Abstract
Objective:
To identify patterns of clinical presentation, imaging findings, and etiologies in a cohort of hospitalized patients with localized nontraumatic convexal subarachnoid hemorrhage.
Methods:
Twenty-nine consecutive patients with atraumatic convexal subarachnoid hemorrhage were identified using International Classification of Diseases–9 code from 460 patients with subarachnoid hemorrhage evaluated at our institution over a course of 5 years. Retrospective review of patient medical records, neuroimaging studies, and follow-up data was performed.
Results:
There were 16 women and 13 men between the ages of 29 and 87 years. Two common patterns of presentations were observed. The most frequent presenting symptom in patients ≤60 years (n = 16) was a severe headache (n = 12; 75%) of abrupt onset (n = 9; 56%) with arterial narrowing on conventional angiograms in 4 patients; 10 (p = 0.003) were presumptively diagnosed with a primary vasoconstriction syndrome. Patients >60 years (n = 13) usually had temporary sensory or motor symptoms (n = 7; 54%); brain MRI scans in these patients showed evidence of leukoaraiosis and/or hemispheric microbleeds and superficial siderosis (n = 9; 69%), compatible with amyloid angiopathy (n = 10; p < 0.0001). In a small group of patients, the presentation was more varied and included lethargy, fever, and confusion. Four patients older than 60 years had recurrent intracerebral hemorrhages in the follow-up period with 2 fatalities.
Conclusion:
Convexal subarachnoid hemorrhage is an important subtype of nonaneurysmal subarachnoid bleeding with diverse etiologies, though a reversible vasoconstriction syndrome appears to be a common cause in patients 60 years or younger whereas amyloid angiopathy is frequent in patients over 60. These observations require confirmation in future studies.
GLOSSARY
- CAA
= cerebral amyloid angiopathy;
- cSAH
= convexal subarachnoid hemorrhage;
- FLAIR
= fluid-attenuated inversion recovery;
- HELLP
= hemolysis, elevated liver enzymes, and low platelets;
- ICH
= intracerebral hemorrhage;
- IE
= infective endocarditis;
- PRES
= posterior reversible leukoencephalopathy syndrome;
- RCVS
= reversible cerebral vasoconstriction syndrome.
Atraumatic localized convexal subarachnoid hemorrhage (cSAH) is an unusual presentation of subarachnoid bleeding, in which the bleeding is localized to the convexities of the brain without involvement of the adjacent parenchyma or extension into the interhemispheric fissures, basal cisterns, or ventricles. Since most saccular aneurysms arise from the circle of Willis, aneurysmal rupture is an unlikely source of cSAH. Diverse etiologies have been posited for its occurrence, including cortical vein occlusions, posterior reversible leukoencephalopathy syndrome (PRES), reversible cerebral vasoconstriction syndrome (RCVS), coagulopathy, cocaine use, lupus vasculitis, cavernoma, brain abscesses, and cerebral amyloid angiopathy (CAA).1–13 Existing information about this condition is largely derived from case reports and small case series, which carry inherent referral and diagnostic biases. We undertook this study to systematically evaluate and review the potential causes and patterns of clinical and radiologic presentation of localized cSAH from the inpatient population at our institution.
METHODS
All patients with a diagnosis of SAH were identified from hospital records, using the International Classification of Diseases–9 code (430), from October 1, 2003, to September 30, 2008. The radiology reports of these patients were reviewed to screen those with potential cSAH. We retrospectively reviewed medical records including hospital notes, laboratory data, and imaging studies to exclude those with traumatic hemorrhages; patients who showed spread of blood into the sylvian or interhemispheric fissures or basal cisterns or involvement of the adjoining brain parenchyma on imaging were also excluded. Information on patient demographics, clinical presentation, and imaging studies was recorded and analyzed.
Standard protocol approvals, registrations, and patient consents.
This study was conducted with the approval of our hospital institutional review board.
