Abstract
Absolute and relative concentrations of estrogens and estrogen metabolites (EM) are important for clinical decisions, as well as for epidemiologic, experimental, and clinical research on hormonal carcinogenesis. Radioimmunoassays (RIA) and enzyme-linked immunosorbent assays (ELISA) are routinely used for measuring EM in blood and urine due to efficiency and low cost. Here we compare absolute and ranked concentrations of estrone, estradiol, and estriol measured by indirect RIA and of 2-hydroxyestrone and 16α-hydroxyestrone measured by ELISA to the concentrations obtained using a novel liquid-chromatography-tandem mass spectrometry (LC-MS/MS) method which measures 15 EM concurrently. We used overnight urine samples collected from control women (362 premenopausal, 168 postmenopausal) participating in a population-based case-control study of breast cancer among Asian-American women ages 20–55 years. When comparing RIA or ELISA levels to LC-MS/MS, absolute concentrations for the five EM ranged from 1.6–2.9 and 1.4–11.8 times higher in premenopausal and postmenopausal women, respectively, (all p<0.0001). However, LC-MS/MS measurements were highly correlated [Spearman r (rs) =0.8–0.9] with RIA and ELISA measurements in premenopausal women, and moderately correlated (rs=0.4–0.8) in postmenopausal women. Measurements of the 2-hydroxyestrone:16α-hydroxyestrone ratio, a putative biomarker of breast cancer risk, were moderately correlated in premenopausal women (rs=0.6–0.7) but only weakly correlated in postmenopausal women (rs=0.2). LC-MS/MS had higher intraclass correlation coefficients (≥99.6%) and lower coefficients of variation (≤9.4%) than ELISA (≥97.2% and ≤14.2%) and RIA (≥95.2% and ≤17.8%). Comparison with the LC-MS/MS method suggests that the widely used RIA and ELISA EM measures may be problematic, especially at low EM levels characteristic of postmenopausal women.
Keywords: estrone, estradiol, LC-MS, RIA, ELISA
Introduction
Numerous epidemiologic studies have shown that higher levels of estrogens are associated with greater risk of breast cancer in postmenopausal women (1–4). Much effort through epidemiologic, laboratory, and clinical studies has been devoted to quantifying and characterizing this association and the mechanisms that may be involved. All of these studies have largely focused on measuring the most abundant estrogens in blood and urine, including estrone (E1), estradiol (E2) and estriol (E3). Of these, E2 is the most commonly measured because it is considered to be the most biologically active form of estrogen.
E1 and E2 can be metabolized into 16α-hydroxyestrone (16α-OHE1) and 2-hydroxyestrone (2-OHE1) through two separate and competing pathways whereby hydroxylation occurs predominantly at either the C-2 or C-16α position. 16α-OHE1 and 2-OHE1 can then be further modified to form additional 2-hydroxylated and 16α-hydroxylated estrogen metabolites (EM). A third pathway for estrogen metabolism occurs via hydroxylation of C-4 to produce 4-hydroxyestrone (4-OHE1). EM from these three pathways can contribute to tumorigenesis through increased cell proliferation and by binding directly to DNA and inducing DNA damage. 16α-hydroxyestrone (16α-OHE1) has been shown to bind covalently to the estrogen receptor and to have estrogenic properties, while 2-hydroxyestrone (2-OHE1) may range from only mildly estrogenic to antiestrogenic (5–7). Components of the 4-pathway have been shown to be potent inducers of genotoxic damage (7–9).
The 2-OHE1:16α-OHE1 ratio has been proposed to be a biomarker of breast cancer risk as it may demonstrate which hydroxylation pathway is more active in an individual, with a high ratio, reflecting predominance of the 2-pathway, being more favorable (5–7). Measurement of only 16α-OHE1 and 2-OHE1 levels has been hypothesized as sufficient to represent all the components of the 16- and 2-pathways, respectively (5–7). Results of epidemiology studies examining the 2-OHE1:16α-OHE1 ratio with breast cancer risk have been mixed (5, 6, 10–20). In contrast to the evaluation of the 2-OHE1 and 16α-OHE1 pathways in epidemiology studies, relatively little research has focused on 4-hydroxyestrone or the 4-pathway.
To add to the complexity of determining which EM to focus on for evaluating breast cancer risk, there are numerous platforms by which to measure the various EM. Currently, EM often are measured using radioimmunoassays (RIA) and enzyme-linked immunosorbent assays (ELISA) in epidemiologic and clinical studies due to efficiency in processing samples and cost. However, these widely accepted methods have been shown to lack the specificity and sensitivity necessary to accurately measure low estrogen concentrations, such as those found in postmenopausal women (21, 22). Furthermore, since most epidemiologic studies use only either RIA or ELISA and there is no standardization across assays, it is difficult to compare results across studies that use different assay platforms. This conundrum was described by Stanczyk and colleagues in an elegant commentary on the lack of standardization of steroid hormone assays (23). Stanczyk, et al. wrote, “No gold standard exists to allow objective validation and cross comparisons among various assays to ensure maximal quality control.” This commentary prompted us to examine the correlation of absolute and relative EM concentrations determined using a novel, advanced high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) method we recently established for measuring 15 urinary EM concurrently (24–26) compared to those obtained using indirect RIA methods for E1, E2, and E3, which are reported to more closely correlate with values obtained from gas chromatography mass spectrometry (21), and improved ELISA methods for 2-OHE1 and 16α-OHE1 (27). For this comparison across assays, we used urine samples collected from control participants in a breast cancer case-control study conducted among Asian-American women.
Materials and Methods
Study population and sample collection
During 1983–1987, we conducted a population-based study of incident breast cancer among women of Chinese, Japanese, or Filipino ancestry, aged 20–55 years, and living in the San Francisco-Oakland CA Metropolitan Statistical Area (MSA), the Los Angeles CA MSA, or Oahu HI. More details of the study design and population have been reported elsewhere (28).
