Abstract
Objectives
Abacavir (ABC) oral clearance, adjusted for body size, is approximately 2 times higher for children than adults with a corresponding difference in dose regimens. However, there are limited data available in the adolescent population. The pharmacokinetics (PKs) of ABC and primary metabolites were determined in HIV-1–infected children and adolescents to evaluate age and patient characteristics as a basis for adjusting ABC dose regimens and to assess the influence of metabolite formation on PK parameters.
Methods
Pediatric subjects 9–18 years of age receiving antiretroviral therapy for HIV-1 infection were stratified by Tanner stage and given a single 8 mg/kg dose of ABC oral solution. Blood samples (n = 10) were obtained over 8 hours and measured for ABC, glucuronide, and carboxylate metabolites using high-performance liquid chromatography. PK parameters for children (Tanner stages 1–2; TS1) and adolescents (Tanner stages 3–5; TS2) were compared.
Results
Twenty-five subjects were enrolled. ABC mean (range) maximum concentration (Cmax; μg/mL), area under the curve (μg·hr/mL), half-life (hours), and apparent clearance (CL/F; mL/min per kg) for TS1 and TS2 were 3.5 (1.2–5.6) vs 3.4 (1.8–5.9), 8.0 (2.1– 18.6) vs 8.9 (3.1–17.2), 1.3 (0.7–2.5) vs 1.4 (0.9–1.9), and 22.1 (7.0– 59.2) vs 18.4 (7.7–42.9) and not significantly different. Age, Tanner stage, and sex were not correlated with ABC clearance by univariate analysis. The ratios of metabolites to ABC area under the curve were correlated with ABC clearance as were the ratios of metabolites to ABC concentrations at the 6-hour time point.
Conclusions
ABC oral clearance in HIV-1-infected pediatric patients does not change during puberty, is similar to younger children, and is higher than previously published in adults. Therefore, dosing adolescents as adults should be reexamined. Intersubject PK variability is substantial and is not correlated with body size or age but more likely due to differences in metabolite formation that may be genetic in origin.
Keywords: abacavir, adolescents, children, HIV, pharmacokinetics
Introduction
Abacavir (ABC) is a highly active guanosine analogue widely used in the treatment of HIV-infected children and adults as part of combination antiretroviral therapy.1 When given at a dose of 8 mg/kg twice daily in combination with either zidovudine (AZT) or lamivudine (3TC) to previously untreated children, it produced a larger reduction in viral load than children receiving an AZT/3TC combination.2 Consistent with other nucleoside analogues, ABC antiretroviral activity requires intracellular metabolism to the active moiety, carbovir triphosphate.3 However, plasma concentrations have been shown to be relevant to clinical outcome.4 Metabolism occurs through 2 primary hepatic pathways, carboxylation and glucuronidation. A minimal amount (<5%) is eliminated unchanged in the urine. Because ABC is not a substrate or inhibitor of the cytochrome P450 system, drug interactions are uncommon. ABC has been well tolerated in pediatric patients with a hypersensitivity reaction being the most common treatment-limiting adverse effect. This reaction can become increasingly severe with subsequent dosing and thus requires discontinuation of the drug.
Physiologic differences often determine the dosage changes based on body weight that are seen between children and adults. Previous pharmacokinetic (PK) studies of ABC in adults and children have revealed some interesting disparities between the 2 populations.1,5–9 When given the same dose referenced to body weight (ie, milligrams per kilogram), systemic exposure [eg, area under the curve (AUC)] in children is approximately 50% lower than adult values, with correspondingly higher oral clearances (CL/F). Fittingly, the current recommended dosing regimen is 2-fold higher in pediatric patients (8 mg/kg per dose twice daily) than in adults (approximately 4 mg/kg per dose twice daily for an average adult weight of 75 kg). Given that the recommended pediatric dose has an upper limit of 300 mg, the possibility exists of underdosing those children/adolescents who weigh more than 37.5 kg once the suggested maximum dose is attained.
Although the PK parameters of ABC have been well described in the adult and pediatric populations, limited data exist in the adolescent population. We conducted a single-dose PK study of ABC in HIV-infected children and adolescents, stratified by Tanner stage (Tanner 1–2 vs Tanner ≥3) and balanced for sex. A common method used in the assessment of the onset and progression of puberty, Tanner staging10 is composed of 5 stages, ranging from prepuberty (stage 1) to full physical maturity (stage 5). After describing the PK parameters of ABC, we set out to determine a dosing regimen for children and adolescents, which would yield a similar systemic exposure previously shown to be safe and effective in adults receiving 300 mg twice daily. Secondarily, we sought to explore the relationships between ABC PKs and the glucuronide (GLU) and carboxylate (CAR) metabolites.
