Abstract
Caveolae are specialized membrane microdomains enriched in cholesterol and sphingolipids which are present in multiple cell types including cardiomyocytes. Along with the essential scaffolding protein caveolin-3, a number of different ion channels and transporters have been localized to caveolae in the heart including L-type Ca2+ channels (Cav1.2), Na+ channels (Nav1.5), pacemaker channels (HCN4), Na+/Ca2+ exchnager (NCX1) and others. Closely associated with these channels are specific macromolecular signaling complexes that provide highly localized regulation of the channels. Mutations in the caveolin-3 gene (CAV3) have been linked with the congenital long QT syndrome (LQT9), and mutations in caveolar-localized ion channels may contribute to other inherited arrhythmias. Changes in the caveolar microdomain in acquired heart disease may also lead to dysregulation and dysfunction of ion channels, altering the risk of arrhythmias in conditions such as heart failure. This review highlights the existing evidence identifying and characterizing ion channels localized to caveolae in cardiomyocytes and their role in arrhythmogenesis.
1. Introduction
The cardiac action potential is generated by the highly orchestrated activity of dozens of ion channel proteins as well as membrane transporters and exchangers. These transmembrane proteins govern the flux of ions across the sarcolemma of cardiomyocytes generating the ionic currents responsible for excitation. Abnormalities in the function or regulation of the ion channel proteins, whether acquired or inherited, underlie many different forms of arrhythmias. A critical factor for understanding the function and regulation of ion channels is their subcellular localization. The purpose of this review is to examine the contribution of ion channels localized to caveolae in normal cardiac physiology and in the genesis of arrhythmias.
2. Membrane microdomains
The cell surface membrane is a heterogeneous mixture of proteins, cholesterol, and lipids including glycero-, phospho- and sphingolipids. The sphingolipids and cholesterol laterally associate with one another within the plasma membrane and form liquid-ordered microdomains, popularly known as lipid rafts (Brown and Petersen, 1998; Jiao et al., 2008; Simons and Ikonen, 1997). These cholesterol and sphingolipid-enriched membrane microdomains are believed to coordinate multiple cellular processes including second messenger signaling and recycling of proteins to the membrane. In addition, lipid rafts have been implicated in the modulation of multiple different types of ion channel proteins. Various types of lipid rafts have been proposed based on different protein markers, morphological features, and their relative cholesterol to sphingolipid content (Echarri et al., 2007; Hanzal-Bayer and Hancock, 2007). A subset of lipid rafts present in cardiac muscle are caveolae which are morphologically distinct structures that will be the focus of this review.
2.1 Caveolae and caveolins
Caveolae were first identified in 1953 by Palade using electron microscopy to examine the endothelial cells of rat capillaries (Palade, 1953). Caveolae were named based on their morphological appearance on electron micrographs as ‘little caves.’ Typically, caveolae exhibit an invaginated flask-shaped structure of 50–100 nm in diameter which is contiguous with the surface plasmalemma; however, the shape of caveolae can also be modulated by the physiological state of the cells (Bruns and Palade, 1968a; Bruns and Palade, 1968b; Palade, 1961; Palade and Bruns, 1968; Simionescu et al., 1975). Caveolae are found in the plasma membrane in most cell types including the muscle cells, but not all cell types such as neurons which lack caveolae (Gabella, 1976; Mobley and Eisenberg, 1975; Napolitano, 1963). Different cell types possess different densities of caveolae in their plasma membrane. For example, approximately 50% of the surface plasmalemma of the adipocyte consists of caveolae (Thorn et al., 2003), while only 5% of fibroblast plasma membrane is made up of caveolae (Guillot et al., 1990). A major defining and essential feature of caveolae is the presence of caveolin proteins (Murata et al., 1995; Rothberg et al., 1992), which are scaffolding proteins that interact with cholesterol. Cholesterol is a major component of caveolae (Simionescu, 1983), and cholesterol depletion from caveolae makes them flat shaped and can reduce or eliminate caveolae from the cell (Rothberg et al., 1992). It is suggested that a critical amount of cholesterol is required for the formation and maintenance of caveolae (Chang et al., 1992); however, the exact relationship between the caveolins and cellular cholesterol is not yet clear. The oligomerization of the caveolins and interaction with cholesterol help generate the invaginated shape of these entities.
Three caveolin-encoding genes (CAV1, CAV2, and CAV3) are translated to express six known subtypes of the protein [caveolin-1α and -1β, caveolin-2α, -2β, -2γ, and caveolin-3] (Parton and Simons, 2007; Razani et al., 2002). Caveolins (18 – 20 kDa proteins) possess a unique hairpin structure with cytoplasmic N- and C-termini. Caveolin-1 (Cav-1) is expressed in most cell types including adipocytes, endothelial, epithelial, fibroblast and smooth muscle cells (Rothberg et al., 1992; Smart et al., 1999). Caveolin-3 (Cav-3) is specifically expressed in cardiac muscle as well as skeletal and smooth muscle (Song et al., 1996). Cavs-1 and -3 are expressed independently of each other and are required for the formation of caveolae, and caveolin-2 (Cav-2) associates with Cav-1 or -3 but is not involved in caveolae formation (Das et al., 1999; Li et al., 1998; Mora et al., 1999; Scherer et al., 1997).
2.2 Caveolae in cardiomyocytes
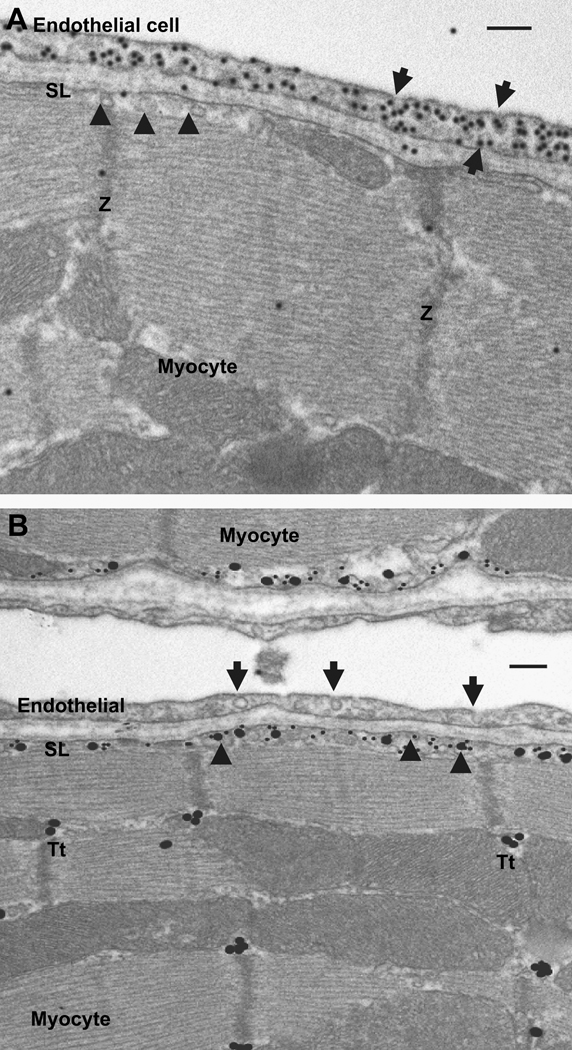
In the heart, caveolae are abundantly present in ventricular, atrial and the nodal cells. Cav-3 has been identified as an essential for formation of caveolae in cardiomyocytes based on knock-out mice studies or knock-down studies (Balijepalli et al., 2006; Galbiati et al., 2001). Expression of the other caveolin genes is variably reported in the cardiomyocytes depending on the stage of development and potential disease conditions. Cav-3 expression has been reported to be low at birth, but expression increases postnatally to reach maximum levels by day 5 followed by a decrease to expression levels seen in the adult cardiomyocytes (Ratajczak et al., 2005). Expression of Cav-3 parallels the rate of cardiac growth, which increases just after birth and attains a maximum value on postnatal day 4 (Ostadal et al., 1999). A recent report also indicates coexpression of Cav-2 and -3 in neonatal cardiac myocytes (Rybin et al., 2003) Adult cardiac myocytes predominantly express Cav-3 as homo-oligomers (Kawabe et al., 2001; Scherer et al., 1997; Tang et al., 1996). Recent studies have suggested Cav-1 is also expressed in adult cardiomyocytes (Patel et al., 2007; Robenek et al., 2008), but we failed to detect Cav-1 in adult mouse ventricular myocytes using immunogold electron microscopy (Fig. 1). Although as shown in Fig. 1, Cav-1 is robustly expressed in endothelial cells and likely other cell types in the myocardium, we could not detect evidence for expression in mouse cardiomyocytes. Studies have likewise failed to detect Cav-2 expression in rat atrial or ventricular myocytes using immunolabeling techniques (Eldstrom et al., 2006), and similarly Cav-2 was not detected in mouse ventricular myocytes in contrast to its presence neighboring endothelial and endocardial cells (Woodman et al., 2002). Similarly, others have failed to detect Cav-1 and Cav-2 in lysates made from rat ventricular lysates (Yarbrough et al., 2002).
Figure 1.
Electron microscopic images of adult mouse ventricular tissue. Panel A, immunogold staining of caveolin-1. Staining for caveolin-1 is observed only in the caveolae (arrows) of endothelial cell. In contrast the ventricular myocyte caveolae (arrow heads) had no specific caveolin-1 staining (SL, sarcolemma; Z, z line of the myofibril). Panel B, immunogold co-localization of the Cav1.2 subunit of L-type Ca2+ channel and caveolin-3. Cav1.2 (large gold particles) and caveolin-3 (small gold particle) are co-localized relative to myocyte caveolae (arrow heads) in sarcolemma (SL) and not in the endothelial caveolae (arrows). Cav1.2 staining is also noticed in the T-tubules (Tt), where Cav3 staining is absent. Scale bar, 100 nm.
A relationship of caveolae to the transverse-tubular system in cardiomyocytes has been suggested but not clearly defined. An early study hypothesized that the formation of transverse-axial tubular system appears to be derived from the repetitive generation of caveolae, which form "beaded tubules" (Forbes et al., 1984). The Cav-3 knockout mice develop a progressive cardiomyopathy characterized by myocyte hypertrophy; however, the cardiac T-tubule system has not been specifically examined (Woodman et al., 2002). On the other hand, in skeletal muscle Cav-3 transiently associates with T-tubules during development and may be involved in the early biogenesis of the T-tubule system (Carozzi et al., 2000; Lee et al., 2002; Parton et al., 1997). Additionally the Cav-3 null mice show loss of caveolae and abnormalities in the organization of the T-tubule system in the skeletal muscle tissue (Galbiati et al., 2001). Further investigation is needed to clearly establish the role of caveolins in the development and maintenance of the T-tubule system in myocardium and changes in this relationship in disease.
3. Caveolar cardiac ion channels
The subcellular localization of ion channels to caveolae allows the integration of these channels into specific macromolecular signalling complexes in a distinct lipid microenvironment. Thus, the localization of these channels provides for their precise regulation and helps define their specific contributions to cellular physiology. Several ion channels and exchangers have been localized to caveolae, and other channels have been associated with non-caveolar lipid rafts in different cells types, including cardiomyocytes. We will describe available data describing caveolar ion channels and their functional properties.
