Abstract
Background
The goals were to compare morbidity and mortality between primary and revisional bariatric surgery and to identify clinical predictors of adverse outcome among patients undergoing revisional surgery in the LABS consortium.
Setting
University hospitals, United States
Methods
Data from the LABS-1 (safety) cohort were analyzed, excluding primary gastric banding patients. There were 3802 LABS-1 patients included: 3577 primary surgery and 225 revisional surgery patients. Demographic, clinical, operative, and 30-day outcome data were compared between groups. A non-linear mixed effects logit model was used to identify independent risk factors for adverse outcome (death, DVT, PE, reintubation, reoperation, or discharge after day 30).
Results
Compared to those undergoing revisional surgery, primary surgery patients were younger (median age 44 vs. 49 years, p<0.0001), more likely to be male (20.5 vs. 12.7%, p=0.006), heavier (median BMI 47.3 vs. 41.2 kg/m2, p<0.0001), and had more co-morbidities (p<0.0001), including hypertension (56.0 vs. 46.0%, p=0.0044), diabetes (35.7 vs. 20.0%, p<0.0001) and sleep apnea (50.3 vs. 27.2%, p<0.0001). Revisional procedure operative time was longer (median 181 vs. 135 min, p<0.0001) and associated with greater blood loss (median 100 vs. <50 ml, p<0.0001). Adverse outcome was more likely after revisional surgery (15.1 vs. 5.3%, p<0.0001, OR 2.4, 95% CI 1.6–3.6). After adjusting for patient characteristics previously shown to be associated with adverse outcome, this difference remained statistically significant (OR = 2.3, 95% CI 1.5–3.8). Thirty day mortality was similar in the two groups (0.4%).
Conclusions
Revisional surgery was performed without substantial mortality but with greater incidence of adverse outcome than primary bariatric surgery.
Keywords: bariatric surgery, revision, failed restrictive procedure, gastric bypass, complications
Background
The prevalence of obesity continues to increase at an alarming rate. From 2004–06, more than one third of the adult population in the United States was found to have a body mass index (BMI) greater than 30 kg/m2 including 33.3% of men and 35.3% of women in 20061, 2. Parallel to the obesity epidemic, the number of primary bariatric surgery procedures has also increased. In 1998, 12,775 bariatric operations were performed compared to 70,256 in 20023. The American Society for Metabolic and Bariatric Surgery estimates that in 2008, 220,000 weight loss procedures were performed in the United States4. Revisional surgery is indicated to treat severe side effects or complications from previous weight loss surgery procedures but more patients are seeking revisional surgery due to inadequate weight loss from the primary procedure. Since revisional surgery mandates operating on previously manipulated tissue and often in the setting of long-term complications, significantly higher morbidity than with first time procedures has been reported 5–12.
Most published series on revisional bariatric surgery derive from single surgeon or institution studies with small cohorts of patients. The Longitudinal Assessment of Bariatric Surgery (LABS) study offers an opportunity to analyze data from a large cohort of bariatric surgery patients from a multicenter, prospectively maintained data registry representing a wide demographic profile from the United States13. The primary aim of this study was to compare the outcome between first time and revisional bariatric cases. The secondary aim was to determine independent risks factors for adverse outcome in patients undergoing revisional bariatric surgery.
Methods
Participants
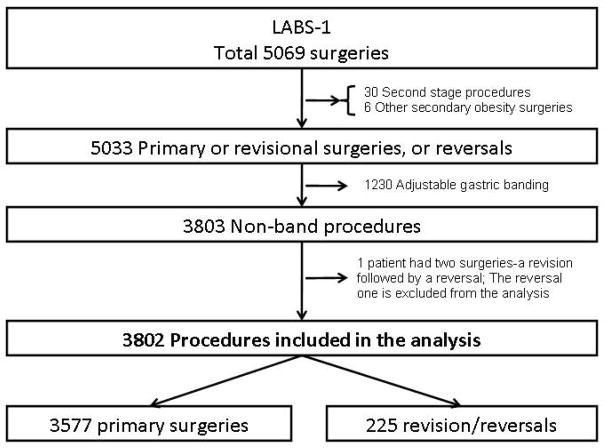
LABS-1 was a 30-day safety study in consecutive participants 18 years or older who underwent bariatric surgical procedures between March 11, 2005 and December 31, 2007. Details of the study have been described elsewhere 14. In brief, by December 31, 2007, 5069 bariatric surgery procedures were performed, of which 30 were second stage procedures, 6 other secondary obesity operations and 5033 primary or revisions/reversals of prior bariatric operations. Of the 5033 primary/revision/reversal operations, 1230 were laparoscopic adjustable gastric banding (LAGB) procedures which were removed from this analysis cohort. Outcome data on the primary lap Band patients were recently published in the main LABS-1 paper15. Also, 1 patient had two operations (a revision followed by a reversal), and the reversal was excluded from the analysis. Thus, the analysis was based on 3802 procedures (3577 primary, 203 revisions, and 22 reversals) [Figure 1]. Procedures that were started laparoscopically and “converted” to open surgery were considered as open15… Participants having primary surgery will be referred to as “primary participants” while those having revision or reversal will be referred to as “revisional participants”.