Methods
Patients
Subjects between the ages of 9 and 18 years with HIV infection were eligible for study. HIV infection was defined as 2 positive results from 2 different samples by 1 or more assays (DNA polymerase chain reaction, RNA polymerase chain reaction, p24 antigen, or HIV culture). Inclusion criteria included a CD4 cell count >200 cells per microliter, plasma HIV RNA <100,000 copies per milliliter, weight for age between the 5th and 95th percentiles, and a stable antiretroviral regimen within the 4 weeks before study entry.
Exclusion criteria included (1) current or prior ABC therapy; (2) surgical or medical problems affecting gastrointestinal motility or absorption; (3) presence of an acute opportunistic or serious bacterial infection; (4) pregnancy or breast-feeding; (5) current chemotherapy for active malignancy; and (6) current grade 3 or greater laboratory toxicity at screening as defined by the Division of AIDS Toxicity Table for Grading Severity of Pediatric Adverse Experiences with the exceptions of aspartate aminotransferase/alanine aminotransferase >3 times the upper limits of normal, bilirubin >2 mg/dL, or serum creatinine (mg/dL) greater than 1.5 times the upper limit of normal for age. Interferons, interleukins, HIV vaccines, hepatic enzyme–inducing agents (ie, rifampin, rifabutin, phenytoin, carbamazepine, or phenobarbital), and experimental therapy within 30 days before study entry were also disallowed.
Patients were enrolled at 11 centers affiliated with the Pediatric AIDS Clinical Trials Group (PACTG). Written informed consent was obtained from the subjects (or parent or legal guardian of subjects when appropriate) before enrollment. Assent was also obtained in subjects younger than 18 years who were able to understand the nature, significance, and risks of the study. This protocol was approved by the institutional review boards of all participating sites before enrolling patients.
Study Design
This was an open-label PK study (PACTG protocol 1018) of ABC in prepubescent and postpubescent HIV-infected adolescents. Subjects received a single oral dose of ABC oral solution 8 mg/kg of body weight (maximum 600 mg) followed by 6–8 oz of water. ABC was supplied as the commercially available oral solution Ziagen (GlaxoSmithKline, Research Triangle Park, NC) containing 20 mg of drug per milliliter of solution. Twenty-four (24) subjects were to be enrolled and evenly stratified into 1 of 2 groups based on physical development as assessed by Tanner staging with each group further balanced for sex. Group 1 (TS1) consisted of subjects who were Tanner stage 1 or stage 2, whereas group 2 (TS2) included subjects of Tanner stage 3, stage 4, or stage 5.
A screening evaluation was performed within 14 days before study entry and included a medical history, physical exam, and laboratory measurements (complete blood count [CBC] with differential, chemistries, and pregnancy test for females). Subjects were evaluated for tolerability of ABC solution and for adverse events/toxicities during the study period. If no toxicities were observed, then no further laboratory workup was required. On the day of study, subjects were asked to refrain from taking food at least 1 hour before and 2 hours after ABC administration. In addition, no other medications were allowed within 1 hour before or after ABC dosing.
