Abstract
Background
The authors have shown that rats can be retrained to swim after a moderately severe thoracic spinal cord contusion. They also found that improvements in body position and hindlimb activity occurred rapidly over the first 2 weeks of training, reaching a plateau by week 4. Overground walking was not influenced by swim training, suggesting that swimming may be a task-specific model of locomotor retraining.
Objective
To provide a quantitative description of hindlimb movements of uninjured adult rats during swimming, and then after injury and retraining.
Methods
The authors used a novel and streamlined kinematic assessment of swimming in which each limb is described in 2 dimensions, as 3 segments and 2 angles.
Results
The kinematics of uninjured rats do not change over 4 weeks of daily swimming, suggesting that acclimatization does not involve refinements in hindlimb movement. After spinal cord injury, retraining involved increases in hindlimb excursion and improved limb position, but the velocity of the movements remained slow.
Conclusion
These data suggest that the activity pattern of swimming is hardwired in the rat spinal cord. After spinal cord injury, repetition is sufficient to bring about significant improvements in the pattern of hindlimb movement but does not improve the forces generated, leaving the animals with persistent deficits. These data support the concept that force (load) and pattern generation (recruitment) are independent and may have to be managed together with respect to postinjury rehabilitation.
Keywords: Spinal cord injury, Swimming, Task-specific learning, Rat, Locomotor retraining, Rehabilitation
Activity-based rehabilitation, in the form of body weight-supported treadmill training, has been used clinically to promote walking following all but the most severe cervical spinal cord injuries.1,2 This approach developed, in part, from research using the fully transected “spinal cat” model.3 In this model, repetitive exposure of the spinal cord to appropriate patterns of afferent information from the hindlimbs is thought to retrain the lumbar central pattern generating circuitry to produce a coherent and organized stepping pattern.4,5 Repetitive exposure to this activity in patients, over a period of weeks or months, can result in significant improvements in autonomous stepping on the treadmill and, in some cases, patterns of muscle activation and limb kinematics that approach normal.5
Both clinical and basic studies have shown that loading of the limbs and cutaneous input from the foot/paw are important components of the afferent pattern6,7 during successful retraining. Also inherent to the retraining process is task specificity, as demonstrated by Hodgson et al,8 who showed that training to stand brings about improvements in weight bearing without significantly influencing the generation of a stepping pattern. Although the majority of clinical reports describe slow but significant improvements in walking over a period of weeks (in the chronically injured), a recent study by Boyce et al9 suggests that application of a growth factor cocktail in an animal model can uncover latent or hidden capabilities within hours or days, thereby drastically reducing the time required to reach maximal functional recovery. Importantly, they found that the combination of growth factors and retraining is more effective than either approach alone. Needless to say, the mechanisms underlying the process of retraining after spinal cord injury, either in the acute or chronic situation, are still unknown.
We showed recently that rats with moderately severe T9 contusive spinal cord injuries can be retrained to swim by exposure to water for 24 minutes per day (6 × 4 minute sessions) starting at 2 weeks postinjury. We further showed that supplemental cutaneous feedback in the form of buoyant inverted centrifuge tubes suspended from the pool bottom can enhance hindlimb activity during the retraining process,10 as was previously shown for chicks by Muir and Steeves.11 In our experiments, rats were acclimated to the swimming pool for several days preinjury and appeared to become comfortable rapidly. Postinjury rats demonstrated little distress in the water and rapidly learned to swim using their forelimbs for propulsion, which are not normally active during swimming. Injured animals also had some difficulty retaining a normal body position in the water. These observations formed the basis for the Louisville Swim Scale (LSS), a tool we developed for assessing injured animals while swimming based on forelimb dependency, hindlimb activity, and body position.12 We found that injured animals retrained to swim starting 2 weeks postinjury transitioned from relying completely on their forelimbs for forward motion to exhibiting frequent to consistent hindlimb kicking (50% to 95% of the time) over a 3- to 4-week period and that their hindlimb movements were more consistent in the presence of supplemental cutaneous feedback. Few untrained animals recovered even occasional hindlimb kicking, and they remained entirely dependent on their forelimbs for forward motion.10
The kinematics of swimming and walking have been described and compared for adult rats by Gruner and colleagues.13,14 They reported that the relative duration of the 2 phases of the stroke cycle are reversed in swimming when compared with walking: the power stroke phase is short and fairly consistent in duration, whereas the recovery phase is longer and varies with swimming speed and acceleration. The stance phase of walking is generally longer in duration than the swing phase and varies significantly with walking speed. The shorter swing phase remains relatively constant in duration as walking speed varies. They also reported that the durations of the swing (walking) and recovery (swimming) phases were just less than 200 ms and were not significantly different when the 2 activities were compared. In contrast, the power stroke during swimming had a duration of around 50 ms, whereas the stance phase of walking had a mean duration of almost half a second (435 ± 77 ms).
The reversal of relative phase durations between swimming and walking in rats suggests that swimming may be more analogous to bipedal running than to walking. In particular, the stance/swing duration ratio for fast running is commonly 0.25 to 0.30,15 which is comparable to the stroke/recovery duration ratios exhibited by swimming rats.13,14 It is interesting to note that walking and running are differentiated by the presence of a double-limb support in walking that is absent in the flight phase (running). This distinction is directly associated with the swing/stance duration ratios being above and below unity (1.0) for walking and running, respectively.
The early work by Gruner and colleagues13,14 used video tape and manual measurements of the limb parameters in a labor-intensive study. They focused on the characteristics of swimming, relative to walking on a treadmill, in normal adult rats and did not assess either mode of locomotion until the experimental animals had become well acclimated to the activity. To extend on these findings, the current study had 2 primary goals. First, to determine if adult rats refine or alter their hindlimb movements when first exposed to the activity of swimming as adults, and second, to compare how adult rats swim before and after a moderately severe spinal cord injury, during the process of postinjury retraining. To accomplish these goals, we develop a streamlined and efficient kinematic analysis of swimming based on a 2-angle, 3-segment hindlimb model that allows hindlimb movements during swimming to be quantitatively described by a single angle–angle plot, plus a measure of peak limb velocity.
Methods
Learning to Swim
All procedures involving experimental animals were performed according to the guidelines of the University of Louisville Institutional Animal Care and Use Committee. Seven young adult female Sprague-Dawley rats (200-225 g) that had not previously been exposed to water were handled for several minutes each day for 2 weeks prior to the start of the experiment. Each animal was placed in the swimming pool, and the first 3 passes (lengths of the pool) were filmed for analysis (see below). Each swimming session was 4 minutes in duration, and each animal swam once each morning and afternoon, for 2 days, and digital video was captured for at least 3 passes from each session.