3.1 Pacemaker Channels
The hyperpolarization-activated, cyclic nucleotide-gated (HCN) family of ion channels are responsible for generating the pacemaker current (funny current, If) which contributes to phase 4 depolarization in nodal cells and hence automaticity. The HCN4 channel is the predominant isoform expressed in nodal cells of the heart and is directly activated by cAMP (DiFrancesco and Tortora, 1991; Marionneau et al., 2005; Wainger et al., 2001). A number of laboratories including ours have provided evidence that HCN4 channels are localized to caveolae based on the presence of HCN4 in low density membrane fractions along with Cav-3 as well as the specific interaction of HCN4 with Cav-3 (Barbuti et al., 2004; Scherer et al., 1997; Ye et al., 2008). Disruption of caveolae by reducing membrane cholesterol using methyl-β-cyclodextrin (MβCD) alters the gating of HCN4 channels shifting the voltage dependence of activation of the channels by approximately 10 mV in the positive direction. In addition, the β2-adrenergic receptor modulation of the HCN4 channel is lost when caveolae are disrupted using the cholesterol chelating drug MβCD consistent with a co-localization of β2-adrenergic receptors and HCN4 channels in caveolae (Barbuti et al., 2007). Thus, the function and regulation of HCN4 channels in pacemaker cells in the heart are impacted by caveolar localization.
3.2 L-type Ca2+ channels
L-type Ca2+ channels are present in all types of cardiomyocytes and provide the critical influx of Ca2+ that triggers excitation-contraction coupling. Likewise, the influx of Ca2+ through these channels may impact other Ca2+-dependent signaling pathways including gene regulation. Inward current through L-type Ca2+ channels contributes to the plateau phase of the ventricular action potential and also is the major contributor to the upstroke of the action potential in nodal cells. L-type Ca channels are multi-subunit complexes composed of a pore forming α1 subunit and auxiliary subunits. Multiple genes encode α1 subunits, and in the heart CACNA1C and CACNA1D genes are expressed. The CACNA1C endcoded Cav1.2 channel being the predominant isoform in working myocardium.
The first suggestion that caveolae may be important in Ca2+ signaling in cardiomyocytes came from a study in which depletion of caveolae by cholesterol chelation using MβCD resulted in a dose-dependent decrease in the frequency, amplitude and size of Ca2+ sparks (Lohn et al., 2000). These Ca2+ sparks reflect the primary Ca2+ release events in excitation-contraction coupling and require the highly localized crosstalk between sarcolemmal L-type Ca2+ channels and sarcoplasmic reticulum-localized ryanodine receptors. Because MβCD treatment did not directly impact the density or gating properties of L-type Ca2+ currents, the authors concluded that the localization of L-type Ca2+ channels to caveolae was essential for appropriate coupling to ryanodine receptors in the neonatal rat ventricular cardiomyocytes studied (Lohn et al., 2000). However, this study did not directly demonstrate that L-type Ca2+ channels are present in caveolae, and an alternative interpretation of the results could be that caveolae are essentially modulating sarcoplasmic reticulum function and localization independent of effects on L-type Ca2+ channels.
More recently, it has been demonstrated using a variety of techniques, including immunogold electron microscopy, that a subpopulation of Cav1.2 L-type Ca2+ channels are localized to caveolae in ventricular cardiomyocytes (Balijepalli et al., 2006; Shibata et al., 2006). Figure 1B shows an immunogold labeled electron micrograph of adult mouse ventricular myocardium demonstrating a colocalization of immunogold labeled Cav-3 and Cav1.2 along in surface sarcolemmal caveolae. In addition, immunoprecipitation studies have demonstrated that Cav-3 associates specifically with Cav1.2 channels and an associated macromolecular signaling complex composed of various molecules in the β2-adrenergic/AC/PKA signaling pathway. Although disruption of caveolae in neonatal mouse ventricular myocytes using either MβCD or siRNA did not alter the ionic currents carried by L-type Ca2+ channels, it did disrupt β2-adrenergic regulation of the channels (Balijepalli et al., 2006). Furthermore, studies focused on the regulation of L-type Ca2+ channels have provided supportive evidence that channels are co-localized with critical regulatory molecules in caveolae as will be discussed in detail later (Barouch et al., 2002; Wang et al., 2005; Warrier et al., 2007). Thus, there is strong evidence that a subpopulation of L-type Ca2+ channels are present in caveolae which is essential for their precise regulation, but questions remain as to the specific role of these channels in cardiac physiology and disease.
3.3 K+ channels
Multiple different types of K+ channels are expressed in cardiomyocytes each with distinct subunit composition and gating properties which uniquely contribute to cardiac excitability. Cardiac K+ channels are divided into three main classes based on their subunit composition and functional properties: 1) voltage gated (Kv) responsible for Ito, IKur, IKr, and IKs; 2) inward rectifiers (Kir) responsible for IK1, IKACh, and IKATP, and 3) background K+ currents (TASK-1, TWIK1/2). There is significant variability in the expression of K+ channels in myocytes from different chambers of the heart as well as transmurally across the wall of the myocardium. In general the K+ channels are key components for repolarization of the myocardium during the action potential and contribute to the unique shape of the cardiac AP in different myocytes cell types. Specific subcellular localization and targeting to lipid raft domains has been investigated for a subset of these cardiac K+ channels. Some K+ channels have been localized to noncaveolar lipid rafts (Kv11.1 and Kir2.1) while other K+ channels (Kv1.5 and Kir6.2) have been localized specifically to caveolae.
The voltage gated Kv11.1 (hERG) channel is expressed in all types of cardiomyocytes throughout the heart and is responsible for the rapid component of the delayed rectifier current, IKr. Membrane fractionation studies using canine ventricular myocytes have demonstrated that Kv11.1 channels are present in cholesterol and sphingolipid-enriched membrane fractions, but these channels are not associated with Cav-3 based on a lack of co-immunoprecipitation (Balijepalli et al., 2007). Thus, Kv11.1 channels are present in lipid raft domains in myocytes but not specifically present in caveolae. Nevertheless, alterations of sarcolemmal cholesterol levels using MβCD clearly modulate the gating of Kv11.1 channels suggesting that the channels are sensitive to the lipid composition of the membrane microdomain where they are present in cardiomyocytes (Balijepalli et al., 2007).
Inward rectifier K+ channels are encoded by the Kir2.x family of genes and the IK1 maintains the resting membrane potential and contributes to terminal repolarization. There is evidence that the membrane cholesterol content alters plasma membrane expression and the Kir2.1 current densities; however, these channels are not localized to caveolar lipid rafts based on a lack of interaction with Cav-3 (McEwen et al., 2008; Romanenko et al., 2002).
3.3.1 Kv1.5
The Kv1.5 K+ channel is expressed predominantly in the atrial myocardium and is responsible for the ultrarapid component of the delayed rectifier K+ current, IKur. The function of this channel is regulated by multiple different proteins including auxiliary Kvβ subunits, protein kinases such as protein kinase A and MAGUKs such as SAP97 (Godreau et al., 2003). Studies in mouse L cell fibroblasts transfected with Kv1.5 first suggested that the Kv1.5 channel can preferentially localize to caveolae (Martens et al., 2001). Subsequent studies provided evidence that in cardiac muscle, Kv1.5 was present in caveolae based on membrane fractionation and immunogold electron microscopy of membrane sheets from rat ventricular myocytes (Shibata et al., 2006). Another study reported that Cav-3 and SAP97, a MAGUK protein, interact and recruit Kv1.5 into caveolae to form a tripartite complex that selectively regulates the expression of currents encoded by a glycosylation-deficient Kv1.5 mutant channel (Folco et al., 2004). More recently McEwen et al. (McEwen et al., 2008) demonstrated Kv1.5 associates with Cav-3, and this association is proposed to recruit Kv1.5 channels from non-lipid raft regions of the membrane to caveolae and in so doing modifying their gating. Furthermore, the association with Cav-3 has been suggested to involve the amino terminus of Kv1.5 based on two independent studies (Folco et al., 2004; McEwen et al., 2008).
Despite a significant body of literature from primarily heterologous expression systems demonstrating an association of Kv1.5 channels and Cav-3 in caveolae, there is controversy regarding the localization of these Kv1.5 channels to caveolae in cardiac muscle. Careful studies have provided evidence that Kv1.5 channels are not preferentially localized to caveolae in cardiomyocytes utilizing a variety of biochemical and molecular imaging techniques. A study using canine and rat atrial and ventricular myocardium argued that Kv1.5 is not localized to lipid rafts and does not co-immunoprecipitate with Cav-3 nor co-localize with Cav-3 either with confocal microscopy or immunogold electron microscopy (Eldstrom et al., 2006). Another study using rat atrial myocytes provided evidence that Kv1.5 channels can be localized to lipid rafts with their gating modulated by membrane cholesterol levels, but the channels did not significantly co-localize with Cav-3 (Abi-Char et al., 2007). In myocytes, Kv1.5 appeared to be localized in the greatest concentration to intercalated disc regions of the myocytes independent of caveolae as well as some cell surface localization (Abi-Char et al., 2007; Eldstrom et al., 2006) Nevertheless, questions remain regarding the localization of the Kv1.5 channel to caveolae in the cardiomyocytes given issues related to antibody specificity, accessibility of epitopes with different fixation procedures (Bush et al., 2006), and potentially the dynamic nature of Kv1.5 localization.
3.3.2 Kv7.1
The KCNQ1 gene encodes the pore forming subunit of the K+ channel Kv7.1 or KvLQT1, which is responsible for the slow component of the delayed rectifier current, IKs. Membrane fractionation studies of cardiac muscle have indicated that Kv7.1 localizes to low-density membrane fractions together with Cav-3. This suggests that these channels are localized to lipid rafts and possibly caveolae (Balijepalli et al., 2007; Nakamura et al., 2007). However, further studies are needed to support the localization of Kv7.1 channels to caveolae such as co-immunoprecipitation with Cav-3.
3.3.3 KATP
In the adult heart, the ATP-sensitive K+ channels, KATP consist of Kir6.2 and the sulfonylurea receptor 2 (SUR2) subunits. IK,ATP, responds to the metabolic state of the cell and plays a role in shortening of the action potential during ischemia (Zingman et al., 2007)(. Additionally, these channels are integral to the protective response of the myocardium in ischemic preconditioning (Patel et al., 2005). Data from studies of vascular smooth muscle have shown that the related Kir6.1 vascular K(ATP) channel is regulated by caveolae and associates with Cav-3 as well as Cav-1 (Jiao et al., 2008; Sampson et al., 2004). Limited data are available describing the localization of KATP channels in the cardiac sarcolemma, but a preliminary study has provided evidence that Kir6.2/SUR2A channels associate with Cav-3 in rat cardiomyocytes (Garg et al., 2008).
