Abstract
Cell fate determination in many systems is based upon inductive events driven by cell-cell interactions. Inductive signaling regulates many aspects of Drosophila compound eye development. Accumulating evidence suggests that the color sensitivity of the R8 photoreceptor cell within an individual ommatidium is regulated by an inductive signal from the adjacent R7 photoreceptor cell. This signal is thought to control an induced versus default cell-fate switch that coordinates the visual pigment expression and color sensitivities of adjacent R7 and R8 photoreceptor cells. Here we describe a disruption in R7 and R8 cell patterning in Scutoid mutants that is due to inappropriate signals from Rh4-expressing R7 cells inducing Rh5 expression in adjacent R8 cells. This dominant phenotype results from the misexpression of the transcriptional repressor snail, which with the co-repressor C-terminal-Binding-Protein represses rhomboid expression in the developing eye. We show that loss of rhomboid suppresses the Scutoid phenotype. However in contrast to the loss of rhomboid alone, which entirely blocks the normal inductive signal from the R7 to the R8 photoreceptor cell, Scutoid rhomboid double mutants display normal Rh5 and Rh6 expression. Our detailed analysis of this unusual dominant gain-of-function neomorphic phenotype suggests that the induction of Rh5 expression in Scutoid mutants is partially rhomboid independent.
Keywords: Drosophila, retina, photoreceptor, cell-fate determination, Scutoid, rhomboid, dominant, neomorph, allele
Introduction
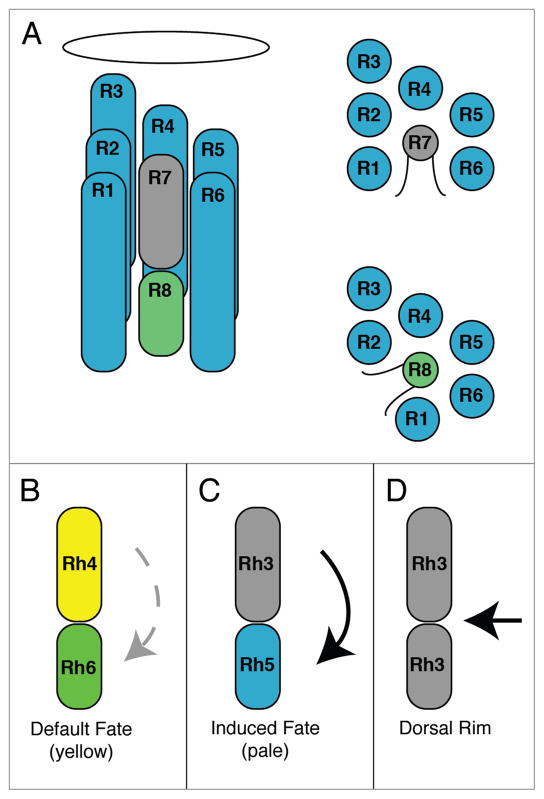
The compound eye of Drosophila melanogaster is a highly ordered structure composed of ~750 ommatidia, each containing eight photoreceptor cells (R1–R8).1,2 As in other organisms that are capable of color vision, different photoreceptor cells in the fly retina are sensitive to different colors of light (Fig. 1). The R1–R6 photoreceptor cells express the blue absorbing visual pigment Rhodopsin 1 (Rh1) encoded by the ninaE gene.3–5 The R7 and R8 cells within an individual ommatidium express visual pigments in a precisely paired manner (Fig. 1B–D). The two major types of ommatidia are referred to as yellow and pale, based on their appearance under blue illumination.6,7 Yellow ommatidia contain UV sensitive Rh4-expressing R7yellow (R7y) cells and green sensitive Rh6-expressing R8yellow (R8y) cells and occupy ~65% of the eye (Fig. 1B).8–11 Pale ommatidia contain UV sensitive Rh3-expressing R7pale (R7p) cells and blue sensitive Rh5-expressing R8pale (R8p) cells and occupy ~35% of the eye (Fig. 1C)).8,11–13 The organization of the R7y and R7p cells is statistically random,14 consistent with the idea that the R7y versus R7p cell fate is determined by a stochastic process that requires the gene spineless.15 A third class of ommatidia along the dorsal rim (DR) is thought to be polarization sensitive and express Rh3 in both the R7 and R8 cells (Fig. 1D). DR ommatidia are specified by wingless, the iroquois genes, and homothorax.16–18 All of these patterning events are likely to take place during the period of late larval through pupal development prior to the onset of opsin gene expression.19
Figure 1.