RESULTS
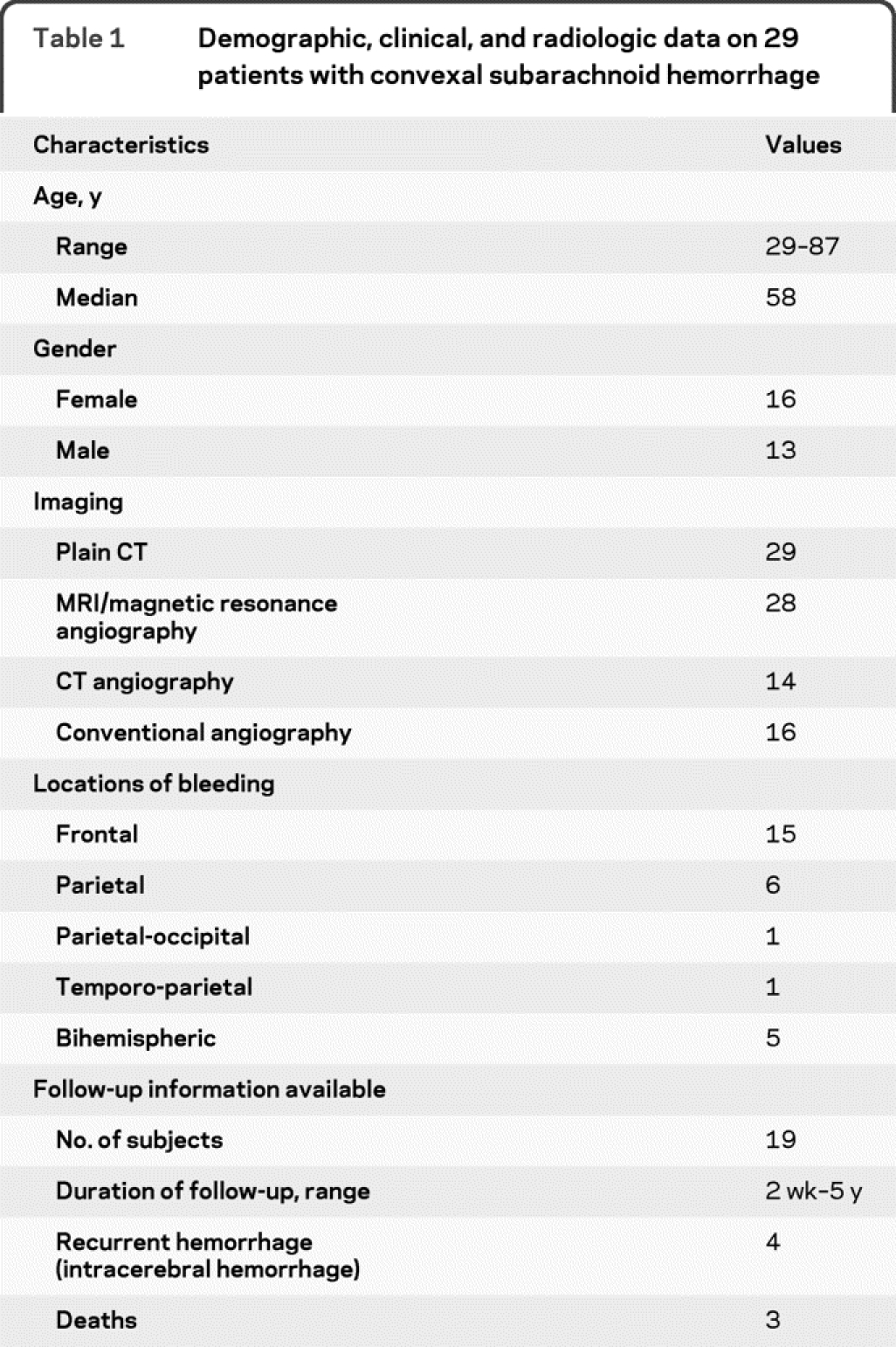
We identified 460 patients with SAH using our search strategy, of whom 389 had a spontaneous SAH. Among these, 29 fulfilled our criteria for atraumatic, localized cSAH. The baseline characteristics of our patient cohort are summarized in table 1.
Table 1 Demographic, clinical, and radiologic data on 29 patients with convexal subarachnoid hemorrhage
Clinical presentation.
Detailed clinical histories were available in all but 1 patient. The most common symptom prompting medical attention was either a headache or transient sensory or motor symptoms. The presentation varied in others.
Headache.
Headache was the presenting symptom in 18 (62%) patients. Among these, 8 had a history of migraine and 1 previously had postcoital headaches. Headaches were severe in 13 (45%) and described as the “worst ever.” Ten patients (34.4%) reported an acute onset of explosive pain characteristic of a thunderclap headache (headache intensity maximal at onset or peaked in ≤1 minute). In the remaining 3, headaches intensified gradually. One patient had a gradual onset of a severe headache 4 days postpartum and developed hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome. Another patient developed low-pressure headache following a lumbar puncture, requiring an epidural blood patch; a head CT obtained later for persistent headaches revealed a small cSAH. All but 1 patient with severe headaches were younger than 60 years. Headaches were typically prolonged, with an average duration of 6.5 days (range, 4 hours–17 days).
