Abstract
High-resolution imaging of human autopsy tissues may improve our understanding of in vivo MRI findings, but interpretation is complicated because samples are obtained by immersion fixation following a postmortem interval (PMI). This study tested the hypotheses that immersion fixation and PMI's from 0 - 24 hours would alter the water relaxation and diffusion properties in rat cortical slice and spinal cord models of human nervous tissue. Diffusion data collected from rat cortical slices at multiple diffusion times (10 - 60 ms) and b-values (7 - 15,000 s/mm2) were analyzed using a two-compartment model with exchange. Rat spinal cords were characterized with standard diffusion tensor imaging (21 directions, b = 1250 s/mm2). Switching from perfusion- to immersion-fixation at 0-hrs PMI altered most MRI properties of rat cortical slices and spinal cords, including a 22% decrease in fractional anisotropy (P < 0.001). After 4 hrs PMI, cortical slice T1 and T2 increased 22% and 65% respectively (P < 0.001), transmembrane water exchange decreased 23% (P < 0.001) and intracellular proton fraction increased 25% (P = 0.002). After 6 hrs PMI, spinal cord white matter fractional anisotropy had decreased 38% (P < 0.001). MRI property changes were observed for PMIs up to 24 hours. The MRI changes correlated with protease activity and histopathological signs of autolysis. Thus, immersion fixation and/or even short PMIs (4-6 hours) altered the MRI properties of rat nervous tissue. This suggests comparisons between in vivo clinical MRI and MRI data from human autopsy tissues should be interpreted with caution.
Keywords: Immersion, Perfusion, Formaldehyde, Exchange, Tensor, Anisotropy, Fixative
Introduction
High-resolution MRI datasets that require high signal-to-noise can be obtained from chemically-fixed human autopsy tissues (Shepherd et al., 2007) to facilitate the interpretation of in vivo imaging studies and help validate MRI characterizations in animal models of disease (Benveniste and Blackband, 2006). It is not possible to collect this data in vivo due to restrictions on the imaging time tolerated by patients, and due to current clinical magnet gradient and field strength limitations. Autopsy tissues for these MRI studies, however, are obtained via immersion-fixation at variable times following the patient's somatic death and the cessation of tissue perfusion; this time period is defined as the postmortem interval (PMI).
The rapid removal, refrigeration and chemical fixation of human autopsy tissues is invariably delayed because the patient's death is unwitnessed, or family grieving, logistics and inadequate staffing delay the tissue procurement process. Hence, most autopsy tissues begin immersion-fixation in formalin solutions many hours after the patient's demise such that a PMI less than 4 hours is rare. Further, autopsy samples may not complete chemical fixation for many additional hours because aldehyde fixatives penetrate tissue slowly (Hayat, 1981). Previous studies have described significant early molecular and morphological changes to nervous tissues that occur during the PMI (Oehmichen and Gencic, 1980; Schulz et al., 1980; Seaman, 1987). Also, human autopsy tissues are immersion-fixed, whereas animal tissues for MRI experiments are typically acquired via exsanguination and the intracardiac perfusion of fixative solutions. Because these differences are largely unavoidable, it becomes important to understand how PMI and immersion-fixation affect the MRI properties of human autopsy tissues.
Damadian, in fact, suggested that an overnight PMI had no impact on T1 in his original report (1971). Other studies, however, suggested that increasing PMI reduced the T1 and T2 (Blamire et al., 1999; Moseley et al., 1984; Nagara et al., 1987; Pfefferbaum et al., 2004), or reduced the diffusivity and fractional anisotropy of nervous tissue (D'Arceuil and de Crespigny, 2007; Kim et al., 2007; Schmierer et al., 2007). The external validity of these studies was limited by the use of long PMIs (20 hours or longer), acquiring data from only a limited number of human samples and/or the confounding MRI effects of formaldehyde solutions (Shepherd et al., 2008). Further, potential differences between the MRI properties of tissue after immersion or perfusion fixation have not been explored. To address these concerns, this study tested the hypotheses that the MRI water relaxation and diffusion properties of rat cortical slice and spinal cord models of human autopsy tissue would differ with 1) increasing PMI during the first 24 hours and 2) between tissues chemically-fixed by perfusion or immersion in formaldehyde solutions.
Materials and Methods
Simulation of Postmortem Interval
Laboratory animal use was approved by the Institutional Animal Care and Use Committee. Male, 250-g Long-Evans rats were euthanized and 3 × 4 mm, 500-μm thick coronal rat cortical slices procured using previous methods (Conners and Gluck, 1984). Some rat cortical slices were immediately immersion-fixed in a >50:1 volume excess of 4% formaldehyde in phosphate buffered saline (PBS)(pH 7.4). These slices represented the immersion-fixation or “0-hours” postmortem interval in the subsequent analysis. The remaining cortical slices were placed on microscope slides inside sealed, humidified chambers to prevent tissue desiccation. Slices then were left undisturbed at room temperature and removed for immersion-fixation in formaldehyde (as above) at postmortem intervals of 2, 4, 8, 12, 24 or 36 hours (7 or 8 slices per timepoint). Simultaneously, 3-cm segments of rat cervical spinal cord were removed by dorsal laminectomy. The treatment of rat spinal cords then paralleled the treatment of rat cortical slices described above, but samples were only chemically-fixed at postmortem intervals of 0, 6, 12 or 24 hours (3 cords per timepoint). Less timepoints were employed in the spinal cord arm of the study to minimize the number of rats sacrificed. Additional rat cortical slices and spinal cords were obtained from 3 transaortic perfusion-fixed rats (Shibutani, 2000). Thus prepared, all samples were stored in 4% formaldehyde for 10-12 days at 4°C prior to the MRI measurements. Additional rat cortical slices were frozen, then used to characterize autolytic enzyme cascade activation with increasing PMI using semi-quantitative Western immunoblot analysis of non-erythroid αII-spectrin and its calpain-specific cleavage fragments (Pike et al., 2001).