Patterning of the R7 and R8 photoreceptor cells. (A) Longitudinal diagram of a Drosophila ommatidium on the left, composed of eight neuronal photoreceptor cells having rhabdomeres containing the visual pigments. The rhabdomeres of the outer photoreceptor cells (R1–R6) surround the apical and basal rhabdomeres of the inner R7 and R8 cells, respectively, as show in the cross sectional diagrams on the right. The oval is the lens. (B and C) Longitudinal diagrams of the two major types of ommatidia, yellow (B) and pale (C), expressing Rh4 and Rh6 or Rh3 and Rh5 in adjacent R7 and R8 cells. Expression of Rh6 in R8yellow cells is not dependent on the presence of an adjacent R7 cell and likely reflects a default fate (faint broken arrow). Expression of Rh5 in R8pale cells is dependent upon the presence of an adjacent Rh3-expressing R7 cell and likely reflects an inductive signal (bold arrow). (D) Ommatidia along the dorsal margin of the eye express Rh3 in both R7 and R8. The specification of ommatidia in the dorsal margin occurs from signaling events in the eye periphery and the dorsal eye that are regulated by wingless, the iroquois genes, and homothorax.16–18
Our knowledge of the molecular basis for the specification of R7 and R8 pairs in yellow and pale ommatidia is incomplete. However there is genetic evidence that the R8 cell fate in an individual ommatidium is established by a switch between induced (Rh5, blue sensitive) versus default fates (Rh6, green sensitive) (Fig. 1B and C, respectively). This induced versus default switch is dependent on the phenotype of the adjacent R7 cell.10,13,20 A presumptive signal from R7 to R8 is thought to regulate the genes melted and warts that are required for proper Rh5 and Rh6 expression.21 Here we show that heterozygous Scutoid (nocSco/+) mutants have a very specific defect in the coordination of the R7 and R8 photoreceptor cell subtypes in an individual ommatidium, that appears to result from the inappropriate induction of Rh5 expression in R8 cells adjacent to Rh4-expressing R7 cells.
The dominant mutation Scutoid was first described by Krivshenko in 195922 and identified in a female offspring of X-irradiated females. The mutation causes the loss of scutellar bristles, and the ocellar and humeral bristles are often lost as well. The mutation has high expressivity and is located on the second chromosome between black and purple.22,23 Subsequent studies suggested that the mutation is due to a pair of reciprocal translocations and the fusion of the no-ocelli (noc) and snail (sna) genes.24,25 This was confirmed in molecular analyses,26 and sna was later shown to be ectopically expressed in the developing notum of nocSco/+ animals, and the nocSco/+ phenotype could be mimicked by such expression.27
sna is required for numerous aspects of development and differentiation such as mesoderm formation, CNS development, cell migration and apoptosis and has been implicated in the biology of cancer.28 Molecular analysis has shown that the sna protein contains five zinc fingers (all C2H2) and functions as a DNA-binding transcriptional regulator.28–31 sna is a short-range repressor, which operates over distances of less than 100 base pairs to quench upstream activators or the core transcription complex,32 and these effects are mediated by the interaction of sna with other transcriptional regulators. Similar to the well-characterized short-range repressor knirps, sna binds to the Drosophila C-terminal Binding Protein (CtBP) via conserved P-DLS-K and P-DLS-R motifs.32
During the early blastoderm stage of embryogenesis, sna acts to restrict neurectoderm and neural fates in the invaginating mesoderm (Fig. 5C). sna expression at this stage is regulated by Dorsal. sna is normally expressed in ventral regions where it helps to establish the limit of the presumptive mesoderm by repressing target genes such as single-minded (sim), ventral nervous system defective (vnd), short gastrulation (sog) and rhomboid (rho).33–35 sna repression of these target genes is dependent upon its interaction with the CtBP co-repressor.
Figure 5.
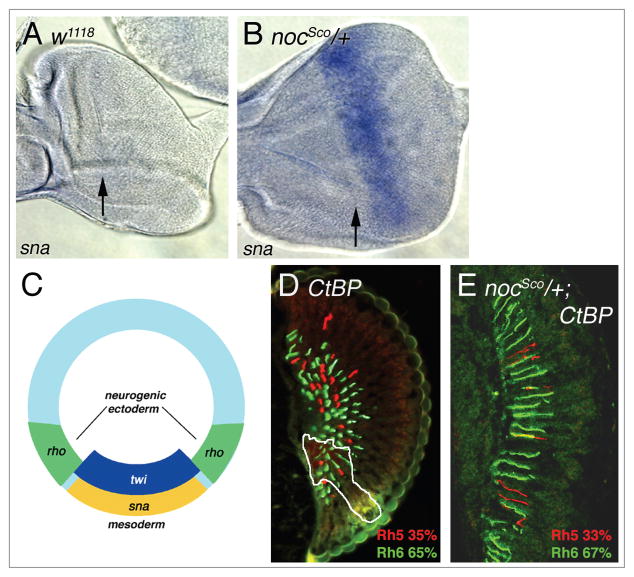
snail is ectopically expressed in Scutoid mutants and requires the co-repressor C-terminal Binding Protein to alter R7/R8 cell patterning. (A) WT (w1118) third instar eye imaginal disc lacks sna expression. The arrow indicates the position of the morphogenetic furrow. (B) sna transcript is ectopically expressed in nocSco/+ eye imaginal discs posterior to the morphogenetic furrow (black arrow). (C) During embryogenesis, twist (twi) and snail (sna) are responsible for mesoderm differentiation. sna represses rho ventrally and establishes the boundary between the mesoderm and the neurogenic ectoderm (modified from ref. 35). (D) In a largely CtBP homozygous mutant retina with a small heterozygous region (outlined in white), loss of CtBP does not significantly affect the proportion of R8 cells expressing Rh5 and Rh6. (E) In a completely mutant nocSco/+; CtBP retina, loss of CtBP dramatically suppresses the nocSco/+ phenotype, producing a WT percentage of Rh5 and Rh6 expression in R8 cells. Compare with nocSco/+ alone in Figure 3B.