Blood samples (5 mL each) were collected in heparin-containing vacutainers immediately before dosing (time 0) and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, and 8 hours (n = 10) after administration. Blood samples were centrifuged within 2 hours of collection and kept at freezing temperatures for storage and shipping to the core pharmacology laboratory site. Plasma ABC and metabolite concentrations were determined by reversed-phase high-performance liquid chromatography (HPLC) assay on a Shimadzu HPLC system (Tokyo, Japan). The HPLC system consisted of an SCL-10Avp system controller, SPD-10Avp UV-visible detector, 2 LC-10ATvp HPLC pumps, and an SIL-10A autoinjector. The system was controlled remotely using Shimadzu Class VP HPLC software. Column temperature was controlled with an Eppendorf column heater CH-30 and TC-50 controller. The assay had a lower limit of quantitation of 0.025 μg/mL (25 ng/mL) and an upper limit of quantitation of 10 μg/mL (10,000 ng/mL). The assay was linear over this range. Plasma samples were assayed for ABC and its metabolites by extracting 100-μL aliquots with methanol (HPLC grade; Fisher Scientific, Pittsburgh, PA), transferring to a fresh tube, and evaporating to dryness. The resultant pellets were suspended with 100 μL of mobile phase A and subjected to gradient separation on a 250 × 4.6-mm with a 5 μm particle size Kromasil C-18 reverse phase column (Phenomenex, Torrance, CA). The separation was performed at a flow rate of 0.75 mL/min and a temperature of 30°C. Mobile phase A consisted of 25 mM sodium acetate (Sigma Chemical Company, St. Louis, MO) and 5% acetonitrile (HPLC grade; Burdick and Jackson, Muskegon, MI) at pH 4.0 and mobile phase B consisted of 25 mM sodium acetate and 50% acetonitrile at pH 7.0. The separation gradient was a 30-minute linear gradient from 0% to 30% B followed by 8 minutes at 100% B. Analytes were detected via UV spectrophotometry at 254 nm for the first 14.5 minutes and at 295 nm for the remainder of the assay. For each set of samples assayed, a standard curve and quality control samples were run. Quality control concentrations were 0.075, 0.75, and 7.5 μg/mL assayed in duplicate. The standard curve was linear and had to have a minimum r2 of 0.98 to be acceptable. Interassay and intra-assay variability was assessed by measuring each analyte at 4 concentrations (0.06, 0.30, 1.5, and 7.5 μg/mL) in replicates of 6 over 6 different days. Overall interassay/intra-assay variability was equal to or better than −16.7% error and 9.8% coefficient of variation. Assay validation was compliant with Food and Drug Administration guidelines and reviewed by the PACTG. ABC and metabolite standards were provided by GlaxoSmithKline and the internal standard (2′,3′-dideoxyuridine) was purchased from Aldrich Chemicals (St. Louis, MO).
PK Analysis
Each subject's concentration data were analyzed separately using the ADAPT II version 4.0 PK/PD analysis program (University of Southern California, Los Angeles, CA).11 A 1-compartment zero-order absorption model was fit to each patient's plasma ABC concentration-time profile using weighted least squares estimation. The model estimated volume of distribution (V/F) and the elimination rate constant (kel). Apparent oral clearance (CL/F) was calculated as the product of V/F and kel. The overall goodness of fit was assessed for precision and bias by calculating the mean absolute error and mean error, presented as the percentage of predicted and the correlation coefficient of the model fit.12 The area under the concentration-versus-time curve from 0 to 8 hours (AUC) was determined by the equation AUC = dose/CL and by the trapezoidal method for comparison. The maximum concentration (Cmax) and time to maximum concentration (Tmax) were determined by inspection of the concentration-time profile.
Statistical Analysis
Results are summarized as mean ± SD unless otherwise stated to allow for comparisons with previously published data. Nonparametric (Wilcoxon rank sum) tests are used to compare distributions by sex, Tanner stage, and race/ethnicity. Spearman correlations based on ranks were used to explore relationships between ABC PK parameters and metabolite data. Differences were considered statistically significant at a P value less than 0.05.
Results
ABC data were obtained from 25 (12 males, 13 females) HIV-infected prepubescent and postpubescent adolescents. All subjects completed the study. Demographic and baseline characteristics are summarized in Table 1. Twenty subjects were receiving at least 1 protease inhibitor plus 1 other nucleoside reverse transcriptase inhibitor (NRTI), 4 subjects were receiving dual-NRTI regimens, and 1 subject was not on any antiretroviral therapy at the time of study enrollment. As expected, subjects in TS2 were, on average, older and weighed more than those of TS1. ABC oral solution was tolerated by all subjects, with no serious adverse events reported.
TABLE 1.
Demographics and Characteristics of Subjects
| Characteristic | All Subjects N = 25 |
Tanner Stages 1–2 n = 13 |
Tanner Stages 3–5 n = 12 |
|---|---|---|---|
| Age (yrs) | |||
| Mean ± SD | 13.2 ± 3.1 | 10.8 ± 0.9 | 15.7 ± 2.4 |
| Range | 9.3–18.3 | 9.3–12.1 | 11.5–18.3 |
| Sex | |||
| Male:female | 12:13 | 6:7 | 6:6 |
| Race/ethnicity (%) | |||
| White | 5 (20) | 2 (15) | 3 (25) |
| African American | 13 (52) | 10 (77) | 3 (25) |
| Hispanic | 7 (28) | 1 (8) | 6 (50) |
| Weight (kg) | |||
| Mean ± SD | 45.4 ± 14.1 | 34.2 ± 8.3 | 57.4 ± 7.4 |
| Range | 25.2–66.7 | 25.2–53.6 | 44.7–66.7 |
| BSA (m2) | |||
| Mean ± SD | 1.37 ± 0.27 | 1.15 ± 0.16 | 1.61 ± 0.12 |
| Range | 0.96–1.78 | 0.96–1.51 | 1.35–1.78 |
| ABC dose (mg) | |||
| Mean ± SD | 360 ± 117 | 268 ± 67 | 460 ± 61 |
| ABC dose (mg/kg) | |||
| Mean ± SD | 7.9 ± 0.2 | 7.8 ± 0.2 | 8.0 ± 0.2 |
BSA, body surface area.