Figure 1.
The study cohort
Data definitions
Details of the LABS-1 pre-operative, operative, and post-operative data have been previously reported15. The study collected demographic and clinical features such as height, weight, comorbid conditions (self-reported) and some measure of severity based on associated healthcare utilization. The primary safety outcome was defined as a composite endpoint (CE) of any of the following occurring within 30 days of surgery: death; deep vein thrombosis (DVT) or venothromboembolism (VTE); reintervention using percutaneous, endoscopic, or operative techniques; reintubation; or failure to discharge from the hospital within 30 days of surgery.
Statistical methods
Descriptive patient characteristics are reported using summary statistics such as frequency distribution, mean, confidence interval, median and quartiles, as appropriate. Characteristics across subgroups (i.e. primary vs. revisional surgeries) were compared using Pearson’s chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. Thirty day adverse outcomes across primary and revisional procedures were compared using Fisher’s exact test. Univariate and multivariate generalized linear logistic regression models were used to evaluate the association between baseline patient characteristics and the odds of 30-day adverse outcome. Candidate variables to appear in the multivariate model were first screened based on the p-value of <0.20 in the univariate analysis and variables with lowest contribution to the response variability (type III sums of squares) were sequentially eliminated from the model. Once the model included variables that reached the significance cut-off of p = 0.10, all other variables were included one at a time to see whether they became significant or whether they had any impact on the strength or significance of the variables that were already in the model. Results are presented in terms of odds ratios (OR) and 95% confidence intervals (CI). Since the unadjusted relationship between 30-day adverse outcome and the BMI showed a quadratic pattern, both linear and quadratic terms of BMI were considered as predictors in the model. For all tests a p-value of <0.05 was considered to be statistically significant. For all statistical analyses, SAS 9.1.3 (SAS Institute Inc., Cary, NC) was used.
Results
Participant characteristics
The comparative baseline patient characteristics for revisional and primary surgeries are presented in Table 1. Compared to those undergoing revisional surgery, primary surgery participants were younger (median age 44 vs. 49 years, p<0.001), more likely to be male (20 vs. 13%, p=0.01) and heavier (median BMI 47.3 vs. 41.2 kg/m2, p<0.001).
Table 1.
Patient characteristics (frequency and percentage, unless otherwise noted)
| Characteristic | Total (N=3802) | Primary participants (N=3577) | Revisional participants (n = 225) | p value* | |||
|---|---|---|---|---|---|---|---|
| Patient age (years), mean, median | 44.22 | 43.98 | 47.95 | <.001 | |||
| 44.0 | 44.0 | 49.0 | |||||
| Patient age (years) | <.001 | ||||||
| <30 | 390 | 10.3 | 380 | 10.6 | 10 | 4.4 | |
| 30–39 | 990 | 26 | 952 | 26.6 | 38 | 16.9 | |
| 40–49 | 1108 | 29.1 | 1043 | 29.2 | 65 | 28.9 | |
| 50–59 | 991 | 26.1 | 899 | 25.1 | 92 | 40.9 | |
| 60–64 | 238 | 6.3 | 225 | 6.3 | 13 | 5.8 | |
| 65+ | 85 | 2.2 | 78 | 2.2 | 7 | 3.1 | |
| BMI (kg/m^2), mean, median | 48.51 | 48.98 | 40.95 | <.001 | |||
| 47.0 | 47.3 | 41.2 | |||||
| BMI (kg/m^2) | <.001 | ||||||
| <35 | 89 | 2.3 | 25 | 0.7 | 64 | 28.6 | |
| 35–<40 | 391 | 10.3 | 353 | 9.9 | 38 | 17 | |
| 40–<50 | 1919 | 50.5 | 1844 | 51.6 | 75 | 33.5 | |
| 50–< 60 | 1019 | 26.8 | 983 | 27.5 | 36 | 16.1 | |
| 60+ | 383 | 10.1 | 372 | 10.4 | 11 | 4.9 | |
| Male | 761 | 20 | 732 | 20.5 | 29 | 12.9 | 0.01 |
| Patient race white | 3365 | 89.3 | 3159 | 89.1 | 206 | 92.4 | 0.13 |
| missing, n | 34 | 32 | 2 | ||||
| Hispanic | 240 | 6.3 | 227 | 6.3 | 13 | 5.8 | 0.73 |
| missing, n | 1 | 1 | 0 | ||||
| Smoker within last | 0.29 | ||||||
| year | 584 | 15.4 | 555 | 15.5 | 29 | 12.9 | |
| missing, n | 1 | 1 | 0 | ||||
Chi-square test for categorical variables (with continuity correction for categorical data), Kruskal-Wallis test for continuous variables
As shown in Table 2, primary participants had more co-morbidities compared to revisional participants (1 or more comorbidities in 84% in primary and 68% in revisional surgeries, 2 or more comorbidities in 56% of the primary and 39% of the revisional surgeries, respectively; p < 0.001). Participants having primary surgery had a higher prevalence of major comorbidities such as hypertension (56 vs. 46 p=0.01), diabetes (36 vs. 20%, p<0.001), and sleep apnea (50 vs. 27%, p<0.001) compared to those having a revision or reversal, except that history of DVT was significantly more common among participants undergoing revisional surgeries (4 vs. 8%, p=0.001) [Table 2]. Use of narcotics (28% vs. 17%, p < 0.001) and antidepressant medications (48% vs. 41%, p < 0.03) were more common among revisional participants compared to primary participants.