3.4 Sodium Channels
The voltage-gated Na+ channel (Nav1.5 encoded by SCN5A) is responsible for the initial upstroke of the action potential in atrial and ventricular myocytes and contributes to propagation of electrical impulses in the heart. The importance of this channel in cardiac excitability has been illustrated by the reports of various mutations in SCN5A that are linked to cardiac arrhythmia syndromes such as conduction block, congenital long QT syndrome, Brugada syndrome and sudden cardiac death (Lehnart et al., 2007). The cardiac Nav1.5 channel was one of the first ion channels to be detected in caveolae in the heart (Yarbrough et al., 2002). The localization of a pool of Nav1.5 channels to caveolar membranes is particularly important for the βAR regulation of the channels. βAR stimulation of the cardiac Nav1.5 channels occurs by both PKA-dependent phosphorylation of Nav1.5 channels which typically results in increases in both current amplitude and the rate of current decay (Frohnwieser et al., 1997; Matsuda et al., 1992), and by direct Gαs modulation of the channel increasing current density (Lu et al., 1999). Based Gαs-mediated increase in INa in ventricular myocytes results from the presentation of a pool of caveolar Nav1.5 channels to the surface membrane (Shibata et al., 2006; Yarbrough et al., 2002). This recruitment of Nav1.5 channels and caveolae to the continuity of the sarcolemma significantly increases the amplitude of INa which reaches steady state in less than five minutes (Shibata et al., 2006). The increase in INa is readily reversible with removal of βAR stimulation. Further studies have suggested that a functional interaction between Gαs and Cav-3 is responsible for the PKA-independent enhancement of INa (Palygin et al., 2008).
3.5 Na+ / Ca2+ exchanger
The cardiac Na+/Ca2+ exchanger (NCX) is an important Ca2+ signaling protein involved primarily in extrusion cytosolic Ca2+, and NCX1 is the predominant isoform expressed in heart. NCX1 regulates cardiac excitation-contraction coupling by its major role in the decay phase of the intracellular Ca2+ transient and by modulating SR Ca2+ load and release (Bers et al., 1989; Goldhaber et al., 1999; Leblanc and Hume, 1990; Litwin et al., 1998; Vites and Wasserstrom, 1996). The NCX is an electrogenic exchanger with a stoichiometry of 3 Na+:1 Ca2+, and thus NCX1 contributes to the ionic currents responsible for the action potential and under pathological conditions to the genesis of delay after depolarizations and resulting arrhythmias (Fozzard, 1992). An initial study reported that NCX1 has several caveolin binding motifs and that the NCX1 protein associates with Cav-3 (Bossuyt et al., 2002). Another study has provided evidence that annexin A5 directly interacts with NCX1 as part of multimolecular complexes including Cav-3 localized to caveolae in human ventricular myocytes (Camors et al., 2006). However, another study was unable to detect co-immunoprecipitation of NCX1 and Cav-3 from rat ventricular myocardium, nor was significant co-localization of NCX1 and Cav-3 detected in immunolabeled rat ventricular myocytes (Cavalli et al., 2007). Further studies are needed to clarify the localization of NCX1 to caveolae and the functional implication of this localization. Given that NCX protein density and function are known to be upregulated in pathological conditions such as cardiac hypertrophy and heart failure, defining the role of NCX1 and its potential caveolar localization will be important for understanding the pathophysiological processes including arrhythmias such as those associated with heart failure.
4. Regulation of caveolar ion channels
Caveolar localization of ion channels can regulate their functional properties in at least three distinct ways. First, the unique lipid environment of caveolae rich in sphingolipids and cholesterol can directly impact the biophysical properties of some ion channels. Second, caveolae provide localized macromolecular complexes that enable various signalling pathways to specifically regulate ion channels in a highly localized fashion. Third, caveolae can regulate the availability of ion channels by controlling their trafficking and thus access to the extracellular environment.
4.1 Lipid microenvironment regulation
Caveolae have a distinct lipid composition including cholesterol, sphingolipids (sphingomyelin and glycosphingolipids), and phosphatidylinositols (Liu et al., 1997), and this unique caveolar lipid microenvironment can significantly influence the functional properties of ion channels. In addition, the lipid microenvironment is dynamic and lipid-based signaling molecules can also directly impact channel function.
4.1.1 Ceramide regulation of K+ channels
Depending on the cell type, caveolae may contain up to 95% of total cell sphingomyelin with the key enzyme, sphingomyelinase, responsible for sphingomyelin regulation also localized to caveolae microdomains (Bilderback et al., 1997; Liu and Anderson, 1995). Sphingomyelinase hydrolyzes sphingomyelin to form ceramide, a second messenger that stimulates multiple signaling cascades (Kolesnick and Kronke, 1998; Levade and Jaffrezou, 1999). A recent finding demonstrated that sphingomyelinase-D treatment resulted in activation of the Kv2.1 channels at negative potentials at which the channels usually remain deactivated (Ramu et al., 2006). However, such regulation has not yet been clearly described for caveolar localized channels.
4.1.2 Polyunsaturated fatty acids regulation of Na+ and Ca2+ channels
Another class of important lipids relevant to cardiac arrhythmias are the polyunsaturated fatty acids (PUFAs) that can regulate the properties of the ion channels. It is known that PUFAs are components of caveolae and can profoundly alter the biochemical makeup of cell membrane lipid rafts/caveolae microdomains (Darios and Davletov, 2006; Ma et al., 2004; Seo et al., 2006). In cardiac myocytes, PUFAs have caused a reduction in cellular excitability and shortening of the action potential by altering the gating properties of Na+ channels and reducion in INa (Kang et al., 1995; Xiao et al., 1995). Similarly, the PUFAs directly modulate ICa,L, and reduce the Ca2+-sparks and Ca2+ transients and Ca2+ -activated membrane current (Xiao et al., 1997). Thus, given these effects on cardiac ion channels, PUFAs have been suggested to exhibit antiarrhythmic properties (Xiao et al., 2005).
4.1.3 PIP2 regulation of channels
Another family of lipids, the phosphoinositides, reside within the intracellular leaflet of the plasmamembrane, interact with membrane proteins and have prominent roles in signaling (McLaughlin and Murray, 2005). Phosphatidylinositol 4,5-bisphosphate (PIP2) is a negatively charged membrane phospholipid present in caveolae and capable of regulating multiple different ion channels and transporters. In addition, PIP2 is the precursor for the important second messengers inositol 1,4,5-trisphosphate (IP3) and sn-1,2-diacylglycerol (DAG) which are involved in multiple signaling cascades ranging from receptor activation, activation of protein kinases, ion channel/exchanger activity to changes in intracellular Ca2+ levels (Woodcock and Matkovich, 2005). Activation of phospholipase C (PLC) leads to the rapid breakdown of PIP2 to IP3 and DAG, and all of these lipid second messengers have the ability to directly or indirectly influence cardiomyocyte electrical activity and can contribute to cardiac arrhythmogenesis (Woodcock et al., 2008).
PIP2 levels in the surface membrane regulate the function of a variety of different ion channels and transporters (Suh and Hille, 2005). The first characterized transmembrane proteins directly regulated by PIP2 were the Na/Ca exchanger and the KATP channel (Kir6.2/SUR2) (Fan and Makielski, 1997; Hilgemann and Ball, 1996), and both of these proteins are likely present in cardiac caveolae. Subsequent studies have demonstrated that multiple member of the Kir family of channels including Kir2, Kir3, and Kir6 are regulated by PIP2 levels. In general, increased levels of PIP2 activate Kir channels and decreased levels lead to lower levels of activity (Suh and Hille, 2005). The importance of PIP2 regulation of cardiac Kir2.1 channels in cardiac excitability was highlighted by the finding that an Andersen-Tawil syndrome mutation which alters the ability of PIP2 to regulate the channel can lead to ventricular arrhythmias (Ma et al., 2007). In case of cardiac Kir6.2 channels which respond to the metabolic state of the cell, PIP2 binding to the channels reduces their affinity for ATP, apparently enabling the channels to respond to metabolic stress (Haider et al., 2007). Finally, it is important to appreciate that PIP2 levels can be rapidly modulated specifically in caveolae in response to neurohormonal stimulation such as α1-adrenergic stimulation in cardiomyocytes (Morris et al., 2006).
The diffusible phosphoinositide IP3 generated by PLC hydrolysis of PIP2 in caveolae may specifically regulate nearby IP3-receptors on the sarcoplasmic reticulum. Co-localization of extrajunctional RyR2 receptors and caveolae has been demonstrated in adult rat ventricular myocytes making such localized signaling possible (Scriven et al., 2005). Predominantly type 2 IP3-receptors are present in atrial and ventricular myocytes (Lipp et al., 2000), and IP3 binding triggers the release of intracellular Ca2+ stores, generating Ca2+ signals that subsequently activate protein kinase cascades and contribute to Ca2+-induced Ca2+ release (CICR) mechanisms influencing inotropy and arrhythmias (Domeier et al., 2008; Proven et al., 2006; Zima and Blatter, 2004). Thus, caveolar microdomains may provide a unique spatial microenvironment conducive for lipid-mediated signaling which both directly modulate ion channels in caveolae as well as providing localized signals that regulate nearby RyRs and hence Ca2+ signaling which may feedback on other caveolar signaling pathways and ion channels.
4.2 Macromolecular signalling complexes
Caveolins can serve as scaffolding proteins organizing macromolecular complexes to enable highly localized and efficient signalling. A spectrum of molecules involved in cellular signalling cascades can be found localized to caveolae including the upstream transmembrane receptors, intermediates such as G-proteins and kinases, and many targets of the signalling such as ion channels. Signalling molecules that have been localized to caveolae include G-protein coupled receptors (GPCRs), heterotrimeric G-proteins, steroid hormone receptors, receptor tyrosine kinases, nonreceptor tyrosine kinases, protein kinases A and C, phosphatidylinositol-3-kinase/protein kinase B, MAP Kinase (p42/44 MAPK), and a range of other downstream signalling molecules (Patel et al., 2008; Steinberg, 2004). A number of different GPCRs responsible for regulation of cardiac function have been detected in caveolae including β1-, β2-, and α1-adrenergic, muscarinic cholinergic (M2 and M4), and adenosine (A1) receptors (Patel et al., 2008; Steinberg, 2004). In addition, downstream molecules including Gαs, Gαi, Gαq, Gβγ adenylyl cyclase types 5 and 6, some phospholipase C isoforms, and NOS3 have been detected in caveolae in cardiomyocytes (Patel et al., 2008; Steinberg, 2004). Thus, caveolae provide an important domain where sympathetic and parasympathetic inputs to the heart are integrated to precisely regulate cardiac function.
What enables the formation of specific macromolecular signaling complexes in caveolae? There are likely multiple interacting parts that come together to form these complexes. Cav-3 itself has a scaffolding domain which as previously described is important for binding with interacting proteins. Binding to the caveolin scaffolding domain by effector enzymes (e.g., NOS3, ACVI) has been proposed to maintain these proteins in an inactive state, and this provides a ready pool of effector molecules available for rapid activation with the appropriate stimulation (Cohen et al., 2004; Okamoto et al., 1998). Additionally, other scaffolding proteins such as membrane associated guanylate kinase (MAGUK) proteins may also contribute to formation of these complexes. For example, a MAGUK protein, SAP97, has been suggested to be instrumental in the localization of Kv1.5 to caveolae (Folco et al., 2004). Furthermore, post-translational modification of signalling proteins such as palmitoylation or myristoylation may contribute to caveolar compartmentalization (Grubb et al., 2008; Resh, 2004; Robbins et al., 1995). The unique lipid environment of the caveolae may also contribute to localization of proteins to caveolae such as binding affinity for PIP2 as PLC exhibits Additionally, it has been suggested that the phosphatidyl serine-binding annexin, A5, may help localize NCX1 to caveolae (Camors et al., 2006). Ultimately, the formation of these macromolecular signaling complexes involves a wide variety of different interacting partners which may also vary in a dynamic fashion in response to stimulation.