Here we show that nocSco/+ mutants ectopically express sna in the developing eye imaginal disc, and that the disruption of photoreceptor cell patterning caused by nocSco/+ is dependent upon the presence of the CtBP co-repressor in the eye. This suggested that sna and CtBP may repress transcription of specific target genes in nocSco/+ mutants and that this could be the basis for the disruption in photoreceptor cell patterning. Interestingly, we show that the expression of rhomboid (rho), a component of the Epidermal Growth Factor Receptor (EGFR) pathway, is disrupted in nocSco/+ mutant flies and that loss of rho can suppress the nocSco/+ retinal patterning phenotype. These findings led us to determine that rho is required for the induction of Rh5 expression in R8 cells.36 These results, obtained from a historically important dominant gain of function mutant (nocSco), have revealed an unexpected function for rho in specifying R8 photoreceptor cell fate.
Results
Scutoid disrupts retinal patterning through inappropriate signaling
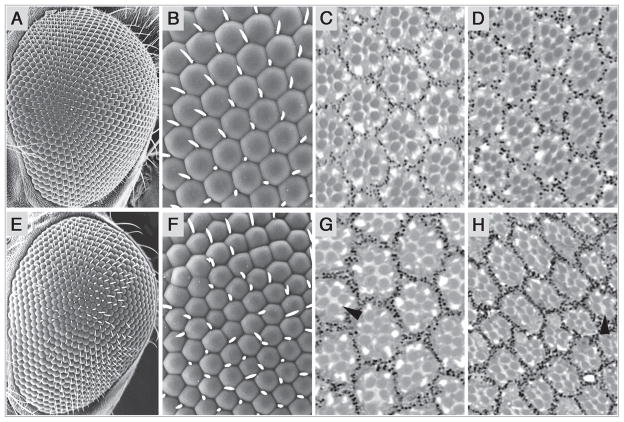
To identify genes that regulate the differentiation of Rh5- and Rh6-expressing R8 photoreceptor cells and establish R7/R8 cell adjacency (Fig. 1B and C), we examined the coordination of opsin gene expression in existing mutants having other defects in eye morphology. We found that animals heterozygous for the Scutoid mutation (nocSco/+) have very specific defects in both photoreceptor cell organization and R7/R8 photoreceptor cell subtype specification. Wild type Canton-S (CS) eyes have regularly spaced ommatidia with mechanosensory bristles located in alternating ommatidial junctions (Fig. 2A and B). However, as noted previously, nocSco/+ ommatidia are misshapen and ommatidial junctions often lack bristles or contain double bristles (Fig. 2E and F).27 Here we show for the first time that in contrast to CS (Fig. 2C and D), nocSco/+ eyes also contain ommatidia lacking one or more outer photoreceptor cell rhabdomeres and central R7 and R8 rhabdomeres (Fig. 2G and H, arrowheads). This likely reflects the loss of these photoreceptor cells, but could potentially result from the failure to build or maintain the rhabdomere alone.
Figure 2.
Scutoid mutant eyes are rough and lack some photoreceptor cells. Wild type, canton S (CS) flies are shown in (A–D). nocSco/+ mutant flies are shown in (E–H). (A) Scanning electron micrographs (SEM) of a CS eye shows the regular arrangement of ommatidia. (B) Increased magnification showing the regular array of facets and bristle placement. (C) An apical cross section of a CS retina shows the outer R1–R6 photoreceptor cells and the inner R7 cell in each ommatidium. (D) A basal cross section of a CS retina shows the outer R1–R6 photoreceptor cells and the inner R8 cell in each ommatidium. The rhabdomeres are organized as in Figure 1A. (E) nocSco/+ mutant eyes are slightly rough. (F) nocSco/+ ommatidia are misshapen and often contain no bristles or double bristles at alternating ommatidial junctions. (G) Many nocSco/+ ommatidia in this apical section are missing one or more outer photoreceptor cells and may also lack R7 cells (arrowhead). (H) Many nocSco/+ ommatidia in this basal section are missing one or more outer photoreceptor cells and may also lack R8 cells (arrowhead). In all panels, dorsal is toward the top and anterior is to the left. The left eye of a female is shown in all cases.