Figure 1 shows the plasma concentration–time profiles for ABC in this study population. After oral administration, ABC seemed to be rapidly absorbed with a mean Tmax of 1.0 hour (range 0.5–2.5 hours). The PK parameter estimations are presented in Table 2. In the compartmental analysis, a 1-compartmental model fit the data well without a large bias as indicated by the mean absolute error of 16.2% ± 1.1% (SE), mean error of 6.5% ± 1.5% (SE), and average r2 = 0.92. Oral clearance (CL/F) referenced to body weight varied by more than 8-fold with a mean (range) of 20.3 (7.0–59.2) mL/min per kg. CL/F did not differ significantly between the 2 groups (P = 0.68) or between males and females (P = 0.12). Similarly, there were no statistically significant differences in AUC, Cmax, V/F, and t½ between sex, age, and Tanner stage groups.
FIGURE 1.
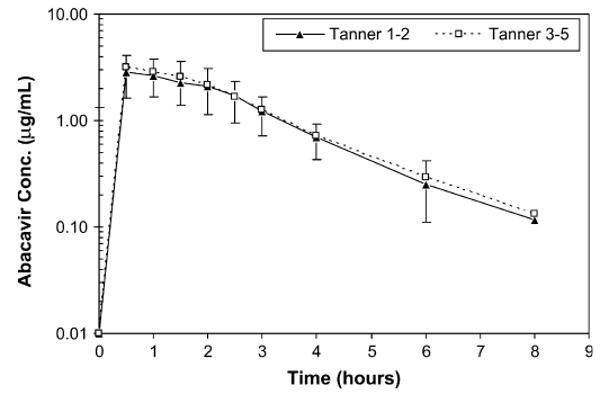
Geometric mean (±SD) plasma ABC concentration– time profiles for all subjects as stratified by Tanner staging [Tanner stages 1–2 (n = 13); Tanner stages 3–5 (n = 12)].
TABLE 2.
Mean ± SD (Range) PK Parameter Estimates for ABC
| Parameter | All Subjects N = 25 |
Tanner Stages 1–2 n = 13 |
Tanner Stages 3–5 n = 12 |
P* |
|---|---|---|---|---|
| CL/F (mL/min per kg) | 20.3 ± 12.8 (7.0–59.2) | 22.1 ± 15.2 (7.0–59.2) | 18.4 ± 9.9 (7.7–42.9) | 0.68 |
| Cmax (μg/mL) | 3.45 ± 1.27 (1.18–5.92) | 3.46 ± 1.39 (1.18–5.57) | 3.43 ± 1.18 (1.83–5.92) | 0.91 |
| Tmax (h) | 1.0 ± 0.6 (0.5–2.5) | 1.2 ± 0.8 (0.5–2.5) | 0.9 ± 0.4 (0.5–1.5) | 0.73 |
| t½ (h) | 1.32 ± 0.39 (0.69–2.51) | 1.30 ± 0.46 (0.69–2.51) | 1.35 ± 0.32 (0.86–1.94) | 0.38 |
| V/F (L/kg) | 2.1 ± 1.1 (1.3–6.5) | 2.2 ± 1.4 (1.4–6.5) | 2.0 ± 0.6 (1.3–3.5) | 0.79 |
| AUC (μg·hr/mL) | 8.43 ± 3.97 (2.10–18.59) | 8.02 ± 4.20 (2.10–18.59) | 8.87 ± 3.85 (3.06–17.15) | 0.68 |
| AUC ratio of CAR:ABC | 0.57 ± 0.21 (0.17–1.07) | 0.58 ± 0.24 (0.17–1.07) | 0.57 ± 0.18 (0.43–1.07) | 0.94 |
| AUC ratio of GLU:ABC | 1.44 ± 0.79 (0.84–4.70) | 1.21 ± 0.42 (0.84–2.28) | 1.68 ± 1.02 (0.89–4.70) | 0.09 |
AUC, area under the concentration–time curve; CL/F, apparent oral clearance; Cmax, maximum concentration; t½, half-life; Tmax, time to maximum concentration; V/F, apparent volume of distribution.