In general, when the animals were first introduced to the water, they swam in place for a few seconds in a tail-down position and then rapidly adopted a normal horizontal swimming position and traversed the length of the pool to the exit ramp. By placing the animals within the camera's field of view, we were able to capture the very first hindlimb kicks made by each animal once it achieved a horizontal body position. Over the following 4 weeks, each animal swam twice each morning and afternoon, for a total of 16 minutes per day, 4 days a week, prior to receiving a spinal cord injury.
Seven days after injury, animals were reintroduced to the swimming pool for their initial LSS assessment, and they began swim training with supplemental cutaneous feedback 8 days postinjury with 2 × 4 minute training sessions on days 8, 9, and 10. They received no training on postinjury days 11, 12, and 13, and they received their week 2 LSS assessment on day 14. Starting on day 14 (after LSS assessment), the animals received 6 × 4 minute swim training sessions for 4 days each week as described previously.10 LSS assessments were performed weekly, and kinematic assessments were done on weeks 3 and 6 (terminal).
Spinal Cord Injury
Rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneal) and were given prophylactic gentamicin (antibiotic, 15 mg/kg, subcutaneous). Each eye was treated with lacri-lube to prevent dryness. A dorsal midline incision was made over the thoracic spinal cord, and a single-level laminectomy was performed at the T9 vertebra to expose the T10 spinal cord. The spine was immobilized using clamps applied to the T8 and T10 spinous processes, and the NYU Impactor (W. Young, Rutgers University, Piscataway, NJ) was used to produce either a moderate (12.5 g cm) or moderately severe (25 g cm) contusion injury. After injury, the incision was closed in layers, and a topical antibiotic ointment was applied to the incision. Each animal was given the analgesic buprenorphine (5 mg/kg, intraperitoneal) on the day of surgery and again the next morning. The animals were placed in recovery cages on heating pads until they recovered from the anesthesia. Injured animals were housed individually and received daily postoperative care, including manual bladder expression until adequate spontaneous voiding occurred.
Kinematic Set-Up
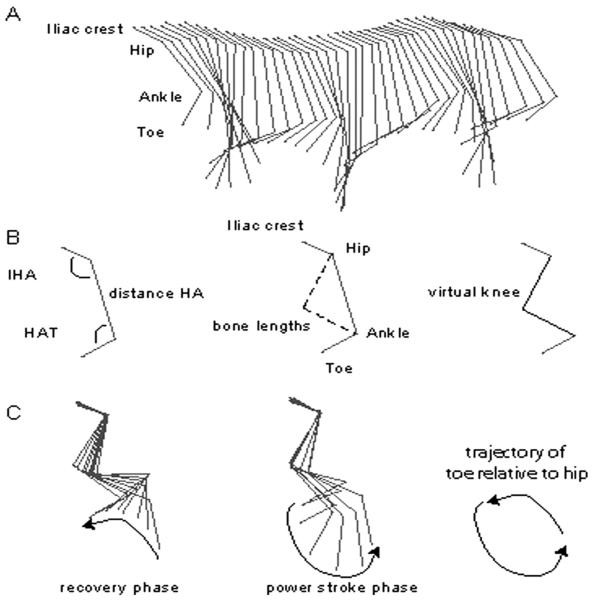
Although the kinematics of stepping and swimming in rats have been described,13,14,16 kinematic assessment itself remains very labor intensive, relies heavily on expensive hardware and software packages, and is sometimes difficult to interpret. Kinematic analysis of rodents is also prone to inaccuracy due to the movement of skin overlying the joints, in particular for the knee.13,17 We have developed a relatively streamlined system for the kinematic analysis of swimming using 2-dimensional (2D) stick figures representing the limb as 2 angles derived from 3 segments (Figure 1). The 3 segments are iliac crest (I) to hip (H), H to ankle (A), and A to toe (T). The 2 angles formed by the 3 segments describe the movement of the hindlimb as proximal (IHA; hip and knee) and distal components (HAT; knee and ankle; Figure 2). During swimming in uninjured animals, the cyclic changes in IHA and HAT angles are almost out of phase, and the angle–angle plot forms an ellipsoid (Figure 3). The area of the ellipsoid describes the out-of-phase excursion of the 2 angles. The peak and trough of each axis describe the angular excursions, and the centroid of the ellipsoid describes the relative relationship of the angular changes during the movement. Thus, a quantitative description of hindlimb movement during swimming can be made using the area, peak and trough of each angle, and the centroid of the angle–angle plot. Changes to the movements can be sensitively detected, and different swimming patterns will appear as different shapes on the angle–angle plot and can be objectively quantified.
Figure 1. Two-Dimensional Stick Figures Represent the Hindlimb.
Note: A, Stick figures representing more than 2 complete stroke cycles using the 3-segment and 2-angle (IHA and HAT) model. B, Stick figures representing the 3-segment and 4-segment models, the latter including the virtual knee calculated using the known bone lengths for the femur and tibia, the known hip-to-ankle distance (HA), and the known angles “a” and “b” (IHA and HAT). C, A complete swimming stroke cycle broken down into the power stroke and recovery phases using the 4-segment model. Also shown is the elliptical trajectory of the toe relative to the hip for 1 stroke cycle. IHA indicates iliac crest–hip–ankle; HAT, hip–ankle–toe; HA, hip–ankle.
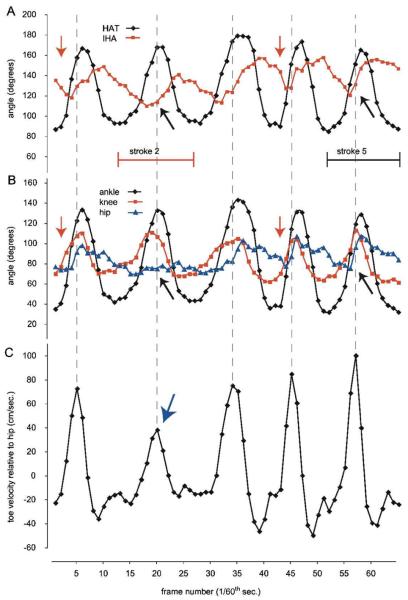
Figure 2. Angular Excursion and Toe Velocity for Stick Figures Representing the Hindlimb.