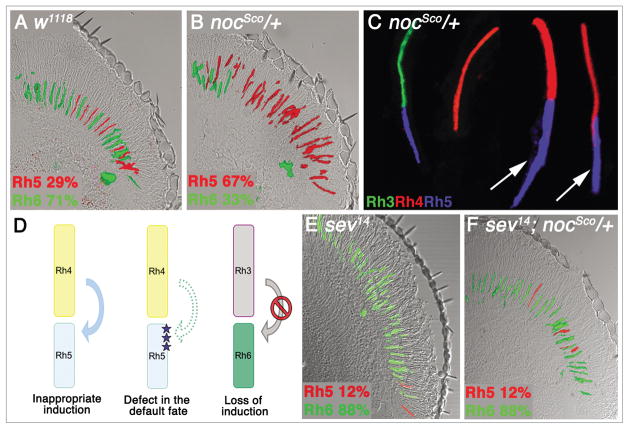
nocSco/+ mutants also show a profound disruption in R8 photoreceptor cell differentiation over the majority of the eye, but not along the dorsal rim. nocSco/+ mutants display a dramatic increase in the proportion of blue sensitive R8 cells that express Rh5, compared to a white eyed control (white, w) (Fig. 3A and B and Table 1). This is accompanied by mispaired expression of Rh4 and Rh5 in adjacent R7 and R8 cells (Fig. 3C and arrows), which suggests that R8 cells in these ommatidia are inappropriately assuming the induced fate and expressing Rh5. There are two different models that may explain the mutant phenotype observed (Fig. 3D). First, the R8 cell may be responding to an inappropriate signal that induces the expression of Rh5. Alternatively there may be a defect in the R8 cell, and it is unable to assume the default fate in the absence of an inductive signal. To distinguish between these alternative models, we examined sevenless (sev) mutant animals, which lack R7 cells. If the nocSco/+ phenotype is due to an inappropriate signal from R7 cells, then Rh5 expression in sev; nocSco/+ double mutants should be dramatically reduced. Alternatively, if the nocSco/+ phenotype is the result of a defect in the R8 cell default pathway, then Rh5 expression should remain broadly expressed. Interestingly, removal of R7 cells in nocSco/+ mutants results in a dramatic decrease in Rh5 expression (Fig. 3F and Table 1), similar to the phenotype of sev mutants that lack a source for the inductive signal (Fig. 3D and E). These findings suggest that the nocSco/+ phenotype results from a normally non-signaling Rh4-expressing R7 cell inappropriately signaling to and inducing the adjacent R8 cell to express Rh5. One caveat to this result is that not all of the R8 cells are reverted to the Rh6-expressing default fate. There are a small number of R8 cells that continue to express Rh5, similar to that observed in sev mutants alone (Fig. 3E).10,13,20 Nonetheless, the degree of suppression of Rh5 expression in sev; nocSco/+ double mutants is substantial and shows that a defect in the default pathway is clearly not the basis for the nocSco/+ phenotype.
Figure 3.
Scutoid mutants show increased Rh5 expression and mis-pairing of Rh4 with Rh5. (A) WT flies (w1118) express Rh5 in 29% of R8 cells and express Rh6 in 71%. (B) In a longitudinal section from a nocSco/+ retina, there is a dramatic increase in the number of R8 cells that express Rh5 (67%). (C) Dissociated ommatidia from nocSco/+ mutants show that the increase in Rh5 expression is coupled with mis-pairing of Rh4 and Rh5 expression in adjacent R7 and R8 cells (arrows). (D) This phenotype could result from an inappropriate inductive signal, or from a defect in the default fate. By contrast, a loss of the inductive signal would be expected to produce a decrease in Rh5 expression and mis-pairing of Rh3 and Rh6 expression in adjacent R7 and R8 cells. (E) In the absence of R7 cells (sev) virtually all R8 cells assume the default fate and express Rh6. This corresponds to a loss of induction from the putative signaling R7 cell. (F) Loss of R7 cells suppresses the nocSco/+ phenotype in the sev; nocSco/+ double mutant and causes a dramatic decrease in Rh5 expression (12%).
Table 1.
Opsin expression in different genetic backgrounds*
| Genotype | R8 cells expressing Rh5 % (n) | R7 cells expressing Rh3 % (n) | Mis-pairing | Figure |
|---|---|---|---|---|
| CS | 33% (359) | 38% (139) | NO | |
| w1118 | 29% (214) | 34% (140) | NO | 1B–D, 3A |
| nocSco/+ | 67% (233) SDF CS, p < 10−15 |
44% (341) | Yes (Rh4/Rh5) | 3B and c |
| sev14 | 12% (585) SDF CS, p = 2.0 × 10−14 |
NA | NA | 3E |
| sev14; nocSco/+ | 12% (269) SDF nocSco/+, p < 10−15 |
NA | NA | 3F |
| b1 nocSco pr1 c1 px1/+ | 60% (238) SDF CS, p = 9.2 × 10−11 |
ND | ND | |
| b1 nocSco pr1px1/+ | 57% (227) SDF CS, p = 1.7 × 10−8 |
ND | ND | |
| al1 dpov1 b1 nocSco pr1 c1 px1/+ | 58% (456) SDF CS, p = 1.3 × 10−12 |
ND | ND | |
| nocSco-rv8/+ | 63% (265) not SDF nocSco/+ |
52% (242) not SDF nocSco/+ |
