (IIb) with iron.
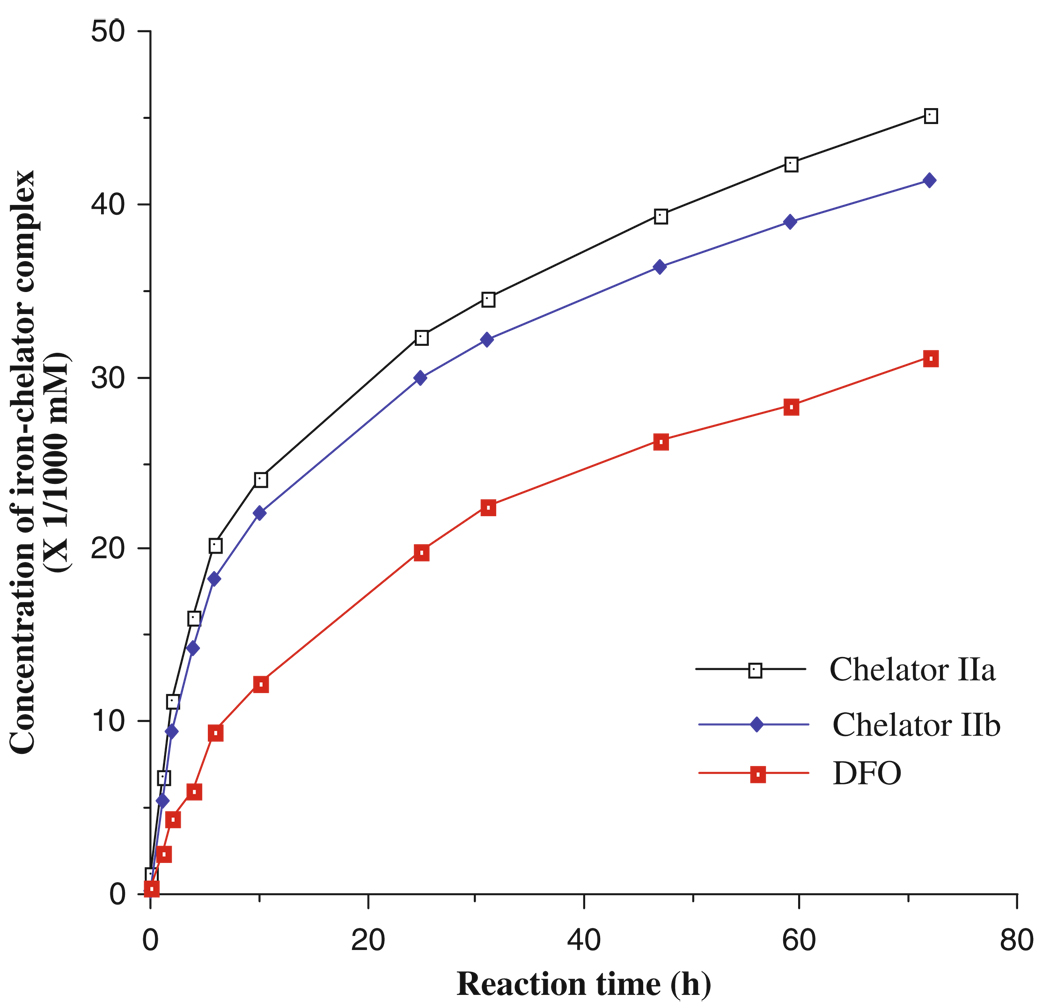
Fig. 8.4.
Removal of iron from ferritin by the chelators of MHEMHP (IIa), EHEMHP (IIb), and DFO.
Fig. 8.5.
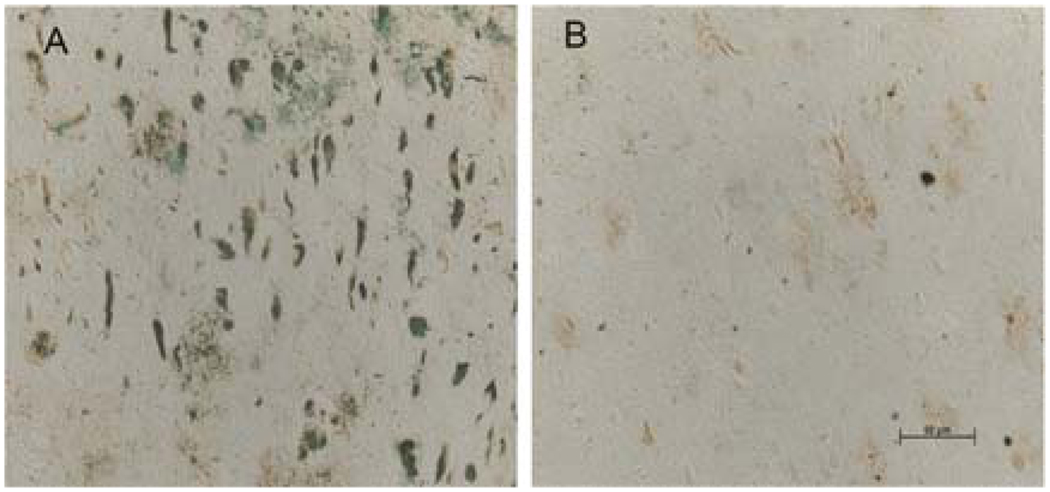
Lesion-associated chelatable iron in AD brain sections was depleted with iron chelator (MAEHP as a prototypal chelator), which was detected histochemically with a modified Perl Stain. Saline- (a) and MAEHP-treated (b) sections.
Fig. 8.6.