These analyses presented here include the control population of 362 premenopausal women and 168 postmenopausal women. All of these women provided 12-hour overnight urine samples. Urine samples were stored on ice or in a refrigerator during overnight collection. The following day, the urine samples were mixed, decanted, and aliquotted and stored at −70°C for long-term storage.
Women taking menopausal hormone therapy, oral contraceptives, or other exogenous steroid hormones were excluded from this study. For women still menstruating, the collection was scheduled for mid-luteal phase of the menstrual cycle (between days 19 and 26) since estrogen levels are relatively stable during this interval. Women were asked by phone to schedule a home visit based on the day their most recent menstrual period had begun, and to return a postcard indicating the day their subsequent menstrual period had started. Determination of menstrual/menopausal status was based on 1.) information collected in study interview on cessation of menstrual periods (naturally and via surgery); 2.) updated information at time of urine collection, including returned postcards; 3.) serum measurements of follicle stimulating hormone, progesterone, and E2; and 4.) age at urine collection. Women were classified as premenopausal luteal phase (n=264), premenopausal not luteal phase (n=98), postmenopausal (n=168), and missing/inconsistent menstrual/menopausal status (n=41) at the time of the urine collection.
Laboratory assays
The urine samples were sent to three different laboratories for analysis of EM. In addition, creatinine was measured (Quest Diagnostics, San Juan Capistrano, CA) to adjust EM from all three assays for urine volume (29). In 1996, E1, E2, and E3 were measured by Quest Diagnostics (Nichols Institute) using RIA methods specific for each EM. For each RIA, a hydrolysis step was included to remove sulfate and glucuronide residues. The EM were then extracted using ethyl acetate:hexane and purified with celite chromatography. Internal standards of [3H] labeled E1, E2, or E3 were used for the respective assays. The linear range for the assays was 7.5–4800 pg/ml for E1, 1.5–150 pg/ml for E2 and 250–2500 pg/ml for E3. All samples were analyzed in duplicate and the average of the measures used for the analyses presented here.
The 2-OHE1 and 16α-OHE1 metabolites in the urine samples were measured using an improved commercially available ELISA (Immunacare, Inc., Bethlehem, PA). The assays were performed at the Strang Cancer Research Laboratory (New York, NY) from 1997–1998. The ELISAs have been described in detail previously (30, 31), including the improvements to the original assay, which included enhancing sensitivity by modifying antibody levels and alkaline phosphatase activity (27). Similar to the RIA and LC-MS/MS protocols used in this study, the ELISA methods also included a hydrolysis step prior to measuring EM. The measurement of 2-OHE1 and 16α-OHE1 in the control population presented here has been reported previously (20). The lower limit of quantitation of each assay was 650 pg/ml. All samples were assayed in triplicate and the average used for the analyses presented here.
Urine samples also were sent to SAIC-Frederick, Inc. (Frederick, MD) for measurement of 15 EM using high performance liquid chromatography tandem mass spectrometry (LC-MS/MS). Details of the analytic method for urinary EM, which includes hydrolysis, extraction, derivatization, and LC-MS/MS with stable isotope-labeled internal standards, have been previously published (26). The enzymatic hydrolysis step removed sulfate and glucuronide residues from EM. Using one 0.5 mL urine sample, the LC-MS/MS method concurrently measures E1, E2, E3, 2-OHE1 and 16α-OHE1, as well as 2-hydroxyestradiol (2-OHE2), 2-methoxyestrone (2-MEOE1), 2-methoxyestradiol(2-MEOE2), 2-hydroxyestrone-3-methyl-ether or, alternatively, 2-hydroxy 3-methoxy-estrone (3-MEOE1), 4-hydroxyestrone (4-OHE1), 4-methoxyestrone (4-MEOE1), 4-methoxyestradiol (4-MEOE2), 17-epiestriol (17-epiE3), 16-epiestriol (16-epiE3), and 16-ketoestradiol (16-ketoE2). The lower limit of quantitation of the assay for each EM is 40 pg/ml. Each sample was assayed once using the LC-MS/MS methods.
Quality control of laboratory assays
For all assays, 10% blinded, quality-control samples (from both pre- and postmenopausal women) were included in each assay batch. The coefficients of variation (CV), and intraclass correlation coefficients (ICC) for E1, E2, E3, 2-OHE1, and 16α-OHE1 measured by LC-MS/MS and either RIA or ELISA can be found in Table 1. Blinded quality control samples for RIA methods included three premenopausal (luteal phase) women and three postmenopausal women; for ELISA methods included three premenopausal (two luteal phase, one follicular phase) women and three postmenopausal women; and for LC-MS/MS methods, which utilized smaller batches, included two premenopausal (luteal phase) women and two postmenopausal women.
Table 1.
Coefficients of variation (CV) and intraclass correlation coefficients (ICC) for RIA, ELISA, and LC-MS/MS methods.