Yes (Rh4/Rh5) | 4 |
| nocSco-rv17 b pr/+ | 42% (205) SDF nocSco/+, p = 4.3 × 10−7 |
46% (220) | NO | 4 |
| nocSco-rv11/+ | 45% (278) SDF nocSco/+, p = 10−6 |
51% (226) SDF CS, p = 0.023 |
NO | 4 |
| Df(2L)nocSco-rv25/+ | 30% (230) SDF nocSco/+, p = 3.8 × 10−15 |
48% (270) | NO | 4 |
| b Df(2L)nocSco-rv19 pr/+ | 44% (208) SDF nocSco/+, p = 2.5 × 10−6 |
42% (214) | NO | 4 |
| sna1 FRT | 28% (163) | ND | ND | |
| CtBP87De-10 FRT | 35% (362) | ND | ND | 5D |
| nocSco/+; CtBP87De-10 FRT | 33% (554) SDF nocSco/+, p < 10−15 |
ND | ND | 5E |
| nocSco/+; rhopΔ5 FRT | 41% (59) SDF nocSco/+, p = 3.7 × 10−4 |
ND | ND | 7E |
| rhopΔ5 FRT | 6.0% (300) SDF CS, p < 10−15 |
35% (280) | Yes (Rh3/Rh6) | 7F# |
Statistical comparisons of strains were carried out as described in the Methods; n = the number of ommatidia counted; Unless indicated, the observed percentages are not significantly different from CS; Strains compared to another control are indicated; Abbreviations are as follows: Significantly Different From (SDF) the strain indicated, at the p value shown; Not Determined (ND); Not applicable (NA);
Quantitative data for this mutant is from Birkholz et al.36
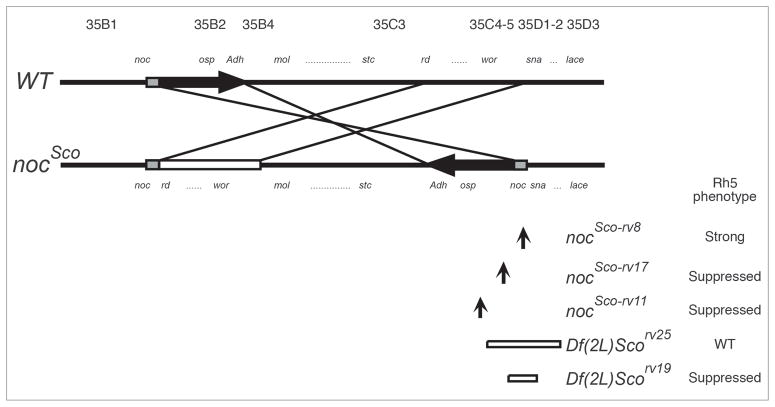
To insure that the retinal patterning defect in nocSco/+ mutants was due to the nocSco/+ mutation rather than some other mutation within the background of the strain, we generated a series of recombinant chromosomes from nocSco and al1 dpov1 b1 pr1 c1 px1 sp1. In each case, we found that animals heterozygous for the recombinant chromosome containing nocSco retained an elevated percentage of Rh5-expressing R8 cells compared to controls (Table 1). In addition, we examined the retinal patterning of a group of nocSco/+ revertants and found that in all but one revertant (nocScorv8/+) the pattern of increased Rh5 expression was either dramatically suppressed or restored to wild-type levels (Fig. 4 and Table 1). These results are consistent with the idea that the molecular defect in the nocSco/+ mutation is the basis for the disruption of rhodopsin expression observed in these animals.
Figure 4.
Diagram of the wild-type, Scutoid and Scutoid revertant chromosomes. In nocSco two segments of the left arm of the second chromosome, nocB-Adh and rd-wor, are reciprocally transposed.52 The segment of noc-Adh has been inverted and translocated in front of the sna gene. The order of the rd-wor segment is not known. A 5 kb segment, shown as the gray box, is duplicated in the nocSco chromosome. The breakpoints of the inversions in nocSco-rv8, nocSco-rv17 and nocSco-rv11 are shown by arrows. The segments deleted in nocSco-rv25 and nocSco-rv19 are shown as open bars. Among the revertants analysed, only nocSco-rv8 demonstrates an abnormal R7 and R8 photorecptor cell patterning phenotype. The proximal direction to the centromere is to the right. moladietz (mol), shuttle craft (stc) and worniu (wor), correspond to the loci previously referred to as l(2)35Bb, l(2)35Cb and l(2)35Da, respectively. 53,54 noc includes l(2)35Ba, nocA, nocB and nocC.54 The figure is adapted from.26,46
Ectopically expressed snail in Scutoid mutants requires the co-repressor C-terminal binding protein to disrupt R8 cell differentiation
nocSco results from a pair of reciprocal intrachromosomal transpositions affecting the second chromosome in the region containing noc and sna (Fig. 4).26 Additionally, noc is expressed weakly in the presumptive ocellar region of the eye-imaginal disc in WT animals and its expression is unchanged in nocSco/+ mutants (data not shown). Although the loss of sna has no affect on the induction of Rh5 expression (Table 1), the nocSco/+ mutation is known to cause the sna gene to be ectopically expressed in the wing, and eye-antennal imaginal discs.27 We confirmed that sna is not expressed in WT eye imaginal discs, but in the nocSco/+ mutant, it is expressed posterior to the morphogenetic furrow (Fig. 5A and B). This suggests that ectopic expression of sna is the basis for the retinal patterning defect, as is thought to be the case for the bristle defects of the mutant.