MRI of Rat Cortical Slices
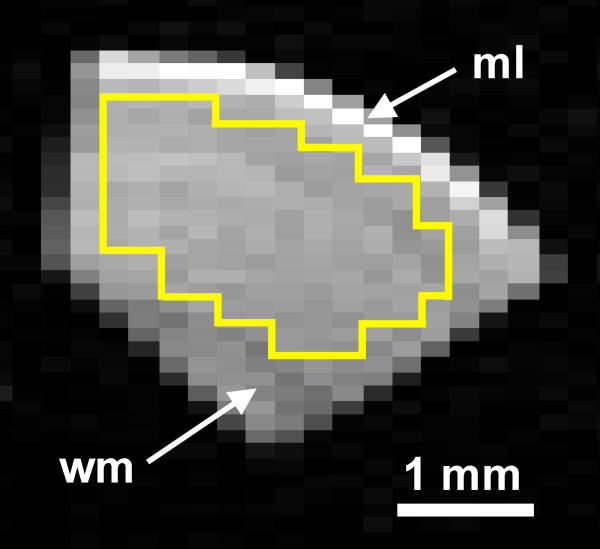
After immersion fixation, cortical slices were equilibrated to room temperature, washed overnight in PBS, then imaged in a 10-mm birdcage coil interfaced to a 14.1-T vertical magnet (Oxford Instruments, Abingdon, England) and console with 3000 mT/m imaging gradients (Bruker, Billerica, MA) using previous methods (Shepherd et al., 2002). Water diffusion measurements employed a pulsed-gradient spin-echo multislice sequence with diffusion times of 10, 20, 30, 45 and 60 ms (gradient duration = 3 ms) and 12 diffusion-weighted images with b-values between 7 and 15,000 s/mm2. These measurements had 2 averages with a 1.5-s repetition time, while echo time was minimized with respect to diffusion time (23.3 - 72.3 ms). The mean signal-to-noise ratio (SNR) of the diffusion-weighted images for cortical slices was 16.1 ± 1.1 at 15005 s/mm2 using the longest diffusion and echo times (60 ms and 72.3 ms respectively). Slice T1 values were determined with a saturation recovery experiment using 10 logarithmically-spaced repetition times (150 ms - 10 s) and T2 values were determined with a multi-echo sequence using 30 consecutive 10-ms echoes. Complete MRI data acquisition took 4 hours. All images had modest in-plane resolution (128 × 64 matrix, 1.5 cm × 1.5 cm FOV) to improve SNR for robust model analysis. Regions-of-interest (ROI) were drawn over the cortical slices to exclude PBS, the molecular cortical layer and white matter from the corpus callosum or external capsule (Fig. 1). The relative proton density of cortical slices was determined based on SNR in images with echo and repetition times of 10 ms and 10 s respectively. After the MRI measurements, cortical slices from different postmortem intervals were processed for histology sections, then stained with hematoxylin and eosin. Slice MRI data were analyzed using a two-compartment diffusion model with transmembrane water exchange that assumes restricted diffusion in the intracellular space and extracellular water diffusion mediated by tortuosity (Stanisz, 2003). The model estimates the extracellular apparent diffusion coefficient (ADCex), mean restriction size (a), transmembrane exchange rate (k) and intracellular magnetization fraction (Min).
Figure 1.
Representative diffusion-weighted image of a coronal rat cortical slice used for this study (117 × 234 μm in-plane resolution, TR/TE = 1500/43.3 ms, diffusion time = 30 ms, b-value = 2005 s/mm2). The region-of-interest (yellow) used for MRI data analysis excluded the acellular molecular layer (ml) and the anisotropic white matter (wm) of the external capsule.
Diffusion Tensor Imaging of Rat Spinal Cord
After fixation, rat spinal cords were equilibrated to room temperature and washed overnight in PBS. Spinal cords then were placed into an NMR tube and imaged in the same 10-mm radiofrequency coil and system. Diffusion tensor data were collected using a pulsed-gradient spin-echo pulse sequence (TR/TE = 1500/28.3 ms). For each cord, images were obtained without diffusion-weighting and from 21 unique diffusion gradient orientations. Diffusion gradient strength was 415 mT/m, gradient separation and duration were 17.8 and 2.4 ms respectively (diffusion time = 17 ms and b = 1250 s/mm2). Continuous 300-μm thick axial slices were obtained through the spinal cord segment with a 9.6-mm square field-of-view and a 64 × 64 matrix (150-μm in-plane resolution). Each dataset required 10.8 hours. The mean SNR for individual diffusion-weighted images (b = 1250 s/mm2) of spinal cord gray and white matter was 43.6 ± 11.7 and 31.3 ± 14.2 respectively. A rank-2 diffusion tensor model was fitted to the data to derive separate images of mean diffusivity, fractional anisotropy (FA) and color fiber orientation (Basser and Jones, 2002). ROI's were segmented manually over ventral gray matter, anterolateral and dorsal white matter regions to extract mean diffusivity and FA values. All quantitative data from different postmortem intervals were compared statistically with Sigmastat 2.03 (Systat Software, San Rafael, CA) using a 1-way ANOVA with posthoc Tukey multiple comparisons tests.