Transient sensory or motor symptoms.
Six patients (21%) presented with sensory symptoms, involving numbness that spread from one body part to another over a few minutes (range 5–20 minutes), mimicking a migrainous march. Three patients had multiple stereotyped episodes of spreading numbness. One reported a mild headache with numbness and another repeatedly became aphasic during these attacks. All individuals with this presentation except one were older than 60. Only 1 of the 6 reported a history of past headaches.
Two patients had transient, focal motor symptoms suspicious for TIA. One 87-year-old man developed temporary leg weakness followed by hand clumsiness. Another 79-year-old woman presented with 2 brief episodes of gait unsteadiness and slurred speech; on the second hospital day, she became lethargic, with gaze deviation, dysarthria, and hemiparesis. A repeat head CT showed a large intracerebral hemorrhage (ICH). The patient died despite hematoma evacuation.
Other presentations.
Three other patients presented with lethargy and decreased alertness, prompting a brain CT which led to the detection of hemorrhage. Among this group, a 52-year-old man had a 5-day history of malaise, headache, fever, and lethargy and was subsequently diagnosed with infective endocarditis (IE). A 57-year-old woman with a history of Still disease developed ITP with bronchoalveolar hemorrhage and underwent a CT scan for agitation, revealing a small cSAH. The third patient was a 79-year-old man with dementia who was hospitalized for evaluation of somnolence and confusion who underwent a head CT, revealing a small cSAH.
Imaging findings.
SAH was detected on a head CT scan in all patients except the 79-year-old woman mentioned above, in whom it was recognized on fluid-attenuated inversion recovery (FLAIR) MRI sequences and later confirmed with CSF analysis. All patients had small hemorrhages restricted to the convexities of the brain. The hemorrhage was typically unilateral (83%), localized between 1 and 3 neighboring sulci; 5 patients had bihemispheric involvement with small but separate foci of SAH. The subarachnoid space over the frontal lobes was most often involved (51% of patients) followed by anterior parietal regions (21%).
MRI findings.
All except 1 patient had a cranial MRI. Of these 28 patients, 12 had MRI with contrast which did not show any abnormal enhancement; 12 (41%) did not have any brain lesions and 3 others had scattered white matter abnormalities. One 45-year-old woman with multiple sclerosis, depression, and headaches lasting 17 days had small, scattered acute subcortical infarcts on diffusion-weighted imaging and apparent diffusion coefficient sequences in the occipital lobes and cerebellar hemispheres. Extensive white matter lesions on FLAIR and T2-weighted sequences were observed in 8 patients; all were older than 60 and all except 1 had hypertension. The extents of these abnormalities were further graded using the scale of Kapeller et al.14; 6 out of 8 patients who scored 2 or more each on the number and extent of deep white matter and periventricular changes were classified as having leukoaraiosis. Gradient echo sequences showed small microbleeds in 7 patients (cortical in 3; cortical and deep in 4). Superficial siderosis was seen in 5 cases; 4 were associated with cortical microbleeds and 1 with both cortical and deep microhemorrhage. Most patients with microbleeds/superficial siderosis were >60 years of age (figure 1); only one 34-year-old subject with IE had microbleeds on MRI. One 45-year-old woman with HELLP syndrome had bilateral occipital lobe hyperintensities on FLAIR/T2-weighted sequences indicating PRES; repeat MRI performed 6 weeks later showed complete resolution.
Figure 1 Superficial subarachnoid hemorrhage (SAH) in a patient with amyloid angiopathy
(A) Admission CT scan of a 70-year-old man with repeated episodes of right-sided numbness and speech difficulty demonstrates a left frontal SAH (arrow). (B) Brain MRI shows evidence of superficial siderosis (arrow) and cortical microbleeds (arrows) involving the frontal lobes.
Vascular imaging.
All patients had vascular imaging with magnetic resonance angiography, CT angiography, and/or conventional brain angiograms. The latter was performed in 16 patients (55%), including all patients with severe headaches. Four (14%) demonstrated arterial narrowing affecting the cortical branches of the anterior cerebral artery, middle cerebral artery, and posterior cerebral artery territories; all such patients had thunderclap headaches and were <60 years of age (figure 2).