As described in the Introduction, sna functions as a transcriptional repressor in combination with the CtBP co-repressor.28–35 If ectopic sna expression is the basis for the disruption in rhodopsin expression in the R7 and R8 photoreceptor cells, we predict that this may be mediated by CtBP dependent repression of specific target genes. To test whether the disruption of blue and green sensitive R8 cell differentiation in nocSco/+ is CtBP dependent, we used the FLP/FRT system to generate homozygous CtBP mutant clones in WT and nocSco/+ mutant animals by site-specific recombination.37,38 We used a FLP recombinase driven by an eye-specific enhancer of the eyeless (ey) gene to generate clones restricted to the eye, and a cell lethal mutation on the homologous wild-type chromosome to eliminate the twin spot.39 This method generated almost entirely homozygous mutant eyes. The removal of CtBP alone had no effect on retinal patterning or eye morphology (Fig. 5D). However, removal of CtBP dramatically suppressed the nocSco/+ mutant phenotype. In nocSco/+; CtBP double mutants, retinal patterning was reverted to normal with respect to Rh5 and Rh6 expression (Fig. 5E). This indicates that the co-repressor CtBP is required to disrupt R8 cell differentiation in the nocSco/+ mutant. We believe that an inappropriate signal is generated in nocSco/+ mutant flies, which switches R8 cells from the Rh6-expressing default fate to the Rh5-expressing induced fate. Inappropriate signaling most likely results from sna and CtBP binding to, and repressing the transcription of, one or more target genes that are required for generating or responding to this signal.
rhomboid is repressed by snail in Scutoid mutants
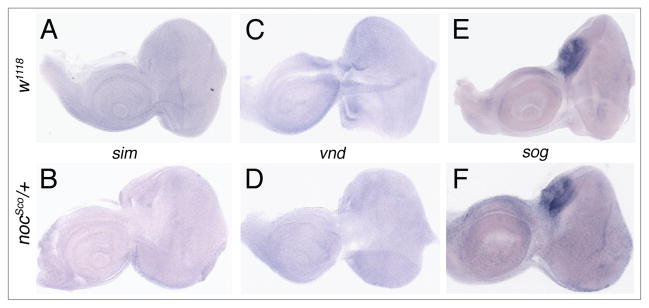
To test whether genes regulated by sna repression during embryogenesis may be targets for sna repression in nocSco/+ mutant eyes, we compared the expression of sim, vnd and sog in WT and nocSco/+ eye imaginal discs. sim and vnd are not expressed in either WT or nocSco/+ eye-imagnal discs (Fig. 6A–D). sog is expressed in a small region that gives rise to the ocelli and is expressed equally in both WT and nocSco/+ eye imaginal discs (Fig. 6E and F). These experiments reveal that none of these three genes are targeted for transcriptional repression by sna and CtBP in the nocSco/+ mutant.
Figure 6.
The expression of single-minded, ventral nervous system defective and short gastrulation in wild type and Scutoid mutant eye-antennal imaginal discs. In situ hybridization analysis of sim (A and B), vnd (C and D) and sog (E and F). Neither sim nor vnd is expressed in w1118 (A and C) or nocSco/+ (B and D) eye-antennal imaginal discs. sog is expressed only in the region that gives rise to the ocelli, and is found at similar levels in w1118 (E) and nocSco/+ (F) eye imaginal discs.
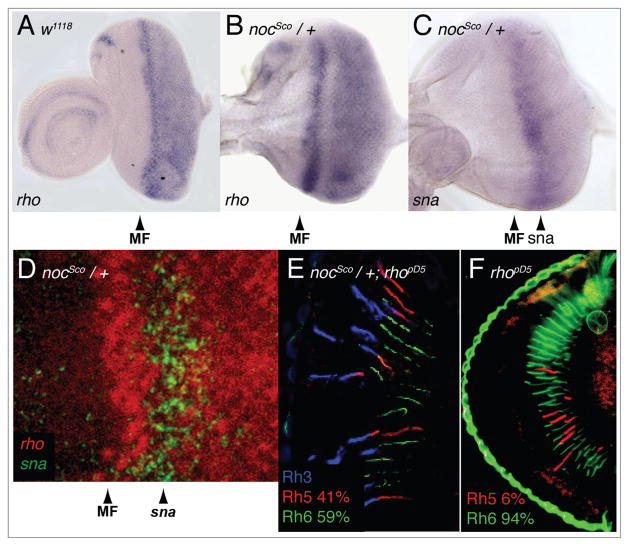
A fourth target of sna repression during embryonic development is rho, which has been extensively characterized in eye development, but whose role in R8 photoreceptor cell differentiation has not been investigated. In WT eye discs, rho expression is intense in the morphogenetic furrow and continues to be uniformly expressed posterior to the furrow (Fig. 7A). In nocSco/+ mutant eye discs, rho expression is also intense in the furrow; however behind the furrow there is a gap in expression. Posterior to this gap, rho expression returns and is maintained uniformly to the posterior edge of the eye disc (Fig. 7B). Remarkably, the gap in rho expression in the nocSco/+ mutant eye discs corresponds precisely to the region of ectopic sna expression (Fig. 7B–D), suggesting that the alteration of rho expression in the nocSco/+ mutant eye imaginal disc is the basis for the disruption of R7/R8 cell subtype coordination.