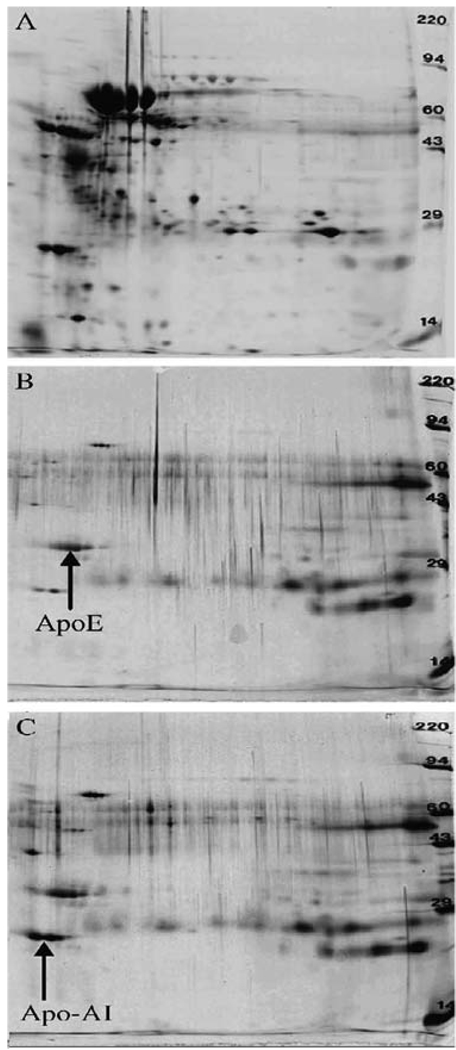
Images of plasma protein patterns examined by 2D PAGE. (a) Plasma; (b) CNPS (MAPHP conjugated) coated with polysorbate 80; and (c) ICNPS.
Scheme 8.1.
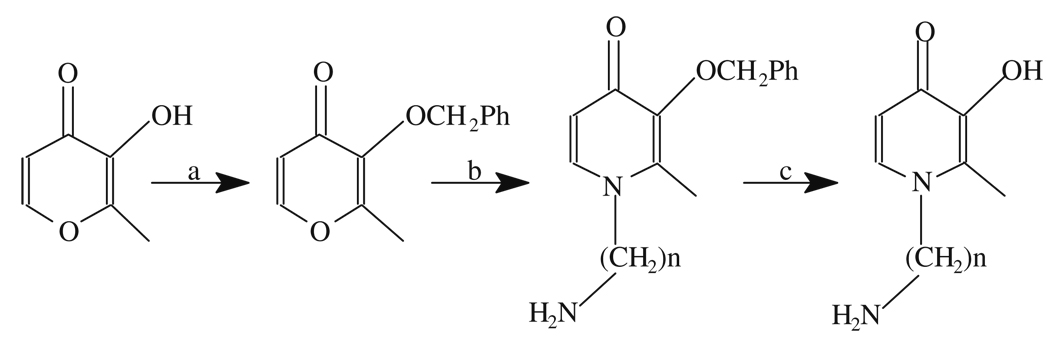
Synthesis of 2-methyl-N-(2′-aminoethyl (n = 2) or 3′-aminopropyl (n = 3))-3-hydroxy-4-pyridinone: (a). benzylchloride/NaOH; (b) NH2(CH2) nNH2, n = 2, 3; and (c). BBr3 in CH2Cl2 at 4°C or hydrogenation with H2/Pt on active carbon.
Scheme 8.2.
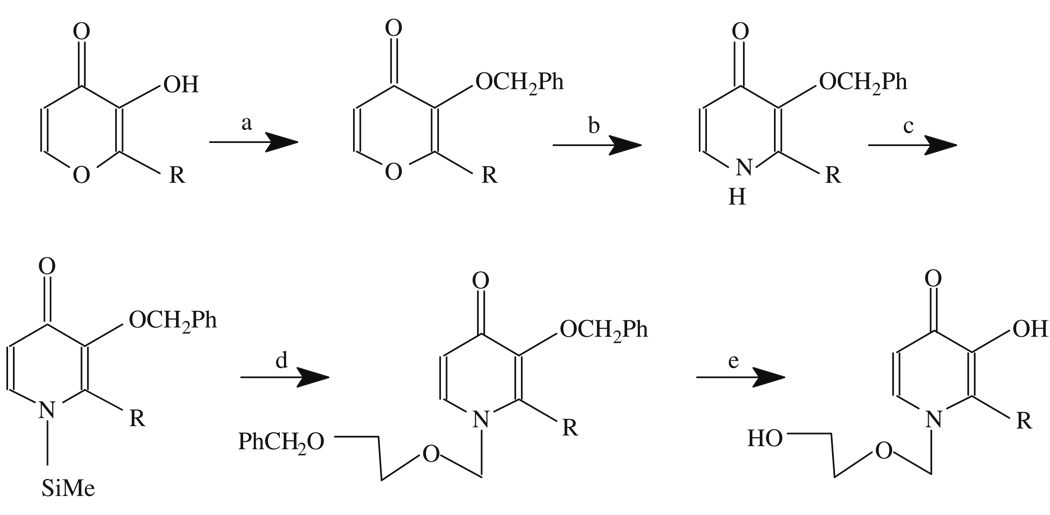
Synthesis of 2-methyl (or ethyl)-N-(2′-hydroxyethoxy)methyl-3-hydroxy-4-pyridinone: (a). PhCH2Cl/NaOH/refluxing/6 h; (b). NH4OH/rt./48 h; (c). hexamethyldisilazane/chlorotrimethylsilane; (d). Benzyloxyethoxy-methylchloride, trimethylsilyl trifluoromethanesulfonate in 1,2-dichloroethane; and (e) H2, Pd/C, AcOH in 95% EtOH. R = Me or Et.