| Premenopausal women | Postmenopausal women | |||
|---|---|---|---|---|
| Estrogens and Estrogen Metabolites |
ICC (%) | CV (%) | ICC (%) | CV (%) |
| Estrone | ||||
| RIA | 98.4 | 9.4 | 95.2 | 17.8 |
| LC-MS/MS | 99.9 | 2.0 | 99.8 | 3.2 |
| Estradiol | ||||
| RIA | 96.7 | 12.6 | 98.5 | 11.8 |
| LC-MS/MS | 99.9 | 2.1 | 99.8 | 4.9 |
| Estriol | ||||
| RIA | 98.6 | 10.4 | 97.7 | 14.0 |
| LC-MS/MS | 99.9 | 1.9 | 99.9 | 2.9 |
| 2-Hydroxyestrone | ||||
| ELISA | 97.2 | 13.5 | 97.9 | 11.5 |
| LC-MS/MS | 99.9 | 2.5 | 99.6 | 3.9 |
| 16α-Hydroxyestrone | ||||
| ELISA | 98.7 | 9.1 | 98.2 | 14.2 |
| LC-MS/MS | 99.6 | 5.1 | 99.8 | 9.4 |
Statistical analysis
The individual EM measurements obtained using the LC-MS/MS, ELISA, and RIA methods were converted from nanograms (ng) or micrograms (µg) to picomoles (pmol) using the molecular weight for the unconjugated form each EM. The conversion to picomoles enabled comparisons across the three assays and also allowed for the creation of “pathway” variables from the LC-MS/MS measures. To examine the 2-, 4-, and 16-pathways from the LC-MS/MS data, individual EM in each pathway in pmol were summed. The 2-pathway variable included the sum of 2-OHE1, 2-OHE2, 2-MEOE1, 2-MEOE2, and 3-MEOE1 levels in picomoles. The 4-pathway variable included the sum in picomoles of the 4-OHE1, 4-MEOE1, and 4-MEOE2 levels. The 16-pathway included 16α-OHE1, E3, 17-epiE3, 16-epiE3, and 16-ketoE2. All EM measures were log transformed and the geometric mean in picomoles/mg creatinine was determined. All comparisons of RIA or ELISA measures to the LC-MS/MS measures utilized Spearman rank correlation and were conducted in SAS v9.1 (Cary, NC). The CV and ICC calculations were obtained using components of variance analysis (proc varcomp) in SAS v9.1 (Cary, NC).
Results
The study population consisted of 264 premenopausal (luteal phase), 98 premenopausal (non-luteal phase), and 168 postmenopausal women. At time of urine collection, the median age for the premenopausal (luteal phase) women was 41.8 years (range 22–54), premenopausal (nonluteal phase) was 44.7 years (range 25–55), and for postmenopausal women was 54.6 years (range 45–65). The premenopausal (luteal phase) subgroup was approximately 23% Filipino, 45% Japanese, and 31% Chinese while the premenopausal (nonluteal phase) subgroup was 40%, 34%, and 27%, respectively. Postmenopausal women were 29% Filipino, 43% Japanese, and 27% Chinese.
The intraclass correlation coefficients (ICC) and coefficients of variation (CV) for the ELISA, RIA, and the LC-MS/MS assay are presented in Table 1 for pre- and postmenopausal women. The assays all have very high ICCs with all the ICCs for the LC-MS/MS assays ≥99.6% and the ICCs for the RIA and ELISA methods ≥95%. For all EM, the CVs calculated for LC-MS/MS are noticeably lower than those calculated for ELISA or RIA. The CVs for LC-MS/MS are ≤5% for premenopausal women and ≤9% for postmenopausal women, while the corresponding CVs for RIA and ELISA are ≤14% and ≤18%, respectively. With the LC-MS/MS methods, the CV is slightly but consistently increased in pre- vs. postmenopausal women.
The geometric mean concentrations and 95% CI, in pmol/mg creatinine, of the urinary EM measured by both LC-MS/MS and either ELISA or RIA for premenopausal luteal phase, premenopausal non-luteal phase, and postmenopausal women are presented in Table 2. Overall, the absolute measurements obtained using RIA or ELISA were all significantly higher than those from LC-MS/MS. Measurements of E1, E2, E3 by RIA were 1.4–2.6 fold higher than the concentrations measured by LC-MS/MS. There was even greater discrepancy between the concentrations of 2-OHE1 and 16α-OHE1 measured by ELISA and LC-MS/MS. Among premenopausal women, the 2-OHE1 and 16α-OHE1 concentrations measured by ELISA were approximately three-fold higher than the concentrations measured by LC-MS/MS. In postmenopausal women the 2-OHE1 concentration was 6-fold higher and the 16α-OHE1 concentration was 12-fold higher by ELISA. However, the ratio of 2-OHE1:16α-OHE1 was only modestly higher using the LC-MS/MS measures than the ELISA measures, and relatively consistent among the three menstrual/menopausal groups (1.3–1.5 for ELISA and 2.1–2.4 for LC-MS/MS). All EM measurements presented in Table 2 were significantly different (p<0.001) across the three menstrual groups. The three subgroups of women were evaluated separately in subsequent analyses.
Table 2.
Geometric mean urinary1 concentrations and 95% confidence intervals (CI) of estrogens and estrogen metabolites (EM) measured by LC-MS/MS and either ELISA or RIA.
| EM measure (assay) | Mean urinary concentration (pmol/mg creatinine) (95% CI) |
||
|---|---|---|---|
| Premenopausal women Luteal Phase n=264 |
Premenopausal women Non-luteal phase n=98 |
Postmenopausal women n=168 |
|
| Parent estrogens | |||
| Estrone (RIA) | 41.9 (39.1, 45.0) |
27.9 (23.1, 33.6) |
6.92 (6.4, 7.5) |
| Estrone (LC-MS/MS) | 23.4 (21.7, 25.3) |
14.6 (12.0, 17.9) |
2.6 (2.4, 2.9) |
| Estradiol (RIA) | 17.6 (16.4, 19.0) |
12.0 (9.9, 14.6) |
2.1 (1.9, 2.3) |
| Estradiol (LC-MS/MS) | 10.9 (10.2, 11.7) |
7.7 (6.4, 9.2) |
1.5 (1.3, 1.7) |
| 2-Hydroxylation pathway | |||
| 2-Hydroxyestrone (ELISA) | 47.83 (44.7, 51.2) |
31.04 (26.6, 36.2) |
18.65 (16.9, 20.5) |
| 2-Hydoxyestrone (LC-MS/MS) | 24.6 (22.4, 26.9) |
13.8 (11.1, 17.1) |
2.9 (2.6, 3.3) |
| 16α-Hydroxylation pathway | |||
| 16α-Hydroxyestrone (ELISA) | 32.26 (30.6, 33.9) |
23.8 (21.3, 26.7) |
14.1 (13.2, 15.0) |
| 16α-Hydroxyestrone (LC-MS/MS) | 11.0 (10.2,11.9) |
6.5 (5.3, 8.0) |
1.2 (1.1, 1.4) |
| Estriol (RIA) | 77.2 (71.6, 83.3) |
50.1 (42.3, 59.4) |
12.97 (12.0, 14.0) |
| Estriol (LC-MS/MS) | 55.5 (50.7, 60.9) |
31.2 (25.3, 38.5) |
5.7 (5.0, 6.4) |
| 2-Hydroxyestrone:16α-Hydroxyestrone | |||
| ELISA ratio | 1.58 (1.4, 1.6) |
1.34 (1.2, 1.5) |
1.35 (1.2, 1.4) |
| LC-MS/MS ratio | 2.2 (2.0, 2.5) |
2.1 (1.8, 2.5) |
2.4 (2.1, 2.8) |
Early morning, 12 hr urines.