Table 2.
Health Status of patients in the cohort (frequency and percentage, unless otherwise noted)
| Characteristic | Total (N=3802) | Primary participants (N=3577) | Revisional participants (N=225) | p value* | |||
|---|---|---|---|---|---|---|---|
| Hypertension | 2104 | 55.3 | 2001 | 55.9 | 103 | 45.8 | 0.00 |
| Medication: | 0.76 | ||||||
| No medication | 246 | 11.9 | 232 | 11.8 | 14 | 13.6 | |
| Single medication | 897 | 43.3 | 851 | 43.2 | 46 | 44.7 | |
| Multiple medication | 929 | 44.8 | 886 | 45 | 43 | 41.7 | |
| Diabetes | 1323 | 34.8 | 1278 | 35.7 | 46 | 20 | <.0001 |
| Medication: | 0.09 | ||||||
| No medication | 203 | 15.4 | 192 | 15 | 11 | 24.4 | |
| Single oral medication | 395 | 29.9 | 382 | 29.9 | 13 | 28.9 | |
| Multiple oral medication | 324 | 24.5 | 319 | 25 | 6 | 11.1 | |
| Insulin (w/or w/o oral meds) | 399 | 30.2 | 383 | 30 | 16 | 35.6 | |
| Congestive heart failure | 76 | 2 | 69 | 1.9 | 7 | 3.1 | 0.21 |
| missing, n | 2 | 0 | 2 | ||||
| Asthma | 911 | 24 | 853 | 23.8 | 58 | 25.9 | 0.49 |
| missing, n | 1 | 0 | 1 | ||||
| Cant walk 200 ft | 67 | 1.8 | 61 | 1.7 | 6 | 2.7 | 0.29 |
| missing, n | 1 | 1 | 0 | ||||
| History of DVT or PE | 149 | 3.9 | 131 | 3.7 | 18 | 8 | 0.001 |
| missing, n | 1 | 0 | 1 | ||||
| Sleep apnea | 1860 | 48.9 | 1799 | 50.3 | 61 | 27.2 | <.0001 |
| missing, n | 1 | 0 | 1 | ||||
| CPAP | 1504 | 80.9 | 1467 | 81.5 | 37 | 60.7 | <.0001 |
| Supplemental oxygen dependent | 74 | 4 | 67 | 3.7 | 7 | 11.5 | 0.00 |
| Ischemic heart disease | 153 | 4 | 138 | 3.9 | 15 | 6.7 | 0.03 |
| missing, n | 2 | 0 | 2 | ||||
| Pulmonary hypertension | 45 | 1.2 | 40 | 1.1 | 5 | 2.2 | 0.14 |
| Venous edema w/ulcerations | 163 | 4.6 | 155 | 4.7 | 8 | 3.8 | 0.55 |
| missing, n | 287 | 273 | 14 | ||||
| Number of comorbidities, mean, median | 1.80, 2.0 | 1.82, 2.0 | 1.43, 1.0 | <.0001 | |||
| Number of comorbidities, n (%) | <.0001 | ||||||
| 1 or more | 3149 | 82.9 | 2998 | 83.8 | 151 | 68 | |
| 2 or more | 2101 | 55.3 | 2014 | 56.3 | 87 | 39.2 | |
| 3 or more | 1038 | 27.3 | 998 | 27.9 | 40 | 18 | |
| 4 or more | 394 | 10.4 | 374 | 10.5 | 20 | 9 | |
| missing, n | 4 | 1 | 3 | ||||
| Beta-blocker | 687 | 18.4 | 642 | 18.3 | 45 | 20.3 | 0.45 |
| missing, n | 64 | 61 | 3 | ||||
| Statin/lipid-lowering agent | 1008 | 26.5 | 969 | 27.1 | 39 | 17.4 | 0.002 |
| missing, n | 1 | 0 | 1 | ||||
| Therapeutic anticoagulation | 174 | 4.6 | 164 | 4.6 | 10 | 4.5 | 0.93 |
| missing, n | 1 | 0 | 1 | ||||
| Narcotic | 657 | 17.3 | 594 | 16.6 | 63 | 28.1 | <.0001 |
| missing, n | 1 | 0 | 1 | ||||
| Anti-depressant | 1558 | 41.7 | 1450 | 41.2 | 108 | 48.4 | 0.03 |
| missing, n | 63 | 61 | 2 | ||||
Chi-square test for categorical variables (with continuity correction for categorical data), Kruskal-Wallis test for continuous variables
Intra-operative characteristics
Intra-operative characteristics significantly differed across primary and revisional surgeries (Table 3). Among revisional participants, the most common prior bariatric procedure was gastric bypass (38%) followed by other, vertical banded gastroplasty and gastric banding (22%, 21% and 19% respectively). Gastric bypass was the most commonly performed revisional procedure (65%). Twenty-one percent of the revisional procedures were classified as other, including 48 separate procedures such as reversal of mini gastric bypass, reversal of jejunoileal bypass, closure of gastro-gastric fistula, etc. Operative time for revisional surgery was longer (median 181 vs. 135 min, p<0.001) and associated with greater blood loss (75% of surgeries participants lost at least 200 ml in the revisional group compared to 75 ml in the primary group).