Scheme 8.3.
Conjugation of iron chelators (MAEHP, n = 2 and MAPHP, n = 3) with particles.
Acknowledgments
The work in the authors’ laboratories is supported by the National Institutes of Health, the Alzheimer’s Association and Philip Morris USA Inc. and Philip Morris International.
Footnotes
The synthetic procedures are straightforward and product yields are high. The chelators have been characterized using standard methods such as 1H-NMR, MS, UV-vis, and elemental analysis.
The chelators are prepared using a modified procedure as described in Scheme 8.1 (91, 95).
These chelators are synthesized using established methods (Scheme 8.2) (90, 96).
(2-Acetoxyethoxy)methyl bromide can be used to replace benzyloxyethoxymethylchloride (97).
SnCl4 could also be used as catalyst in the alkylation reaction but might result in separation difficulties and low yields (98).
To evaluate whether the linked (2′-hydroxyethoxy)methyl moiety affected the geometry of the iron binding site in the chelators, molecular and crystal structures of EHEMHP were determined by X-ray crystallographic analysis. A piece of colorless crystal (0.33 × 0.33 × 0.11 mm) formed in methanol-ethyl acetate solution was used for X-ray measurement with an Enraf-Nonius CAD-4 diffractometer equipped with a graphite monochromator of Mo Kα (0.71073 Å) (90). The results indicate that there is no significant change in the geometry of iron binding site. An ORTEP stereo-view of the EHEMHP molecular structure was depicted in Fig. 8.2.
Typical titration curves using MHEMHP and EHEMHP as prototype are presented in Fig. 8.3. The endpoints of the titration indicate the formation of chelator/iron (3:1) complexes (89, 90).
The concentrations of iron–chelator complexes are estimated from εmax values at the wavelength of λmax of the complexes (89, 90). Fig. 8.4 shows the iron removal from ferritin by MHEMHP and EHEMHP as a prototype compared with DFO. It also shows that the chelators are more effective to remove iron from ferritin than DFO.
The use of methacarn instead of formalin for fixation can provide more accurate results (93, 102).
The results show that chelators are capable of depleting iron from the AD brain tissue sections (Fig. 8.5), which depends on the chelator chemical structures and concentrations used (80, 103). This method also provides a useful tool to screen potential chelators for mobilization of iron from the AD brain.
A variety of covalent bonds including amido, amino, ether, and thioether can be easily formed for linking chelators and particles, which are dependent on the existing functional groups located on chelator side chains and on the surface of particles (94, 104, 105).
The preparation of the chelator–particle conjugates is presented in Scheme 8.3 (103).
The particles are rapidly washed with cool Mill-Q water since the active intermediate ester is unstable and undergoes hydrolysis. Alternatively, a water-soluble N-hydroxyl compound like sulfo-N-hydroxysuccinimide (NHS) could be added to increase the coupling yield. This is because NHS is known to form a more stable intermediate ester by replacing the oacylisourea intermediate formed by carbodiimide. The NHS-formed ester is less susceptible to hydrolysis but still highly reactive toward amino groups (106, 107).
Interestingly, this bi-dentate iron chelator converts to hexandentate chelators after conjugation to particles because the particles provided backbone linkages. This phenomenon greatly improved the metal binding stability and lowered toxicity associated with metal–chelator complexes. DFO still retains its hexadentate iron binding property after conjugation to particles (103).
These studies show that the protein absorption pattern on the iron chelator particle systems is totally different from that of the human plasma proteins (Fig. 8.6a). Through changing the system-surface properties, such as chelators and surfactants, the chelator–particle systems can preferentially absorb ApoE (Fig. 8.6b). With the same kind of changes, it is also found that the chelator–particle systems after binding metals can preferentially absorb Apo A-I (Fig. 8.6c). Such preferential absorptions allow the systems to mimic the ApoE or Apo A-I nanoparticles and to cross the BBB through LDL transport mechanisms (76, 86). Uniform coating of the systems with ApoE, B, or A-I can also be achieved by overcoating of these apolipoproteins, which may enable the systems to cross the BBB with high efficiency (74). Studies indicate the potential to obtain chelator–nanoparticle systems with optimal surface properties via changing chelators, linkages, coating materials, and nanoparticles with different surfaces. The particles can be made of biocompatible synthetic or natural macromolecules (60, 73, 108) with functional groups on their surface for covalent bonding with chelators (94, 104).