n=152;
n=252;
n=97;
n=164;
n=263;
n=163;
n=251
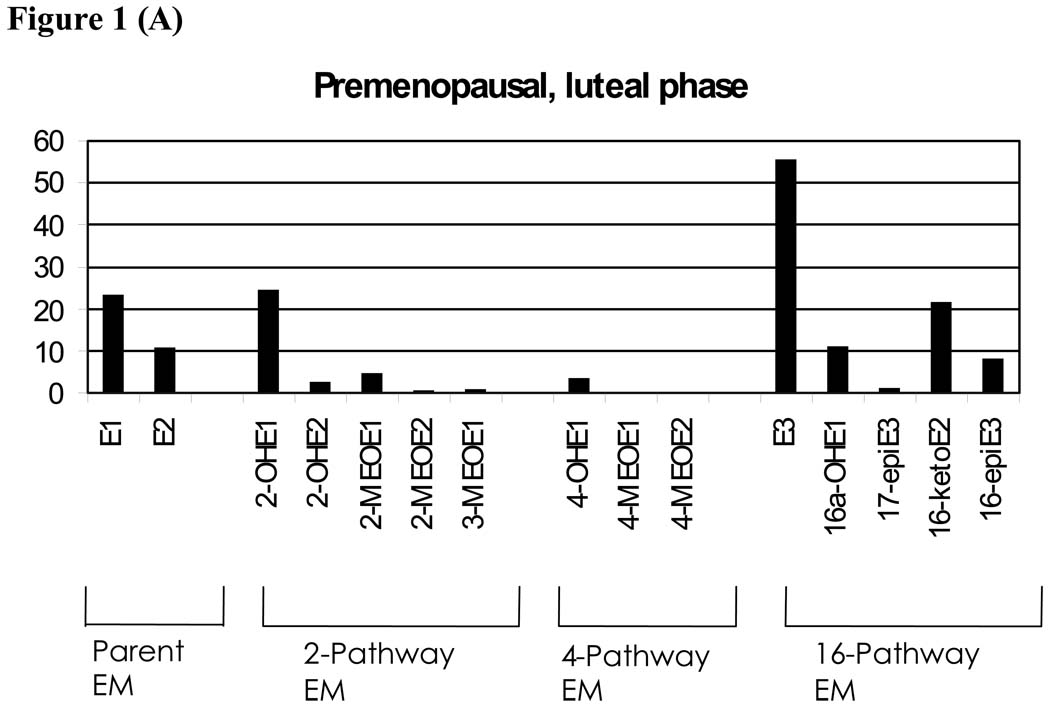
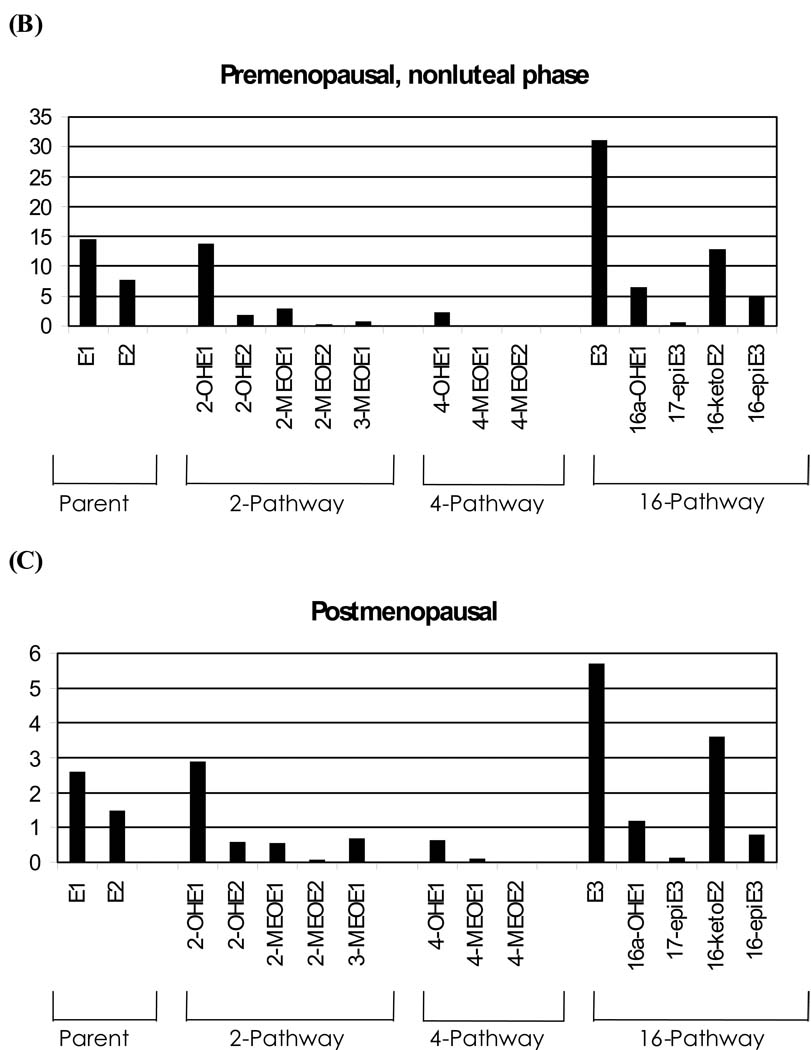
All 15 EM measurements obtained by LC-MS/MS, including those not measured by other methods, are presented in Figure 1A–C. This figure shows the geometric mean EM levels in pmol/mg creatinine for each of the three menstrual/menopausal groups. EM values were significantly different (p<0.001) across the three menstrual subgroups, with a few exceptions. The 4-methoxyestrone, 4-methoxyestradiol, and 3-methoxyestrone measurements for the two premenopausal groups were not different from each other but were statistically different at p<0.0001 from the postmenopausal women. Therefore, the menstrual/menopausal groups are shown separately and with different y-axes in Figure 1A–C. While the absolute concentrations vary by phase of cycle and menopausal status, the pattern of relative abundance is largely consistent across the three groups.