Table 3.
Pre- and Intra-operative characteristics (frequency and percentage, unless otherwise mentioned)
| Characteristic | Total (N=3802) | First surgery (N=3577) | Revision/reversal (N=225) | p value* | ||||
|---|---|---|---|---|---|---|---|---|
| Prior obesity/foregut surgery | 292 | 7.6 | 67 | 1.9 | 225 | 100 | <.001 | |
| missing, n | 1 | 1 | 0 | |||||
| Gastric bypass | 84 | 29.2 | 0 | 0 | 84 | 38 | ||
| Biliopancreatic diversion | 1 | 0.3 | 0 | 0 | 1 | 0.5 | ||
| Biliopancreatic diversion switch | 11 | 3.8 | 0 | 0 | 11 | 5 | ||
| Adjustable gastric band | 42 | 14.6 | 0 | 0 | 42 | 19 | ||
| Vertical banded gastroplasty | 47 | 16.3 | 0 | 0 | 47 | 21.3 | ||
| Sleeve gastrectomy | 4 | 1.4 | 0 | 0 | 4 | 1.8 | ||
| Prior foregut | 84 | 29.2 | 67 | 100 | 17 | 7.7 | ||
| Other previous obesity surgery | 49 | 17 | 0 | 0 | 49 | 22.2 | ||
| Surgery performed | <.001 | |||||||
| RYGB | 3557 | 93.6 | 3411 | 95.4 | 146 | 64.9 | ||
| Biliopancreatic diversion | 4 | 0.1 | 2 | 0.1 | 2 | 0.9 | ||
| Biliopancreatic diversion switch | 53 | 1.4 | 45 | 1.3 | 8 | 3.6 | ||
| Sleeve gastrectomy | 136 | 3.6 | 117 | 3.3 | 19 | 8.4 | ||
| Vertical banded gastroplasty | 1 | 0 | 1 | 0 | 0 | 0 | ||
| Other | 49 | 1.3 | 1 | 0 | 48 | 21.1 | ||
| Banded RYGB | 2 | 0.1 | 0 | 0 | 2 | 0.9 | ||
| Mins. incision - skin close, mean, median | 146.3, 137 | 143.9, 135 | 183.0, 180.5 | <.001 | ||||
| Missing, n | 7 | 6 | 1 | |||||
| Crystalloid fluids ml, mean, median | 2878.2, 2800 | 2845.1, 2700 | 3403, 3300 | <.001 | ||||
| missing, n | 42 | 40 | 2 | |||||
| Colloid fluids, ≥500 ml | 258 | 6.9 | 233 | 6.6 | 25 | 11.2 | 0.01 | |
| missing, n | 46 | 44 | 2 | |||||
| Blood loss ml, median (Q1, Q3)** | 0 (0,75) | 0 (0,60) | 100 (0,200) | <.001 | ||||
| missing, n | 39 | 38 | 1 | |||||
Chi-square test for categorical variables (with continuity correction for categorical data), Kruskal-Wallis test for continuous variables
Any blood loss of 50ml or less was treated as zero.
Adverse outcomes
Death within the first 30 days following surgery occurred in 16 (0.4%) primary and 1 (0.4%) revisional participants (Table 4). The percentage of participants diagnosed with DVT/PE within 30 days of surgery was significantly higher in revisional participants (1.8%) compared to primary participants (0.5%, p = 0.02). Participants were more likely to have a CE after revisional surgery (15.1% vs. 5.3%, p<0.001) compared to primary surgery. The unadjusted odds of having a CE after a revisional surgery was more than double that after a primary surgery (OR 2.4, 95% CI 1.6–3.6). Other characteristics that were significantly associated with higher odds of CE in univariate analysis included longer operative time, 75 cc or more blood loss, higher BMI, history of DVT, congestive heart failure, sleep apnea, inability to walk 200 ft and having a procedure other than laparoscopic RYGB (Table 5).