Figure 2 Convexal subarachnoid hemorrhage (SAH) in a patient with cerebral vasoconstriction syndrome
(A) CT scan of a 56-year-old man with severe headaches showing presence of superficial convexal SAH along bilateral posterior frontal lobes (arrows). (B) Conventional angiogram performed a day later shows multiple vessel narrowings (arrows) involving the branches of the posterior and anterior cerebral arteries. A repeat angiogram performed 4 weeks later showed restitution of normal arterial caliber (not shown).
Three patients had arterial dissection, confirmed by conventional angiography. One presented with thunderclap headaches, retching, and vomiting, and had a small dissecting aneurysm of the distal right intracranial vertebral artery; another patient with bilateral extensive internal carotid artery dissection extending from the upper cervical to cavernous segments had symptoms of mild headache with multiple episodes of face, arm, and leg numbness akin to a migrainous march; the third patient had headaches in setting of HELLP syndrome and was found to have a small right internal carotid artery dissection at the level of the first cervical vertebra and changes suggestive of fibromuscular dysplasia. Two other patients had small intracranial aneurysms believed to be unrelated to the SAH.
Other studies.
Surface EEGs were performed in 8 patients with transient neurologic symptoms. One patient with multiple episodes of sensory attacks and speech abnormality had a prolonged EEG telemetry recording. None of these studies captured any epileptiform discharges. Seven patients had lumbar puncture with CSF analysis including cytology. Most patients had normal or slightly elevated protein levels and no xanthochromia; one patient showed markedly elevated protein (564 mg/dL) with xanthochromia. This patient later developed a fatal hemorrhage and underwent a necropsy revealing amyloid-beta–related angiitis.
Follow-up information was available in 19 patients. There were 4 recurrent hemorrhages; all were ICH and occurred in the older age group, with 2 fatalities. Another elderly patient who presented with lethargy died in the hospital from sepsis. Only one patient with thunderclap headache had a recurrent headache 4 weeks after initial presentation but repeat head CT and brain angiogram were normal. There were no residual neurologic symptoms or deaths in the younger age group of patients.
Diagnosis.
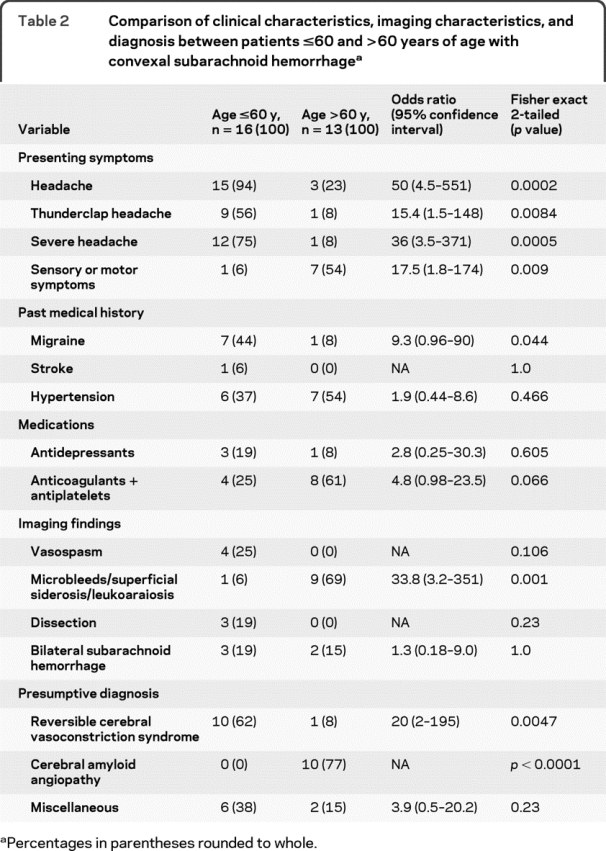
All medical records were reviewed for patient diagnosis. In situations where the diagnosis was unclear, a presumptive diagnosis was made by the study authors through consensus by reviewing the patient records and imaging studies. In the absence of any validated criteria, diagnosis of RCVS was made based on features proposed by Calabrese and colleagues.15 Since patients with RCVS often do not have angiographically evident vasoconstriction early during their clinical course,13 we relied on a history of severe or thunderclap headache with normal conventional angiograms and laboratory studies, absence of other potential causes of bleeding, and signs of clinical reversibility at discharge or during early follow-up, for a presumptive clinical diagnosis. Similarly in the absence of pathologic confirmation, patients were diagnosed with CAA if they were ≥55 years of age, had extensive leukoaraiosis or microhemorrhages, superficial siderosis on the gradient echo MRI sequences,16–18 and no other potential explanation for hemorrhage. The results are summarized in table 2. Patients were initially dichotomized according to the median sample age of 58 but subsequently changed to 60 to improve clinical interpretability once it was clear that this had no impact on the results. Of the 8 patients in miscellaneous category, 1 was diagnosed with HELLP/PRES, 1 with IE, 1 with ITP, 1 with intracranial hypotension, and 4 others remained undiagnosed. Two-tailed Fisher exact test was used to compare binomial proportions between the 2 groups using 2 × 2 contingency tables; odds ratios with 95% confidence intervals were also calculated.