Figure 1.
Geometric mean levels of urinary estrogen metabolites (EM) in pmol/mg creatinine for premenopausal, luteal phase (A); premenopausal, nonluteal phase (B), and postmenopausal women (C). EM are grouped as either parent estrogens or by hydroxylation pathway.
Spearman correlations were used to compare the rank order of EM measurements by assay type (Table 3). The correlation coefficients for the RIA and LC-MS/MS assay of E1, E2, and E3 ranged from 0.91 to 0.96 in premenopausal women, but decreased to 0.63–0.79 in postmenopausal women. The correlation coefficients for the ELISA and LC-MS/MS assays of 2-OHE1 and 16α-OHE1 were also higher for premenopausal women (0.81 to 0.89) and noticeably lower in postmenopausal women (0.37–0.62). For the 2-OHE1:16α-OHE1 ratio, the correlation between the ELISA and LC-MS/MS measures was 0.60–0.68 for premenopausal women, but only 0.17 for postmenopausal women (p=0.03). All correlations, except the one noted above, were significant at p<0.0001.
Table 3.
Spearman correlation analysis of LC-MS/MS and either ELISA or RIA assays for urinary* estrogen and estrogen metabolite (EM) measures.
| Spearman correlation coefficients | |||
|---|---|---|---|
| EM measure, in pmol (assays compared) |
Premenopausal, luteal phase women |
Premenopausal, non-luteal phase women |
Postmenopausal women |
| Estrone (LC-MS/MS and RIA) | 0.94 | 0.96 | 0.79 |
| Estradiol ( LC-MS/MS and RIA) | 0.91 | 0.95 | 0.63 |
| Estriol ( LC-MS/MS and RIA) | 0.94 | 0.94 | 0.73 |
| 2-hydroxyestrone (LC-MS/MS and ELISA) | 0.81 | 0.89 | 0.37 |
| 16α-Hydroxyestrone (LC-MS/MS and ELISA) | 0.86 | 0.89 | 0.62 |
| 2-Hydroxyestrone:16α-Hydroxyestrone (LC-MS/MS and ELISA) | 0.68 | 0.60 | 0.17 |
| 2-Hydroxyestrone:16α-Hydroxyestrone (ELISA) and 2-pathway:16α-pathway** (LC-MS/MS) | 0.60 | 0.55 | 0.25 |
| 2-Hydroxyestrone:16α-Hydroxyestrone (LC-MS/MS) and 2-pathway:16α-pathway3(LC-MS/MS) | 0.78 | 0.81 | 0.65 |
Early morning, 12 hr urines
2-pathway is the sum in pmol of 2-OHE1, 2-OHE2, 2-MEOE1, 2-MEOE2, and 3-MEOE1 levels. 16-pathway is the sum in pmol of 16α-OHE1, E3, 17-epiE3, 16-epiE3, and 16-ketoE2.
Others have proposed that the ratio of the 2-OHE1 and 16α-OHE1 measurements from the ELISA assays may be sufficient to indicate the relevance of the entire 2-hydroxylation and 16α-hydroxylation pathways, respectively (5–7). Therefore, the correlations of the 2-OHE1:16α-OHE1 ratios measured by ELISA or by LC-MS/MS with the composite ratio of 2-pathway EM:16-pathway EM, measured by LC-MS/MS, were examined. When the ratio of the individual EM (2-OHE1:16α-OHE1) measured by LC-MS/MS is compared to the ratio of all pathway EM (2-pathway EM:16-pathway EM) determined by LC-MS/MS, the values were highly correlated in premenopausal women (r=0.78–0.81), but only moderately correlated in postmenopausal women (r=0.65). When ELISA measures of the 2-OHE1:16α-OHE1 ratio were compared with the LC-MS/MS ratio of 2-pathway EM:16-pathway EM, all the correlations were noticeably reduced (r=0.55–0.60) in premenopausal women and only r=0.25 (p=0.001) in postmenopausal women. For pathway comparisons, again all were significant at p<0.0001 with the one exception noted previously.
The hypothesis that the 2-OHE1 and 16α-OHE1 ELISA measures may be representative of the 2-pathway and 16-pathway was explored further by comparing the absolute concentrations of these two EM measured by ELISA, with the absolute concentrations of individual EM and the sum of the 2-, 4-, and 16-pathways, measured using LC-MS/MS (Table 4 and Table 5). In premenopausal women, the 2-OHE1 ELISA measure was most strongly correlated with components of the 2-pathway measured by LC-MS/MS. For example, the correlation coefficient for 2-OHE1 measured by ELISA with levels of 2-OHE1 obtained by LC-MS/MS was 0.81 in premenopausal women in the luteal phase. Among postmenopausal women, the correlation coefficients were similar when comparing the 2-pathway or 16-pathway components measured by LC-MS/MS to 2-OHE1 measured by ELISA. There was not as much distinction between the correlation coefficients from the two pathways as observed in premenopausal women.