Figure 7.
rhomboid is repressed in Scutoid mutants and is required for induction of Rh5 expression in R8 cells. (A) In situ hybridization shows that rho is normally expressed in the eye imaginal disc beginning at the morphogenetic furrow (MF, arrowhead) and continuing to the posterior edge of the disc. (B) In a nocSco/+ mutant eye disc, rho expression is normal at the furrow (MF, arrowhead), repressed directly posterior to the furrow and then expressed again to the posterior edge of the eye disc. (C) sna is ectopically expressed in the nocSco/+ mutant eye disc posterior to the furrow (sna, arrowhead). (D) Double labeled in situ demonstrates that the repression of rho posterior to the furrow occurs in the region of sna expression. (E) Triple labeled longitudinal section of a retina from an animal double mutant for nocSco/+ and rhopΔ5 demonstrates a dramatic suppression in the nocSco/+ phenotype. (F) An ey-FLP FRT generated rhopΔ5 mutant eye shows near complete elimination of Rh5 expression.
Loss of rhomboid suppresses the Scutoid mutant phenotype and is required for the induction of Rh5 expression
To test whether rho is required for the inappropriate induction of Rh5 expression found in nocSco/+ mutants, we generated rhopΔ5 mutant clones in a nocSco/+ mutant background using the ey-FLP/FRT system as described above. We found that the loss of rho in these mutant animals dramatically reduced the number of Rh5 expressing R8 cells compared to nocSco/+ mutants alone (Fig. 7E and Table 1). As was the case with CtBP, this demonstrates that the nocSco/+ mutant phenotype is dependent, at least in part, upon the presence of rho. As we have previously reported, in animals that are mutant for rho alone the expression of Rh5 in R8 cells is almost completely eliminated, despite there being no effect on the expression of Rh3 and Rh4 (Fig. 7F and Table 1).36 This demonstrates that rho is required for the induction of Rh5 expression (Fig. 3D, right diagram) and suggests that the disruption of rho expression (rather than rho loss) in nocSco/+ mutants is the basis for inappropriate induction of Rh5 expression. The effect of nocSco/+ in double mutant animals is to suppress the effect of rho loss, producing an eye having Rh5 expression that is not significantly different from CS (41% vs. 33%, respectively, p = 0.3). This demonstrates that while rho is essential for normal paired expression of rhodopsin genes in the R7 and R8 cells, at least part of the induction of Rh5 expression by nocSco/+ is rho independent.
Discussion
In the present study we examined the precise pairing of adjacent R7 and R8 photoreceptor cells that confers the fly’s unique sensitivity to different colors and polarized light.2,6,40 We showed that this pattern is disrupted in nocSco/+ mutants, leading to an increase in the expression of Rh5 and a mispairing of Rh5 expressing R8 cells and Rh4 expressing R7 cells. This inappropriate induction of Rh5 expression is dependent upon the presence of the R7 cell and appears to result from the ectopic expression of the transcriptional repressor sna, which represses the expression of rho in the developing eye imaginal disc. We show that the dominant effect of nocSco/+ on Rh5 expression is dependent upon the presence of the co-repressor CtBP and is suppressed by the loss of rho, although part of the nocSco/+ effect is rho independent. Interestingly, loss of rho alone severely impairs the induction of Rh5 expression.36 Elsewhere we show that rho is both necessary and sufficient for the induction of Rh5 expression and that rho is specifically required in the R8 cell as well as non-cell autonomously elsewhere in the eye.36 Furthermore, we also previously reported that the Epidermal Growth Factor Receptor (EGFR) is required for the induction of Rh5 expression and that EGFR activation is sufficient to overcome the requirement for rho, consistent with the idea that rho and EGFR play a role in establishing the competency of Rh3-expressing R7 cells to induce Rh5 expression in R8 cells.36
The studies reported here highlight how the ectopic expression of a gene normally expressed during embryonic development (sna) can disrupt gene expression in a tissue and developmental stage where it is not normally expressed, which reiterates its normal function to produce an abnormal phenotype. These phenotypes include the disrupted patterning of R8 photoreceptor cell rhodopsin gene expression that we have examined in detail and also a range of additional photoreceptor cell phenotypes, including loss of outer photoreceptor cell rhabdomeres (R1–6) and loss of R7 and R8 cell rhabdomeres. As mentioned previously, these phenotypes likely reflect photoreceptor cell loss, but could potentially result from the failure to build or maintain the rhabdomere. Although rhabdomere or photoreceptor cell loss has not been the primary focus of the current work, they raise a number of interesting issues. This phenotype is reminiscent of that observed in animals misexpressing seven-up (svp), in which loss of outer photoreceptor cells, loss of R8 and R7 cells, formation of extra R7 cells, and the transformation of an R7 cell to an outer photoreceptor cell has been observed.41,42
svp encodes a transcription factor belonging to the chicken ovalbumin upstream transcription factor (COUP-TF) subgroup of the nuclear receptor superfamily. svp and many of the members of this family are “orphan receptors” with no identified ligand and are thought to act in a ligand-independent manner.43 Remarkably, misexpression of svp in the R8 cells under the control of the scabrous driver results in the loss of both R7 and R8 photoreceptor cells.42 Furthermore, expression of svp in R2 and R5 photoreceptor cells under the control of the rough driver leads to the loss of one or more outer photoreceptor cells and may also cause the loss of some R7 cells.42 Our results with the loss of some R7, R8 and outer rhabdomeres or photoreceptor cells in nocSco/+ flies suggests that ectopic expression of sna in the eye imaginal disc may also repress expression of genes that are negatively regulated or downstream of svp, such as ultraspiracle and rigor mortis.44,45
Finally, nocSco/+ is also associated with an olfactory phenotype showing diminished extracellular electroantennogram responses to the odorants ethyl acetate and acetone.46 In a manner that is reminiscent of the pairing of rhodopsin gene expression in adjacent R7 and R8 photoreceptor cells, there is also a characteristic pairing of odorant sensitivities and odorant receptor gene expression within neurons of the same olfactory sensilla.47,48 Furthermore, asymmetric Notch activation has been shown to play an important role in specifying olfactory receptor neuron cell-type and regulating odorant receptor gene expression.49 Thus our results suggest that the olfactory phenotype of nocSco/+ may result from a similar sna mediated alteration in gene expression in the developing antenna, and that rho may play a role in patterning olfactory receptor gene expression in chemosensory sensilla.