References
- 1.Smith MA. Alzheimer disease. Int. Rev. Neurobiol. 1998;42:1–54. doi: 10.1016/s0074-7742(08)60607-8. [DOI] [PubMed] [Google Scholar]
- 2.Gutteridge JM. Hydroxyl radicals, iron, oxidative stress, and neurodegeneration. Ann. NY Acad. Sci. 1994;738:201–213. doi: 10.1111/j.1749-6632.1994.tb21805.x. [DOI] [PubMed] [Google Scholar]
- 3.Evans PH. Free radicals in brain metabolism and pathology. Br. Med. Bull. 1993;49:577–587. doi: 10.1093/oxfordjournals.bmb.a072632. [DOI] [PubMed] [Google Scholar]
- 4.Perry G, Castellani RJ, Hirai K, Smith MA. Reactive oxygen species mediate cellular damage in Alzheimer disease. J. Alzheimer’s Dis. 1998;1:45–55. doi: 10.3233/jad-1998-1103. [DOI] [PubMed] [Google Scholar]
- 5.Smith MA, Sayre LM, Monnier VM, Perry G. Radical AGEing in Alzheimer’s disease. Trends Neurosci. 1995;18:172–176. doi: 10.1016/0166-2236(95)93897-7. [DOI] [PubMed] [Google Scholar]
- 6.Prasad KN, Hovland AR, Cole WC, Prasad KC, Nahreini P, Edwards-Prasad J, Andreatta CP. Multiple antioxidants in the prevention and treatment of Alzheimer disease: Analysis of biologic rationale. Clin. Neuropharmacol. 2000;23:2–13. doi: 10.1097/00002826-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Pitchumoni SS, Doraiswamy PM. Current status of antioxidant therapy for Alzheimer’s disease. J. Am. Geriatr. Soc. 1998;46:1566–1572. doi: 10.1111/j.1532-5415.1998.tb01544.x. [DOI] [PubMed] [Google Scholar]
- 8.Christen Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- 9.Kennard ML, Feldman H, Yamada T, Jefferies WA. Serum levels of the iron binding protein p97 are elevated in Alzheimer’s disease. Nat. Med. 1996;2:1230–1235. doi: 10.1038/nm1196-1230. [DOI] [PubMed] [Google Scholar]
- 10.Jefferies WA, Food MR, Gabathuler R, Rothenberger S, Yamada T, Yasuhara O, McGeer PL. Reactive microglia specifically associated with amyloid plaques in Alzheimer’s disease brain tissue express melanotransferrin. Brain Res. 1996;712:122–126. doi: 10.1016/0006-8993(95)01407-1. [DOI] [PubMed] [Google Scholar]
- 11.Harman D. Free radical theory of aging: Alzheimer’s disease pathogensis. Age. 1995;18:97–119. [Google Scholar]
- 12.Casadesus G, Smith MA, Zhu X, Aliev G, Cash AD, Honda K, Petersen RB, Perry G. Alzheimer disease: Evidence for a central pathogenic role of iron-mediated reactive oxygen species. J. Alzheimer’s Dis. 2004;6:165–169. doi: 10.3233/jad-2004-6208. [DOI] [PubMed] [Google Scholar]
- 13.Olanow CW. An introduction to the free radical hypothesis in Parkinson’s disease. Ann. Neurol. 1992;32 Suppl:S2–S9. doi: 10.1002/ana.410320703. [DOI] [PubMed] [Google Scholar]
- 14.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. New York: Oxford University; 1999. [Google Scholar]
- 15.Markesbery WR, Ehmann WD. Oxidative stress in Alzheimer disease. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer Disease. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 401–414. [Google Scholar]
- 16.Ohtawa M, Seko M, Takayama F. Effect of aluminum ingestion on lipid peroxidation in rats. Chem. Pharm. Bull. (Tokyo) 1983;31:1415–1418. doi: 10.1248/cpb.31.1415. [DOI] [PubMed] [Google Scholar]
- 17.Evans PH, Klinowski J, Yano E, Urano N. Alzheimer’s disease: A pathogenic role for aluminosilicate-induced phagocytic free radicals. Free Radic. Res. Commun. 1989;6:317–321. doi: 10.3109/10715768909055157. [DOI] [PubMed] [Google Scholar]
- 18.Garrel C, Lafond JL, Guiraud P, Faure P, Favier A. Induction of production of nitric oxide in microglial cells by insoluble form of aluminium. Ann. NY Acad. Sci. 1994;738:455–461. doi: 10.1111/j.1749-6632.1994.tb21837.x. [DOI] [PubMed] [Google Scholar]
- 19.Kong S, Liochev S, Fridovich I. Aluminum(III) facilitates the oxidation of NADH by the superoxide anion. Free Radic. Biol. Med. 1992;13:79–81. doi: 10.1016/0891-5849(92)90168-g. [DOI] [PubMed] [Google Scholar]
- 20.Bondy SC, Guo-Ross SX, Truong AT. Promotion of transition metal-induced reactive oxygen species formation by beta-amyloid. Brain Res. 1998;799:91–96. doi: 10.1016/s0006-8993(98)00461-2. [DOI] [PubMed] [Google Scholar]
- 21.Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998;158:47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 22.Markesbery WR, Carney JM. Oxidative alterations in Alzheimer’s disease. Brain Pathol. 1999;9:133–146. doi: 10.1111/j.1750-3639.1999.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Multhaup G, Schlicksupp A, Hesse L, Beher D, Ruppert T, Masters CL, Beyreuther K. The amyloid precursor protein of Alzheimer’ disease in the reduction of copper(II) to copper(I) Science. 1996;271:1406–1409. doi: 10.1126/science.271.5254.1406. [DOI] [PubMed] [Google Scholar]
- 24.Sayre LM, Perry G, Smith MA. Redox metals and neurodegenerative disease. Curr. Opin. Chem. Biol. 1999;3:220–225. doi: 10.1016/S1367-5931(99)80035-0. [DOI] [PubMed] [Google Scholar]