Results
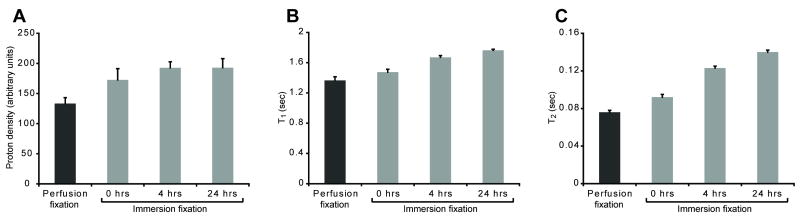
Simply switching from perfusion to immersion fixation methods increased rat cortical slice relative proton density by 30%, T1 by 8% and T2 by 21% (all, P < 0.01) (Fig. 2). Slice relative proton density increased by 45% after 4 hours PMI (P < 0.001), did not change further up to 24 hours PMI, but was 66% higher by 36 hours PMI (data not shown, P < 0.001). In contrast, cortical slice T1 and T2 values increased significantly throughout the first 24 hours PMI (all comparisons, P < 0.001)(Fig. 2B & C). At 4 hours PMI, slice T1 and T2 increased 22% and 62% respectively compared with perfusion-fixed slices, or increased 13% and 34% respectively compared with slices immersion-fixed at 0 hours PMI. After 24 hours, slice T1 and T2 had increased 20 and 52% respectively compared with slices prepared by immediate immersion fixation.
Figure 2.
Immersion fixation and postmortem interval alter the MRI relaxation properties of rat cortical slices [Mean ± SD, 7-8 slices per timepoint]. Relative proton density (A)(TR/TE = 10,000/10 ms) in the perfusion fixation treatment was lower than all other groups (P < 0.001). There were trends towards increasing proton density with immersion fixation from 0 to 4 hours (P = 0.111) or from 0 to 24 hours PMI (P = 0.104). PMI progressively increased cortical slice T1 (B) and T2 (C) (all groups statistically different, P < 0.001). Further individual comparisons between different PMIs are discussed in the results.
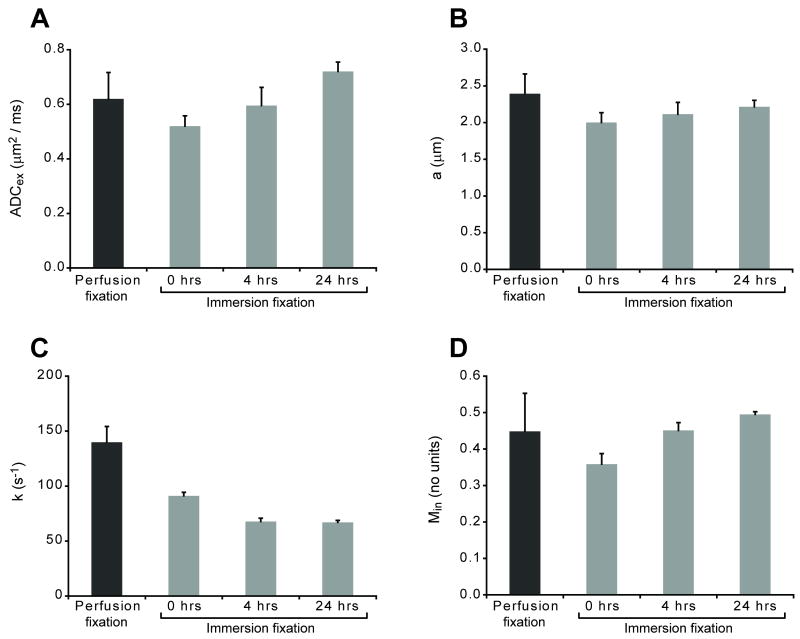
The biophysical changes to rat cortical slices with immersion fixation or increasing PMI were further characterized using a two compartment analytical model of water diffusion (Stanisz, 2003). This analytical model fitted the diffusion data well (Fig. 3). Chemically-fixed cortical slices again demonstrated increased transmembrane exchange compared with in vitro cortical slice data due to formaldehyde fixation (Shepherd et al., 2008). Comparisons between perfusion and immersion fixation at 0 hours PMI, and with further increasing PMI demonstrated significant changes to the biophysical parameters of the analytical model (Fig. 4). Switching from perfusion to immersion fixation without a PMI decreased mean restriction size 16% (P < 0.001), transmembrane water exchange 35% (P < 0.001) and the intracellular magnetization fraction 20% (P = 0.002). Cortical slice transmembrane water exchange continued to decrease significantly with increasing PMI and was reduced 52% compared with perfusion-fixed samples at 24 hours PMI (P < 0.001). The extracellular apparent diffusion coefficient decreased 16% after switching from perfusion to immersion fixation (P = 0.064), but subsequently increased from 0 to 24 hours immersion fixation by 38% (P < 0.001). After initial decreases with immersion fixation at 0 hours PMI (above), the intracellular magnetization fraction increased 25% and 36% after 4 or 24 hours PMI respectively (P ≤ 0.002).