Table 4.
Spearman correlation analysis of 2-hydroxyestrone measured by ELISA with 15 estrogen and estrogen metabolites (EM) measured by LC-MS/MS.
| Spearman correlation coefficient | ||||
|---|---|---|---|---|
| EM measure, in pmol | Premenopausal Luteal Phase women |
Postmenopausal women |
||
| Parent estrogens | ||||
| Estrone | 0.56 | 0.28 | ||
| Estradiol | 0.48 | 0.11 | ||
| 2-pathway* | 0.84 | 0.48 | ||
| 2-Hydoxyestrone | ||||
| 0.81 | 0.37 | |||
| 2-Hydroxyestradiol | ||||
| 0.81 | 0.59 | |||
| 2-Methoxyestrone | ||||
| 0.73 | 0.33 | |||
| 2-Methoxyestradiol | ||||
| 0.70 | 0.31 | |||
| 2-Hydroxyestrone-3-methyl-ether | ||||
| 0.56 | 0.32 | |||
| 4-pathway* | 0.70 | 0.35 | ||
| 4-Hydroxyestrone | 0.70 | 0.48 | ||
| 4-Methoxyestrone | 0.33 | 0.48 | ||
| 4-Methoxyestradiol | 0.50 | 0.48 | ||
| 16-pathway* | 0.52 | 0.33 | ||
| 16α-Hydroxyestrone | 0.54 | 0.37 | ||
| Estriol | 0.48 | 0.21 | ||
| 17-Epiestriol | 0.44 | 0.42 | ||
| 16-Ketoestradiol | 0.47 | 0.38 | ||
| 16-Epiestriol | 0.54 | 0.40 | ||
2-pathway, 4-pathway, and 16-pathway are the sum in pmol of all EM listed under each pathway.
Table 5.
Spearman correlation analysis of 16α-hydroxyestrone measured by ELISA with all estrogens and estrogen metabolites (EM) measured by LC-MS/MS
| Spearman correlation coefficient | ||||
|---|---|---|---|---|
| EM measure, in pmol | Premenopausal Luteal Phase women |
Postmenopausal women |
||
| Parent estrogens | ||||
| Estrone | 0.67 | 0.43 | ||
| Estradiol | 0.60 | 0.28 | ||
| 2-pathway* | 0.58 | 0.33 | ||
| 2-Hydoxyestrone | 0.59 | 0.28 | ||
| 2-Hydroxyestradiol | 0.48 | 0.23 | ||
| 2-Methoxyestrone | 0.50 | 0.37 | ||
| 2-Methoxyestradiol | 0.51 | 0.41 | ||
| 2-Hydroxyestrone-3-methyl-ether | 0.39 | 0.36 | ||
| 4-pathway* | 0.51 | 0.27 | ||
| 4-Hydroxyestrone | 0.51 | 0.22 | ||
| 4-Methoxyestrone | 0.33 | 0.29 | ||
| 4-Methoxyestradiol | 0.38 | 0.29 | ||
| 16-pathway* | 0.75 | 0.55 | ||
| 16α-Hydroxyestrone | 0.86 | 0.62 | ||
| Estriol | 0.68 | 0.43 | ||
| 17-Epiestriol | 0.63 | 0.42 | ||
| 16-Ketoestradiol | 0.70 | 0.58 | ||
| 16-Epiestriol | 0.72 | 0.59 | ||
2-pathway, 4-pathway, and 16-pathway are the sum in pmol of all EM listed under each pathway.
Conversely, in Table 5, 16α-OHE1 measured by ELISA correlated most strongly with components of the 16-pathway in both pre- and postmenopausal women. The correlation of 16α-OHE1 measured by ELISA with the 16α-hydroxylation pathway was stronger among premenopausal women compared to postmenopausal women, but both pre- and postmenopausal women showed higher association of the 16α-OHE1 measured by ELISA with the 16-pathway compared to the 2-pathway.
4-hydroxylated EM were evaluated as this pathway also produces estrogens capable of interacting with and mutating DNA. In premenopausal women, 4-hydroxylated EM were moderately correlated with 2-OHE1 (rs=0.3 to 0.7) and 16α-OHE1 (rs=0.3 to 0.5) ELISA measurements. In postmenopausal women, 4-hydroxylated EM were also associated with both the 2-OHE1 and 16α-OHE1 ELISA measures with rs=0.2 to 0.3 for the components of the 4-hydroxylation pathway. For example, among postmenopausal women, the correlation between 4-hydroxyestrone and 2-OHE1 (rs=0.33) was of similar magnitude as associations between 2-OHE1 and other 2-hydroxylated EM.
Discussion
Here we demonstrate that both RIA and ELISA measures of urinary EM correlate well with LC-MS/MS measures, especially in premenopausal women. However, in postmenopausal women the correlation of both RIA and ELISA measures with LC-MS/MS measures is considerably weaker. The absolute concentrations as measured using RIA and ELISA also are significantly higher than those obtained using LC-MS/MS. In premenopausal women, the geometric mean concentrations using RIA for E1, E2, and E3 were approximately two times higher and ELISA for 2-OHE1 and 16α-OHE1 were approximately three times greater than measures of these same EM by LC-MS/MS. In postmenopausal, the differences in the concentrations were even more striking. For example, levels of 16-OHE1 measured by ELISA were approximately 12-fold higher than levels obtained using LC-MS/MS. These results are consistent with the suggestion these widely used and accepted ELISA for 2-hydroxyestrone and 16α-hydroxyestrone and RIA for E1, E2, and E3 may exhibit some cross-reactivity with other EM and thus report overall higher levels of the EM concentration (21–23). The LC-MS/MS method also provides a more complete picture of the estrogen metabolite profile by being able to provide specific measures for all 15 EM present in urine and serum.