- 25.Linder MC, Hazegh-Azam M. Copper biochemistry and molecular biology. Am. J. Clin. Nutr. 1996;63:797S–811S. doi: 10.1093/ajcn/63.5.797. [DOI] [PubMed] [Google Scholar]
- 26.Bush AI, Pettingell WH, Multhaup G, d Paradis M, Vonsattel JP, Gusella JF, Beyreuther K, Masters CL, Tanzi RE. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science. 1994;265:1464–1467. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- 27.Hensley K, Carney JM, Mattson MP, Aksenova M, Harris M, Wu JF, Floyd RA, Butterfield DA. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: Relevance to Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1994;91:3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butterfield DA. beta-Amyloid-associated free radical oxidative stress and neurotoxicity: Implications for Alzheimer’s disease. Chem. Res. Toxicol. 1997;10:495–506. doi: 10.1021/tx960130e. [DOI] [PubMed] [Google Scholar]
- 29.Pratico D, Clark CM, Liun F, Rokach J, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: A possible predictor of Alzheimer disease. Arch. Neurol. 2002;59:972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- 30.Atwood CS, Scarpa RC, Huang X, Moir RD, Jones WD, Fairlie DP, Tanzi RE, Bush AI. Characterization of copper interactions with alzheimer amyloid beta peptides: Identification of an attomolar-affinity copper binding site on amyloid beta1–42. J. Neurochem. 2000;75:1219–1233. doi: 10.1046/j.1471-4159.2000.0751219.x. [DOI] [PubMed] [Google Scholar]
- 31.Pratico D, Uryu K, Sung S, Tang S, Trojanowski JQ, Lee VM. Aluminum modulates brain amyloidosis through oxidative stress in APP transgenic mice. FASEB J. 2002;16:1138–1140. doi: 10.1096/fj.02-0012fje. [DOI] [PubMed] [Google Scholar]
- 32.House E, Collingwood J, Khan A, Korchazkina O, Berthon G, Exley C. Aluminium, iron, zinc and copper influence the in vitro formation of amyloid fibrils of Abeta42 in a manner which may have consequences for metal chelation therapy in Alzheimer’s disease. J. Alzheimer’s Dis. 2004;6:291–301. doi: 10.3233/jad-2004-6310. [DOI] [PubMed] [Google Scholar]
- 33.McLachlan DR, Kruck TP, Lukiw WJ, Krishnan SS. Would decreased aluminum ingestion reduce the incidence of Alzheimer’s disease? CMAJ. 1991;145:793–804. [PMC free article] [PubMed] [Google Scholar]
- 34.Cuajungco MP, Faget KY, Huang X, Tanzi RE, Bush AI. Metal chelation as a potential therapy for Alzheimer’s disease. Ann. NY Acad. Sci. 2000;920:292–304. doi: 10.1111/j.1749-6632.2000.tb06938.x. [DOI] [PubMed] [Google Scholar]
- 35.Richardson DR, Ponka P. Development of iron chelators to treat iron overload disease and their use as experimental tools to probe intracellular iron metabolism. Am. J. Hematol. 1998;58:299–305. doi: 10.1002/(sici)1096-8652(199808)58:4<299::aid-ajh9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 36.Keberle H. The biochemistry of desferrioxamine and its relation to iron metabolism. Ann. NY Acad. Sci. 1964;119:758–768. doi: 10.1111/j.1749-6632.1965.tb54077.x. [DOI] [PubMed] [Google Scholar]
- 37.Hider RC, Hall AD. Clinically useful chelators of tripositive elements. Prog. Med. Chem. 1991;28:41–173. doi: 10.1016/s0079-6468(08)70363-1. [DOI] [PubMed] [Google Scholar]
- 38.Finefrock AE, Bush AI, Doraiswamy PM. Current status of metals as therapeutic targets in Alzheimer’s disease. J. Am. Geriatr. Soc. 2003;51:1143–1148. doi: 10.1046/j.1532-5415.2003.51368.x. [DOI] [PubMed] [Google Scholar]
- 39.Ben-Shachar D, Riederer P, Youdim MB. Iron-melanin interaction and lipid peroxidation: Implications for Parkinson’s disease. J. Neurochem. 1991;57:1609–1614. doi: 10.1111/j.1471-4159.1991.tb06358.x. [DOI] [PubMed] [Google Scholar]
- 40.Floor E. Iron as a vulnerability factor in nigrostriatal degeneration in aging and Parkinson’s disease. Cell Mol. Biol. (Noisy-le-grand) 2000;46:709–720. [PubMed] [Google Scholar]
- 41.Blake DR, Winyard P, Lunec J, Williams A, Good PA, Crewes SJ, Gutteridge JM, Rowley D, Halliwell B, Cornish A, et al. Cerebral and ocular toxicity induced by desferrioxamine. Q. J. Med. 1985;56:345–355. [PubMed] [Google Scholar]
- 42.Kruck TP, Fisher EA, McLachlan DR. A predictor for side effects in patients with Alzheimer’s disease treated with deferoxamine mesylate. Clin. Pharmacol. Ther. 1993;53:30–37. doi: 10.1038/clpt.1993.6. [DOI] [PubMed] [Google Scholar]
- 43.Struck M, Waldmeier P, Berdoukas V. The treatment of iron overload-psychiatric implication. In: Riederer P, Youdim MBH, editors. Iron in Central Nervous System Disorders. Wien: Springer Verlag; 1993. pp. 189–196. [Google Scholar]
- 44.Klaasen CD. Heavy metals and heavy-metalantagonists. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York: McGraw Hill; 1996. pp. 1649–1671. [Google Scholar]
- 45.Jenner P, Olanow CW. Understanding cell death in Parkinson’s disease. Ann. Neurol. 1998;44:S72–S84. doi: 10.1002/ana.410440712. [DOI] [PubMed] [Google Scholar]