L-type Ca2+ channels in cardiomyocytes have been extensively studied for their regulation by caveolar signaling pathways. The prominent β-AR regulation of L-type Ca2+ channels involves the cAMP/PKA signaling cascade and contributes to the response of the myocardium to sympathetic stimulation. Localized regulation of L-type Ca2+ channels by β-AR stimulation was suggested by early studies in rat and frog ventricular myocytes (Jurevicius and Fischmeister, 1996; Zhou et al., 1997). It was postulated that β2-AR regulation of ICa,L was highly localized in the rat ventricular myocyte in contrast to the more diffuse β1-AR regulation (Zhou et al., 1997), and this was supported by cell-attached single channel studies (Chen-Izu et al., 2000). A role for localized pools of cAMP in the regulation of ICa,L has been demonstrated in guinea pig ventricular myocytes studied using FRET-based biosensors for cAMP (Warrier et al., 2007). Recent studies have put structure with these functional studies suggesting that localized β2-AR regulation involves caveolar L-type Ca2+ channels by identifying a caveolar macromolecular signaling complex including β2-AR, Gαs, Gαi, AC, PKA, PP2a, and Cav1.2 in mouse ventricular myocytes (Balijepalli et al., 2006). Furthermore, disruption of caveolae in neonatal mouse myocytes using siRNA for Cav-3 or MβCD eliminates β2-AR but not β1-AR stimulation of ICa,L (Balijepalli et al., 2006). However, a study of adult rat ventricular myocytes showed that disruption of caveolae with MβCD resulted in a significant enhancement the β2-AR stimulation of the ICa,L without affecting the β1-AR stimulation of ICa,L (Calaghan and White, 2006). A subsequent study by the same group revealed that β2-AR- and cAMP-mediated regulation was highly localized under control conditions, but disruption of caveolae with MβCD treatment resulted in a more global β2-AR stimulated cAMP regulation, that is, loss of compartmentalization (Calaghan et al., 2008). These studies are consistent with a highly localized β2-AR/AC/PKA cascade regulating Cav1.2 channels in caveolae in ventricular myocytes, but there are differences in β2-AR signaling compartmentalization in caveolae between neonatal and adult cardiomyocytes and across species.
Caveolae can also serve as a site for integration of cellular signaling pathways as they regulate various ion channels. Building on the example of the β-AR/AC/PKA regulation of L-type Ca2+ channels described above, another prominent caveolar localized enzyme that can impact this regulation is NOS3 (Feron et al., 1998). NOS3 has specifically been implicated in blunting β-adrenergic stimulation of contractility by reducing β-AR stimulation of ICa,L in caveolae in mouse ventricular myocytes (Barouch et al., 2002; Massion et al., 2004). Additionally, caveolar NOS3 has been implicated in the α1-adrenergic-mediated regulation of ICa,L in cat atrial myocytes (Wang et al., 2005). In this case α1-adrenergic receptor activates the PLC/IP3/IP3R/Ca2+ release pathway which leads to the activation of NOS3. The NO produced then activates guanylate cyclase to produce cGMP which acts to inhibit phosphodiesterase III leading to increased cAMP/PKA stimulating L-type Ca2+ channels. Thus the impact of NOS3 signaling on L-type Ca2+ channel activity likely depends on the other signaling pathways concurrently activated and likely the cardiac cell type studied.
The ability of caveolae to coordinate signaling is not only related to the ability of various signaling pathways to crosstalk, but also a given signaling pathway can impact multiple targets to produce an appropriate cellular response. An example of such coordinated regulation of ion channels is provided by the recent demonstration that progesterone acting via nongenomic pathways through the PI3K/Akt pathway activates NOS3, and the resulting NO release can both increase basal IKs and concurrently blunt β-AR stimulated ICa,L (Nakamura et al., 2007). The combination of these effects helps control cardiac repolarization preventing excessive prolongation of the action potential duration which is associated with a risk of the ventricular arrhythmia torsades de pointes. These cellular findings correlate nicely with clinical studies showing a protective effect of the female hormone progesterone on LQTS-associated arrhythmias (Nakagawa et al., 2006; Rodriguez et al., 2001; Seth et al., 2007). These progesterone-mediated effects are consistent with the findings of different levels of nitrosylation of the L-type Ca2+ channels (see Murphy work)
4.3 Regulated caveolae dynamics
Regulation of the formation of caveolae and their association with the surface sarcolemma is not well understood. Subplasmalemmal pools of caveolae have been identified in some cell types, which may be able to be dynamically recruited to the surface membrane (Pelkmans and Zerial, 2005). Thus, the density of ion channels at the surface sarcolemma could rapidly be altered by the addition or subtraction of caveolae containing defined subsets of ion channels. Such a mechanism of regulation has been described for the cardiac Nav1.5 channel in response to β-AR stimulation (Yarbrough et al., 2002). In this case, it was proposed that a PKA-independent direct Gαs interaction with Cav-3 promotes the presentation of Nav1.5 containing caveolae to the surface membrane (Palygin et al., 2008; Shibata et al., 2006). Further investigation is required to understand this mechanism of regulation of channel density and to determine if other channels in caveolae are subject to this type of dynamic regulation in response to neurohormonal regulation.
5. Caveolae and arrhythmias
Given that a variety of different ion channels are localized to caveolae, it is likely that caveolar ion channels can contribute to the genesis of arrhythmias. Both inherited arrhythmia syndromes and acquired arrhythmias in conditions such as heart failure can theoretically involve dysregulation or alterations in the function of caveolar ion channels. The extent to which caveolar ion channels contribute to various arrhythmia syndromes is only beginning to be investigated.
5.1 Inherited arrhythmias
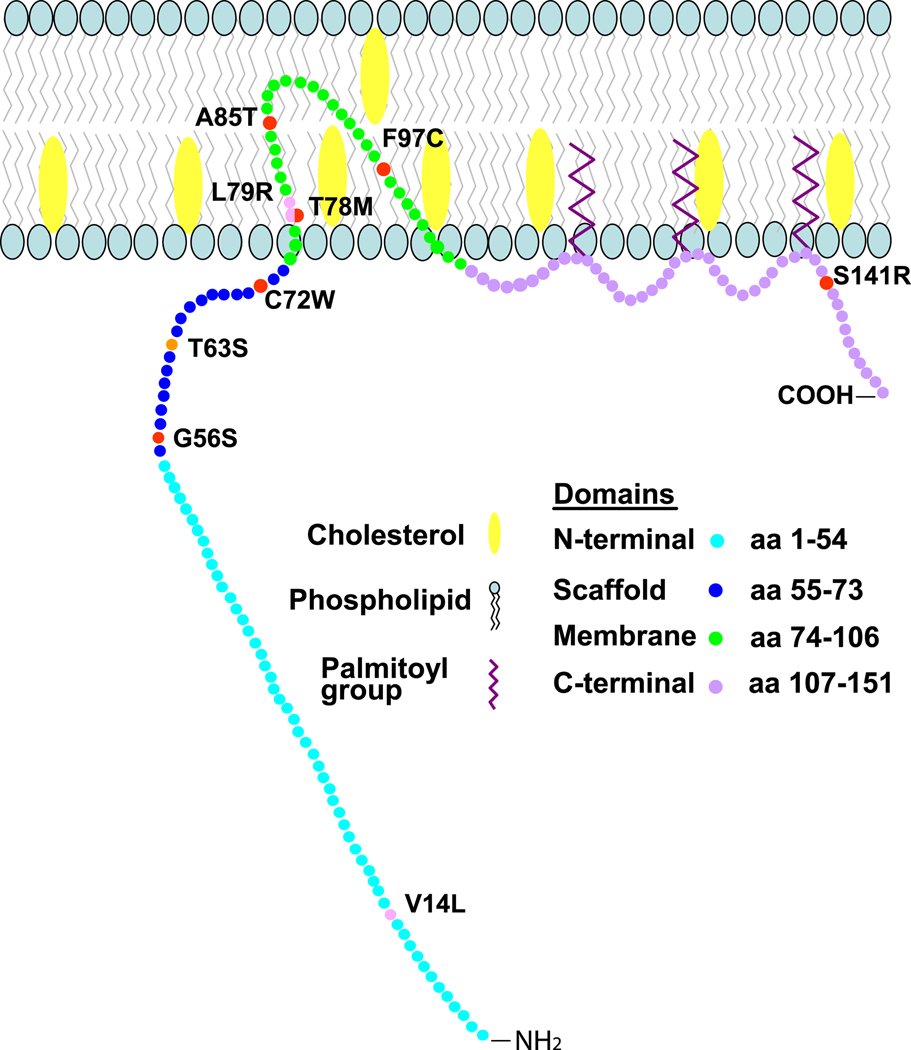
Inherited arrhythmia syndromes are caused by mutations in a rich variety of ion channels and associated proteins (Lehnart et al., 2007), and some of these diseases involve channels which are present in caveolae. For example, Nav1.5 channels are known to have a presence in caveolae, and various mutations in Nav1.5 have been implicated in type 3 long QT syndrome, Brugada syndrome, idiopathic ventricular fibrillation, sick sinus syndrome (Lehnart et al., 2007). However, the studies characterizing genetic mutations responsible for inherited arrhythmias have typically not evaluated the impact of the mutation relative to caveolae. One report has directly implicated caveolae in an inherited arrhythmia syndrome by linking mutations in CAV3 (see Figure 3) with the congenital long QT syndrome type 9 (LQT9) (Vatta et al., 2006). When the LQT9 CAV3 mutations were co-expressed with the cardiac Nav1.5 sodium channel, an increase in late INa (gain of function effect) was observed (Vatta et al., 2006). The increase in late INa is predicted to delay cardiac repolarization and thus generate the long QT phenotype in a fashion similar to that previously described for gain of function mutations of Nav1.5 responsible for the type 3 long QT syndrome (George et al., 1995; Wang et al., 1995a; Wang et al., 1995b). These observations are consistent with the reported association of Nav1.5 channels and Cav-3 (Vatta et al., 2006; Yarbrough et al., 2002). The finding that mutations in CAV3 can be proarrhythmic is further supported by findings identifying additional CAV3 mutations (see Fig. 2) in a cohort of sudden infant death syndrome (SIDS) post-mortem samples (Cronk et al., 2007). These SIDS CAV3 mutations also induced an increase in late Na current like the LQT9 CAV3 mutations. The impact of the LQT9 and SIDS CAV3 mutations on the function of other caveolar ion channels besides Nav1.5 has not yet been defined and could provide further insight into the pathophysiology. The only other CAV3 mutation reported with a cardiac phenotype is T63S CAV3 mutation found in siblings with hypertrophic cardiomyopathy in a Japanese family. The father died prematurely of sudden cardiac death, but no genotype was available (Hayashi et al., 2004).