Figure 3.
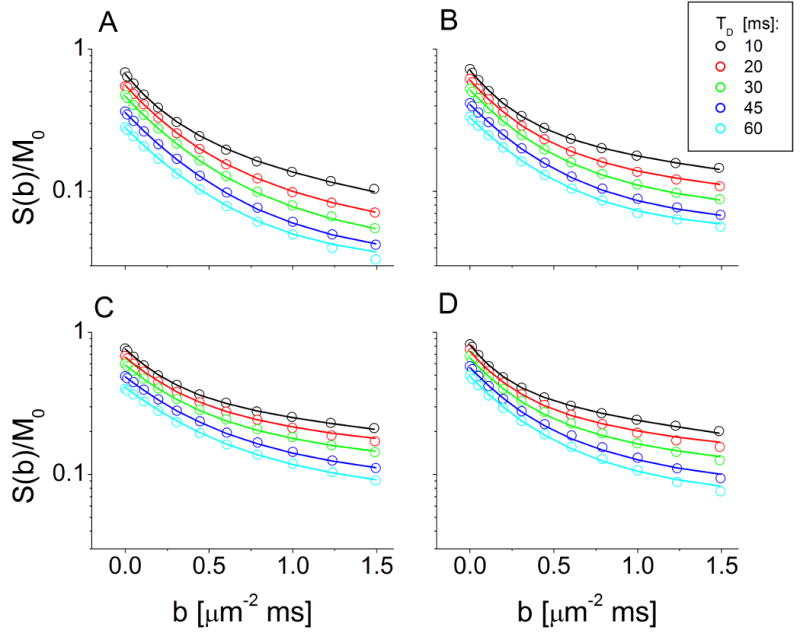
Log-semilog plots of diffusion-weighted signal attenuation curves from representative rat cortical slices at different diffusion times (10-60 ms) after perfusion fixation (A), and immersion fixation at 0 hrs (B), 4 hrs (C) or 24 hrs (D) postmortem interval (PMI). Points represent the MRI data while lines represent fits from the two-compartment analytical model of water diffusion. Note, the data were not normalized such that signal decreases with increasing diffusion time at b = 0 ms/μm2 reflect T2 relaxation effects.
Figure 4.
Immersion fixation and PMI altered the extracellular apparent diffusion coefficient (“ADCex”)(A), mean restriction size (“a”)(B), transmembrane exchange rate (“k”)(C) and intracellular magnetization fraction (“Min”)(D) of rat cortical slices [Mean ± SD, 7-8 slices per timepoint]. Switching from perfusion to immersion fixation at 0-hours PMI significantly reduced mean restriction size, transmembrane exchange and the intracellular magnetization fraction (all comparisons, P ≤ 0.002). Longer PMIs further decreased transmembrane exchange while significantly increasing extracellular ADC and intracellular magnetization fraction (individual comparisons discussed in the results).
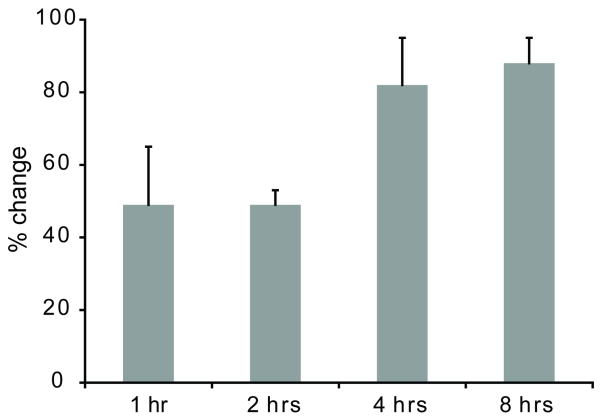
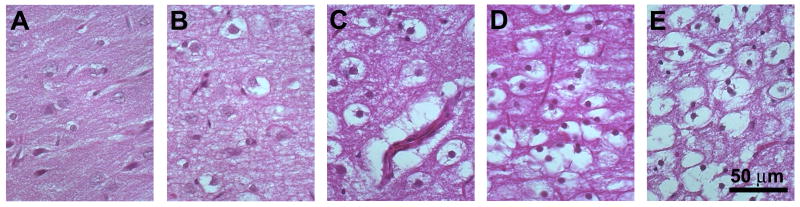
Correlative immunoblot studies of rat cortical slice MRI changes during the first 8 hours of the PMI demonstrated 49% and 82% relative increases in calpain-specific spectrin cleavage products at 1 and 4 hours PMI respectively (Fig. 5). These changes were consistent with autolytic molecular changes associated with oncotic cell death (Zhao et al., 1999). It was not possible to study calpain activity differences between immersion and perfusion-fixed cortical slices because formaldehyde alters the binding affinity of the immunoblot antibodies. However, no significant morphological differences were noted between perfusion and immersion fixation samples. During the first 12 hours PMI, histology of cortical slices demonstrated increasing neuronal retraction artifact, neuropil staining pallor and perinuclear vacuolization (Fig. 6). Early changes noted in the superficial layers subsequently spread to the large pyramidal neurons in cortical layers IV and V. From 12 to 24 hours PMI, cortical slices demonstrated more prominent perinuclear vacuolization and nuclear pyknosis. After 24 to 36 hours PMI, frank autolytic changes became much more obvious with cellular ghosts and pronounced glial pyknosis. At 36 hours PMI, the superficial cortical layers of some slices separated from the deeper layers. The observed morphologic changes in cortical slices were similar to previous descriptions of PMI's impact on in situ rat cortex (Seaman, 1987).
