Abstract
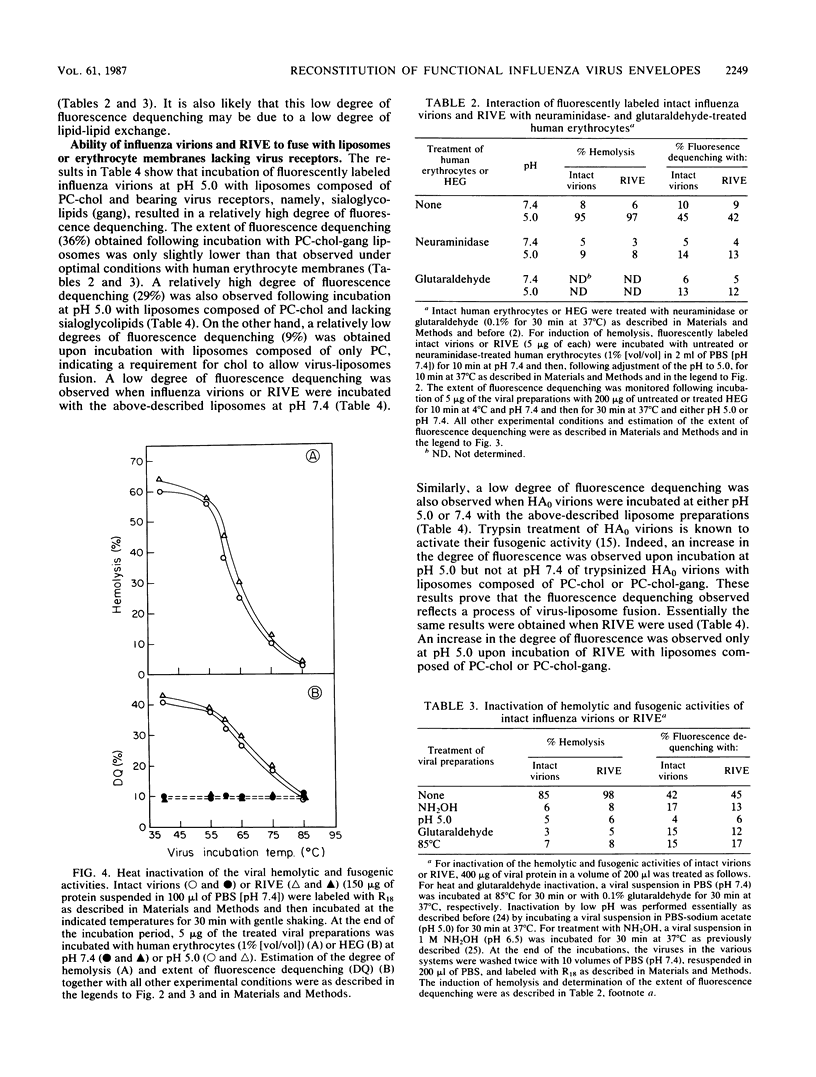
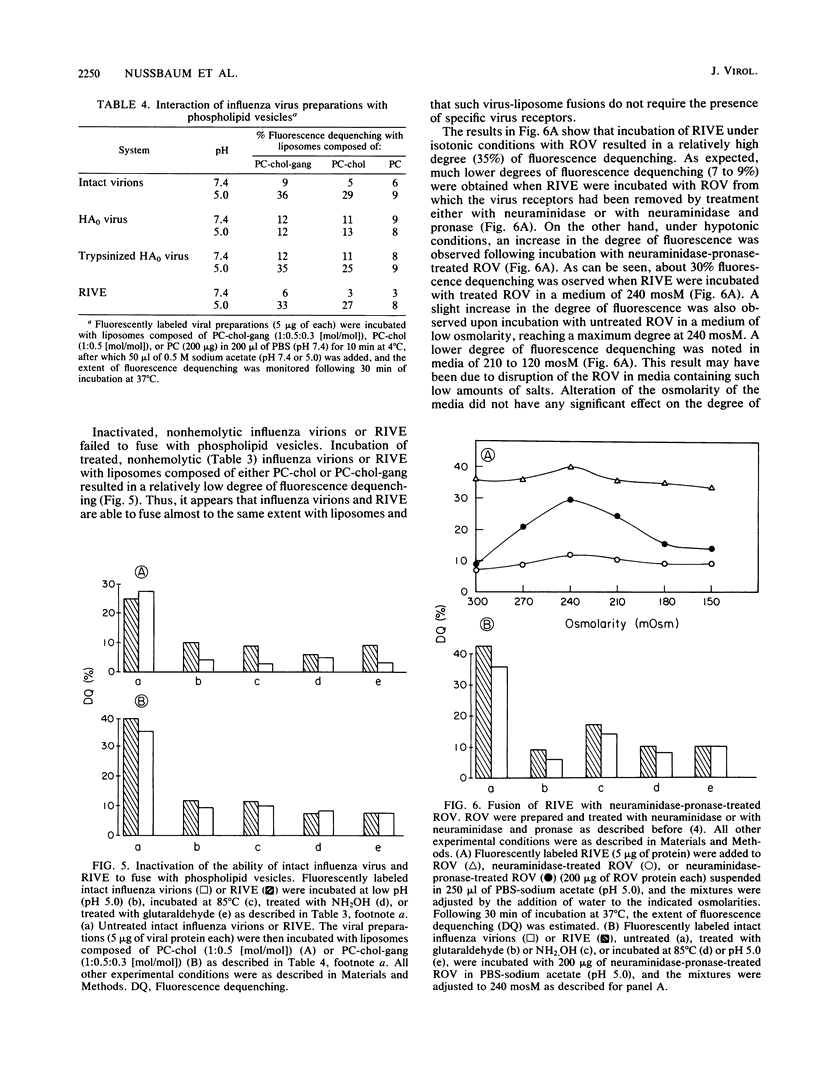
Reconstituted influenza virus envelopes were obtained following solubilization of intact virions with Triton X-100. Quantitative determination revealed that the hemolytic and fusogenic activities of the envelopes prepared by the present method were close or identical to those expressed by intact virions. Hemolysis as well as virus-membrane fusion occurred only at low pH values, while both activities were negligible at neutral pH values. Fusion of intact virions as well as reconstituted envelopes with erythrocyte membranes--and also with liposomes--was determined by the use of fluorescently labeled viral envelopes and fluorescence dequenching measurements. Fusion with liposomes did not require the presence of specific virus receptors, namely sialoglycolipids. Under hypotonic conditions, influenza virions or their reconstituted envelopes were able to fuse with erythrocyte membranes from which virus receptors had been removed by treatment with neuraminidase and pronase. Inactivated intact virions or reconstituted envelopes, namely, envelopes treated with hydroxylamine or glutaraldehyde or incubated at low pH or 85 degrees C, neither caused hemolysis nor possessed fusogenic activity. Fluorescence dequenching measurements showed that only fusion with liposomes composed of neutral phospholipids and containing cholesterol reflected the viral fusogenic activity needed for infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chejanovsky N., Henis Y. I., Loyter A. Fusion of fluorescently labeled Sendai virus envelopes with living cultured cells as monitored by fluorescence dequenching. Exp Cell Res. 1986 Jun;164(2):353–365. doi: 10.1016/0014-4827(86)90034-0. [DOI] [PubMed] [Google Scholar]
- Chejanovsky N., Loyter A. Fusion between Sendai virus envelopes and biological membranes. The use of fluorescent probes for quantitative estimation of virus-membrane fusion. J Biol Chem. 1985 Jul 5;260(13):7911–7918. [PubMed] [Google Scholar]
- Citovsky V., Blumenthal R., Loyter A. Fusion of Sendai virions with phosphatidylcholine-cholesterol liposomes reflects the viral activity required for fusion with biological membranes. FEBS Lett. 1985 Dec 2;193(2):135–140. doi: 10.1016/0014-5793(85)80137-x. [DOI] [PubMed] [Google Scholar]
- Citovsky V., Loyter A. Fusion of Sendai virions or reconstituted Sendai virus envelopes with liposomes or erythrocyte membranes lacking virus receptors. J Biol Chem. 1985 Oct 5;260(22):12072–12077. [PubMed] [Google Scholar]