Note: A, The changes over time in the IHA and HAT angles using the 3-segment model for 5 complete stroke cycles. B, The same 5 stroke cycles are shown as changes over time in the hip, knee, and ankle angles using the 4-segment model. The red arrows indicate that HAT (in A) or knee (in B) extension precedes extension of the other angles for each step cycle. The black arrows indicate the reduced or delayed extension of the IHA (in A) or hip (in B) during the power stroke phase of stroke 2, and for comparison the same points in stroke 5. C, The velocity of the toe relative to the hip, with the rostrocaudal direction (power stroke) being shown as positive velocity. The blue arrow indicates the reduced peak toe velocity that results from the reduced hip extension in stroke 2 when compared with stroke 5. IHA indicates iliac crest–hip–ankle; HAT, hip–ankle–toe.
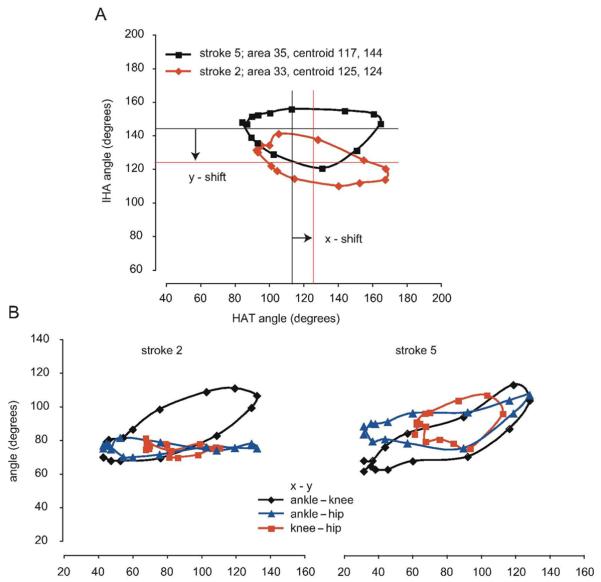
Figure 3. IHA–HAT Angle–Angle Plot for Strokes 2 and 5.
Note: A, The IHA–HAT angle–angle plot for strokes 2 and 5 from Figure 2. It can be seen that the ellipsoid representing stroke 2 has a reduced area and a shifted centroid, indicating that the proximal angle (IHA) is less extended overall and that the distal angle (HAT) is more extended overall. B, Strokes 2 and 5 are also shown as separate angle–angle plots for the ankle–knee, ankle–hip, and knee–hip. The ankle–hip and knee–hip plots are compressed for stroke 2 when compared with stroke 5 because of the reduced extension of the hip. IHA indicates iliac crest–hip–ankle; HAT, hip–ankle–toe.
The swimming pool used was 60-in. long, 6.5-in. wide and 12-in. deep and was constructed of 3/8th-in. thick plexiglas as described previously.10,12 The narrow lane (less than 6 in.) and tendency for the rats to swim down the middle, rather than along the side of the pool, helped reduce the variability in camera–subject distance. The pool has a neoprene-covered ramp at one end, which permits even injured rats to exit the pool with ease. Prior to filming, each rat had its flanks and hindlimbs shaved and received green tattoos (Aramis micro-tattoo punch, Braintree, MA) on the skin overlying the iliac crest and hip (greater tro-chanter). These tattoos allowed the markers, made with black sharpie pens, to be placed accurately for the duration of the experiment without having to reanesthetize the animals. Sharpie marks were also placed on the ankle (lateral malleolus) and the metatarsal phalangeal joint of the toe. The filming was done using a Basler 602f high-resolution digital camera (Basler Vision Technologies, Exton, PA), running at 60 Hz. Digital video was acquired in AVI format using the software package DVR Explorer (Advanced Digital Vision, Natick, MA). The camera was set 18 in. from the side of the pool, which allowed the capture of 6 to 7 complete stroke cycles, per pass, from an uninjured animal. We captured a minimum of 3 complete passes and digitized a minimum of 9 stroke cycles from animals that kicked with their hindlimbs while swimming. For injured animals that were not kicking, a short video segment from each pass was divided into virtual strokes, 15 frames each, and analyzed.
Uninjured animals adopt a very consistent, dorsoventral position during swimming and exhibit little variation in body angle or rotation about the long axis during swimming.12 Thus, the movement of the near-side hindlimb remains within the vertical plane and can be accurately described in 2 dimensions. However, postinjury, animals do experience variable body rotation about the long axis, which can push the hindlimb out of the vertical plane and add variability to the angle–angle plots. To minimize this variability, we attempted to capture and analyze videos of passes where the body rotation was minimal (hindpaws were at a similar depth during the power stroke phase).
AVI files were processed using MaxTraq, a software package developed by Innovision Systems (Columbiaville, MI). The iliac crest and hip markers were identified and digitized semiautomatically using the built-in point recognition algorithm, whereas the ankle and toe markers were digitized manually. For comparison purposes, videos were also analyzed using the plug-in MaxVJR (virtual joint recognition) to include the position of the knee based on known femur and tibial bone lengths. Data files were created that included the iliac crest–hip, hip–ankle, and ankle–toe distances, plus the IHA and HAT angles with the video frame numbers representing increments of 1/60th of a second. MaxTraq output files were further analyzed in MaxMate, a plug-in for Microsoft Excel, also developed by Innovision Systems. Within MaxMate, we generated angle–angle plots for each stroke sequence and used elliptic Fourier analysis to obtain a complete and objective description of each plot. We determined the area and centroid as described by Ferson et al18 using the freeware program EFAW (http://life.bio.sunysb.edu/morph/).
Results
Kinematic Analysis of Normal Swimming
Figure 1A depicts stick figures illustrating hindlimb motions for normal swimming obtained using the 2-angle, 3-segment model (iliac crest–hip–ankle and hip–ankle–toe) hindlimb model. As shown in Figure 1B, the position of the knee for the 3-angle, 4-segment model was calculated using MaxVJR (Innovision Systems). In Figure 1C, stick figures, which include the virtual knee, are shown for a typical swimming (stroke) cycle. The cycle is divided into power stroke and recovery phases, with the segment positions shown relative to the hip. Also shown in Figure 1C is the elliptical trajectory of the toe marker.