Figure 5.
Semi-quantitative densitometry of Western immunoblots demonstrated accumulation of calpain-specific non-erythroid αII-spectrin cleavage products in rat cortical slices from cellular autolysis as the PMI increases [Mean ± SD, 3 slices per timepoint]. Compared with baseline (0 hrs PMI), calpain-specific spectrin cleavage products increased 49% in the first 1-2 hrs (P < 0.001) and 82% after 4-8 hrs PMI (P < 0.001). There were no differences between 1 and 2 hrs PMI (P = 0.917), or between 4 and 8 hrs PMI (P = 0.615).
Figure 6.
Histology demonstrated progressive neuronal morphology changes with increasing PMI (Hematoxylin and eosin stain, 10-μm sections, 400× original magnification). Nervous tissue morphology was well-preserved with immediate immersion fixation (A). By 4 hrs PMI, cortical slices developed some perinuclear vacuolization and staining pallor to the neuropil, but nuclear morphology remained preserved (B). Perinuclear vacuolization involved all neuronal layers of the cortical slice by 12 hrs PMI and the nuclear chromatin appeared less distinct (C). After 24 hrs PMI, slices demonstrated retraction artifacts for all neurons with increased numbers of pyknotic nuclei (D). Autolytic changes became more prominent by 36 hrs PMI, with cellular ghosts containing fluid instead of debris as well as interval development of glial pyknosis (E).
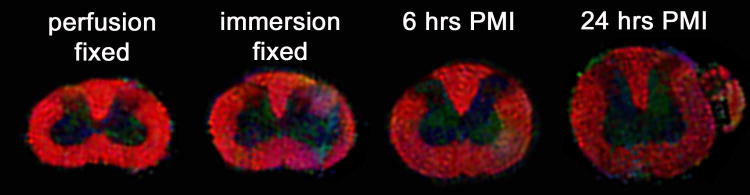
Cortical slices have minimal diffusion fractional anisotropy (FA ∼ 0.05), so the PMI effects on white matter were studied using rat spinal cords (Fig. 7). There were significant reductions to mean diffusivity and FA in the rat spinal cord with immersion fixation or increasing PMIs (Fig. 8). Changing from perfusion to immersion fixation decreased mean diffusivity 20-25% in gray and white matter (P < 0.001). Immersion fixation also reduced FA in spinal cord white matter 22% (P < 0.001). In spinal cords prepared by immersion fixation, there was a further 25% and 54% decrease in mean diffusivity of the gray and white matter respectively between 0 and 24 hrs PMI (P < 0.001). Also, compared to immersion fixation at 0 hours PMI, spinal cord white matter FA decreased 21% and 39% at 6 and 24 hours PMI respectively (P < 0.001). MRI data comparisons were similar for both the anterolateral and dorsal column white matter regions of the rat spinal cord. Further, the mean diffusivity and FA of perfusion-fixed rat spinal cord were similar to previous reports (Berens et al., 2005), but white matter anisotropy appeared slightly lower than in vivo values due to chemical fixation (Madi et al., 2005). Despite the significant decreases to white matter FA and mean diffusivity with increasing PMI, the fiber orientation of the anterolateral and dorsal spinal cord white matter remained cranio-caudal (Fig. 7).
Figure 7.
Axial color fiber orientation maps of rat cervical spinal cords with increasing PMI (fiber orientation: red = through-plane, green = left-right, and blue = up-down, color intensity determined by fractional anisotropy). Anisotropy in the spinal cord white matter decreased when switching from perfusion to immersion fixation techniques, and then progressively decreased with longer PMI's (see Fig. 8). White matter fiber orientation, however, appeared relatively preserved.
Figure 8.