- 46.May PM, Bulman RA. The present status of chelating agents in medicine. Prog. Med. Chem. 1983;20:225–336. doi: 10.1016/s0079-6468(08)70220-0. [DOI] [PubMed] [Google Scholar]
- 47.Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997;89:739–761. [PubMed] [Google Scholar]
- 48.Lynch SG, Fonseca T, Levine SM. A multiple course trial of desferrioxamine in chronic progressive multiple sclerosis. Cell Mol. Biol. (Noisy-le-grand) 2000;46:865–869. [PubMed] [Google Scholar]
- 49.Crowe A, Morgan EH. Effects of chelators on iron uptake and release by the brain in the rat. Neurochem. Res. 1994;19:71–76. doi: 10.1007/BF00966731. [DOI] [PubMed] [Google Scholar]
- 50.Kontoghiorghes GJ. New concepts of iron and aluminium chelation therapy with oral L1 (deferiprone) and other chelators. A review. Analyst. 1995;120:845–851. doi: 10.1039/an9952000845. [DOI] [PubMed] [Google Scholar]
- 51.Ward RJ, Dexter D, Florence A, Aouad F, Hider R, Jenner P, Crichton RR. Brain iron in the ferrocene-loaded rat: Its chelation and influence on dopamine metabolism. Biochem. Pharmacol. 1995;49:1821–1826. doi: 10.1016/0006-2952(94)00521-m. [DOI] [PubMed] [Google Scholar]
- 52.Richardson DR. The therapeutic potential of iron chelators. Expert Opin. Investig. Drugs. 1999;8:2141–2158. doi: 10.1517/13543784.8.12.2141. [DOI] [PubMed] [Google Scholar]
- 53.Medical News Today. FDA grants priority review for Exjade(R) for the treatment of chronic iron overload due to blood transfusions. 2005 http://www.medicalnewstoday.com/medicalnews.php?newsid=26610. [Google Scholar]
- 54.Neufeld EJ. Oral chelators deferasirox and deferiprone for transfusional iron overload in thalassemia major: New data, new questions. Blood. 2006;107:3436–3441. doi: 10.1182/blood-2006-02-002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piga A, Galanello R, Forni GL, Cappellini MD, Origa R, Zappu A, Donato G, Bordone E, Lavagetto A, Zanaboni L, Sechaud R, Hewson N, Ford JM, Opitz H, Alberti D. Randomized phase II trial of deferasirox (Exjade, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overload. Haematologica. 2006;91:873–880. [PubMed] [Google Scholar]
- 56.Hider RC, Porter JB, Singh S. The design of therapeutically useful iron chelators. In: Bergeron RJ, Brittenham GM, editors. The Development of Iron Chelators for Clinical Use. Boca Raton: CRC; 1994. pp. 353–371. [Google Scholar]
- 57.Gassen M, Youdim MB. The potential role of iron chelators in the treatment of Parkinson’s disease and related neurological disorders. Pharmacol. Toxicol. 1997;80:159–166. doi: 10.1111/j.1600-0773.1997.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 58.Lee JY, Friedman JE, Angel I, Kozak A, Koh JY. The lipophilic metal chelator DP-109 reduces amyloid pathology in brains of human beta-amyloid precursor protein transgenic mice. Neurobiol. Aging. 2004;25:1315–1321. doi: 10.1016/j.neurobiolaging.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 59.Porter JB, Morgan J, Hoyes KP, Burke LC, Huehns ER, Hider RC. Relative oral efficacy and acute toxicity of hydroxypyridin-4-one iron chelators in mice. Blood. 1990;76:2389–2396. [PubMed] [Google Scholar]
- 60.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv. Drug Deliv. Rev. 2001;47:65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 61.Raymond KN, Xu J. Siderophore-based hydroxypyridonate sequestering agents. In: Bergeron RJ, Brittenham GM, editors. The Development of Iron Chelators for Clinical Use. Boca Raton: CRC Press; 1994. pp. 354–371. [Google Scholar]
- 62.Hider RC, Choudhury R, Rai BL, Dehkordi LS, Singh S. Design of orally active iron chelators. Acta Haematol. 1996;95:6–12. doi: 10.1159/000203851. [DOI] [PubMed] [Google Scholar]
- 63.Martell AE, Motekaitis RJ, Sun Y, Ma R, Welch MJ, Pajeau T. New chelating-agents suitable for the treatment of iron overload. Inorg. Chim. Acta. 1999;291:238–246. [Google Scholar]
- 64.Caravan P, Orvig C. Tripodal aminophenolate ligand complexes of aluminum(III), gallium(III), and indium(III) in water. Inorg. Chem. 1997;36:237–248. [Google Scholar]
- 65.Faller B, Spanka C, Sergejew T, Tschinke V. Improving the oral bioavailability of the iron chelator HBED by breaking the symmetry of the intramolecular H-bond network. J. Med. Chem. 2000;43:1467–1475. doi: 10.1021/jm990261n. [DOI] [PubMed] [Google Scholar]
- 66.Bergeron RJ, McManis JS. Synthesis and biological activity of hydroxamate-based iron chelators. In: Bergeron RJ, Brittenham GM, editors. The Development of Iron Chelators for Clinical Use. Boca Raton: CRC; 1994. pp. 237–273. [Google Scholar]
- 67.Richardson DR, Ponka P. Pyridoxal isonicotinoyl hydrazone and its analogs: Potential orally effective iron-chelating agents for the treatment of iron overload disease. J. Lab. Clin. Med. 1998;131:306–315. doi: 10.1016/s0022-2143(98)90180-9. [DOI] [PubMed] [Google Scholar]