Wilcoxon rank sum test.
The ratios of metabolite to parent drug were significant predictors of ABC clearance. GLU:ABC AUC ratio varied more than 5-fold, with a mean (range) of 1.44 (0.84–4.7). Subjects with a GLU:ABC ratio ≥1.44 had a mean ABC oral clearance of 30.4 mL/min per kg, whereas those with a ratio <1.44 had a mean clearance of 14.7 mL/min per kg. The CAR:ABC AUC ratio varied more than 6-fold, with a mean (range) of 0.57 (0.17–1.07). Subjects with a CAR:ABC ratio ≥0.57 had a mean ABC oral clearance of 27.3 mL/min per kg, whereas those with a ratio <0.57 had a mean clearance of 15.7 mL/min per kg. Both GLU and CAR AUC ratios were significantly correlated with ABC clearance (r = 0.64, P = 0.001; r = 0.48, P = 0.015, respectively) (Figs. 2A, B).
FIGURE 2.
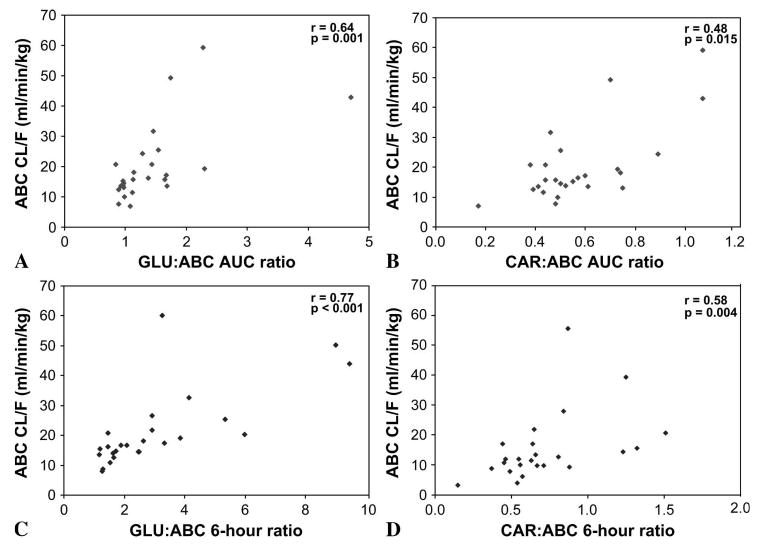
Correlation between ABC clearance (N = 25) and (A) GLU:ABC AUC ratio, (B) CAR:ABC AUC ratio, (C) GLU:ABC 6-hour concentration ratio, and (D) CAR:ABC 6-hour concentration ratio.
Likewise, the ratio of metabolite to ABC at the 6-hour time point was also predictive of ABC clearance. The GLU:ABC ratio at 6 hours varied more than 8-fold, with a mean (range) of 3.04 (1.18–9.45). Subjects with a GLU:ABC ratio ≥3.04 had a mean ABC oral clearance of 32.6 mL/min per kg, whereas those with a ratio <3.04 had a mean clearance of 14.6 mL/min per kg. The CAR:ABC ratio at 6 hours varied more than 10-fold, with a mean (range) of 0.72 (0.15–1.51). Subjects with a CAR:ABC ratio ≥0.72 had a mean ABC clearance of 28.1 mL/min per kg, whereas those with a ratio <0.72 had a mean clearance of 14.7 mL/min per kg. Again, both GLU and CAR ratios at 6 hours were significantly correlated with ABC clearance (r = 0.77, P < 0.001; r = 0.56, P = 0.004, respectively) (Figs. 2C, D).
Discussion
ABC is a useful and effective antiretroviral agent when used as part of combination therapy for the treatment of children with HIV The Paediatric European Network of Treatment of AIDS (PENTA) 5 trial2 compared the activity of 3 randomly assigned dual-NRTI regimens (AZT/3TC, AZT/ABC, and 3TC/ABC) in previously untreated children. The dose of ABC was 8 mg/kg given twice daily, which is consistent with current dosing recommendations. Results demonstrated that ABC-containing dual-NRTI regimens produced larger reductions in viral load than standard AZT/3TC regimens. In particular, the ABC/3TC regimen resulted in a greater and more sustained (up to 48 weeks) viral load reduction of the combinations studied.