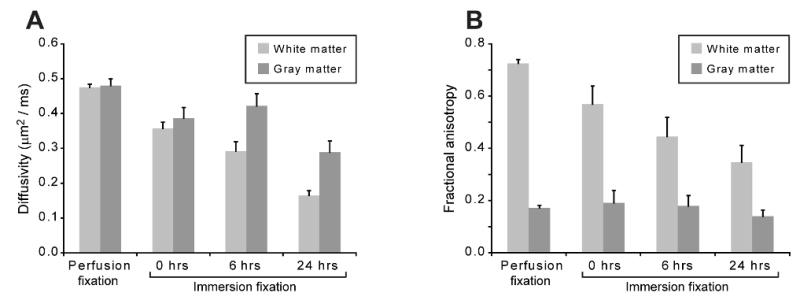
Immersion fixation and postmortem interval alter the mean diffusivity (A) and fractional anisotropy (B) of gray and white matter in the rat spinal cord [Mean ± SD, 3 spinal cords per timepoint]. The anterolateral white matter of the spinal cord showed progressive decreases in mean diffusivity with immersion fixation and increasing PMI (all comparisons, P < 0.001). Mean diffusivity in gray matter also significantly decreased (P < 0.001), except between the 0 and 6 hrs PMI (P = 0.127). Fractional anisotropy in white matter decreased significantly with immersion fixation and increasing PMI (all comparisons, P ≤ 0.007), but did not demonstrate biologically significant changes in gray matter.
Discussion
In rat cortical slices, relative proton density, T1 and T2 values increased significantly with increasing PMI (Fig. 2). In contrast, previous studies attributed T1 or T2 reductions in nervous tissue to increasing PMI (Blamire et al., 1999; Moseley et al., 1984; Nagara et al., 1987; Pfefferbaum et al., 2004). These reductions, however, were confounded by excess tissue dehydration that should not occur in human samples (Finkbeiner et al., 2004), or by formaldehyde-induced T1 and T2 shortening (Thickman et al., 1983). In this study, tissues were stored in chambers to prevent dehydration and washed overnight prior to MRI to remove T2-shortening effects from free formaldehyde solution (Shepherd et al., 2008). The observed progressive increases in nervous tissue proton density, T1 and T2 correlated with histology changes consistent with cytotoxic edema (Fig. 6). These changes are comparable to the MRI changes observed with irreversible ischemia in vivo (Provenzale et al., 2003), but may have different molecular and morphologic etiologies.
PMI-induced early progressive decreases to rat nervous tissue mean diffusivity and fractional anisotropy (FA) also demonstrated some qualitative similarities to the MRI changes observed during irreversible ischemia. Mean diffusivity in rat spinal cord gray and white matter decreased 20% and 39% in the first 6 hours of PMI respectively. Diffusivity decreased progressively within the first 24 hours of PMI (Fig. 8). Only reductions to mean diffusivity after PMI's of 20 hours or greater have been reported previously (D'Arceuil and de Crespigny, 2007; Pattany et al., 1997; Schmierer et al., 2007). The two-compartment analytical model fits suggested that alterations in membrane permeability, redistribution of magnetization between compartments and increased diffusivity within the remaining extracellular space (Fig. 4) may underlie the mean diffusivity changes observed with clinical DTI contrast techniques. Membrane changes also may explain the observed 38% decrease in FA of rat spinal cord white matter in the first 6 hours and 52% decrease in FA by 24 hours PMI (Fig. 8). Diffusion anisotropy also decreased 33% in normal-appearing white matter from multiple sclerosis human brain samples after a 20-hour room-temperature PMI (Schmierer et al., 2007). In contrast, other reports may not have detected early diffusion anisotropy changes during the first 24 hrs of the PMI (D'Arceuil and de Crespigny, 2007; Kim et al., 2007; Pattany et al., 1997) due to immediate sample refrigeration to 4°C or customizing MRI acquisitions to different treatment groups. Although its anisotropy decreased, spinal cord white matter fiber orientation appeared preserved during the first 24 hrs (Fig. 7). Cerebral white matter fiber propagation also did not change significantly after a 24-hour PMI using liberal deterministic tractography criteria (stopping fractional anisotropy of 0.05) when samples were immediately refrigerated (D'Arceuil and de Crespigny, 2007).
Several studies have demonstrated that 4% formaldehyde alters the MRI properties of nervous tissues (Madi et al., 2005; Shepherd et al., 2008; Thickman et al., 1983). However, it is not practical to image human autopsy tissues immediately, so dissected samples are inevitably immersed in formaldehyde solutions for storage prior to MRI experiments. In contrast, animal models of human disease are perfusion-fixed with formaldehyde. Switching from perfusion to immersion fixation increased rat cortical slice relative proton density, T1 and T2, and caused significant diffusion model changes (Figs. 2 & 4). Further, this switch decreased white matter fractional anisotropy 22% in rat spinal cords (Fig. 8). During immersion, the fixative must diffuse from the tissue surface, whereas perfusion allows fixative to diffuse directly from capillary beds within the tissue microstructure. Immersion thus delays complete fixative penetration and allows for further tissue autolysis; a previous report suggests these MRI differences should be more pronounced in whole human brain samples compared with rat cortical slices or spinal cords (Yong-Hing et al., 2005). Possible tissue injury from autopsy dissection (Finkbeiner et al., 2004) also may contribute to the MRI differences between samples prepared by immersion or perfusion fixation.