- Citovsky V., Yanai P., Loyter A. The use of circular dichroism to study conformational changes induced in Sendai virus envelope glycoproteins. A correlation with the viral fusogenic activity. J Biol Chem. 1986 Feb 15;261(5):2235–2239. [PubMed] [Google Scholar]
- Citovsky V., Zakai N., Loyter A. Active function of membrane receptors for enveloped viruses. I. Specific requirement for liposome-associated sialoglycolipids, but not sialoglycoproteins, to allow lysis of phospholipid vesicles by reconstituted Sendai viral envelopes. Exp Cell Res. 1986 Oct;166(2):279–294. doi: 10.1016/0014-4827(86)90477-5. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gitman A. G., Kahane I., Loyter A. Use of virus-attached antibodies or insulin molecules to mediate fusion between Sendai virus envelopes and neuraminidase-treated cells. Biochemistry. 1985 May 21;24(11):2762–2768. doi: 10.1021/bi00332a025. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., de Boer T., Klappe K., Wilschut J. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry. 1984 Nov 20;23(24):5675–5681. doi: 10.1021/bi00319a002. [DOI] [PubMed] [Google Scholar]
- Huang R. T., Rott R., Klenk H. D. Influenza viruses cause hemolysis and fusion of cells. Virology. 1981 Apr 15;110(1):243–247. doi: 10.1016/0042-6822(81)90030-1. [DOI] [PubMed] [Google Scholar]
- Huang R. T., Rott R., Wahn K., Klenk H. D., Kohama T. The function of the neuraminidase in membrane fusion induced by myxoviruses. Virology. 1980 Dec;107(2):313–319. doi: 10.1016/0042-6822(80)90299-8. [DOI] [PubMed] [Google Scholar]
- Huang R. T., Wahn K., Klenk H. D., Rott R. Association of the envelope glycoproteins of influenza virus with liposomes--a model study on viral envelope assembly. Virology. 1979 Aug;97(1):212–217. doi: 10.1016/0042-6822(79)90390-8. [DOI] [PubMed] [Google Scholar]
- Huang R. T., Wahn K., Klenk H. D., Rott R. Fusion between cell membrane and liposomes containing the glycoproteins of influenza virus. Virology. 1980 Jul 30;104(2):294–302. doi: 10.1016/0042-6822(80)90334-7. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lalazar A., Michaeli D., Loyter A. Restoration of the fusion capacity of human erythrocyte ghosts by SH blocking reagents. Exp Cell Res. 1977 Jun;107(1):79–88. doi: 10.1016/0014-4827(77)90388-3. [DOI] [PubMed] [Google Scholar]
- Laster Y., Sabban E., Loyter A. Susceptibility of membrane phospholipids in erythrocyte ghosts to phospholipase C and their refractiveness in the intact cell. FEBS Lett. 1972 Feb 15;20(3):307–310. doi: 10.1016/0014-5793(72)80093-0. [DOI] [PubMed] [Google Scholar]