Table 2 Comparison of clinical characteristics, imaging characteristics, and diagnosis between patients ≤60 and >60 years of age with convexal subarachnoid hemorrhage
DISCUSSION
To our knowledge, the current report represents the largest cohort of patients with cSAH in existing literature. Two recent smaller case series have been published on this topic; one employed a similar methodology to ours and retrospectively identified 20 cases of cSAH from a radiologic database10 whereas another9 reported on 12 cases seen at a neurology clinic. Detailed clinical descriptions were not provided in these reports. In our cohort of 389 consecutive hospitalized patients with nontraumatic SAH, we identified 29 patients with localized cSAH, accounting for 7.45% of all spontaneous SAH seen at our hospital. The prevalence is likely higher in the general population because of underascertainment of cases, making this an important group of nonaneurysmal SAH.
Our study confirms that localized collections of blood in the convexal regions of the subarachnoid space are not related to rupture of saccular aneurysms. However, unlike nonaneurysmal perimesencephalic hemorrhage, the etiology, clinical presentation, and prognosis of cSAH is more heterogeneous. We noted 2 separate patterns that included most of the 29 patients. Younger patients had clinical and imaging findings suggestive of RCVS while older patients likely had CAA. The presentation and etiology varied in others.
Younger patients in our cohort presented with severe headaches similar to an aneurysmal rupture. All such patients had conventional angiograms that excluded vascular malformations but in some showed multifocal arterial narrowing suggestive of vasospasm. It is unlikely that the small quantity of subarachnoid blood provoked arterial spasm in multiple vascular beds. Most of these patients reported improvement in their symptoms at hospital discharge and were later followed up by their primary care physicians; among those reevaluated in the neurology clinic, only 1 had a headache recurrence 4 weeks later, but a repeat angiogram showed resolution of the vasospasm. Vasculitis is an unlikely explanation for these findings in light of the temporary nature of the episodes, absence of any serologic or spinal fluid abnormalities suggestive of an underlying inflammatory disorder, and reversibility of the clinical syndrome within 3 months without immunosuppressive treatment.
The etiology of cSAH as reported in 2 previous case series is summarized in table 3. In the larger series comprising 20 patients,10 15 (75%) presented with headaches and 6 had thunderclap headaches; 8 underwent conventional angiograms, revealing vasoconstriction in 5 patients. The smaller case series9 reported headaches in 6 out of 12 patients of which 3 had thunderclap headaches; 4 underwent angiograms, demonstrating vasoconstriction in 1 and delayed filling of distal cortical arteries in 2 patients. Many of the conditions reported in these studies have also been associated with cerebral vasoconstriction.13,15 Superficial cSAH has been previously reported in patients with RCVS.6,13 In a prospective cohort of 67 patients with angiographically documented RCVS, 22% were found to have cSAH.13 Our younger patients had a lower frequency of vasoconstriction, probably because, unlike the situation in the aforementioned study, presence of documented vasospasm was not a necessary criterion for study inclusion and imaging studies were performed earlier in the clinical course, when they were less likely to demonstrate arterial narrowing.13 The pathophysiologic mechanism of cSAH in patients with RCVS is unsettled but speculated to result from abrupt changes in cerebral arterial tone.13,15
Table 3 Reported etiologies of convexal subarachnoid hemorrhage in 2 previous case series dichotomized according to age

We found that older patients with cSAH had very different presentations, as summarized in table 2. Most elderly patients had focal deficits with transient sensory symptoms, mimicking a migrainous march or motor impairment suggestive of a TIA. The absence of headache was striking. We found a high prevalence of leukoaraiosis, microbleeds, and superficial siderosis, suggestive of CAA.16–18 One patient, who did not exhibit these findings, had amyloid-beta–related angiitis on necropsy.