Table 4.
Adverse outcomes
| Characteristic | Total (N=3802) | Primary participants (N=3577) | Revisional participants (N=225) | p value*** | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | OR** (95% CI) | ||
| Death | 17 | 0.4 | 16 | 0.4 | 1 | 0.4 | 0.61 (0.08, 4.73) | 0.995 |
| DVT or PE | 22 | 0.6 | 18 | 0.5 | 4 | 1.8 | 2.81 (0.91, 8.69) | 0.015 |
| Tracheal reintubation | 25 | 0.7 | 20 | 0.6 | 5 | 2.2 | 2.69 (0.93, 7.78) | 0.003 |
| Endoscopy | 63 | 1.7 | 53 | 1.5 | 10 | 4.4 | 2.69 (1.32, 5.47) | 0.001 |
| Operation | ||||||||
| Tracheostomy | 12 | 0.3 | 12 | 0.3 | 0 | 0 | NA | 0.38 |
| Placement of percutaneous drain | 20 | 0.5 | 17 | 0.5 | 3 | 1.3 | 1.38 (0.39, 4.90) | 0.08 |
| Abdominal re-operation | 134 | 3.5 | 116 | 3.2 | 18 | 8 | 2.39 (1.39, 4.14) | <0.001 |
| Not discharged by day 30 | 25 | 0.7 | 23 | 0.6 | 2 | 0.9 | 0.78 (0.17, 3.56) | 0.66 |
| Composite event* | 224 | 5.9 | 190 | 5.3 | 34 | 15.1 | 2.37 (1.56, 3.62) | <.001 |
Death/DVT/PE/no discharge/intervention/post-bariatric surgery
Odds of corresponding outcome for revisional surgery compared to the primary surgery
Fisher’s exact test
Table 5.
Composite events by patient characteristics
| Characteristics | N | n (%) | OR | 95% LCL | 95% UCL | p |
|---|---|---|---|---|---|---|
| Pre and Intra-operative | ||||||
| Procedure | ||||||
| Primary | 3577 | 190(5.3) | 1 | |||
| Revision/Reversal | 225 | 34(15.1) | 2.374 | 1.555 | 3.625 | <0.001 |
| Prior foregut surgery | ||||||
| No | 3718 | 220(5.9) | 1 | |||
| Yes | 84 | 4(4.8) | 0.481 | 0.17 | 1.358 | 0.17 |
| Mins. incision - skin close** | <.001 | |||||
| 107 or less | 910 | 32(3.5) | 1 | |||
| 108 – 136 | 970 | 41(4.2) | 1.338 | 0.821 | 2.18 | 0.24 |
| 137 – 171 | 958 | 63(6.6) | 2.268 | 1.398 | 3.678 | 0.001 |
| 172 or more | 957 | 88(9.2) | 3.078 | 1.893 | 5.006 | <.001 |
| Incision - skin close (per hour) | 1.594 | 1.388 | 1.83 | <.001 | ||
| Crystalloid fluids ml** | 0.005 | |||||
| Less than 2000 | 714 | 30(4.2) | 1 | |||
| 2001 – 2799 | 1205 | 61(5.1) | 1.103 | 0.695 | 1.75 | 0.68 |
| 2800 – 3499 | 848 | 48(5.7) | 1.354 | 0.819 | 2.239 | 0.24 |
| 3500 or more | 1035 | 85(8.2) | 2.075 | 1.264 | 3.407 | 0.004 |
| Crystalloid fluids ml (per 500ml) | 1.106 | 1.042 | 1.173 | 0.001 | ||
| Colloid fluids ml | ||||||
| Less than 500 | 3498 | 197(5.6) | 1 | |||
| 500 or more | 258 | 24(9.3) | 1.632 | 1.002 | 2.659 | 0.049 |
| Blood loss ml | ||||||
| Less than 75 | 2748 | 120(4.4) | 1 | |||
| 75 or more | 1015 | 102(10) | 2.347 | 1.741 | 3.164 | <.001 |
| Demographics | ||||||
| Age (years) | 0.71 | |||||
| <30 | 390 | 16(4.1) | 1 | |||
| 30–39 | 990 | 53(5.4) | 1.265 | 0.709 | 2.256 | 0.43 |
| 40–49 | 1108 | 66(6) | 1.31 | 0.743 | 2.312 | 0.35 |
| 50–59 | 991 | 65(6.6) | 1.462 | 0.826 | 2.591 | 0.19 |
| 60–64 | 238 | 17(7.1) | 1.499 | 0.729 | 3.079 | 0.27 |
| 65+ | 85 | 7(8.2) | 1.98 | 0.774 | 5.062 | 0.16 |
| Age per year | 1.012 | 0.999 | 1.025 | 0.07 | ||
| BMI (kg/m2) categories | 0.01 | |||||
| <40 | 480 | 40(8.3) | 1.767 | 1.186 | 2.631 | 0.005 |