- Maeda T., Asano A., Okada Y., Ohnishi S. I. Transmembrane phospholipid motions induced by F glycoprotein in hemagglutinating virus of Japan. J Virol. 1977 Jan;21(1):232–241. doi: 10.1128/jvi.21.1.232-241.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Kawasaki K., Ohnishi S. Interaction of influenza virus hemagglutinin with target membrane lipids is a key step in virus-induced hemolysis and fusion at pH 5.2. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4133–4137. doi: 10.1073/pnas.78.7.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum O., Zakai N., Loyter A. Membrane-bound antiviral antibodies as receptors for Sendai virions in receptor-depleted erythrocytes. Virology. 1984 Oct 30;138(2):185–197. doi: 10.1016/0042-6822(84)90344-1. [DOI] [PubMed] [Google Scholar]
- Patel K., Pasternak C. A. Permeability changes elicited by influenza and Sendai viruses: separation of fusion and leakage by pH-jump experiments. J Gen Virol. 1985 Apr;66(Pt 4):767–775. doi: 10.1099/0022-1317-66-4-767. [DOI] [PubMed] [Google Scholar]
- Sato S. B., Kawasaki K., Ohnishi S. Hemolytic activity of influenza virus hemagglutinin glycoproteins activated in mildly acidic environments. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3153–3157. doi: 10.1073/pnas.80.11.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Lambrecht B. On the structure of the acyl linkage and the function of fatty acyl chains in the influenza virus haemagglutinin and the glycoproteins of Semliki Forest virus. J Gen Virol. 1985 Dec;66(Pt 12):2635–2647. doi: 10.1099/0022-1317-66-12-2635. [DOI] [PubMed] [Google Scholar]
- Stegmann T., Hoekstra D., Scherphof G., Wilschut J. Fusion activity of influenza virus. A comparison between biological and artificial target membrane vesicles. J Biol Chem. 1986 Aug 25;261(24):10966–10969. [PubMed] [Google Scholar]
- Vainstein A., Hershkovitz M., Israel S., Rabin S., Loyter A. A new method for reconstitution of highly fusogenic Sendai virus envelopes. Biochim Biophys Acta. 1984 Jun 27;773(2):181–188. doi: 10.1016/0005-2736(84)90081-6. [DOI] [PubMed] [Google Scholar]
- White J., Kartenbeck J., Helenius A. Membrane fusion activity of influenza virus. EMBO J. 1982;1(2):217–222. doi: 10.1002/j.1460-2075.1982.tb01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- van Meer G., Davoust J., Simons K. Parameters affecting low-pH-mediated fusion of liposomes with the plasma membrane of cells infected with influenza virus. Biochemistry. 1985 Jul 2;24(14):3593–3602. doi: 10.1021/bi00335a030. [DOI] [PubMed] [Google Scholar]