A single swimming pass with 5 stroke cycles is illustrated in Figure 2 as joint angle trajectories obtained using the 2-angle (A) and 3-angle (B) models. These data are taken from 1 representative animal after 4 weeks of daily swimming and prior to receiving a spinal cord injury. In Figure 2C, the toe velocity (relative to the hip) is shown for the same cycles, with positive velocity representing the rostrocaudal direction (power stroke). It is of interest to note that in Figure 2B (red arrow) the power stroke is initiated by extension of the knee and ankle, which moves the foot forward prior to hip extension. This sequence is represented in Figure 2A (red arrow) as the initiation of extension of the HAT angle (black diamonds) prior to the initiation of extension of the IHA (red squares). The peak toe velocity, indicated by the vertical dashed line linking the 3 graphs, is achieved part way through each power stroke after which the distal segments decelerate prior to the initiation of the recovery phase.
During the second stroke cycle shown in Figure 2, the animal appeared to attenuate overall limb movement during the power stroke phase. It can be seen that hip excursion was reduced to only a few degrees (blue triangles, indicated by the black arrow in Figure 2B). This attenuation of limb movement can also be seen as a delay in the IHA angle extension (red squares, indicated by the black arrow in Figure 2A), compared with, for example, stroke cycle 5 (as indicated by a second black arrow). As a result of the reduced hip excursion, the peak toe velocity in cycle 2 was reduced by 40%, to less than 40 cm/s (blue arrow in Figure 2C). For comparison, stroke 5 involved an effective pattern of knee and ankle (HAT) extension preceding hip extension resulting in a peak toe velocity of approximately 100 cm/s.
The angle–angle plots for strokes 2 and 5 are shown in Figure 3, with Figure 3A showing HAT–IHA and Figure 3B showing ankle–knee, ankle–hip, and knee–hip plots. As mentioned earlier, stroke 2 involved a reduced hip excursion, which is shown as a shift in the angle–angle plot centroid along the y axis and a compression of the angle–angle plot along the y axis resulting in a slightly reduced plot area (from 35 to 33°2). The angular displacement changes were quantified by a centroid shift of −20° in the y axis and a shift of +8° in the x axis. This indicates that the proximal angle representing the hip and knee is less extended throughout the cycle for stroke 2, whereas the distal angle representing the knee and ankle is slightly more extended throughout the stroke cycle (Figure 3A). These changes in the IHA–HAT angle–angle plots were explored further by quantifying the angular displacement adaptations seen in the ankle–knee, ankle–hip, and knee–hip angle–angle plots, as shown in Figure 3B. These plots are compressed in the y axis for stroke 2 but not for stroke 5.
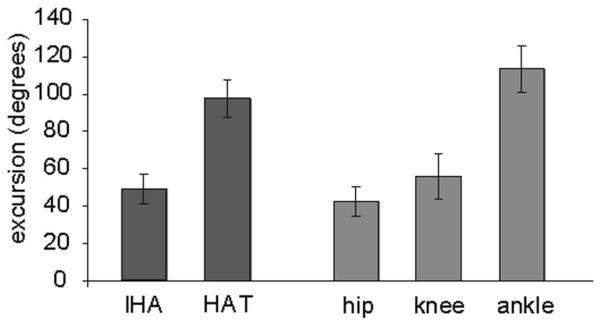
The mean excursions for the IHA and HAT angles across all 7 experimental animals after 4 weeks of swimming, but pre-spinal cord injury, are shown in Figure 4. The IHA angle moves through a much smaller range when compared with the HAT angle. The data are shown as mean ± standard deviation and illustrate that there is only moderate interanimal variability with respect to joint excursions. In particular, it is of interest to note that we did not directly control for swimming velocity, but simply tried to capture and analyze swimming strokes when the animals were “at speed,” in the middle of the pool rather than during acceleration or deceleration. Presumably, if we had controlled for velocity the variability observed would be even lower. In contrast to walking, which involves paw contact and liftoff as 2 distinct points of reference to which other parameters can be compared, swimming involves a freedom to move in 3-dimensional space with the only constraints being the size and shape of the pool and the need to stay at the surface. Despite that freedom, the variability observed in limb movements during forward swimming was quite low. The mean angular excursions for the hip, knee, and ankle across all 7 experimental animals are also presented in Figure 4. The ankle joint has close to twice the mean angular excursion of the other 2 joints. Therefore, we can conclude that the differences between the HAT and IHA angular excursions are due to the greater range of motion exhibited by the ankle joint when compared with the hip and knee. It is worth noting that the variability of these angles, represented by the standard deviation, is also quite low.
Figure 4. Mean Excursions for the IHA and HAT Angles for all 7 Experimental Animals.
Note: The mean excursions (±SD) for the hip, knee, and ankle when compared with those for the IHA and HAT angles representing the 4-segment and 3-segment models, respectively. These are from all 7 animals examined at the 4-week time point after swimming twice a day, 4 days each week for 4 weeks. IHA indicates iliac crest–hip–ankle; HAT, hip–ankle–toe.
Acclimatization Period
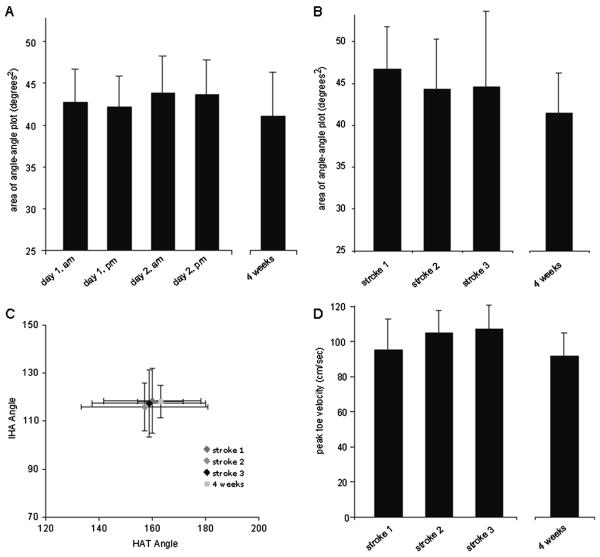
To address the question of whether or not rats alter or refine their hindlimb movements when first exposed to water as adults, presumably during an acclimatization period prior to baseline measurements being taken, we compared mean areas and centroids of HAT–IHA angle–angle plots and the mean peak toe velocities (relative to the hip) for these 7 uninjured rats at different time points. In Figure 5A, we compare the mean areas (±SD) of angle–angle plots for the 4 swimming sessions with the final preinjury session at week 4 (32nd swimming session). The mean area was not different for any 2 sessions and there were no changes in area over time. In Figure 5B, 5C, and 5D, the area and centroid of angle–angle plots and the mean peak toe velocities for the first 3 swimming strokes (hindlimb kicks) taken are compared with the final preinjury session at week 4. There were no differences found in mean area, centroid, or toe velocity for any 2 of the initial 3 swimming strokes, or when each measurement was compared with the final swimming session at week 4. These data indicate that the very first few strokes performed by these adult rats when first introduced to water are very similar to each other and quite similar to those performed 4 weeks later after the expression of more than 1000 strokes in the interim. Please note that 1 of the 7 animals performed only a single stroke with a horizontal body position during the initial pass, which was included in the data. The animal then adopted a tail down escape response for several seconds; consequently, no data were obtained for the second and third strokes.