Our results suggest that the LC-MS/MS methods are preferable for comparisons of either absolute or relative amounts of EM, or change in EM levels, in postmenopausal women, as well as for measuring absolute concentrations of EM in all women. In postmenopausal women, the concentrations of most EM are much lower than in premenopausal women. At these low concentrations, methods that introduce a high level of background or non-specific binding are especially problematic (22). The amount of the concentration measurement derived from non-specific binding may explain the differences not only in the absolute concentrations of the EM but also in the weaker correlations between assays in postmenopausal women. This difference is particularly evident for the measures of E2, 2-OHE1, 16α-OHE1, and the 2-OHE1:16α-OHE1 ratio in postmenopausal women.
There is much interest in determining if the 2-OHE1:16α-OHE1 ratio is a marker of breast cancer risk (5, 6, 10–20). In this study, the 2-OHE1:16α-OHE1 ratio was not significantly different across the subgroups regardless of whether it was determined by ELISA or LC-MS/MS. This consistency in the 2-OHE1:16α-OHE1 ratio across menstrual/menopausal groups suggests that the pattern of estrogen metabolism may remain relatively constant throughout adult life. Similarly, the levels of the 15 EM determined using LC-MS/MS also demonstrate that the pattern of relative abundance of each EM is similar across the three menstrual/menopausal groups.
While the measures of the 2-OHE1:16α-OHE1 ratio were not different across menstrual/menopausal groups, the ratios obtained using ELISA were noticeably lower (1.3–1.5) than the ratios obtained from the LC-MS/MS measures (2.1–2.4) and these ratios showed little correlation in postmenopausal women (rs=0.17). In postmenopausal women, there also was little correlation when comparing the 2-OHE1:16α-OHE1 ratio obtained using ELISA to the ratio of 2-pathway:16-pathway EM measured using LC-MS/MS (rs=0.25). For both pre- and postmenopausal women, slightly higher correlation coefficients were noted when the 2-OHE1 and 16α-OHE1 levels from ELISA were compared with the individual components of the 2-, 4-, and 16-pathways obtained using LC-MS/MS rather than to the aggregate measure of each pathway. The lack of correlation in the 2-OHE1:16α-OHE1 ratios derived from LC-MS/MS measurements compared to those obtained using ELISA indicate that future studies using LC-MS/MS to examine the association of the 2-OHE1:16α-OHE1 ratio with breast cancer risk may lead to much different interpretations than the current studies using ELISA measurements of 2-OHE1 and 16α-OHE1 (6, 10–19). It would be worthwhile to design a future study of breast cancer using prospectively collected urines to measure 2-OHE1 and 16α-OHE1 using both ELISA and LC-MS/MS to examine the differences in risk estimates.
As noted by Stanczyk et al. (23), the LC-MS/MS methods may require re-visiting the current thresholds or references ranges for EM since LC-MS/MS consistently reports lower concentrations of EM when compared to ELISA and RIA methods. Indeed, our LC-MS/MS method is very specific for the measurement of each EM as each metabolite produces a distinct measureable signal (26). This specificity results in a lower absolute concentration for each EM (23, 32, 33). LC-MS/MS assays also may be of interest for treatment or intervention studies where a more precise measure for quantifying change in EM, especially in postmenopausal women, is needed.
The current study was conducted using urine samples, however, the limitations of the ELISA for 2-OHE1 and 16α-OHE1 and RIA assays for E1, E2, and E3 also are relevant to the analysis of plasma and serum (23, 32). Further studies comparing the quantitative results obtained for plasma or serum samples using this LC-MS/MS assay and the results from the commercially available ELISA and RIA kits are necessary to determine how well the measurements correlate for EM levels measured in plasma and serum. The LC-MS/MS method also provides concentrations for 15 EM, which may result in greater refinement of our understanding of the roles of the various EM in breast and other hormone-related cancers.
Acknowledgements
We thank Drs. H. Leon Bradlow and Daniel Sepkovic for conducting the ELISA of 2-OHE1 and 16α-OHE1. We also thank the participants in the Asian-American breast cancer case:control study for their time and willingness to participate in the study.
Grant support (all authors):
This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health (NIH), and by Federal funds from the National Cancer Institute, NIH, under contract NO1-CO-12400 to SAIC-Frederick.