- 68.Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 69.Cherny RA, Legg JT, McLean CA, Fairlie DP, Huang X, Atwood CS, Beyreuther K, Tanzi RE, Masters CL, Bush AI. Aqueous dissolution of Alzheimer’s disease Abeta amyloid deposits by biometal depletion. J. Biol. Chem. 1999;274:23223–23228. doi: 10.1074/jbc.274.33.23223. [DOI] [PubMed] [Google Scholar]
- 70.Loske C, Gerdemann A, Schepl W, Wycislo M, Schinzel R, Palm D, Riederer P, Munch G. Transition metal-mediated glycoxidation accelerates cross-linking of beta-amyloid peptide. Eur. J. Biochem. 2000;267:4171–4178. doi: 10.1046/j.1432-1327.2000.01452.x. [DOI] [PubMed] [Google Scholar]
- 71.Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, Masters CL. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: A pilot phase 2 clinical trial. Arch. Neurol. 2003;60:1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- 72.Doraiswamy PM, Xiong GL. Pharmacological strategies for the prevention of Alzheimer’s disease. Expert Opin. Pharmacother. 2006;7:1–10. doi: 10.1517/14656566.7.1.1. [DOI] [PubMed] [Google Scholar]
- 73.Brem H, Walter KA, Tamargo RJ, Olivi A, Langer R. Drug delivery to the brain. In: Domb AJ, editor. Polymeric Site-Specific Pharmacotherapy. New York: John Wiley & Sons; 1994. pp. 117–139. [Google Scholar]
- 74.Kreuter J, Shamenkov D, Petrov V, Ramge P, Cychutek K, Koch-Brandt C, Alyautdin R. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J. Drug Target. 2002;10:317–325. doi: 10.1080/10611860290031877. [DOI] [PubMed] [Google Scholar]
- 75.Schroeder U, Sommerfeld P, Ulrich S, Sabel BA. Nanoparticle technology for delivery of drugs across the blood-brain barrier. J. Pharm. Sci. 1998;87:1305–1307. doi: 10.1021/js980084y. [DOI] [PubMed] [Google Scholar]
- 76.Alyautdin RN, Tezikov EB, Ramge P, Kharkevich DA, Begley DJ, Kreuter J. Significant entry of tubocurarine into the brain of rats by adsorption to polysorbate 80-coated polybutylcyanoacrylate nanoparticles: An in situ brain perfusion study. J. Microencapsul. 1998;15:67–74. doi: 10.3109/02652049809006836. [DOI] [PubMed] [Google Scholar]
- 77.Siegemund T, Paulke BR, Schmiedel H, Bordag N, Hoffmann A, Harkany T, Tanila H, Kacza J, Hartig W. Thioflavins released from nanoparticles target fibrillar amyloid beta in the hippocampus of APP/PS1 transgenic mice. Int. J. Dev. Neurosci. 2006;24:195–201. doi: 10.1016/j.ijdevneu.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 78.Cui Z, Lockman PR, Atwood CS, Hsu CH, Gupte A, Allen DD, Mumper RJ. Novel D-penicillamine carrying nanoparticles for metal chelation therapy in Alzheimer’s and other CNS diseases. Eur. J. Pharm. Biopharm. 2005;59:263–272. doi: 10.1016/j.ejpb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 79.Shea TB, Ortiz D, Nicolosi RJ, Kumar R, Watterson AC. Nanosphere-mediated delivery of vitamin E increases its efficacy against oxidative stress resulting from exposure to amyloid beta. J. Alzheimer’s Dis. 2005;7:297–301. doi: 10.3233/jad-2005-7405. [DOI] [PubMed] [Google Scholar]
- 80.Liu G, Men P, Harris PL, Rolston RK, Perry G, Smith MA. Nanoparticle iron chelators: A new therapeutic approach in Alzheimer disease and other neurologic disorders associated with trace metal imbalance. Neurosci. Lett. 2006;406:189–193. doi: 10.1016/j.neulet.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 81.Ravi Kumar MN. Nano and microparticles as controlled drug delivery devices. J. Pharm. Sci. 2000;3:234–258. [PubMed] [Google Scholar]
- 82.Dehouck B, Fenart L, Dehouck MP, Pierce A, Torpier G, Cecchelli R. A new function for the LDL receptor: Transcytosis of LDL across the blood-brain barrier. J. Cell Biol. 1997;138:877–889. doi: 10.1083/jcb.138.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muller RH, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy. Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001;47:3–19. doi: 10.1016/s0169-409x(00)00118-6. [DOI] [PubMed] [Google Scholar]
- 84.Ramge P, editor. Untersuchungen zur Ueberwindung der Blut-Hirn-Schranke mit Hilfe von Nanopartileln. Aachen: Shaker Verlag; 1998. [Google Scholar]
- 85.Fenart L, Casanova A, Dehouck B, Duhem C, Slupek S, Cecchelli R, Betbeder D. Evaluation of effect of charge and lipid coating on ability of 60-nm nanoparticles to cross an in vitro model of the blood-brain barrier. J. Pharmacol. Exp. Ther. 1999;291:1017–1022. [PubMed] [Google Scholar]
- 86.Davson H, Segal MB. Physiology of the CSF and Blood-Brain Barriers. Boca Raton, FL: CRC Press; 1996. [Google Scholar]
- 87.Porter JB, Gyparaki M, Burke LC, Huehns ER, Sarpong P, Saez V, Hider RC. Iron mobilization from hepatocyte monolayer cultures by chelators: The importance of membrane permeability and the iron-binding constant. Blood. 1988;72:1497–1503. [PubMed] [Google Scholar]