Figure 5. Mean Angle-Angle Plot Data.
Note: A, The mean areas of the HAT–IHA angle–angle plots are shown for the morning and afternoon swimming sessions of days 1 and 2, and these values are compared with the mean area from the 4-week preinjury baseline session. B, The mean area and centroids for the angle–angle plots from the first strokes performed during the first pass (pool length), comparing them to the preinjury baseline taken at 4 weeks. C, The mean HAT and IHA centroids of the angle–angle plots illustrating the limb position during swimming did not change from stroke 1 to stroke 3 or to a stroke performed after 4 weeks of preinjury swimming. D, The mean toe velocities (relative to the hip) for the first 3 stroke cycles performed when compared with that from a swimming session after 4 weeks of preinjury swimming. There were no significant changes in any kinematic parameter over time with daily sessions of preinjury swim training. IHA indicates iliac crest–hip–ankle; HAT, hip–ankle–toe.
Postinjury Swimming
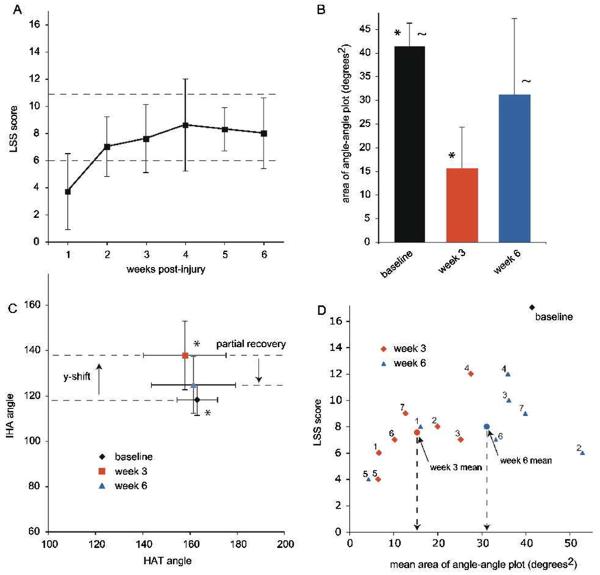
Figure 6A shows the mean LSS scores for each postinjury assessment, which increased significantly over time (P < .05), as we reported previously.10 Interestingly, the LSS scores show no significant improvement after week 2, at which point all but one of the animals scored in the 6 to 11 (moderate) range of the scale, indicating that they had frequent to consistent hindlimb kicking but were still dependent on their forelimbs for forward motion.10 Figure 6B shows that the mean area of the HAT–IHA angle–angle plot was significantly lower than baseline at week 3 postinjury (P < .05), and recovered toward baseline by week 6. In Figure 6C, the HAT–IHA angle–angle plot centroids show a significant y shift following injury (P < .05), indicating that the IHA angle (hip and knee) is more extended. This y shift shows a partial recovery toward baseline by week 6. Figure 6D shows a scatterplot of the mean area of the HAT–IHA angle–angle plots for individual animals at weeks 3 and 6 versus their LSS scores at those time points. The means, shown as red and blue circles (indicated by arrows), illustrate that continued training brings about significant improvements in swimming kinematics, reflected as angle–angle plots with increased areas and limb positions closer to normal, despite the fact that the LSS scores do not significantly increase after postinjury week 2.
Figure 6. Postinjury Assessments: Louisville Swim Scale and Angle-Angle Plots.
Note: A, The mean Louisville Swim Scale (LSS) scores for all 7 animals, over time postinjury. The dashed lines indicate the transition points in the scale when most animals acquire frequent to consistent hindlimb movement with some retained reliance on forelimbs for forward motion (6-7) and when animals show consistent hindlimb alternation with only occasional reliance on forelimbs for forward motion (11-12). B, The mean areas of the angle–angle (HAT–IHA) plots are shown for preinjury baseline and weeks 3 and 6 postinjury. There is a significant difference in area at week 3 compared with baseline (*; P < .001) and at week 6 compared with week 3 (~; P < .05). C, The mean x and y centroids for the angle–angle plots are shown for preinjury baseline and postinjury weeks 3 and 6. There is a significant shift in the y position (IHA) at week 3 (*; P < .05) compared with baseline and a recovery toward baseline by week 6. D, Weeks 3 and 6 postinjury LSS scores are plotted against the area of the angle–angle plots for individual animals. The mean angle–angle plot area increases significantly from week 3 to week 6 despite the fact that the mean LSS scores do not change over that time period. IHA indicates iliac crest–hip–ankle; HAT, hip–ankle–toe.
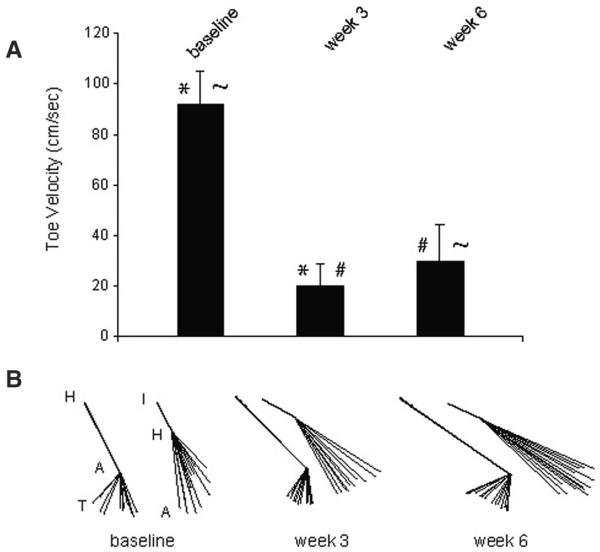
The improvement in angular excursion was associated with a modest but statistically significant increase in toe velocity (relative to the hip) from 20.0 ± 8.5 at week 3 to 29.6 ± 14.3 cm/s at week 6 (mean ± SD; P < .05). However, the velocity remains significantly reduced when compared with the preinjury level of 91.8 ± 13.1 cm/s (P < .001). Figure 7B shows stick figures taken from a single animal representative of the group at preinjury baseline and at weeks 3 and 6 postinjury. The excursion of the HAT and IHA angles is illustrated by registering the hip–ankle and iliac crest–hip segments of stick figures comprising 1 complete stroke cycle. These illustrate the recovery toward baseline of the excursion of the HAT angle, in particular, and also illustrate a persistent deficit in overall limb position even following 4 weeks of swim training (postinjury week 6).