Focal cSAH has been described previously with CAA.11 Pathologic studies of patients with CAA show that many amyloid-laden arteries are present in the leptomeninges.19,20 Human autopsy studies have revealed that involvement of the leptomeningeal arteries occurs in early stages of CAA followed by extension of CAA to arterioles in the allocortex and the midbrain.20 Patients with suspected amyloid angiopathy in our study had cSAH as their initial clinical hemorrhagic event and 5 others had evidence of prior superficial bleeding in the form of superficial siderosis. Patients over 60 also had higher intake of antiplatelets and anticoagulants but it is unclear whether this played a contributory role or merely occurred by association due to greater vascular comorbidities in this group.
The basis of transitory symptoms in older patients with presumed CAA is unclear. Transient attacks resembling TIAs have been reported with CAA although large brain infarcts are rare.21,22 Blood in the subarachnoid space can also produce cortical spreading depression, an important pathophysiologic mechanism underlying migrainous auras.23,24 Symptoms akin to a migrainous aura were recently reported in 4 elderly patients with cSAH; all had cerebral microbleeds and/or superficial siderosis on brain MRI.25 The semiology of these events appears atypical for seizures and no epileptiform activity was detected on EEGs in our patients. One patient underwent prolonged EEG monitoring during which he had multiple episodes of right-sided sensory loss and aphasia without any EEG correlate.
The low frequency of seizures in our study contrasts with earlier case series where 20% and 58% of patients had seizures though details of seizure semiology and EEG findings were not provided.9,10 This may reflect differing case mix such as higher number of patients with PRES in these cohorts. The incidence of seizures in these reports is also much higher than observed with aneurysmal SAH,5 suggesting that they were probably provoked by the underlying disorder rather than hemorrhage itself.
An intriguing finding in our series was the presence of arterial dissection in 3 patients. Two had clinical findings consistent with RCVS and the third had a radiologic appearance of fibromuscular dysplasia. Occurrence of arterial dissection has been observed by others in a minority of cases with cSAH though a direct causal relationship has not been established.6,13,26 It is possible that edema of arterial wall and sudden surges in blood pressure predisposes to dissection.
Infective endocarditis, with or without associated mycotic aneurysms, has been reported to produce cSAH.27 We encountered only one patient with IE and cSAH who also had parenchymal microbleeds on brain MRI. Other causes for cSAH seen in our study included ITP with markedly reduced platelet counts and HELLP syndrome with postpartum angiopathy and thrombocytopenia. The clinical presentation of these patients was quite different from the others and included other systemic manifestations of an underlying disorder. Despite careful review of all imaging studies, we found no evidence of cortical vein thrombosis or vascular malformations in any of our patients. Although small arteriovenous malformations or cavernomas can result in superficial cortical hemorrhages, they are an unlikely source of isolated subarachnoid bleeding without parenchymal involvement.28 Similarly, presence of small mycotic aneurysms cannot be formally eliminated in those patients who did not undergo cerebral angiograms, though we believe that this would be rare in the absence of any associated risk factors or clinical and hematologic stigmata of IE.
These observations reflect experience from a single tertiary care center and are not free from bias. Another limitation of this study is its retrospective nature with limited follow-up information. We were, however, fortunate to have access to a wealth of detailed clinical and imaging information. The lack of systematic follow-up makes it difficult to infer accurate prognostic information. Despite these constraints, we found that cSAH carries significant risks of recurrent ICH and mortality in the elderly, in contrast to previous observations.9,10 Another potential weakness of the study is that contrast MRI and conventional angiograms were not performed in all patients and was done based on the judgment of treating physicians. It is thus possible that some small vascular malformations, mycotic aneurysms, or cortical vein thrombosis may have escaped detection. However, we believe this to be of low likelihood given that all the patients had alternative vascular imaging studies and brain MRI.
We would like to reemphasize the descriptive connotation of the term cSAH, which encompasses different pathophysiologic mechanisms and does not represent a singular clinical syndrome. Our findings should be considered preliminary and warrant further investigation.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Sandeep Kumar.