Figure 2.
Schematic of CAV3 mutations associated with cardiac phenotype genetic diseases. Shown are locations of Cav-3 mutations identified in of Long QT syndrome type 9 (LQT9; red), sudden infant death syndrome (SIDS; pink) and hypertrophic cardiomyopathy (orange) patients. Mutation T78M in the membrane insertion region of the Cav3 was found to be associated with cases of both LQT9 and SIDS.
A wide variety of other mutations in CAV3 have been described which are causative of skeletal myopathies including limb girdle muscular dystrophy, type 1C (LGMD-1C), in some cases of rippling muscle disease, distal myopathy, and idiopathic hyper-CKemia (Woodman et al., 2004). However, cardiac pathology is not typically observed for these CAV3 mutations, and no propensity to arrhythmias has clearly been described. This suggests that the caveolae and compensatory mechanisms adapting to Cav-3 mutations are quite different in cardiac and skeletal muscle, but the basis for this difference is unknown.
5.2 Acquired arrhythmias
Many different kinds of arrhythmias result from acquired heart disease. In these acquired arrhythmias, cardiomyocytes may exhibit changes in the abundance or composition of caveolae, but few studies have directly examined for such alterations. In a canine tachycardia-induced cardiomyopathy model, there is an increase in Cav-3 protein levels and in morphological caveolae which is associated with increased NO production (Hare et al., 2000). Other studies have identified changes in caveolar NOS3 in hypertrophy and post-myocardial infarction (Piech et al., 2003; Ratajczak et al., 2003). A potential role of alterations in NOS3 in the generation of arrhythmias was suggested by genetic studies in mice. NOS3−/− mice exhibit a higher incidence of arrhythmias in a cell culture model in the presence of ouabain which was attributed to delayed afterdepolarizations (Kubota et al., 2000), and this was supported by in vivo mouse electrophysiology studies showing a higher incidence of inducible ventricular tachycardia in NOS3−/− mice (Rakhit et al., 2001). Likewise, isolated myocytes from NOS3−/− mice exposed to β-AR stimulation with isoproterenol exhibited an increased incidence of early afterdepolarizations and aftercontractions (Wang et al., 2008). Overexpression of NOS3 in transgenic mice conversely leads to a lower incidence of spontaneous arrhythmias in a neonatal ventricular myocyte culture model (Massion et al., 2004). The underlying mechanism by which NOS3 regulates these effects on arrhythmogenesis likely involves alterations in β-AR stimulation of cardiac excitability specifically in the caveolar microdomain (Barouch et al., 2002). Specifically, NO produced by NOS3 is hypothesized to act to blunt β-AR stimulation of currents through L-type Ca2+ channels (Barouch et al., 2002; Wahler and Dollinger, 1995; Wang et al., 2008). Reduction or elimination of the NOS3-produced NO brake on β-AR stimulation of ICa,L leads to greater influx of Ca2+ which can contribute two potent arrhythmia triggers: early afterdepolarizations and delayed afterdepolarizations (Fozzard, 1992; January and Riddle, 1989). This is consistent with data localizing the relevant molecules to caveolae including L-type Ca2+ channels, NOS3, β2-AR, and multiple components of the cAMP/PKA signaling cascade (Balijepalli et al., 2006; Feron et al., 1998; Head et al., 2005; Xiang et al., 2002). In addition, Ca2+/Calmodulin-mediated NOS3 regulation of IKs has also been described which importantly contributes to action potential durations and the associated risk of arrhythmias (Bai et al., 2005).
6. Conclusions and future directions
Although caveolae were morphologically identified by electron microscopy more than fifty years ago as defined invaginations of the surface membrane, it is only in the past 15 years that their composition and functional significance have begun to be understood. Caveolae are distinct in their membrane lipid composition, being highly enriched in cholesterol and sphingolipids, and caveolins are signature proteins essential for the formation of caveolae. Specifically Cav-3 is expressed in muscle cells including the cardiomyocytes. A subset of cardiac ion channels have been proposed to reside in caveolae in cardiomyocytes including HCN4, Cav1.2 Kv1.5 channels, Kir6.2/Sur2a channels, Nav1.5, and NCX. These caveolar ion channels are regulated by a variety of signaling pathways in a highly localized fashion in part due to the localization of multiple components of specific signaling pathways to the caveolar microdomain. Dysfunction of caveolar ion channels or their regulation have been implicated in the genesis of inherited arrhythmias such as LQT3 and LQT9 and potentially in acquired arrhythmias in conditions such as heart failure. However, understanding the composition and functional roles of caveolae in the heart as well as their contribution to arrhythmia syndromes is only beginning. Many critical questions remain to be answered.
Limitations in the techniques used to determine whether channel proteins are present in caveolae remain and need to be carefully considered. Early studies oftentimes identified proteins as localized to caveolae based on biochemical membrane fractionation studies revealing a co-distribution of the protein with Cav-3 in low density fraction sucrose gradients. Although this is supportive evidence, it is possible that these proteins reside in noncaveolar lipid rafts or are nonspecifically present in these membrane fractions. Co-immunoprecipitation studies demonstrating that proteins associate with Cav-3 are commonly employed, but these studies share the limitations of all immunoprecipitation studies of possible nonspecific associations and require careful control studies. Imaging approaches are useful, but the spatial resolution of standard light microscopy including confocal microscopy cannot definitively localize proteins to the small 50–100 nm caveolae. Electron microscopy studies can provide spatially defined localization of proteins to caveolae, but these studies are technically challenging and limited by the available specific antibodies that can be used. Approaches to disrupt caveolae using MβCD and evaluating the impact on channel localization can be helpful, but as a nonspecific chelator of cholesterol, MβCD can exert a wide range of effects in addition to disrupting caveolae which can confuse data interpretation. Reducing or eliminating Cav-3 expression is another technique using transgenic technology or siRNA approaches which may offer a more specific manner to disrupt caveolae. The most definitive studies have used a variety of techniques to demonstrate caveolar localization of the ion channels or signaling molecules to caveolae. It is likely that as additional research is performed, the list of caveolar proteins will continue to be refined.
Even more challenging than localizing a protein to caveolae, is isolating the functional properties of that protein in the caveolar microdomain in the intact cell. In the case of ion channels, determining the ionic current properties specifically of caveolar-localized channels is difficult. The loss of function studies using MβCD have the limitations previously described in specificity for caveolae. Disrupting caveolae by knocking down Cav-3 expression can be accomplished, but whether this will disrupt the function of the ion channel previously present in caveolae is not a certainty, as the channel may simply redistribute to other membrane domains. The interpretation of these results can be challenging. The most common supportive finding in caveolar channel functional studies is a dysregulation of the channels by signaling pathways following disruption of caveolae. Future efforts are needed to more specifically define the functional roles of caveolar ion channels to cardiac electrophysiology and pathology.
There are many unanswered fundamental questions regarding ion channels in caveolae and cardiac arrhythmias. How are ion channels targeted to caveolae, and how are these channels trafficked distinctly to the surface membrane? Are there multiple types of caveolae in a single cell type which may have different compositions and functional properties? For any given ion channel, what is the distribution of the channel in caveolar membranes vs. noncaveolar membranes? Is the caveolar distribution of a given ion channel dynamic and how is it regulated? How do various disease processes impact the function and regulation of caveolae that may alter the risk of arrhythmias? These are among the many interesting and important questions that will be addressed by future research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abi-Char J, Maguy A, Coulombe A, Balse E, Ratajczak P, Samuel JL, Nattel S, Hatem SN. Membrane cholesterol modulates Kv1.5 potassium channel distribution and function in rat cardiomyocytes. J Physiol. 2007;582:1205–1217. doi: 10.1113/jphysiol.2007.134809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai CX, Namekata I, Kurokawa J, Tanaka H, Shigenobu K, Furukawa T. Role of nitric oxide in Ca2+ sensitivity of the slowly activating delayed rectifier K+ current in cardiac myocytes. Circ Res. 2005;96:64–72. doi: 10.1161/01.RES.0000151846.19788.E0. [DOI] [PubMed] [Google Scholar]
- Balijepalli RC, Delisle BP, Balijepalli SY, Foell JD, Slind JK, Kamp TJ, January CT. Kv11.1 (ERG1) K+ channels localize in cholesterol and sphingolipid enriched membranes and are modulated by membrane cholesterol. Channels (Austin) 2007;1:263–272. doi: 10.4161/chan.4946. [DOI] [PubMed] [Google Scholar]
- Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca2+ channels to a caveolar macromolecular signaling complex is required for {beta}2-adrenergic regulation. Proc Natl. Acad. Sci. U.S.A. 2006 doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbuti A, Gravante B, Riolfo M, Milanesi R, Terragni B, DiFrancesco D. Localization of pacemaker channels in lipid rafts regulates channel kinetics. Circulation Research. 2004;94:1325–1331. doi: 10.1161/01.RES.0000127621.54132.AE. [DOI] [PubMed] [Google Scholar]