Figure 7. Toe Velocities and Stick Figures.
Note: A, The mean toe velocities (relative to the hip) for the entire group at baseline (after 4 weeks of preinjury training), at 3 weeks (after 8 days of postinjury training), and at 6 weeks (after 20 days of postinjury training). There is a significant decrease in toe velocity at week 3 compared with baseline (*; P < .001), a significant improvement in velocity from week 3 to 6 with continued training (#; P < .05), but the velocity remains significantly below baseline (~; P < .05). B, Stick figures representing single strokes taken from a representative animal at baseline, week 3, and week 6. These have been registered for the hip–ankle segment to show the range of motion of the HAT angle and for the iliac crest–hip segment to show the range of motion of the IHA angle.
Discussion
Studies on locomotor retraining in animal models of spinal cord injury have primarily focused on complete transections and treadmill step-training with weight support,6,19 approaches that do not translate into improved open-field locomotion and that are not particularly relevant to the clinical situation where the vast majority of injuries are contusive. When clinically relevant contusive injuries are used in adult rat models, retraining is not very successful, at best resulting in very modest improvements in overground stepping,20,21 which are additive to the already remarkable functional recovery shown following even moderate to severe injuries with as little as 10% to 20% spared white matter at the injury epicenter.22 Based on the hypothesis that a retraining strategy involving a large number of “step” cycles generated where weight support is provided will bring about improvements in locomotion, we used swimming, with or without supplementary cutaneous feedback, to retrain adult rats starting 10 days after a moderately severe contusive spinal cord injury at T9.10 We found that hindlimb movement and body position improves rapidly with training and that the majority of animals exhibit at least frequent hindlimb movement (51% to 95% of the time) by 4 weeks postinjury, after only 12 days of training. Despite the improvements in hindlimb movement during swimming, no improvements in overground locomotion were observed, leading us to suggest that swim training after spinal cord injury is a task-specific model of plasticity in the spinal cord. To date, our swim training studies have relied on the LSS, an assessment tool developed in our laboratory based on the characteristics of swimming before and after a thoracic contusion spinal cord injury.12 Although the LSS appears to be both sensitive and robust, it is by nature subjective and nonlinear and would benefit from being paired with a sensitive and quantitative kinematic assessment.
Walking and swimming are normal locomotor activities for rats in the wild, but for laboratory rodents swimming is restricted to experimental paradigms. Although swimming and walking share certain key characteristics such as hindlimb and flexor/extensor alternation, there are several important differences. Swimming is normally a bipedal activity for uninjured rats, where the forelimbs are used exclusively for steering and for making contact with the walls of the pool or objects in the water. It lacks limb loading (weight bearing) and the associated cutaneous afferent feedback from the plantar surface of the paw. Intralimb coordination is also distinct for swimming because the extension phase (power stroke) is rapid and consistent in duration, whereas the flexion phase (recovery) is longer and more variable. This relative duration (duration ratio) is opposite for walking, where the extension phase (stance) is longer in duration and more variable than the flexion (swing) phase.13,16 Paw contact and liftoff are kinematic events that clearly mark the phase transitions from swing to stance and stance to swing during stepping and also provide a dimensional constraint where the paw, normally marked by the metatarsal phalangeal joint, is essentially stationary with respect to the walking surface during stance. In contrast, during swimming the hindlimbs are never stationary with respect to their surroundings and experience no dimensional constraints. Clear transitions between the extension-dominated power stroke phase and the flexion-dominated recovery phases of swimming are difficult to discern, but it does appear that knee flexion and extension initiate the recovery and power stroke phases, respectively.13,23
In addition to these functional and kinematic distinctions, both Gruner and Altman13 and de Leon et al16 noted that while the order of hindlimb muscle recruitment during walking and swimming were similar, the patterns of recruitment were very different. In particular, de Leon et al16 noted that knee extensor recruitment was biased toward the soleus during walking and the medial gastrocnemius during swimming. Both groups also noted that tibialis anterior, the primary ankle flexor, was activated for a greater proportion of the overall cycle and with a greater amplitude during swimming than during walking and attributed this to the resistance to flexion of the water. These and other observations lead to the suggestion that the differences in recruitment pattern reflect a significant reorganization within the pattern-generating circuitry, which may involve either or both central mechanisms alone and/or central responses to peripheral input. It is important to note that for each of these studies, and a number of others,20,24 the experimental animals were extensively pre-trained on the treadmills and in the swimming pools prior to kinematic and electrophysiological assessment.
Taken together, these observations suggest that whereas walking and swimming share some key characteristics, the intralimb coordination, patterns of motoneuron recruitment, and, by inference, patterns of interneuron activity and role(s) of afferent input are very different. These suggestions lead us to hypothesize that laboratory rats would need to go through a learning process when first introduced to the water as adults and that the acclimatization process would involve changes, over time, in hindlimb movement during swimming. In other words, is the task specificity of swim and step (or stand) training observed after spinal cord injury observable in some form in the uninjured animal? Thus, one of the main goals of the current study was to describe the hindlimb movements of rats when first exposed to the water as adults and to determine if they go through a period of learning to swim, which would be inferred by changes in their kinematics over time. As mentioned earlier, a number of previous publications have already provided comprehensive and comparative descriptions of the kinematics of walking and swimming following an acclimatization period, so this comparison was not undertaken.13,23
Learning to Swim
Our results illustrate that the first few kicks made by adult rats when first introduced to the water are kinematically very similar to those made later in the same swimming session, to those made in subsequent swimming sessions, and to those made after 4 weeks of daily swimming activity (16 minutes per day, 5 days per week). We found no significant changes in the angular excursions and phase relationship of the iliac crest–hip–ankle (IHA) and hip–ankle–toe (HAT) angles (which are represented in the angle–angle plots), the average limb positions throughout the swimming cycle (which are represented as the x and y centroids of the angle–angle plots), or the peak toe velocity (relative to the hip), which directly reflects the force of limb extension during the power stroke phase. These observations suggest that adult rats express a mature and complete swimming pattern immediately on immersion and do not go through a learning process. Furthermore, because our comparisons included swimming strokes over the first 4 swimming sessions (2-day period) and a swimming session after 4 weeks of daily swimming, we showed that significant changes in the pattern of limb movement do not result when the activity is no longer novel or when the animal is given ample opportunity to develop a more efficient strategy.