The PK parameters of the present study are comparable to previously published data in children receiving 8 mg/kg of ABC.6,13 Hughes et al and Jullien et al reported mean CL/F values of 18.9 and 15.8 mL/min per kg and mean AUC values of 8.1 and 8.5 μg·hr/mL, respectively, in populations that were limited primarily to prepubescent subjects. The current study revealed mean CL/F and AUC values of 20.3 mL/min per kg and 8.4 μg·hr/mL overall with no appreciable differences between the 2 study groups. As shown in Table 2, despite the large variability in oral clearance and AUC, subjects in Tanner stages 3–5 had similar values (within 20%) to the younger cohort of Tanner stages 1–2. Other PK parameters were also not discernibly different, and in the regression analysis, there were no associations found between PK parameters (weight adjusted) and age or Tanner stage.
Comparing the results of the present study with those previously reported in adults4,8 generated some interesting observations. Kumar et al and Weller et al studied ABC PKs in adults (average age 38 and 39 years, respectively) receiving 600 mg (approximately 8 mg/kg) and reported mean CL/F values of 10.2 and 11.5 mL/min per kg, respectively. The recommended adult dose of 300 mg twice daily has been shown to produce AUCs in the range of 5–6 μg·hr/mL,1,4,8,14–16 whereas single-dose studies in adults given 600 mg have generated AUCs in the range of 11–16 μg·hr/mL.4,8,14 Our present study yielded a mean AUC of 8.4 μg·hr/mL, which falls in between the 4 and 8 mg/kg doses studied in adults. It is apparent that oral clearance and systemic exposure of ABC in adolescents are more comparable to values in children than in adults at the same milligram per kilogram dose (ie, 8 mg/kg; Table 3).
TABLE 3.
Comparison of Mean PK Parameters of Single-Dose ABC
| PK Parameter | Dose | P1018 N = 25 (9–18 yrs) |
Children6 n = 22 (3 mo to 13 yrs) |
Adults8 n = 12 (24–48 yrs) |
|---|---|---|---|---|
| Cmax (μg/mL) | 4 mg/kg | — | 1.7 | 2.9 |
| 8 mg/kg | 3.5 | 3.9 | 4.7 | |
| AUC (μg·hr/mL) | 4 mg/kg | — | 2.8 | 6.0 |
| 8 mg/kg | 8.4 | 8.1 | 15.7 | |
| CL/F (mL/min per kg) | 4 mg/kg | — | 27.4 | 13.4 |
| 8 mg/kg | 20.3 | 18.9 | 10.2 | |
| t½ (h) | 4 mg/kg | — | 0.9 | 1.2 |
| 8 mg/kg | 1.3 | 1.1 | 1.7 |
AUC, area under the concentration–time curve; CL/F, apparent oral clearance; Cmax, maximum concentration; P1018, PACTG protocol 1018; t½, half-life.
In all the postpubescent adolescents in this study, the 8 mg/kg dose exceeded 300 mg, yet the overall ABC exposure was similar to younger children. Assuming dose linearity, if the dose had been limited to a maximum of 300 mg, the AUC in the adolescent group (Tanner ≥ 3) would have been reduced by about a third and the resulting AUC of 5.7 ± 2.2 would have been lower than the younger group (Tanner 1–2) but similar to adults. Therefore, as older adolescents continue to develop and gain weight, a 300-mg dose will steadily provide less drug on a milligram per kilogram basis. If clearance normalized to body size remains constant during this time of growth, then further reductions in AUC would be expected; thus, potentially increasing the likelihood of virologic failure.
This study demonstrated that ABC clearance is highly correlated with the extent of metabolite formation. After oral administration, ABC undergoes rapid and extensive absorption and is eliminated primarily by metabolism to the GLU and CAR metabolites with less than 5% eliminated unchanged in the urine. The enzymes UDP-glucuronosyltransferases, alcohol dehydrogenase, and aldehyde dehydrogenase-2 account for approximately 70% of ABC metabolism.14 These primary enzymatic pathways have known genetic polymorphisms17–19 shown to substantially alter clearance of known substrates. Moreover, there are differences in ethnic prevalence for genotype and phenotype for UDP-glucuronosyltransferases,20,21 alcohol dehydrogenase, and aldehyde dehydrogenase-2.22 We found that the ratios of metabolites to parent compound, expressed as either AUC or 6-hour concentrations, show a relationship with ABC CL/F. Although measuring systemic exposure (AUC) of all 3 compounds is not practical in a clinical setting, the possibility exists that a single blood sample at the 6-hour time point could be useful in predicting subjects with high ABC clearance, more so with the GLU:ABC ratio.