- Barbuti A, Terragni B, Brioschi C, DiFrancesco D. Localization of f-channels to caveolae mediates specific beta2-adrenergic receptor modulation of rate in sinoatrial myocytes. J Mol Cell Cardiol. 2007;42:71–78. doi: 10.1016/j.yjmcc.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O'Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- Bers DM, Bridge JH, Spitzer KW. Intracellular Ca2+ transients during rapid cooling contractures in guinea-pig ventricular myocytes. J Physiol. 1989;417:537–553. doi: 10.1113/jphysiol.1989.sp017817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilderback TR, Grigsby RJ, Dobrowsky RT. Association of p75(NTR) with caveolin and localization of neurotrophin-induced sphingomyelin hydrolysis to caveolae. J Biol Chem. 1997;272:10922–10927. doi: 10.1074/jbc.272.16.10922. [DOI] [PubMed] [Google Scholar]
- Bossuyt J, Taylor BE, James-Kracke M, Hale CC. The cardiac sodium-calcium exchanger associates with caveolin-3. Ann.N.Y.Acad.Sci. 2002;976:197–204. doi: 10.1111/j.1749-6632.2002.tb04741.x. [DOI] [PubMed] [Google Scholar]
- Brown CM, Petersen NO. An image correlation analysis of the distribution of clathrin associated adaptor protein (AP-2) at the plasma membrane. J Cell Sci. 1998;111(Pt 2):271–281. doi: 10.1242/jcs.111.2.271. [DOI] [PubMed] [Google Scholar]
- Bruns RR, Palade GE. Studies on blood capillaries I. General organization of blood capillaries in muscle. J Cell Biol. 1968a;37:244–276. doi: 10.1083/jcb.37.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns RR, Palade GE. Studies on blood capillaries II. Transport of ferritin molecules across the wall of muscle capillaries. J Cell Biol. 1968b;37:277–299. doi: 10.1083/jcb.37.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush WS, Ihrke G, Robinson JM, Kenworthy AK. Antibody-specific detection of caveolin-1 in subapical compartments of MDCK cells. Histochem Cell Biol. 2006;126:27–34. doi: 10.1007/s00418-006-0144-y. [DOI] [PubMed] [Google Scholar]
- Calaghan S, Kozera L, White E. Compartmentalisation of cAMP-dependent signalling by caveolae in the adult cardiac myocyte. J Mol Cell Cardiol. 2008;45:88–92. doi: 10.1016/j.yjmcc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Calaghan S, White E. Caveolae modulate excitation-contraction coupling and beta2-adrenergic signalling in adult rat ventricular myocytes. Cardiovasc Res. 2006;69:816–824. doi: 10.1016/j.cardiores.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Camors E, Charue D, Trouve P, Monceau V, Loyer X, Russo-Marie F, Charlemagne D. Association of annexin A5 with Na+/Ca2+ exchanger and caveolin-3 in non-failing and failing human heart. J Mol Cell Cardiol. 2006;40:47–55. doi: 10.1016/j.yjmcc.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Carozzi AJ, Ikonen E, Lindsay MR, Parton RG. Role of cholesterol in developing T-tubules: analogous mechanisms for T-tubule and caveolae biogenesis. Traffic. 2000;1:326–341. doi: 10.1034/j.1600-0854.2000.010406.x. [DOI] [PubMed] [Google Scholar]
- Cavalli A, Eghbali M, Minosyan TY, Stefani E, Philipson KD. Localization of sarcolemmal proteins to lipid rafts in the myocardium. Cell Calcium. 2007;42:313–322. doi: 10.1016/j.ceca.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WJ, Rothberg KG, Kamen BA, Anderson RG. Lowering the cholesterol content of MA104 cells inhibits receptor-mediated transport of folate. J. Cell Biol. 1992;118:63–69. doi: 10.1083/jcb.118.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Izu Y, Xiao RP, Izu LT, Cheng H, Kuschel M, Spurgeon H, Lakatta EG. G(i)-dependent localization of beta(2)-adrenergic receptor signaling to L-type Ca(2+) channels. Biophysical Journal. 2000;79:2547–2556. doi: 10.1016/S0006-3495(00)76495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- Cronk LB, Ye B, Kaku T, Tester DJ, Vatta M, Makielski JC, Ackerman MJ. Novel mechanism for sudden infant death syndrome: persistent late sodium current secondary to mutations in caveolin-3. Heart Rhythm. 2007;4:161–166. doi: 10.1016/j.hrthm.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darios F, Davletov B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature. 2006;440:813–817. doi: 10.1038/nature04598. [DOI] [PubMed] [Google Scholar]
- Das K, Lewis RY, Scherer PE, Lisanti MP. The membrane-spanning domains of caveolins-1 and -2 mediate the formation of caveolin hetero-oligomers. Implications for the assembly of caveolae membranes in vivo. J Biol Chem. 1999;274:18721–18728. doi: 10.1074/jbc.274.26.18721. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- Domeier TL, Zima AV, Maxwell JT, Huke S, Mignery GA, Blatter LA. IP3 receptor-dependent Ca2+ release modulates excitation-contraction coupling in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H596–H604. doi: 10.1152/ajpheart.01155.2007. [DOI] [PubMed] [Google Scholar]
- Echarri A, Muriel O, DelPozo MA. Intracellular trafficking of raft/caveolae domains: insights from integrin signaling. Semin Cell Dev Biol. 2007;18:627–637. doi: 10.1016/j.semcdb.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Eldstrom J, Van Wagoner DR, Moore ED, Fedida D. Localization of Kv1.5 channels in rat and canine myocyte sarcolemma. FEBS Lett. 2006;580:6039–6046. doi: 10.1016/j.febslet.2006.09.069. [DOI] [PubMed] [Google Scholar]
- Fan Z, Makielski JC. Anionic phospholipids activate ATP-sensitive potassium channels. J Biol Chem. 1997;272:5388–5395. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- Feron O, Dessy C, Opel DJ, Arstall MA, Kelly RA, Michel T. Modulation of the endothelial nitric-oxide synthase-caveolin interaction in cardiac myocytes Implications for the autonomic regulation of heart rate. J Biol Chem. 1998;273:30249–30254. doi: 10.1074/jbc.273.46.30249. [DOI] [PubMed] [Google Scholar]
- Folco EJ, Liu GX, Koren G. Caveolin-3 and SAP97 form a scaffolding protein complex that regulates the voltage-gated potassium channel Kv1.5. Am J Physiol Heart Circ Physiol. 2004;287:H681–H690. doi: 10.1152/ajpheart.00152.2004. [DOI] [PubMed] [Google Scholar]
- Forbes MS, Hawkey LA, Sperelakis N. The transverse-axial tubular system (TATS) of mouse myocardium: its morphology in the developing and adult animal. Am J Anat. 1984;170:143–162. doi: 10.1002/aja.1001700203. [DOI] [PubMed] [Google Scholar]
- Fozzard HA. Afterdepolarizations and triggered activity. Basic Res Cardiol. 1992;87 Suppl 2:105–113. doi: 10.1007/978-3-642-72477-0_10. [DOI] [PubMed] [Google Scholar]
- Frohnwieser B, Chen LQ, Schreibmayer W, Kallen RG. Modulation of the human cardiac sodium channel alpha-subunit by cAMP-dependent protein kinase and the responsible sequence domain. J Physiol. 1997;498(Pt 2):309–318. doi: 10.1113/jphysiol.1997.sp021859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G. Quantitative morphological study of smooth muscle cells of the guinea-pig taenia coli. Cell Tissue Res. 1976;170:161–186. doi: 10.1007/BF00224297. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, Hou H, Jr, Kneitz B, Edelmann W, Lisanti MP. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. Journal of Biological Chemistry. 2001;276:21425–21433. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- Garg V, Jiao J, Hu K. ATP-sensitive K+ channels are regulated by caveolin-enriched microdomains in cardiac myocytes. Circulation. 2008;118 doi: 10.1093/cvr/cvp039. [DOI] [PubMed] [Google Scholar]
- George AL, Jr, Varkony TA, Drabkin HA, Han J, Knops JF, Finley WH, Brown GB, Ward DC, Haas M. Assignment of the human heart tetrodotoxin-resistant voltage-gated Na+ channel alpha-subunit gene (SCN5A) to band 3p21. Cytogenet Cell Genet. 1995;68:67–70. doi: 10.1159/000133892. [DOI] [PubMed] [Google Scholar]
- Godreau D, Vranckx R, Maguy A, Goyenvalle C, Hatem SN. Different isoforms of synapse-associated protein, SAP97, are expressed in the heart and have distinct effects on the voltage-gated K+ channel Kv1.5. J Biol Chem. 2003;278:47046–47052. doi: 10.1074/jbc.M308463200. [DOI] [PubMed] [Google Scholar]
- Goldhaber JI, Lamp ST, Walter DO, Garfinkel A, Fukumoto GH, Weiss JN. Local regulation of the threshold for calcium sparks in rat ventricular myocytes: role of sodium-calcium exchange. J Physiol. 1999;520(Pt 2):431–438. doi: 10.1111/j.1469-7793.1999.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb DR, Vasilevski O, Huynh H, Woodcock EA. The extreme C-terminal region of phospholipase Cbeta1 determines subcellular localization and function; the "b" splice variant mediates alpha1-adrenergic receptor responses in cardiomyocytes. FASEB J. 2008;22:2768–2774. doi: 10.1096/fj.07-102558. [DOI] [PubMed] [Google Scholar]
- Guillot FL, Audus KL, Raub TJ. Fluid-phase endocytosis by primary cultures of bovine brain microvessel endothelial cell monolayers. Microvasc Res. 1990;39:1–14. doi: 10.1016/0026-2862(90)90055-v. [DOI] [PubMed] [Google Scholar]
- Haider S, Tarasov AI, Craig TJ, Sansom MS, Ashcroft FM. Identification of the PIP2-binding site on Kir6.2 by molecular modelling and functional analysis. EMBO J. 2007;26:3749–3759. doi: 10.1038/sj.emboj.7601809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzal-Bayer MF, Hancock JF. Lipid rafts and membrane traffic. FEBS Lett. 2007;581:2098–2104. doi: 10.1016/j.febslet.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Hare JM, Lofthouse RA, Juang GJ, Colman L, Ricker KM, Kim B, Senzaki H, Cao S, Tunin RS, Kass DA. Contribution of caveolin protein abundance to augmented nitric oxide signaling in conscious dogs with pacing-induced heart failure. Circulation Research. 2000;86:1085–1092. doi: 10.1161/01.res.86.10.1085. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Arimura T, Ueda K, Shibata H, Hohda S, Takahashi M, Hori H, Koga Y, Oka N, Imaizumi T, Yasunami M, Kimura A. Identification and functional analysis of a caveolin-3 mutation associated with familial hypertrophic cardiomyopathy. Biochem.Biophys.Res.Commun. 2004;313:178–184. doi: 10.1016/j.bbrc.2003.11.101. [DOI] [PubMed] [Google Scholar]
- Head BP, Patel HH, Roth DM, Lai NC, Niesman IR, Farquhar MG, Insel PA. G-protein-coupled receptor signaling components localize in both sarcolemmal and intracellular caveolin-3-associated microdomains in adult cardiac myocytes. J Biol.Chem. 2005;280:31036–31044. doi: 10.1074/jbc.M502540200. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Ball R. Regulation of cardiac Na +, Ca 2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- January CT, Riddle JM. Early afterdepolarizations: Mechanaism of induction and block. A role for L-type Ca 2+ current. Coronary Art Dis. 1989;74:977–990. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- Jiao J, Garg V, Yang B, Elton TS, Hu K. Protein kinase C-epsilon induces caveolin-dependent internalization of vascular adenosine 5′-triphosphate-sensitive K+ channels. Hypertension. 2008;52:499–506. doi: 10.1161/HYPERTENSIONAHA.108.110817. [DOI] [PubMed] [Google Scholar]