These observations suggest that the swimming pattern of activity is either “hardwired” in the spinal cord neural circuitry or that it represents a baseline or “default” pattern that is expressed in a final or mature form immediately on presentation of a given set of descending and afferent input. As mentioned earlier, given what we know about the locomotor activities of swimming as compared with overground stepping13,16,23 and running,15 swimming must involve a unique combination of neural pathways and recruitment patterns. For example, because swimming in uninjured rats is a bipedal activity, the involvement of the interenlargement pathways that mediate hindlimb–forelimb coordination during walking or running must be absent or significantly altered. The “clock” circuitry controlling the cycle frequency must run faster during swimming, and the interneurons responsible for phase duration ratio (extension–flexion switching) must respond to a very different pattern of afferent input that lacks the plantar cutaneous and extensor loading information that normally accompanies paw contact and weight support during the stance phase of stepping or running. Despite these fundamental differences between overground locomotion and swimming, the adult rat central nervous system and musculoskeletal system are capable of expressing a novel and complex locomotor activity in a mature and final form instantly, when first exposed to the water. Even with extensive practice, the initial kinematic strategy chosen remained unchanged, suggesting that uninjured animals do not adopt more efficient strategies over time but appear to rely on the hardwired pattern they expressed with their very first swimming strokes.
Swimming Following Spinal Cord Injury
A secondary goal of the current study was to evaluate the changes in hindlimb swimming activity during post–spinal cord injury swim training. We showed previously that swim trained animals with moderately severe contusion injuries at T9 transition from relying almost entirely on their forelimbs for forward motion (LSS scores <6) to a combination of forelimb and hindlimb kicking at 2 to 3 weeks postinjury (LSS scores >6 but <11). Using the 3-segment, 2-angle model, we compared the kinematics of animals undergoing swim training at 3 and 6 weeks postinjury with their baseline (preinjury) measures. These animals had LSS scores of 8 by week 3 and showed no further improvement at week 6. However, at 6 weeks postinjury, these animals demonstrated an increase in the angle–angle plot area and a negative shift in the y position of the centroid, compared with week 3, indicating greater out-of-phase joint excursions and a more flexed average limb position that approaches the preinjury baseline assessment (Figure 6). These results suggest that the rapid increase in LSS score observed over the first few training sessions is followed by continued improvement in hindlimb movements that go beyond the largely qualitative changes assessed by the LSS of occasional, frequent, or consistent hindlimb movement and alternation. These data suggest that swim training initiated at 1 to 2 weeks postinjury is sufficient to improve the amount of hindlimb movement during swimming at 3 weeks but that training from 3 to 6 weeks postinjury brings additional improvements in the angular excursions and average limb position that are not significantly different from baseline measures.
Despite these obvious improvements in limb excursion resulting from post–spinal cord injury swim training, the effectiveness of the hindlimb strokes remained poor because the limb velocity being generated, measured as toe velocity relative to the hip, remained significantly below baseline levels (Figure 7A). This observation suggests that the mechanisms underlying the task-specific improvement in the pattern of hindlimb activity brought about by repetitive training are distinct from those that would bring about an improvement in the forces generated by the extensor muscles and may reflect the fact that swim training involves very little loading of the extensor muscle groups.
Conclusion
These experiments were designed to document changes in kinematics that we hypothesized would occur over time with exposure to the novel but normal activity of swimming in adult rats. The data show that the kinematics of the first swimming stroke are very similar to every subsequent stroke generated. This observation suggests that a complex locomotor pattern that is normal for a given animal can be established and maintained in the central nervous system during development even if the activity is not experienced until adulthood. Arguably, this conclusion may be questioned if the pattern can be thought of as baseline or “default” for the circuitry involved. However, if this were the case for swimming, one would anticipate that some form of adaptation (plasticity) might allow for minor adjustments to the pattern over time to improve efficiency. No changes in the pattern were observed, however, even following extensive exposure to the activity. Following a mid-thoracic, incomplete spinal cord injury, the hindlimb activity associated with swimming was extinguished but returned with repeated exposure to the activity in a rehabilitation setting. The process of retraining appeared to involve 2 phases with hindlimb movement being established over the first 1 to 2 weeks of training, as we described previously,10 and a later phase leading to improvements in overall limb position and angular excursion during the stroke cycle. The mean velocity of the toe, relative to the hip, remained significantly below normal despite showing some improvement between 3 and 6 weeks postinjury. This greatly reduces the effectiveness of the swimming stroke, so the retrained animals remained far below normal animals in their ability.
Based on these and earlier findings,10,20 we propose that swimming provides a novel model of locomotor learning and post–spinal cord injury activity-based retraining where, unlike for stepping, exposure to the retrained activity is controlled entirely by the experimenter. Swimming and the recovery of swimming with retraining can be easily and quantitatively assessed using a combination of the LSS12 and 2D kinematics of a 3-segment, 2-angle model of the hindlimb and the quantification of the HAT–IHA angle–angle plot plus toe velocity. Whereas the activity of swimming is very different from stepping, adult female Sprague-Dawley rats do not refine their swimming pattern during initial exposure to the activity but instead express a mature and complete swimming pattern, suggesting that the neural pathways and recruitment patterns are relatively hardwired. After spinal cord injury, the persistent deficit in toe velocity, despite the significant improvement in limb excursion, suggests that the lack of load applied to the limb during swim training may specifically limit the recovery of force during the extension phase of the stroke cycle. These data support the suggestion made by numerous authors25,26 that load (weight support) and pattern generation (recruitment order) may be independent with respect to post–spinal cord injury rehabilitation.
In the clinical setting, task specificity has been demonstrated for standing and stepping;8 however, the potential improvements that might be brought about by employing a strategy where limb loading is reduced while cycle number and frequency are increased are presently unknown. In total, our observations using swim training in adult rats with incomplete contusion injuries suggest that swimming and overground stepping are task specific. However, as suggested previously,10,20 the retraining that occurs “spontaneously” postinjury as the animals move about in their cages may render any additional improvements due to swim training as indistinguishable. It is tempting to speculate that a combinatorial approach using both limb loaded and unloaded strategies may prove effective by enhancing extensor function and weight support in addition to improving pattern generation.