There are some limitations of this study worth mentioning. First, this study used the ABC oral solution to provide a flexible means to dose subjects over a wide range of body size. Administering the solution aided in giving a more accurate milligram per kilogram dose than could be done using the commercially available tablet, which only exists in a 300 mg strength. Although the solid oral dosage form is more commonly used in adolescents, the bioavailability of the solution has been shown to be equivalent to the tablet.5 It is also worth noting that intracellular concentrations of carbovir triphosphate, the active moiety, were not measured in this study. Currently, it is impractical and time consuming to measure carbovir triphosphate in a clinical setting.
Rapid ABC absorption, resulting in 10 subjects with the first concentration being the Cmax, limited the ability to characterize the absorption phase with a high level of precision. This means that the true peak concentrations may have been missed and thus AUC would be underestimated by the noncompartmental method. However, comparing AUC estimates by 2 separate methods yielded similar results (data not shown). A related concern is that AUC was determined over an 8-hour sampling period for a medication commonly given twice daily. However, it is estimated that <3% of the total AUC can be accounted for in the period after 8 hours (for all subjects: mean 8-hour concentration = 0.124 μg/mL and mean kel = 0.57/h).
This study was not powered to detect modest age-related PK differences. Thus, although we saw no differences between the 2 subject groups, we cannot rule out the possibility that a moderate difference exists. Although there was a frequent use of protease inhibitors and other nonnucleoside reverse transcriptase inhibitors, there is no indication that these concomitant drugs affect ABC disposition in this population.
In conclusion, the results of this study describe the PKs of 8 mg/kg ABC in an HIV-infected adolescent population. We found that both GLU and CAR metabolites:ABC ratios are able to discriminate subjects with high and low ABC clearance. This finding needs further confirmation but could potentially have clinical relevance for ABC and other similarly metabolized drugs. PK data from the present study compared with previously published data of ABC reveal that CL/F in adolescents is comparable to that in children, and lower AUCs would be expected in older adolescents if given the adult-referenced maximum dose of 300 mg twice daily. As a result, further study of efficacy, safety, and tolerability is warranted in an older adolescent population and young adult population with doses of 8 mg/kg (maximum 600 mg) twice daily.
Acknowledgments
We thank all the children, adolescents, and their families for participating in this trial. We gratefully acknowledge all the staff at the participating PACTG sites for enrolling subjects and collecting data. We also would like to thank Nancy Carter for data management and Edmund Capparelli, PharmD, for his helpful insight and review of the article.
Supported in part by the Pediatric AIDS Clinical Trials Group of the National Institute of Allergy and Infectious Diseases, the Pediatric/Perinatal HIV Clinical Trials Network of the National Institute of Child Health and Human Development, GlaxoSmithKline, Inc, a Center of Excellence grant from the University of Tennessee, and the American Lebanese Syrian Associated Charities. The project described was supported by the National Institute of Allergy and Infectious Diseases.
Footnotes
Presented as a poster presentation at the 2002 Annual Meeting of the American College of Clinical Pharmacy, October 20–23, 2002, Albuquerque, NM.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
We dedicate this article to the memory of John H. Rodman, our colleague, mentor, and friend, who passed away unexpectedly in April 2006. Through his hard work and dedication, he made immeasurable contributions to the field of clinical pharmacy.
References
- 1.Staszewski S, Katlama C, Harrer T, et al. A dose-ranging study to evaluate the safety and efficacy of abacavir alone or in combination with zidovudine and lamivudine in antiretroviral treatment-naive subjects. AIDS. 1998;12:F197–F202. doi: 10.1097/00002030-199816000-00001. [DOI] [PubMed] [Google Scholar]
- 2.PENTA. Comparison of dual nucleoside-analogue reverse-transcriptase inhibitor regimens with and without nelfinavir in children with HIV-1 who have not previously been treated: the PENTA 5 randomised trial. Lancet. 2002;359:733–740. doi: 10.1016/S0140-6736(02)07874-1. [DOI] [PubMed] [Google Scholar]