- Jurevicius J, Fischmeister R. cAMP compartmentation is responsible for a local activation of cardiac Ca2+ channels by beta-adrenergic agonists. Proc Natl Acad Sci USA. 1996;93:295–299. doi: 10.1073/pnas.93.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JX, Xiao YF, Leaf A. Free, long-chain, polyunsaturated fatty acids reduce membrane electrical excitability in neonatal rat cardiac myocytes. Proc Natl Acad Sci U S A. 1995;92:3997–4001. doi: 10.1073/pnas.92.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe JI, Grant BS, Yamamoto M, Schwencke C, Okumura S, Ishikawa Y. Changes in caveolin subtype protein expression in aging rat organs. Mol Cell Endocrinol. 2001;176:91–95. doi: 10.1016/s0303-7207(01)00472-5. [DOI] [PubMed] [Google Scholar]
- Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- Kubota I, Han X, Opel DJ, Zhao YY, Baliga R, Huang P, Fishman MC, Shannon RP, Michel T, Kelly RA. Increased susceptibility to development of triggered activity in myocytes from mice with targeted disruption of endothelial nitric oxide synthase. J Mol Cell Cardiol. 2000;32:1239–1248. doi: 10.1006/jmcc.2000.1158. [DOI] [PubMed] [Google Scholar]
- Leblanc N, Hume JR. Sodium current-induced release of calcium from cardiac sarcoplasmic reticulum. Science. 1990;248:372–376. doi: 10.1126/science.2158146. [DOI] [PubMed] [Google Scholar]
- Lee E, Marcucci M, Daniell L, Pypaert M, Weisz OA, Ochoa GC, Farsad K, Wenk MR, De Camilli P. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science. 2002;297:1193–1196. doi: 10.1126/science.1071362. [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Ackerman MJ, Benson DW, Jr, Brugada R, Clancy CE, Donahue JK, George AL, Jr, Grant AO, Groft SC, January CT, Lathrop DA, Lederer WJ, Makielski JC, Mohler PJ, Moss A, Nerbonne JM, Olson TM, Przywara DA, Towbin JA, Wang LH, Marks AR. Inherited arrhythmias: a National Heart, Lung, and Blood Institute and Office of Rare Diseases workshop consensus report about the diagnosis, phenotyping, molecular mechanisms, and therapeutic approaches for primary cardiomyopathies of gene mutations affecting ion channel function. Circulation. 2007;116:2325–2345. doi: 10.1161/CIRCULATIONAHA.107.711689. [DOI] [PubMed] [Google Scholar]
- Levade T, Jaffrezou JP. Signalling sphingomyelinases: which, where, how and why? Biochim Biophys Acta. 1999;1438:1–17. doi: 10.1016/s1388-1981(99)00038-4. [DOI] [PubMed] [Google Scholar]
- Li S, Galbiati F, Volonte D, Sargiacomo M, Engelman JA, Das K, Scherer PE, Lisanti MP. Mutational analysis of caveolin-induced vesicle formation Expression of caveolin-1 recruits caveolin-2 to caveolae membranes. FEBS Lett. 1998;434:127–134. doi: 10.1016/s0014-5793(98)00945-4. [DOI] [PubMed] [Google Scholar]
- Lipp P, Laine M, Tovey SC, Burrell KM, Berridge MJ, Li W, Bootman MD. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Curr Biol. 2000;10:939–942. doi: 10.1016/s0960-9822(00)00624-2. [DOI] [PubMed] [Google Scholar]
- Litwin SE, Li J, Bridge JH. Na-Ca exchange and the trigger for sarcoplasmic reticulum Ca release: studies in adult rabbit ventricular myocytes. Biophys J. 1998;75:359–371. doi: 10.1016/S0006-3495(98)77520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Oh P, Horner T, Rogers RA, Schnitzer JE. Organized endothelial cell surface signal transduction in caveolae distinct from glycosylphosphatidylinositol-anchored protein microdomains. J Biol Chem. 1997;272:7211–7222. doi: 10.1074/jbc.272.11.7211. [DOI] [PubMed] [Google Scholar]
- Liu P, Anderson RG. Compartmentalized production of ceramide at the cell surface. J Biol Chem. 1995;270:27179–27185. doi: 10.1074/jbc.270.45.27179. [DOI] [PubMed] [Google Scholar]
- Lohn M, Furstenau M, Sagach V, Elger M, Schulze W, Luft FC, Haller H, Gollasch M. Ignition of calcium sparks in arterial and cardiac muscle through caveolae. Circulation Research. 2000;87:1034–1039. doi: 10.1161/01.res.87.11.1034. [DOI] [PubMed] [Google Scholar]
- Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD, MacDonald JF. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat.Neurosci. 1999;2:331–338. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- Ma D, Tang XD, Rogers TB, Welling PA. An andersen-Tawil syndrome mutation in Kir2.1 (V302M) alters the G-loop cytoplasmic K+ conduction pathway. J Biol Chem. 2007;282:5781–5789. doi: 10.1074/jbc.M608776200. [DOI] [PubMed] [Google Scholar]
- Ma DW, Seo J, Davidson LA, Callaway ES, Fan YY, Lupton JR, Chapkin RS. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J. 2004;18:1040–1042. doi: 10.1096/fj.03-1430fje. [DOI] [PubMed] [Google Scholar]
- Marionneau C, Couette B, Liu J, Li H, Mangoni ME, Nargeot J, Lei M, Escande D, Demolombe S. Specific pattern of ionic channel gene expression associated with pacemaker activity in the mouse heart. J Physiol. 2005;562:223–234. doi: 10.1113/jphysiol.2004.074047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens JR, Sakamoto N, Sullivan SA, Grobaski TD, Tamkun MM. Isoform-specific localization of voltage-gated K+ channels to distinct lipid raft populations Targeting of Kv1.5 to caveolae. Journal of Biological Chemistry. 2001;276:8409–8414. doi: 10.1074/jbc.M009948200. [DOI] [PubMed] [Google Scholar]
- Massion PB, Dessy C, Desjardins F, Pelat M, Havaux X, Belge C, Moulin P, Guiot Y, Feron O, Janssens S, Balligand JL. Cardiomyocyte-restricted overexpression of endothelial nitric oxide synthase (NOS3) attenuates beta-adrenergic stimulation and reinforces vagal inhibition of cardiac contraction. Circulation. 2004;110:2666–2672. doi: 10.1161/01.CIR.0000145608.80855.BC. [DOI] [PubMed] [Google Scholar]
- Matsuda JJ, Lee H, Shibata EF. Enhancement of rabbit cardiac sodium channels by beta-adrenergic stimulation. Circ Res. 1992;70:199–207. doi: 10.1161/01.res.70.1.199. [DOI] [PubMed] [Google Scholar]
- McEwen DP, Li Q, Jackson S, Jenkins PM, Martens JR. Caveolin regulates kv1.5 trafficking to cholesterol-rich membrane microdomains. Mol Pharmacol. 2008;73:678–685. doi: 10.1124/mol.107.042093. [DOI] [PubMed] [Google Scholar]
- McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- Mobley BA, Eisenberg BR. Sizes of components in frog skeletal muscle measured by methods of stereology. J Gen Physiol. 1975;66:31–45. doi: 10.1085/jgp.66.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora R, Bonilha VL, Marmorstein A, Scherer PE, Brown D, Lisanti MP, Rodriguez-Boulan E. Caveolin-2 localizes to the golgi complex but redistributes to plasma membrane, caveolae, and rafts when co-expressed with caveolin-1. J Biol Chem. 1999;274:25708–25717. doi: 10.1074/jbc.274.36.25708. [DOI] [PubMed] [Google Scholar]
- Morris JB, Huynh H, Vasilevski O, Woodcock EA. Alpha1-adrenergic receptor signaling is localized to caveolae in neonatal rat cardiomyocytes. J Mol Cell Cardiol. 2006;41:17–25. doi: 10.1016/j.yjmcc.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci U S A. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Ooie T, Takahashi N, Taniguchi Y, Anan F, Yonemochi H, Saikawa T. Influence of menstrual cycle on QT interval dynamics. Pacing Clin Electrophysiol. 2006;29:607–613. doi: 10.1111/j.1540-8159.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kurokawa J, Bai CX, Asada K, Xu J, Oren RV, Zhu ZI, Clancy CE, Isobe M, Furukawa T. Progesterone regulates cardiac repolarization through a nongenomic pathway: an in vitro patch-clamp and computational modeling study. Circulation. 2007;116:2913–2922. doi: 10.1161/CIRCULATIONAHA.107.702407. [DOI] [PubMed] [Google Scholar]
- Napolitano L. The Differentiation of White Adipose Cells. An Electron Microscope Study. J Cell Biol. 1963;18:663–679. doi: 10.1083/jcb.18.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing "preassembled signaling complexes" at the plasma membrane. Journal of Biological Chemistry. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- Ostadal B, Ostadalova I, Dhalla NS. Development of cardiac sensitivity to oxygen deficiency: comparative and ontogenetic aspects. Physiol Rev. 1999;79:635–659. doi: 10.1152/physrev.1999.79.3.635. [DOI] [PubMed] [Google Scholar]
- Palade GE. An electron microscope study of the mitochondrial structure. J Histochem Cytochem. 1953;1:188–211. doi: 10.1177/1.4.188. [DOI] [PubMed] [Google Scholar]
- Palade GE. Blood capillaries of the heart and other organs. Circulation. 1961;24:368–388. doi: 10.1161/01.cir.24.2.368. [DOI] [PubMed] [Google Scholar]
- Palade GE, Bruns RR. Structural modulations of plasmalemmal vesicles. J Cell Biol. 1968;37:633–649. doi: 10.1083/jcb.37.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palygin OA, Pettus JM, Shibata EF. Regulation of caveolar cardiac sodium current by a single Gsalpha histidine residue. Am J Physiol Heart Circ Physiol. 2008;294:H1693–H1699. doi: 10.1152/ajpheart.01337.2007. [DOI] [PubMed] [Google Scholar]
- Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- Parton RG, Way M, Zorzi N, Stang E. Caveolin-3 associates with developing T-tubules during muscle differentiation. J. Cell Biol. 1997;136:137–154. doi: 10.1083/jcb.136.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel HH, Gross ER, Peart JN, Hsu AK, Gross GJ. Sarcolemmal KATP channel triggers delayed ischemic preconditioning in rats. Am J Physiol Heart Circ Physiol. 2005;288:H445–H447. doi: 10.1152/ajpheart.00031.2004. [DOI] [PubMed] [Google Scholar]
- Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–391. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel HH, Zhang S, Murray F, Suda RY, Head BP, Yokoyama U, Swaney JS, Niesman IR, Schermuly RT, Pullamsetti SS, Thistlethwaite PA, Miyanohara A, Farquhar MG, Yuan JX, Insel PA. Increased smooth muscle cell expression of caveolin-1 and caveolae contribute to the pathophysiology of idiopathic pulmonary arterial hypertension. FASEB J. 2007;21:2970–2979. doi: 10.1096/fj.07-8424com. [DOI] [PubMed] [Google Scholar]
- Pelkmans L, Zerial M. Kinase-regulated quantal assemblies and kiss-and-run recycling of caveolae. Nature. 2005;436:128–133. doi: 10.1038/nature03866. [DOI] [PubMed] [Google Scholar]
- Piech A, Dessy C, Havaux X, Feron O, Balligand JL. Differential regulation of nitric oxide synthases and their allosteric regulators in heart and vessels of hypertensive rats. Cardiovasc Res. 2003;57:456–467. doi: 10.1016/s0008-6363(02)00676-4. [DOI] [PubMed] [Google Scholar]
- Proven A, Roderick HL, Conway SJ, Berridge MJ, Horton JK, Capper SJ, Bootman MD. Inositol 1,4,5-trisphosphate supports the arrhythmogenic action of endothelin-1 on ventricular cardiac myocytes. J Cell Sci. 2006;119:3363–3375. doi: 10.1242/jcs.03073. [DOI] [PubMed] [Google Scholar]