It is impractical to study PMI in human samples systematically, so it is important to examine the validity of the experimental models used. In contrast to human autopsies, rat cortical slice and spinal cord dissections occurred at the start of the postmortem interval. This controlled for the expected increased impact of dissection at later PMIs due to the deteriorating condition of the tissues. However, to minimize potential additional deleterious effects of early dissection, the cut edges of slices and spinal cords were excluded from analysis. Human cadavers usually are refrigerated within 6-12 hours such that PMIs at room temperature beyond 12 hours should be less common. However, other differences suggest the rat tissue models may underestimate how PMI affects human autopsy tissues. Unlike human autopsies, tissues were procured from healthy, young adult rats without an agonal state and after exsanguination. Further, the small tissue dimensions facilitated early complete fixative penetration compared with typical human samples (Hayat, 1981) and accelerated tissue equilibration with room temperature. In contrast, human cadavers equilibrate to room temperature 20 hours after death (Knight and Nokes, 2002). These differences may have improved relative preservation of rat tissues compared to human autopsy samples at the same PMI.
Practical Applications
High-resolution MRI of human autopsy tissues may improve our understanding of in vivo MRI and MRI from animal models of disease, but these samples are obtained following a PMI that is typically 4 hours or greater. Even during this short interval, PMI significantly increased rat nervous tissue T1, T2 and relative proton density while significantly reducing fractional anisotropy and diffusivity. These MRI property changes correlated with cell death enzyme cascade activity and oncotic neuronal morphology changes. The observed changes with increasing PMI were comparable to the MRI changes observed during irreversible ischemic injury, but occurred without tissue reperfusion (White et al., 2000) and at ambient temperatures that are more neuroprotective (Newman et al., 1992). It will be difficult to further reduce the time to human autopsy tissue dissection and fixation in most clinical settings, however previous studies suggest prompt refrigeration may delay PMI changes to nervous tissue water relaxivity and diffusion (D'Arceuil and de Crespigny, 2007; Moseley et al., 1984). Further, the immersion fixation methods used for autopsies (or surgical biopsies) also altered the MRI properties of rat nervous tissue. Wide-scale application of perfusion fixation during autopsy remains unlikely. Given these limitations, it is important that human autopsy samples used for MRI research should be clearly described (and/or matched) in terms of their overall PMI, pre-refrigeration PMI and agonal state.
Acknowledgments
The authors appreciate laboratory assistance from Barbara O'Steen, Daniel Plant and Monica Shepherd. The authors also appreciate a critical manuscript review by Ruedi Thoeni.
Grant Sponsors: RO1 NS36992 and P41 RR16105
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed. 2002;15:456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Blackband SJ. Translational neuroscience and magnetic-resonance microscopy. Lancet Neurol. 2006;5:536–544. doi: 10.1016/S1474-4422(06)70472-0. [DOI] [PubMed] [Google Scholar]
- Berens SA, Colvin DC, Yu CG, Yezierski RP, Mareci TH. Evaluation of the pathologic characteristics of excitotoxic spinal cord injury with MR imaging. AJNR Am J Neuroradiol. 2005;26:1612–1622. [PMC free article] [PubMed] [Google Scholar]
- Blamire AM, Rowe JG, Styles P, McDonald B. Optimising imaging parameters for post mortem MR imaging of the human brain. Acta Radiol. 1999;40:593–597. doi: 10.3109/02841859909175593. [DOI] [PubMed] [Google Scholar]
- Conners Gluck. Neocortex: cellular properties and intrinsic circuitry. In: Dingledine R, editor. Brain Slices. Plenum Press; New York: 1984. pp. 313–340. [Google Scholar]
- D'Arceuil H, de C A. The effects of brain tissue decomposition on diffusion tensor imaging and tractography. Neuroimage. 2007;36:64–68. doi: 10.1016/j.neuroimage.2007.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damadian R. Tumor detection by nuclear magnetic resonance. Science. 1971;171:1151–1153. doi: 10.1126/science.171.3976.1151. [DOI] [PubMed] [Google Scholar]
- Finkbeiner WE, Ursell PC, Davis RL. Autopsy Pathology: A Manual and Atlas. 1st. Churchill Livingstone; Philadelphia, PA: 2004. [Google Scholar]
- Hayat MA. Fixation for electron microscopy. Academic Press; New York: 1981. [Google Scholar]
- Kim JH, Trinkaus K, Ozcan A, Budde MD, Song SK. Postmortem delay does not change regional diffusion anisotropy characteristics in mouse spinal cord white matter. NMR Biomed. 2007;20:352–359. doi: 10.1002/nbm.1138. [DOI] [PubMed] [Google Scholar]