DISCLOSURE
Dr. Kumar has received honoraria for speaking activities not funded by industry; receives partial salary support from the NIH (NINDS 5UO1-NS044876-03 [Site PI]) and (NINDS 5R01-NS057127-01A [Co-I]), and receives research support from the Charles and Irene Goldman Neurology Research Fund. Dr. Goddeau reports no disclosures. Dr. Selim receives research support from the NIH (NINDS 1R01-NS 057127-01A1 [PI], NINDS 1R01-NS 045754-01A2 [Co-I], and 5R01-HL46690-14 [Co-I]). Dr. Thomas reports no disclosures. Dr. Schlaug receives research support from the NIH (NINDS 1R01NS045049 [PI], NIDCD 1RO1 DC008796 [PI], NIDCD 3R01DC008796-02S1 [PI], R01 DC009823-01 [PI], and 1R01-NS 057127 [Co-I]). Dr. Alhazzani and Dr. Searls report no disclosures. Dr. Caplan serves on scientific advisory boards for Boehringer Ingelheim, Genentech, Inc., LifeCycle Pharma A/S, ReNeuron, Novovision, Inc., NeuroLogica Corporation, and Avanir Pharmaceuticals; serves as a consultant to Micromedex, AstraZeneca, Bayer-Schering Pharma, Takeda Pharmaceutical Company Limited, CoAxia, Inc., Millennium Pharmaceuticals, Inc., Jones & Davis L.L.P., and Novo Nordisk; has served on a speakers' bureau for Otsuka Pharmaceutical Co., Ltd.; serves as Co-editor of Reviews in Neurological Diseases and on the editorial board of Archives of Neurology; and receives royalties from the publication of Blueprints in Neurology, 2nd ed. (Lippincott Williams & Wilkins, 2006), Brain Embolism (Informa Healthcare, 2006), Case Studies in Stroke: Common and Uncommon Presentations (Cambridge University Press, 2006), Intracranial Atherosclerosis (Wiley-Blackwell, 2008), Uncommon Causes of Stroke, 2nd ed. (Cambridge University Press, 2008), and Caplan's Stroke: A Clinical Approach, 4th ed. (Elsevier, 2009).
Address correspondence and reprint requests to Dr. Sandeep Kumar, Department of Neurology, Stroke Division, Beth Israel Deaconess Medical Center, Palmer 127, 330 Brookline Ave., Boston, MA 02215 skumar@bidmc.harvard.edu
Editorial, page 874
Disclosure: Author disclosures are provided at the end of the article.
Received May 19, 2009. Accepted in final form November 12, 2009.
REFERENCES
- 1.Ciccone A, Citterio A, Santilli I, Sterzi R. Subarachnoid haemorrhage treated with anticoagulants. Lancet 2000;356:1818. [DOI] [PubMed] [Google Scholar]
- 2.Shah AK. Non-aneurysmal primary subarachnoid hemorrhage in pregnancy-induced hypertension and eclampsia. Neurology 2003;61:117–120. [DOI] [PubMed] [Google Scholar]
- 3.Oshiro S, Motomura K, Fukushima T. Systemic lupus erythematosus manifesting as subarachnoid hemorrhage induced by cortical venous thrombosis and followed by medial medullary infarction. No To Shinkei 2003;55:791–795. [PubMed] [Google Scholar]
- 4.Aggarwal SK, Williams V, Levine SR, et al. Cocaine-associated intracranial hemorrhage: absence of vasculitis in 14 cases. Neurology 1996;46:1741–1743. [DOI] [PubMed] [Google Scholar]
- 5.van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain 2001;124:249–278. [DOI] [PubMed] [Google Scholar]
- 6.Singhal AB. Postpartum angiopathy with reversible posterior leukoencephalopathy. Arch Neurol 2004;61:411–416. [DOI] [PubMed] [Google Scholar]
- 7.Chang R, Friedman DP. Isolated cortical vein thrombosis presenting as subarachnoid hemorrhage: a report of three cases. AJNR Am J Neuroradiol 2004;25:1676–1679. [PMC free article] [PubMed] [Google Scholar]
- 8.Patel KC, Finelli PF. Nonaneurysmal convexity subarachnoid hemorrhage. Neurocrit Care 2006;4:229–233. [DOI] [PubMed] [Google Scholar]
- 9.Spitzer C, Mull M, Rohde V, Kosinski CM. Non-traumatic cortical subarachnoid haemorrhage: diagnostic work-up and aetiological background. Neuroradiology 2005;47:525–531. [DOI] [PubMed] [Google Scholar]