- Rakhit A, Maguire CT, Wakimoto H, Gehrmann J, Li GK, Kelly RA, Michel T, Berul CI. In vivo electrophysiologic studies in endothelial nitric oxide synthase (eNOS)-deficient mice. J Cardiovasc Electrophysiol. 2001;12:1295–1301. doi: 10.1046/j.1540-8167.2001.01295.x. [DOI] [PubMed] [Google Scholar]
- Ramu Y, Xu Y, Lu Z. Enzymatic activation of voltage-gated potassium channels. Nature. 2006;442:696–699. doi: 10.1038/nature04880. [DOI] [PubMed] [Google Scholar]
- Ratajczak P, Damy T, Heymes C, Oliviero P, Marotte F, Robidel E, Sercombe R, Boczkowski J, Rappaport L, Samuel JL. Caveolin-1 and -3 dissociations from caveolae to cytosol in the heart during aging and after myocardial infarction in rat. Cardiovascular Research. 2003;57:358–369. doi: 10.1016/s0008-6363(02)00660-0. [DOI] [PubMed] [Google Scholar]
- Ratajczak P, Oliviero P, Marotte F, Kolar F, Ostadal B, Samuel JL. Expression and localization of caveolins during postnatal development in rat heart: implication of thyroid hormone. J Appl Physiol. 2005;99:244–251. doi: 10.1152/japplphysiol.01292.2004. [DOI] [PubMed] [Google Scholar]
- Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- Resh MD. Membrane targeting of lipid modified signal transduction proteins. Subcell Biochem. 2004;37:217–232. doi: 10.1007/978-1-4757-5806-1_6. [DOI] [PubMed] [Google Scholar]
- Robbins SM, Quintrell NA, Bishop JM. Myristoylation and differential palmitoylation of the HCK protein-tyrosine kinases govern their attachment to membranes and association with caveolae. Mol Cell Biol. 1995;15:3507–3515. doi: 10.1128/mcb.15.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robenek H, Weissen-Plenz G, Severs NJ. Freeze-fracture replica immunolabeling reveals caveolin-1 in the human cardiomyocyte plasma membrane. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA. 2001;285:1322–1326. doi: 10.1001/jama.285.10.1322. [DOI] [PubMed] [Google Scholar]
- Romanenko VG, Rothblat GH, Levitan I. Modulation of endothelial inward-rectifier K+ current by optical isomers of cholesterol. Biophys.J. 2002;83:3211–3222. doi: 10.1016/S0006-3495(02)75323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Rybin VO, Grabham PW, Elouardighi H, Steinberg SF. Caveolae-associated proteins in cardiomyocytes: caveolin-2 expression and interactions with caveolin-3. Am J Physiol Heart Circ. Physiol. 2003;285:H325–H332. doi: 10.1152/ajpheart.00946.2002. [DOI] [PubMed] [Google Scholar]
- Sampson LJ, Hayabuchi Y, Standen NB, Dart C. Caveolae localize protein kinase A signaling to arterial ATP-sensitive potassium channels. Circ Res. 2004;95:1012–1018. doi: 10.1161/01.RES.0000148634.47095.ab. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, Kohtz DS, van Donselaar E, Peters P, Lisanti MP. Cell-type and tissue-specific expression of caveolin-2 Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. Journal of Biological Chemistry. 1997;272:29337–29346. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- Scriven DR, Klimek A, Asghari P, Bellve K, Moore ED. Caveolin-3 is adjacent to a group of extradyadic ryanodine receptors. Biophys.J. 2005;89:1893–1901. doi: 10.1529/biophysj.105.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Barhoumi R, Johnson AE, Lupton JR, Chapkin RS. Docosahexaenoic acid selectively inhibits plasma membrane targeting of lipidated proteins. FASEB J. 2006;20:770–772. doi: 10.1096/fj.05-4683fje. [DOI] [PubMed] [Google Scholar]
- Seth R, Moss AJ, McNitt S, Zareba W, Andrews ML, Qi M, Robinson JL, Goldenberg I, Ackerman MJ, Benhorin J, Kaufman ES, Locati EH, Napolitano C, Priori SG, Schwartz PJ, Towbin JA, Vincent GM, Zhang L. Long QT syndrome and pregnancy. J Am Coll Cardiol. 2007;49:1092–1098. doi: 10.1016/j.jacc.2006.09.054. [DOI] [PubMed] [Google Scholar]
- Shibata EF, Brown TL, Washburn ZW, Bai J, Revak TJ, Butters CA. Autonomic regulation of voltage-gated cardiac ion channels. J Cardiovasc Electrophysiol. 2006;17 Suppl 1:S34–S42. doi: 10.1111/j.1540-8167.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- Simionescu M, Simionescu N, Palade GE. Segmental differentiations of cell junctions in the vascular endothelium. The microvasculature. J Cell Biol. 1975;67:863–885. doi: 10.1083/jcb.67.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N. Cellular aspects of transcapillary exchange. Physiol Rev. 1983;63:1536–1579. doi: 10.1152/physrev.1983.63.4.1536. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. Journal of Biological Chemistry. 1996;271:15160–15165. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- Steinberg SF. beta(2)-Adrenergic receptor signaling complexes in cardiomyocyte caveolae/lipid rafts. J Mol.Cell Cardiol. 2004;37:407–415. doi: 10.1016/j.yjmcc.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. Journal of Biological Chemistry. 1996;271:2255–2261. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- Thorn H, Stenkula KG, Karlsson M, Ortegren U, Nystrom FH, Gustavsson J, Stralfors P. Cell surface orifices of caveolae and localization of caveolin to the necks of caveolae in adipocytes. Mol Biol Cell. 2003;14:3967–3976. doi: 10.1091/mbc.E03-01-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, Tester DJ, Balijepalli RC, Foell JD, Li Z, Kamp TJ, Towbin JA. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation. 2006;114:2104–2112. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- Vites AM, Wasserstrom JA. Ca2+ influx via Na-Ca exchange and ICa can both trigger transient contractions in cat ventricular myocytes. Ann N Y Acad Sci. 1996;779:521–524. doi: 10.1111/j.1749-6632.1996.tb44826.x. [DOI] [PubMed] [Google Scholar]
- Wahler GM, Dollinger SJ. Nitric oxide donor SIN-1 inhibits mammalian cardiac calcium current through cGMP-dependent protein kinase. Am J Physiol. 1995;268:C45–C54. doi: 10.1152/ajpcell.1995.268.1.C45. [DOI] [PubMed] [Google Scholar]
- Wainger BJ, DeGennaro M, Santoro B, Siegelbaum SA, Tibbs GR. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature. 2001;411:805–810. doi: 10.1038/35081088. [DOI] [PubMed] [Google Scholar]
- Wang H, Kohr MJ, Wheeler DG, Ziolo MT. Endothelial nitric oxide synthase decreases beta-adrenergic responsiveness via inhibition of the L-type Ca2+ current. Am J Physiol Heart Circ Physiol. 2008;294:H1473–H1480. doi: 10.1152/ajpheart.01249.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Shen J, Li Z, Timothy K, Vincent GM, Priori SG, Schwartz PJ, Keating MT. Cardiac sodium channel mutations in patients with long QT syndrome, an inherited cardiac arrhythmia. Hum Mol Genet. 1995a;4:1603–1607. doi: 10.1093/hmg/4.9.1603. [DOI] [PubMed] [Google Scholar]
- Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995b;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- Wang YG, Dedkova EN, Ji X, Blatter LA, Lipsius SL. Phenylephrine acts via IP3-dependent intracellular NO release to stimulate L-type Ca2+ current in cat atrial myocytes. J Physiol. 2005;567:143–157. doi: 10.1113/jphysiol.2005.090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier S, Ramamurthy G, Eckert RL, Nikolaev VO, Lohse MJ, Harvey RD. cAMP microdomains and L-type Ca2+ channel regulation in guinea-pig ventricular myocytes. J Physiol. 2007;580:765–776. doi: 10.1113/jphysiol.2006.124891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock EA, Kistler PM, Ju Y. Phosphoinositide signalling and cardiac arrhythmias. Cardiovasc Res. 2008 doi: 10.1093/cvr/cvn283. [DOI] [PubMed] [Google Scholar]
- Woodcock EA, Matkovich SJ. Ins(1,4,5)P3 receptors and inositol phosphates in the heart-evolutionary artefacts or active signal transducers? Pharmacol Ther. 2005;107:240–251. doi: 10.1016/j.pharmthera.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, Shirani J, Tang B, Jelicks LA, Kitsis RN, Christ GJ, Factor SM, Tanowitz HB, Lisanti MP. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. Journal of Biological Chemistry. 2002;277:38988–38997. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- Woodman SE, Sotgia F, Galbiati F, Minetti C, Lisanti MP. Caveolinopathies: mutations in caveolin-3 cause four distinct autosomal dominant muscle diseases. Neurology. 2004;62:538–543. doi: 10.1212/wnl.62.4.538. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Rybin VO, Steinberg SF, Kobilka B. Caveolar localization dictates physiologic signaling of beta 2-adrenoceptors in neonatal cardiac myocytes. Journal of Biological Chemistry. 2002;277:34280–34286. doi: 10.1074/jbc.M201644200. [DOI] [PubMed] [Google Scholar]
- Xiao YF, Gomez AM, Morgan JP, Lederer WJ, Leaf A. Suppression of voltage-gated L-type Ca 2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proc Natl Acad Sci USA. 1997;94:4182–4187. doi: 10.1073/pnas.94.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YF, Kang JX, Morgan JP, Leaf A. Blocking effects of polyunsaturated fatty acids on Na+ channels of neonatal rat ventricular myocytes. Proc Natl Acad Sci U S A. 1995;92:11000–11004. doi: 10.1073/pnas.92.24.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YF, Sigg DC, Leaf A. The antiarrhythmic effect of n-3 polyunsaturated fatty acids: modulation of cardiac ion channels as a potential mechanism. J Membr Biol. 2005;206:141–154. doi: 10.1007/s00232-005-0786-z. [DOI] [PubMed] [Google Scholar]
- Yarbrough TL, Lu T, Lee HC, Shibata EF. Localization of cardiac sodium channels in caveolin-rich membrane domains: regulation of sodium current amplitude. Circulation Research. 2002;90:443–449. doi: 10.1161/hh0402.105177. [DOI] [PubMed] [Google Scholar]
- Ye B, Balijepalli RC, Foell JD, Kroboth S, Ye Q, Luo YH, Shi NQ. Caveolin-3 Associates with and Affects the Function of Hyperpolarization-Activated Cyclic Nucleotide-Gated Channel 4. Biochemistry. 2008 [PMC free article] [PubMed] [Google Scholar]
- Zhou YY, Cheng H, Bogdanov KY, Hohl C, Altschuld R, Lakatta EG, Xiao RP. Localized cAMP-dependent signaling mediated β2 -adrenregic modulation of cardiac excitation-contraction coupling. American Journal of Physiology. 1997;273:H1611–H1618. doi: 10.1152/ajpheart.1997.273.3.H1611. [DOI] [PubMed] [Google Scholar]
- Zima AV, Blatter LA. Inositol-1,4,5-trisphosphate-dependent Ca(2+) signalling in cat atrial excitation-contraction coupling and arrhythmias. J Physiol. 2004;555:607–615. doi: 10.1113/jphysiol.2003.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingman LV, Alekseev AE, Hodgson-Zingman DM, Terzic A. ATP-sensitive potassium channels: metabolic sensing and cardioprotection. J Appl Physiol. 2007;103:1888–1893. doi: 10.1152/japplphysiol.00747.2007. [DOI] [PubMed] [Google Scholar]