- Knight B, Nokes L. Temperature-based Methods I. In: Henssge C, Knight B, Krompecker T, Madea B, Nokes L, editors. The Estimation of the Time Since Death in the Early Postmortem Period. 2nd. Arnold Publishers; London: 2002. pp. 3–42. [Google Scholar]
- Madi S, Hasan KM, Narayana PA. Diffusion tensor imaging of in vivo and excised rat spinal cord at 7 T with an icosahedral encoding scheme. Magn Reson Med. 2005;53:118–125. doi: 10.1002/mrm.20304. [DOI] [PubMed] [Google Scholar]
- Moseley ME, Nishimura MC, Pitts LH, Bartkowski HM, James TL. Proton nuclear magnetic resonance spectroscopy of normal and edematous brain tissue in vitro: changes in relaxation during tissue storage. Magn Reson Imaging. 1984;2:205–209. doi: 10.1016/0730-725x(84)90006-7. [DOI] [PubMed] [Google Scholar]
- Nagara H, Inoue T, Koga T, Kitaguchi T, Tateishi J, Goto I. Formalin fixed brains are useful for magnetic resonance imaging (MRI) study. J Neurol Sci. 1987;81:67–77. doi: 10.1016/0022-510x(87)90184-5. [DOI] [PubMed] [Google Scholar]
- Newman GC, Qi H, Hospod FE, Grundmann K. Preservation of hippocampal brain slices with in vivo or in vitro hypothermia. Brain Res. 1992;575:159–163. doi: 10.1016/0006-8993(92)90438-f. [DOI] [PubMed] [Google Scholar]
- Oehmichen M, Gencic M. Postmortal histomorphologic and histoenzymatic alterations in rat brain. Pathol Res Pract. 1980;169:72–83. doi: 10.1016/s0344-0338(80)80100-2. [DOI] [PubMed] [Google Scholar]
- Pattany PM, Puckett WR, Klose KJ, Quencer RM, Bunge RP, Kasuboski L, Weaver RG. High-resolution diffusion-weighted MR of fresh and fixed cat spinal cords: evaluation of diffusion coefficients and anisotropy. AJNR Am J Neuroradiol. 1997;18:1049–1056. [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Adalsteinsson E, Garrick T, Harper C. Postmortem MR imaging of formalin-fixed human brain. Neuroimage. 2004;21:1585–1595. doi: 10.1016/j.neuroimage.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Pike BR, Flint J, Dutta S, Johnson E, Wang KK, Hayes RL. Accumulation of non-erythroid alpha II-spectrin and calpain-cleaved alpha II-spectrin breakdown products in cerebrospinal fluid after traumatic brain injury in rats. J Neurochem. 2001;78:1297–1306. doi: 10.1046/j.1471-4159.2001.00510.x. [DOI] [PubMed] [Google Scholar]
- Provenzale JM, Jahan R, Naidich TP, Fox AJ. Assessment of the patient with hyperacute stroke: imaging and therapy. Radiology. 2003;229:347–359. doi: 10.1148/radiol.2292020402. [DOI] [PubMed] [Google Scholar]
- Schmierer K, Wheeler-Kingshott CA, Boulby PA, Scaravilli F, Altmann DR, Barker GJ, Tofts PS, Miller DH. Diffusion tensor imaging of post mortem multiple sclerosis brain. Neuroimage. 2007;35:467–477. doi: 10.1016/j.neuroimage.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz U, Hunziker O, Frey H, Schweizer A. Postmortem changes in stereological parameters of cerebral neurons. Pathol Res Pract. 1980;166:260–270. doi: 10.1016/S0344-0338(80)80134-8. [DOI] [PubMed] [Google Scholar]
- Seaman WJ. Postmortem change in the rat: a histologic characterization. Iowa State University Press; Ames, Iowa: 1987. [Google Scholar]
- Shepherd TM, Blackband SJ, Wirth ED., III Simultaneous diffusion MRI measurements from multiple perfused rat hippocampal slices. Magn Reson Med. 2002;48:565–569. doi: 10.1002/mrm.10241. [DOI] [PubMed] [Google Scholar]
- Shepherd TM, Ozarslan E, Yachnis AT, King MA, Blackband SJ. Diffusion tensor microscopy indicates the cytoarchitectural basis for diffusion anisotropy in the human hippocampus. AJNR Am J Neuroradiol. 2007;28:958–964. [PMC free article] [PubMed] [Google Scholar]
- Shepherd TM, Thelwall PE, Stanisz GJ, Blackband SJ. Aldehyde fixative solutions alter the water relaxation and diffusion properties of nervous tissue. Magn Reson Med. 2008 doi: 10.1002/mrm.21977. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani M. Anesthesia, Artificial Ventilation and Perfusion Fixation. In: Krinke GJ, editor. The Laboratory Rat. Academic Press; San Francisco: 2000. pp. 511–521. [Google Scholar]
- Stanisz GJ. Diffusion MR in biological systems: tissue compartments and exchange. Israel J Chem. 2003;43:33–44. [Google Scholar]
- Thickman DI, Kundel HL, Wolf G. Nuclear magnetic resonance characteristics of fresh and fixed tissue: the effect of elapsed time. Radiology. 1983;148:183–185. doi: 10.1148/radiology.148.1.6856832. [DOI] [PubMed] [Google Scholar]
- White BC, Sullivan JM, DeGracia DJ, O'Neil BJ, Neumar RW, Grossman LI, Rafols JA, Krause GS. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- Yong-Hing CJ, Obenaus A, Stryker R, Tong K, Sarty GE. Magnetic resonance imaging and mathematical modeling of progressive formalin fixation of the human brain. Magn Reson Med. 2005;54:324–332. doi: 10.1002/mrm.20578. [DOI] [PubMed] [Google Scholar]
- Zhao X, Pike BR, Newcomb JK, Wang KK, Posmantur RM, Hayes RL. Maitotoxin induces calpain but not caspase-3 activation and necrotic cell death in primary septo-hippocampal cultures. Neurochem Res. 1999;24:371–382. doi: 10.1023/a:1020933616351. [DOI] [PubMed] [Google Scholar]