- 3.Faletto MB, Miller WH, Garvey EP, et al. Unique intracellular activation of the potent anti-human immunodeficiency virus agent 1592U89. Antimicrob Agents Chemother. 1997;41:1099–1107. doi: 10.1128/aac.41.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weller S, Radomski KM, Lou Y, et al. Population pharmacokinetics and pharmacodynamic modeling of abacavir (1592U89) from a dose-ranging, double-blind, randomized monotherapy trial with human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 2000;44:2052–2060. doi: 10.1128/aac.44.8.2052-2060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chittick GE, Gillotin C, McDowell JA, et al. Abacavir: absolute bioavailability, bioequivalence of three oral formulations, and effect of food. Pharmacotherapy. 1999;19:932–942. doi: 10.1592/phco.19.11.932.31568. [DOI] [PubMed] [Google Scholar]
- 6.Hughes W, McDowell JA, Shenep J, et al. Safety and single-dose pharmacokinetics of abacavir (1592U89) in human immunodeficiency virus type 1-infected children. Antimicrob Agents Chemother. 1999;43:609–615. doi: 10.1128/aac.43.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kline MW, Blanchard S, Fletcher CV, et al. A phase I study of abacavir (1592U89) alone and in combination with other antiretroviral agents in infants and children with human immunodeficiency virus infection. AIDS Clinical Trials Group 330 Team. Pediatrics. 1999;103:e47. doi: 10.1542/peds.103.4.e47. [DOI] [PubMed] [Google Scholar]
- 8.Kumar PN, Sweet DE, McDowell JA, et al. Safety and pharmacokinetics of abacavir (1592U89) following oral administration of escalating single doses in human immunodeficiency virus type 1-infected adults. Antimicrob Agents Chemother. 1999;43:603–608. doi: 10.1128/aac.43.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang LH, Chittick GE, McDowell JA. Single-dose pharmacokinetics and safety of abacavir (1592U89), zidovudine, and lamivudine administered alone and in combination in adults with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1999;43:1708–1715. doi: 10.1128/aac.43.7.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanner J. Growth at Adolescence. 2nd. Blackwell Scientific; Oxford, UK: 1962. [Google Scholar]
- 11.D'Argenio DZ, Schumitzky A. ADAPT II User's Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Los Angeles, CA: Biomedical Simulations Resource; 1997. [Google Scholar]
- 12.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9:503–512. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 13.Jullien V, Urien S, Chappuy H, et al. Abacavir pharmacokinetics in human immunodeficiency virus-infected children ranging in age from 1 month to 16 years: a population analysis. J Clin Pharmacol. 2005;45:257–264. doi: 10.1177/0091270004272215. [DOI] [PubMed] [Google Scholar]
- 14.Product Information: Ziagen(R), Abacavir. Research Triangle Park, NC: GlaxoSmithKline; 2000. [Google Scholar]
- 15.McDowell JA, Lou Y, Symonds WS, et al. Multiple-dose pharmacokinetics and pharmacodynamics of abacavir alone and in combination with zidovudine in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2000;44:2061–2067. doi: 10.1128/aac.44.8.2061-2067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staszewski S, Keiser P, Montaner J, et al. Abacavir-lamivudine-zidovudine vs indinavir-lamivudine-zidovudine in antiretroviral-naive HIV-infected adults: a randomized equivalence trial. JAMA. 2001;285:1155–1163. doi: 10.1001/jama.285.9.1155. [DOI] [PubMed] [Google Scholar]
- 17.Fisher MB, Paine MF, Strelevitz TJ, et al. The role of hepatic and extrahepatic UDP-glucuronosyltransferases in human drug metabolism. Drug Metab Rev. 2001;33:273–297. doi: 10.1081/dmr-120000653. [DOI] [PubMed] [Google Scholar]
- 18.Higuchi S, Matsushita S, Masaki T, et al. Influence of genetic variations of ethanol-metabolizing enzymes on phenotypes of alcohol-related disorders. Ann N Y Acad Sci. 2004;1025:472–480. doi: 10.1196/annals.1316.058. [DOI] [PubMed] [Google Scholar]
- 19.Miners JO, McKinnon RA, Mackenzie PI. Genetic polymorphisms of UDP-glucuronosyltransferases and their functional significance. Toxicology. 2002:181–182. 453–456. doi: 10.1016/s0300-483x(02)00449-3. [DOI] [PubMed] [Google Scholar]
- 20.Bhasker CR, McKinnon W, Stone A, et al. Genetic polymorphism of UDP-glucuronosyltransferase 2B7 (UGT2B7) at amino acid 268: ethnic diversity of alleles and potential clinical significance. Pharmacogenetics. 2000;10:679–685. doi: 10.1097/00008571-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Innocenti F, Grimsley C, Das S, et al. Haplotype structure of the UDP-glucuronosyltransferase 1A1 promoter in different ethnic groups. Pharmacogenetics. 2002;12:725–733. doi: 10.1097/00008571-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Shen YC, Fan JH, Edenberg HJ, et al. Polymorphism of ADH and ALDH genes among four ethnic groups in China and effects upon the risk for alcoholism. Alcohol Clin Exp Res. 1997;21:1272–1277. [PubMed] [Google Scholar]