| 40– <50 | 1919 | 85(4.4) | 1 | |||
| 50 – <60 | 1019 | 63(6.2) | 1.291 | 0.916 | 1.819 | 0.14 |
| 60+ | 383 | 36(9.4) | 1.784 | 1.168 | 2.724 | 0.007 |
| BMI per 1 kg/m2 | 1.007 | 0.993 | 1.022 | 0.32 | ||
| Co-morbidities | ||||||
| Hypertension | ||||||
| No | 1698 | 97(5.7) | 1 | |||
| Yes | 2104 | 127(6) | 1.03 | 0.78 | 1.36 | 0.84 |
| Diabetes | ||||||
| No | 2479 | 132(5.3) | 1 | |||
| Yes | 1323 | 92(7) | 1.212 | 0.912 | 1.61 | 0.19 |
| Congestive heart failure | ||||||
| No | 3724 | 214(5.7) | 1 | |||
| Yes | 76 | 10(13.2) | 2.193 | 1.083 | 4.44 | 0.029 |
| Asthma | ||||||
| No | 2890 | 166(5.7) | 1 | |||
| Yes | 911 | 58(6.4) | 1.036 | 0.755 | 1.423 | 0.82 |
| Functional status | ||||||
| Can walk 200 ft | 3734 | 210(5.6) | 1 | |||
| Can not walk 200 feet | 67 | 14(20.9) | 2.555 | 1.323 | 4.934 | 0.005 |
| DVT | ||||||
| No | 3652 | 200(5.5) | 1 | |||
| Yes | 149 | 24(16.1) | 3.212 | 1.996 | 5.168 | <.001 |
| Sleep apnea | ||||||
| No | 1941 | 97(5) | 1 | |||
| Yes | 1860 | 127(6.8) | 1.381 | 1.029 | 1.855 | 0.032 |
| Ischemic heart disease | ||||||
| No | 3647 | 211(5.8) | 1 | |||
| Yes | 153 | 13(8.5) | 1.465 | 0.804 | 2.67 | 0.21 |
| Pulmonary hypertension | ||||||
| No | 3757 | 218(5.8) | 1 | |||
| Yes | 45 | 6(13.3) | 1.765 | 0.705 | 4.418 | 0.22 |
| Venous edema with ulcerations | ||||||
| No | 3639 | 206(5.7) | 1 | |||
| Yes | 163 | 18(11) | 1.577 | 0.923 | 2.692 | 0.10 |
| Number of co-morbidities | 0.037 | |||||
| None | 649 | 25(3.9) | 1 | |||
| 1–2 | 2111 | 120(5.7) | 1.45 | 0.926 | 2.269 | 0.10 |
| 3+ | 1038 | 79(7.6) | 1.853 | 1.145 | 3 | 0.012 |
| Surgical procedure | .005 | |||||
| LRYGB | 3024 | 150(5) | 1 | |||
| ORYGB | 534 | 48(9) | 1.67 | 1.064 | 2.619 | 0.026 |
| Other | 244 | 26(10.7) | 2.073 | 1.197 | 3.589 | 0.009 |
Categorization is based on quartiles
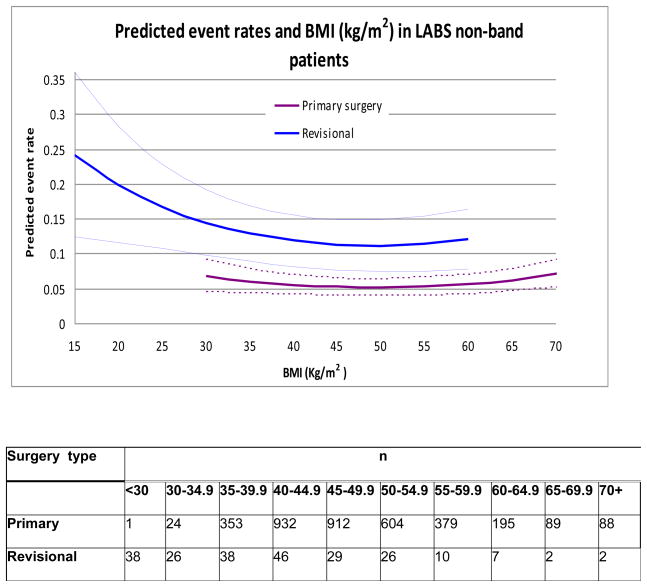
In the multivariable analyses, only baseline demographics, pre-operative characteristics, and their interactions were considered. The analysis identified several factors in addition to revisional/primary procedure type that were independently associated with higher odds of CE.(Table 6). Extreme BMI (Figure 2), being unable to walk 200 ft (OR = 1.92, 95% CI 0.96–3.82), history of DVT/PE (OR = 2.78, 95% CI 1.71–4.53), and history of OSA (OR = 1.45, 95% CI 1.06–1.97) were associated with the CE. After adjusting for these patient characteristics (BMI, functional status [inability to walk 200 ft], history of DVT/PE and OSA), the odds ratio of CE for revisional surgery was similar to the unadjusted odds ratio (OR = 2.3, 95% CI 1.5–3.8) compared to primary procedures. None of the two-way interactions between these factors were statistically significant and hence were excluded from the final model. The multivariable analysis was also repeated excluding 2 patients whose revisional procedure consisted of a banded gastric bypass (band placement over a prior gastric bypass); the results were nearly identical to those presented in Table 6.