Footnotes
Conflict of Interest
L. Keefer, T. Veenstra, X. Xu, R. Ziegler; coinventors on a relevant government patent.
References
- Kaaks R, Rinaldi S, Key TJ, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12:1071–1082. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96:1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- Zeleniuch-Jacquotte A, Toniolo P, Levitz M, et al. Endogenous estrogens and risk of breast cancer by estrogen receptor status: a prospective study in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1995;4:857–860. [PubMed] [Google Scholar]
- Bradlow HL, Telang NT, Sepkovic DW, Osborne MP. 2-hydroxyestrone: the 'good' estrogen. J Endocrinol. 1996;150 Suppl:S259–S265. [PubMed] [Google Scholar]
- Kabat GC, O'Leary ES, Gammon MD, et al. Estrogen metabolism and breast cancer. Epidemiology. 2006;17:80–88. doi: 10.1097/01.ede.0000190543.40801.75. [DOI] [PubMed] [Google Scholar]
- Sepkovic DW, Bradlow HL. Estrogen hydroxylation--the good and the bad. Ann N Y Acad Sci. 2009;1155:57–67. doi: 10.1111/j.1749-6632.2008.03675.x. [DOI] [PubMed] [Google Scholar]
- Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- Tsutsui T, Tamura Y, Yagi E, Barrett JC. Involvement of genotoxic effects in the initiation of estrogen-induced cellular transformation: studies using Syrian hamster embryo cells treated with 17beta-estradiol and eight of its metabolites. Int J Cancer. 2000;86:8–14. doi: 10.1002/(sici)1097-0215(20000401)86:1<8::aid-ijc2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Eliassen AH, Missmer SA, Tworoger SS, Hankinson SE. Circulating 2-hydroxy- and 16alpha-hydroxy estrone levels and risk of breast cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:2029–2035. doi: 10.1158/1055-9965.EPI-08-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho GH, Luo XW, Ji CY, Foo SC, Ng EH. Urinary 2/16 alpha-hydroxyestrone ratio: correlation with serum insulin-like growth factor binding protein-3 and a potential biomarker of breast cancer risk. Ann Acad Med Singapore. 1998;27:294–299. [PubMed] [Google Scholar]
- Kabat GC, Chang CJ, Sparano JA, et al. Urinary estrogen metabolites and breast cancer: a case-control study. Cancer Epidemiol Biomarkers Prev. 1997;6:505–509. [PubMed] [Google Scholar]
- Meilahn EN, De Stavola B, Allen DS, et al. Do urinary oestrogen metabolites predict breast cancer? Guernsey III cohort follow-up. Br J Cancer. 1998;78:1250–1255. doi: 10.1038/bjc.1998.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muti P, Bradlow HL, Micheli A, et al. Estrogen metabolism and risk of breast cancer: a prospective study of the 2:16alpha-hydroxyestrone ratio in premenopausal and postmenopausal women. Epidemiology. 2000;11:635–640. doi: 10.1097/00001648-200011000-00004. [DOI] [PubMed] [Google Scholar]
- Ursin G, London S, Stanczyk FZ, et al. A pilot study of urinary estrogen metabolites (16alpha-OHE1 and 2-OHE1) in postmenopausal women with and without breast cancer. Environ Health Perspect. 1997;105 Suppl 3:601–605. doi: 10.1289/ehp.97105s3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin G, London S, Stanczyk FZ, et al. Urinary 2-hydroxyestrone/16alpha-hydroxyestrone ratio and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1999;91:1067–1072. doi: 10.1093/jnci/91.12.1067. [DOI] [PubMed] [Google Scholar]
- Ursin G, London S, Yang D, et al. Urinary 2-hydroxyestrone/16alpha-hydroxyestrone ratio and family history of breast cancer in premenopausal women. Breast Cancer Res Treat. 2002;72:139–143. doi: 10.1023/a:1014896417653. [DOI] [PubMed] [Google Scholar]
- Ursin G, Wilson M, Henderson BE, et al. Do urinary estrogen metabolites reflect the differences in breast cancer risk between Singapore Chinese and United States African-American and white women? Cancer Res. 2001;61:3326–3329. [PubMed] [Google Scholar]
- Bradlow HL, Davis DL, Lin G, Sepkovic D, Tiwari R. Effects of pesticides on the ratio of 16 alpha/2-hydroxyestrone: a biologic marker of breast cancer risk. Environ Health Perspect. 1995;103 Suppl 7:147–150. doi: 10.1289/ehp.95103s7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk RT, Fears TR, Xu X, et al. Urinary estrogen metabolites and their ratio among Asian American women. Cancer Epidemiol Biomarkers Prev. 2005;14:221–226. [PubMed] [Google Scholar]
- Lee JS, Ettinger B, Stanczyk FZ, et al. Comparison of methods to measure low serum estradiol levels in postmenopausal women. J Clin Endocrinol Metab. 2006;91:3791–3797. doi: 10.1210/jc.2005-2378. [DOI] [PubMed] [Google Scholar]
- Santen RJ, Demers L, Ohorodnik S, et al. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72:666–671. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomarkers Prev. 2007;16:1713–1719. doi: 10.1158/1055-9965.EPI-06-0765. [DOI] [PubMed] [Google Scholar]
- Xu X, Keefer LK, Ziegler RG, Veenstra TD. A liquid chromatography-mass spectrometry method for the quantitative analysis of urinary endogenous estrogen metabolites. Nat Protoc. 2007;2:1350–1355. doi: 10.1038/nprot.2007.176. [DOI] [PubMed] [Google Scholar]
- Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:7813–7821. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- Xu X, Veenstra TD, Fox SD, et al. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem. 2005;77:6646–6654. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- Falk RT, Rossi SC, Fears TR, et al. A new ELISA kit for measuring urinary 2-hydroxyestrone, 16alpha-hydroxyestrone, and their ratio: reproducibility, validity, and assay performance after freeze-thaw cycling and preservation by boric acid. Cancer Epidemiol Biomarkers Prev. 2000;9:81–87. [PubMed] [Google Scholar]
- Ziegler RG, Hoover RN, Pike MC, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- Flores OR, Sun L, Vaziri ND, Miyada DS. Colorimetric rate method for the determination of creatinine as implemented by the Beckman Creatinine Analyzer 2. Am J Med Technol. 1980;46:792–798. [PubMed] [Google Scholar]
- Bradlow HL, Sepkovic DW, Klug T, Osborne MP. Application of an improved ELISA assay to the analysis of urinary estrogen metabolites. Steroids. 1998;63:406–413. doi: 10.1016/s0039-128x(98)00041-5. [DOI] [PubMed] [Google Scholar]
- Klug TL, Bradlow HL, Sepkovic DW. Monoclonal antibody-based enzyme immunoassay for simultaneous quantitation of 2- and 16 alpha-hydroxyestrone in urine. Steroids. 1994;59:648–655. doi: 10.1016/0039-128x(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Hsing AW, Stanczyk FZ, Belanger A, et al. Reproducibility of serum sex steroid assays in men by RIA and mass spectrometry. Cancer Epidemiol Biomarkers Prev. 2007;16:1004–1008. doi: 10.1158/1055-9965.EPI-06-0792. [DOI] [PubMed] [Google Scholar]
- Ziegler RG, Rossi SC, Fears TR, et al. Quantifying estrogen metabolism: an evaluation of the reproducibility and validity of enzyme immunoassays for 2-hydroxyestrone and 16alpha-hydroxyestrone in urine. Environ Health Perspect. 1997;105 Suppl 3:607–614. doi: 10.1289/ehp.97105s3607. [DOI] [PMC free article] [PubMed] [Google Scholar]