- 88.Liu G, Garrett MR, Men P, Zhu X, Perry G, Smith MA. Nanoparticle and other metal chelation therapeutics in Alzheimer disease. Biochim. Biophys. Acta. 2005;1741:246–252. doi: 10.1016/j.bbadis.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 89.Liu G, Bruenger FW, Miller SC, Arif AM. Molecular structure and biological and pharmacological properties of 3-hydroxy-2-methyl-1-(beta-D-ribofuranosyl or pyranosyl)-4-pyridinone: Potential iron overload drugs for oral administration. Bioorg. Med. Chem. Lett. 1998;8:3077–3080. doi: 10.1016/S0960-894X(98)00569-1. [DOI] [PubMed] [Google Scholar]
- 90.Liu G, Men P, Kenner GH, Miller SC, Bruenger FW. Acyclonucleoside iron chelators of 1-(2-hydroxyethoxy)methyl-2-alkyl-3-hydroxy-4-pyridinones: Potential oral iron chelation therapeutics. Nucleosides Nucleotides Nucleic Acids. 2004;23:599–611. doi: 10.1081/NCN-120030718. [DOI] [PubMed] [Google Scholar]
- 91.Liu G, Miller SC, Bruenger FW. Synthesis of lipophilic 3-hydroxy-2-methyl-4-pyridinone derivatives. Syn. Commun. 1995;25:3247–3253. [Google Scholar]
- 92.Blunk T, Hochstrasser DF, Sanchez JC, Muller BW, Muller RH. Colloidal carriers for intravenous drug targeting: Plasma protein adsorption patterns on surface-modified latex particles evaluated by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis. 1993;14:1382–1387. doi: 10.1002/elps.11501401214. [DOI] [PubMed] [Google Scholar]
- 93.Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. USA. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bangs Laboratories, Inc. TechNote 201. 1999. Working with microspheres. [Google Scholar]
- 95.Dobbin PS, Hider RC, Hall AD, Taylor PD, Sarpong P, Porter JB, Xiao G, van der Helm D. Synthesis, physicochemical properties, and biological evaluation of N-substituted 2-alkyl-3-hydroxy-4(1H)-pyridinones: Orally active iron chelators with clinical potential. J. Med. Chem. 1993;36:2448–2458. doi: 10.1021/jm00069a002. [DOI] [PubMed] [Google Scholar]
- 96.Liu G, Miller SC, Bruenger FW. Efficient synthesis of N-[2-hydroxyethoxy)methyl]-2-alkyl-3-hydroxy-4-pyridinone by a modified Hilbert-Johnson reaction. Syn. Commun. 1996;26:2681–2686. [Google Scholar]
- 97.Robins MJ, Hatfield PW. Nucleic acid related compounds. 37. Convenient and high-yield synthesis of N-[(2-hydroxyethoxy)methyl] heterocycles as “acyclic nucleoside” analogues. Can. J. Chem. 1982;60:547–553. [Google Scholar]
- 98.Schaeffer HJ, Gurwara S, Vince R, Bittner S. Novel substrate of adenosine deaminase. J. Med. Chem. 1971;14:367–369. doi: 10.1021/jm00286a024. [DOI] [PubMed] [Google Scholar]
- 99.Streater M, Taylor PD, Hider RC, Porter J. Novel 3-hydroxy-2(1H)-pyridinones. Synthesis, iron(III)-chelating properties, and biological activity. J. Med. Chem. 1990;33:1749–1755. doi: 10.1021/jm00168a033. [DOI] [PubMed] [Google Scholar]
- 100.Nelson WO, Timothy B, Karpishin TB, Retting SJ, Orvig C. Aluminum and gallium compounds of 3-hydroxy-4-pyridinones: Synthesis, characterization, and crystallography of biologically active complexes with unusual hydrogen bonding. Inorg. Chem. 1988;27:1045–1051. [Google Scholar]
- 101.Harris RLN. Potential wool growth inhibitors. Improved synthesis of mimosine and related 4(1H)-pyridones. Australian J. Chem. 1976;29:1329–1334. doi: 10.1071/bi9760189. [DOI] [PubMed] [Google Scholar]
- 102.Sayre LM, Perry G, Harris PL, Liu Y, Schubert KA, Smith MA. In situ oxidative catalysis by neurofibrillary tangles and senile plaques in Alzheimer’s disease: A central role for bound transition metals. J. Neurochem. 2000;74:270–279. doi: 10.1046/j.1471-4159.2000.0740270.x. [DOI] [PubMed] [Google Scholar]
- 103.Liu G, Men P, Perry G, Smith MA. Nanoparticles for the treatment of Alzheimer’s disease: Theoretical rationale, present status and future perspectives. In: Kumar CSSR, editor. Nanomaterials for Medical Diagnosis and Therapy. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA. (Nanotechnologies for the Life Sciences); 2007. pp. 644–706. [Google Scholar]
- 104.Wong SS. Chemistry of Protein Conjugation and Cross-Linking. Boca Raton, Fl: CRC Press; 1991. [Google Scholar]
- 105.Arano Y, Matsushima H, Tagawa M, Koizumi M, Endo K, Konishi J, Yokoyama A. A novel bifunctional metabolizable linker for the conjugation of antibodies with radionuclides. Bioconjug. Chem. 1991;2:71–76. doi: 10.1021/bc00008a001. [DOI] [PubMed] [Google Scholar]
- 106.Anjaneyulu PS, Staros JV. Reactions of N-hydroxysulfosuccinimide active esters. Int. J. Pept. Protein Res. 1987;30:117–124. doi: 10.1111/j.1399-3011.1987.tb03319.x. [DOI] [PubMed] [Google Scholar]
- 107.Staros JV, Wright RW, Swingle DM. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal. Biochem. 1986;156:220–222. doi: 10.1016/0003-2697(86)90176-4. [DOI] [PubMed] [Google Scholar]
- 108.Kreuter J. Influence of the surface properties on nanoparticle-mediated transport of drugs to the brain. J. Nanosci. Nanotechnol. 2004;4:484–488. doi: 10.1166/jnn.2003.077. [DOI] [PubMed] [Google Scholar]