Table 6.
Multivariate model excluding operative characteristics.
| Variable | OR | 95% CI lower | 95% CI upper | p |
|---|---|---|---|---|
| Revision/Reversal vs. primary | 2.34 | 1.45 | 3.77 | 0.001 |
| BMI (kg/m2)[linear]* | See Figure 2 | 0.86 | ||
| BMI (kg/m2) [quadratic]* | 0.001 | |||
| Able to walk 200 ft (No vs yes) | 1.92 | 0.96 | 3.82 | 0.06 |
| History of DVT (yes vs. no) | 2.78 | 1.71 | 4.53 | <.001 |
| History of sleep apnea (yes vs. no) | 1.45 | 1.06 | 1.97 | 0.02 |
Figure 2.
Patient BMI and surgery type (primary/revisional) as predictors of combined event for non-band operations in LABS. The predicted event rate is weighted for the prevalence of history of DVT, OSA and functional status in the LABS sample. The dotted lines represent 95% point-wise confidence intervals. The primary curve is truncated at BMI = 70 kg/m2, and the revisional surgery curve is truncated at BMI = 60 kg/m2 since there were only a few observations beyond these weight categories.
Since 67 of the primary participants had prior foregut surgery, it was of interest to see whether having a prior foregut procedure was related to the composite events rate. As seen in Table 5, having a prior foregut surgery was not significantly associated with CE. A separate multivariable analysis, excluding participants in the primary surgery group with prior foregut surgery was conducted. The results were similar to the ones presented in Table 6, except that the p-value for functional status (inability to walk 200 ft) was reduced to 0.0499.
To identify risk factors of CE for patients undergoing revisional procedures, we conducted a separate multivariable analyses within the revisional surgery group. Only history of DVT/PE (OR = 4.1, 95% CI 1.4–11.9) was significantly associated with CE among the revisional patients.
Discussion
This study is the first prospective, multi-center study to analyze revisional bariatric surgery paying particular attention to composite endpoints and risk factors for adverse outcome. When designing the study, the investigators elected to exclude adjustable gastric banding patients from the study cohort due to the observed low morbidity and mortality of banding procedures compared to stapling bariatric procedures15. The aims of this study were to compare the outcome of primary and revisional bariatric operations and to identify independent risk factors for adverse outcome in patients undergoing revisional surgery.
The results demonstrate that revisional surgery was performed with low mortality but with an increased occurrence of adverse outcome compared to primary surgery. There were interesting differences between primary and revisional surgery patients. Revisional surgery patients were older, weighed less and had less obesity-related comorbidity than primary surgery patients. It is striking that the incidence of three highly prevalent comorbidities – hypertension, diabetes and obstructive sleep apnea – were significantly lower in revisional patients, possibly suggesting that the primary bariatric procedure had some positive health benefits. On the other hand, a history of DVT was higher in patients undergoing revisional surgery.
The mortality in the study was low in each group (0.4%) which is in keeping with other reports of revisional bariatric surgery6, 7, 16. Since the occurrence of any single adverse outcome (such as death) was infrequent by itself, to increase statistical power, a composite endpoint was created by combining the most frequent major complications into one clinically meaningful category (death, DVT, PE, reintubation, reoperation, or discharge after day 30)15. When using this classification scheme, the incidence of adverse outcome was greater in the revisional group (15.1%) than in the primary group (5.3%). After risk adjustment, this difference was maintained with 2.3 times the odds for adverse outcome in revisional procedures compared to primary procedures. As has been demonstrated in the analysis using the entire LABS-1 cohort, there was a quadratic relationship between the predicted event rate and BMI15. It is interesting to note that the point estimate for the predicted risk for an adverse outcome is higher among people with lower BMI (Figure 6).
There are several limitations to this study. First, the indications for revisional bariatric surgery were not recorded and it may be that patients undergoing revisional surgery for a chronic complication may have had a different outcome than those undergoing revisional surgery for other reasons (inadequate weight loss). However, this cannot be addressed in LABS-1. Secondly, the LABS-1 dataset captures only 30-day outcome and does not provide long-term follow-up data (as opposed to the yet-to-be completed LABS-2 data set which will include more long-term data). Thus in this study there are no data on long-term resolution of comordities or degree of weight loss, both of which have been shown to be related to the type of primary procedure that is being revised5.