- 10.Refai D, Botros JA, Strom RG, et al. Spontaneous isolated convexity subarachnoid hemorrhage: presentation, radiological findings, differential diagnosis, and clinical course. J Neurosurg 2008;109:1034–1041. [DOI] [PubMed] [Google Scholar]
- 11.Katoh M, Yoshino M, Asaoka K, et al. A restricted subarachnoid hemorrhage in the cortical sulcus in cerebral amyloid angiopathy: could it be a warning sign? Surg Neurol 2007;68:457–460. [DOI] [PubMed] [Google Scholar]
- 12.Karabatsou K, Lecky BR, Rainov NG, et al. Cerebral amyloid angiopathy with symptomatic or occult subarachnoid haemorrhage. Eur Neurol 2007;57:103–105. [DOI] [PubMed] [Google Scholar]
- 13.Ducros A, Boukobza M, Porcher R, et al. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome: a prospective series of 67 patients. Brain 2007;130:3091–3101. [DOI] [PubMed] [Google Scholar]
- 14.Kapeller P, Barber R, Vermeulen RJ, et al. Visual rating of age-related white matter changes on magnetic resonance imaging: scale comparison, interrater agreement, and correlations with quantitative measurements. Stroke 2003;34:441–445. [DOI] [PubMed] [Google Scholar]
- 15.Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med 2007;146:34–44. [DOI] [PubMed] [Google Scholar]
- 16.Feldman HH, Maia LF, Mackenzie IR, et al. Superficial siderosis: a potential diagnostic marker of cerebral amyloid angiopathy in Alzheimer disease. Stroke 2008;39:2894–2897. [DOI] [PubMed] [Google Scholar]
- 17.Vernooij MW, Ikram MA, Hofman A, et al. Superficial siderosis in the general population. Neurology 2009;73:202–205. [DOI] [PubMed] [Google Scholar]
- 18.Smith EE, Gurol ME, Eng JA, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology 2004;63:1606–1612. [DOI] [PubMed] [Google Scholar]
- 19.Vinters HV, Gilbert JJ. Cerebral amyloid angiopathy: incidence and complications in the aging brain: II: the distribution of amyloid vascular changes. Stroke 1983;14:924–928. [DOI] [PubMed] [Google Scholar]
- 20.Thal DR, Ghebremedhin E, Orantes M, Wiestler OD. Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol 2003;62:1287–1301. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg SM, Vonsattel JPG, Stakes JW, et al. The clinical spectrum of cerebral amyloid angiopathy: presentations without lobar hemorrhage. Neurology 1993;43:2073–2079. [DOI] [PubMed] [Google Scholar]
- 22.Smith DB, Hitchcock M, Philpott PJ. Cerebral amyloid angiopathy presenting as transient ischemic attacks: case report. J Neurosurg 1985;63:963–964. [DOI] [PubMed] [Google Scholar]
- 23.Lauritzen M. Pathophysiology of the migraine aura: the spreading depression theory. Brain 1994;117:199–210. [DOI] [PubMed] [Google Scholar]
- 24.Dreier JP, Woitzik J, Fabricius M, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain 2006;129:3224–3237. [DOI] [PubMed] [Google Scholar]
- 25.Kleinig TJ, Kiley M, Thompson PD. Acute convexity subarachnoid haemorrhage: a cause of aura-like symptoms in the elderly. Cephalalgia 2008;28:658–663. [DOI] [PubMed] [Google Scholar]
- 26.Arnold M, Camus-Jacqmin M, Stapf C, et al. Postpartum cervicocephalic artery dissection. Stroke 2008;39:2377–2379. [DOI] [PubMed] [Google Scholar]
- 27.Chukwudelunzu FE, Brown RD, Jr., Wijdicks EF, Steckelberg JM. Subarachnoid haemorrhage associated with infectious endocarditis: case report and literature review. Eur J Neurol 2002;9:423–427. [DOI] [PubMed] [Google Scholar]
- 28.Aoki N. Do intracranial arteriovenous malformations cause subarachnoid hemorrhage? Review of computed tomography features of ruptured arteriovenous malformations in the acute stage. Acta Neurochir 1991;112:92–95. [DOI] [PubMed] [Google Scholar]