Acknowledgments
The authors would like to acknowledge the excellent technical assistance of Alice Shum-Siu, Ashley Whelan, and Victoria Druzhinina. The authors also wish to thank Kim Fentress, Aaron Puckett, Johnny Morehouse, and Christine Nunn for animal care, behavioral analysis, and surgical support. This work was supported by The Kentucky Spinal Cord and Head Injury Research Trust and the NIH/NINDS (R01 NS052292).
References
- 1.Dobkin B, Apple D, Barbeau H, et al. Weight-supported treadmill vs overground training for walking after acute incomplete SCI. Neurology. 2006;66:484–493. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobkin BH. Confounders in rehabilitation trials of task-oriented training: lessons from the designs of the EXCITE and SCILT multicenter trials. Neurorehabil Neural Repair. 2007;21:3–13. doi: 10.1177/1545968306297329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- 4.Rossignol S, Chau C, Brustein E, Belanger M, Barbeau H, Trevor D. Locomotor capacities after complete and partial lesions of the spinal cord. Acta Neurobiol Exp. 1996;56:449–463. doi: 10.55782/ane-1996-1148. [DOI] [PubMed] [Google Scholar]
- 5.Barriere G, Leblond H, Provencher J, Rossignol S. Prominent role of the spinal central pattern generator in the recovery of locomotion after partial spinal cord injuries. J Neurosci. 2008;28:3976–3987. doi: 10.1523/JNEUROSCI.5692-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timoszyk WK, Nessler JA, Acosta C, et al. Hindlimb loading determines stepping quantity and quality following spinal cord transection. Brain Res. 2005;1050:180–189. doi: 10.1016/j.brainres.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 7.Bouyer LJ, Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. II. Spinal cats. J Neurophysiol. 2003;90:3640–3653. doi: 10.1152/jn.00497.2003. [DOI] [PubMed] [Google Scholar]
- 8.Hodgson JA, Roy RR, de Leon R, Dobkin B, Edgerton VR, et al. Can the mammalian lumbar spinal cord learn a motor task? Med Sci Sports Exerc. 1994;26:1491–1497. [PubMed] [Google Scholar]
- 9.Boyce VS, Tumolo M, Fischer I, Murray M, Lemay MA. Neurotrophic factors promote and enhance locomotor recovery in untrained spinalized cats. J Neurophysiol. 2007;98:1988–1996. doi: 10.1152/jn.00391.2007. [DOI] [PubMed] [Google Scholar]
- 10.Smith RR, Shum-Siu A, Baltzley R, et al. The effects of swimming on functional recovery after incomplete spinal cord injury in rats. J Neurotrauma. 2006;23:908–919. doi: 10.1089/neu.2006.23.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muir GD, Steeves JD. Phasic cutaneous input facilitates locomotor recovery after incomplete spinal injury in the chick. J Neurophysiol. 1995;74:358–368. doi: 10.1152/jn.1995.74.1.358. [DOI] [PubMed] [Google Scholar]
- 12.Smith RR, Burke DA, Baldini AD, et al. The Louisville Swim Scale: a novel assessment of hindlimb function following spinal cord injury in adult rats. J Neurotrauma. 2006;23:1654–1670. doi: 10.1089/neu.2006.23.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruner JA, Altman J. Swimming in the rat: analysis of locomotor performance in comparison to stepping. Exp Brain Res. 1980;40:374–382. doi: 10.1007/BF00236146. [DOI] [PubMed] [Google Scholar]
- 14.Gruner JA, Altman J, Spivack N. Effects of arrested cerebellar development on locomotion in the rat. Cinematographic and electromyographic analysis. Exp Brain Res. 1980;40:361–373. doi: 10.1007/BF00236145. [DOI] [PubMed] [Google Scholar]
- 15.Novacheck TF. The biomechanics of running. Gait Posture. 1998;7:77–95. doi: 10.1016/s0966-6362(97)00038-6. [DOI] [PubMed] [Google Scholar]
- 16.Thota A, Carlson S, Jung R. Recovery of locomotor function after tread-mill training of incomplete spinal cord injured rats. Biomed Sci Instrum. 2001;37:63–67. [PubMed] [Google Scholar]
- 17.Leblond H, L'esperance M, Orsal D, Rossignol S. Treadmill locomotion in the intact and spinal mouse. J Neurosci. 2003;36:11411–11419. doi: 10.1523/JNEUROSCI.23-36-11411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferson S, Rohlf FJ, Koehn RK. Measuring shape variation of two-dimensional outlines. Syst Zool. 1995;34:59–68. [Google Scholar]
- 19.Ichiyama RM, Courtine G, Gerasimenko YP, et al. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci. 2008;28:7370–7375. doi: 10.1523/JNEUROSCI.1881-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fouad K, Metz GA, Merkler D, Dietz V, Schwab ME. Treadmill training in incomplete spinal cord injured rats. Behav Brain Res. 2000;115:107–113. doi: 10.1016/s0166-4328(00)00244-8. [DOI] [PubMed] [Google Scholar]
- 21.Multon S, Franzen R, Poirrier AL, Scholtes F, Schoenen J. The effect of treadmill training on motor recovery after a partial spinal cord compression-injury in the adult rat. J Neurotrauma. 2003;20:699–706. doi: 10.1089/089771503767869935. [DOI] [PubMed] [Google Scholar]
- 22.Schucht P, Raineteau O, Schwab ME, Fouad K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp Neurol. 2002;176:143–153. doi: 10.1006/exnr.2002.7909. [DOI] [PubMed] [Google Scholar]
- 23.de Leon R, Hodgson JA, Roy RR, Edgerton VR. Extensor- and flexor-like modulation within motor pools of the rat hindlimb during treadmill locomotion and swimming. Brain Res. 1994;654:241–250. doi: 10.1016/0006-8993(94)90485-5. [DOI] [PubMed] [Google Scholar]
- 24.Roy RR, Hutchison DL, Pierotti DJ, Hodgson JA, Edgerton VR. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J Appl Physiol. 1991;70:2522–2529. doi: 10.1152/jappl.1991.70.6.2522. [DOI] [PubMed] [Google Scholar]
- 25.Harkema SJ, Hurley SL, Patel UK, Reguejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- 26.Maegele M, Muller S, Wernig A, Edgerton VR, Harkema SJ. Recruitment of spinal motor pools during voluntary movements versus stepping after human spinal cord injury. J Neurotrauma. 2002;19:1217–1229. doi: 10.1089/08977150260338010. [DOI] [PubMed] [Google Scholar]