Many factors influence the outcome of bariatric surgery, including but not limited to the choice of procedure, initial weight, patient compliance and the incidence of short and long-term outcomes. The need for revisional procedures will undoubtedly increase in time as the number of primary bariatric cases grows. This study shows that revisional surgery can be performed with low mortality, though there was a higher incidence of adverse outcome when compared to primary bariatric procedures.
Footnotes
Revision/Reversal COI Disclosure:
| Name | Disclosure |
| Belle, Steven | None |
| Bessler, Marc | None |
| Courcoulas, Anita | None |
| Dellinger, Patchen |
|
| Garcia, Luis | None |
| Inabnet, William B. | Covidien (Educational or Research Grant Recipient) |
| Mitchell, Jim | None |
| O’Rourke, Robert | None |
| Oelschlager, Brant | Covidien (Speakers Bureau) Cook Surgical (Speakers Bureau) |
| Pender, John | Tyco Health Care – consultant, educational grants |
| Pomp, Alfons | None |
| Pories, Walter | Johnson & Johson – Research Grant GlaxoSmith Kline – Research Grant |
| Ramanathan, Ramesh | None |
| Wahed, Abdus | None |
| Wolfe, Bruce | American College of Surgeons Bariatric Centers (Speakers Bureau) Enteromedics (Consultant) Surgical Review Corporation (Speakers Bureau) |
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen NT, Root J, Zainabadi K, et al. Accelerated growth of bariatric surgery with the introduction of minimally invasive surgery. Arch Surg. 2005;140:1198–202. doi: 10.1001/archsurg.140.12.1198. discussion 203. [DOI] [PubMed] [Google Scholar]
- 4.http://www.asbs.org/Newsite07/media/asmbs_fs_surgery.pdf.
- 5.Brolin RE, Cody RP. Weight loss outcome of revisional bariatric operations varies according to the primary procedure. Ann Surg. 2008;248:227–32. doi: 10.1097/SLA.0b013e3181820cdf. [DOI] [PubMed] [Google Scholar]
- 6.Calmes JM, Giusti V, Suter M. Reoperative laparoscopic Roux-en-Y gastric bypass: an experience with 49 cases. Obes Surg. 2005;15:316–22. doi: 10.1381/0960892053576785. [DOI] [PubMed] [Google Scholar]
- 7.Coakley BA, Deveney CW, Spight DH, et al. Revisional bariatric surgery for failed restrictive procedures. Surg Obes Relat Dis. 2008;4:581–6. doi: 10.1016/j.soard.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 8.de Csepel J, Nahouraii R, Gagner M. Laparoscopic gastric bypass as a reoperative bariatric surgery for failed open restrictive procedures. Surg Endosc. 2001;15:393–7. doi: 10.1007/s004640000347. [DOI] [PubMed] [Google Scholar]
- 9.Gumbs AA, Pomp A, Gagner M. Revisional bariatric surgery for inadequate weight loss. Obes Surg. 2007;17:1137–45. doi: 10.1007/s11695-007-9209-9. [DOI] [PubMed] [Google Scholar]
- 10.Ikramuddin S, Kellogg TA, Leslie DB. Laparoscopic conversion of vertical banded gastroplasty to a Roux-en-Y gastric bypass. Surg Endosc. 2007;21:1927–30. doi: 10.1007/s00464-007-9537-9. [DOI] [PubMed] [Google Scholar]
- 11.Lim CS, Liew V, Talbot ML, Jorgensen JO, Loi KW. Revisional Bariatric Surgery. Obes Surg. 2008 doi: 10.1007/s11695-008-9750-1. [DOI] [PubMed] [Google Scholar]
- 12.Sarr MG. Reoperative bariatric surgery. Surg Endosc. 2007;21:1909–13. doi: 10.1007/s00464-007-9536-x. [DOI] [PubMed] [Google Scholar]
- 13.Belle S. The NIDDK Bariatric Surgery clinical Research Consortium (LABS) Surg Obes Relat Dis. 2005;1:145–7. doi: 10.1016/j.soard.2005.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belle SH, Berk PD, Courcoulas AP, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis. 2007;3:116–26. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flum DR, Belle SH, King WC, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. The New England journal of medicine. 2009;361:445–54. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.te Riele WW, Sze YK, Wiezer MJ, van Ramshorst B. Conversion of failed laparoscopic gastric banding to gastric bypass as safe and effective as primary gastric bypass in morbidly obese patients. Surg Obes Relat Dis. 2008;4:735–9. doi: 10.1016/j.soard.2008.03.001. [DOI] [PubMed] [Google Scholar]