Identification and characterization of nocSco/+ provided the initial insight into the convoluted puzzle leading to our identification of rho as a gene required for retinal patterning. These studies highlight the potential difficulty in determining the mechanisms underlying phenotypes identified in misexpression screens. In the absence of a loss-of-function phenotype of the misexpressed gene (sna) in the tissue of interest (retina) one is faced with a substantial challenge. While the alteration of rho expression identified here was critical in determining its role in coordinating the expression of opsin genes in the R7 and R8 photoreceptor cells, it is insufficient to explain the entire nocSco/+ retinal phenotype. Because loss of rho does not completely suppress the nocSco/+ phenotype, it suggests that part of the induction of Rh5 expression in nocSco/+ flies is independent of rho, perhaps through the CtBP-dependent repression of other target genes or the activation of EGFR signaling downstream of rho. Thus despite the important insight that nocSco/+ has provided into this aspect of retinal patterning, at least part of the mechanism underlying the phenotype remains unresolved.
Materials and Methods
Stocks and genetics
Stocks were maintained in humidified incubators on standard cornmeal/molasses/agar media. Unless otherwise specified, all stocks were obtained from the Bloomington Stock Center. rhopΔ5 FRT was generously provided by Mathew Freeman. Homozygous mutant clones in the eye of rhopΔ5 and CtBP87De-10 were generated with the FLP/FRT system by standard techniques38 using the eyeless promoter-driven FLP recombinase (ey-FLP).39 The strains and alleles used were as follows: Canton-S (FBst0000001), nocSco (FBal0013044), w1118 (FBal0018186), al1 dpov1 b1 pr1 c1 px1 sp1 (FBst0000156), sev14 (FBal0015458), nocSco-rv8 (FBal0013048), nocSco-rv17 (FBal0013054), nocSco-rv11 (FBal0013050), Df(2L) Scorv25 (FBab0001552), Df(2L)Scorv19 (FBab0001550), CtBP87De-10 (FBal0010480), rhopΔ5 (FBal0033087).
Scanning electron micrographs
Flies were dehydrated for 12 hours each in a graded ethanol series (25%, 50%, 75% and 100%), followed by incubation in hexamethyldisilazane. Samples were dried under house vacuum and sputter coated with a Gold Palladium Target (Electron Microscopy Sciences, Hatfield, PA), mounted on 12 mm diameter Carbon Adhesive tabs and placed on stubs. Samples were examined with a LEO 435VP scanning electron microscope (LEO Electron Microscopy Ltd., Cambridge, UK).
Histology and immunohistochemistry
Epon embedded retinal cross sections were performed as previously described.10 For immunohistochemistry, 10 μm cryosections were prepared and treated as previously described.10 Antibodies were used at the following dilutions: directly conjugated mouse monoclonal anti-Rh5 (Texas Red, 1:100), anti-Rh6 (FITC, 1:100), anti-Rh3 (clone 2B1, 1:20), anti-Rh4 (clone 11E6, 1:10), FITC-conjugated Fab fragment goat anti-mouse (1:400, Jackson Immunoresearch Laboratories, Inc., West Grove, PA), AlexaFluor 568 goat anti-mouse IgG (H + L) (1:400, Molecular Probes, Eugene, OR). Immunofluorescence images were acquired with an Axioskop plus/AxioCamHRc (Carl Zeiss, Inc., Thornwood, NY) or by confocal microscopy using a Zeiss Pascal LSM (Carl Zeiss, Inc.,). Comparisons of the proportions (percentages) of opsin expression in different genetic backgrounds were performed with a z-score and are shown in Table 1.50 The z-score was calculated using the equation:
p1 and p2 = proportions of marker expression in each of the two different genotypes under comparison. n1. and n2. = number of ommatidia counted for each genotype. p̄ = average proportion for both genotypes combined. q̄ = 1 − p̄. The significance of the difference between the two proportions was determined from the normal distribution as a two-tailed test.
RNA in situ hybridization
Eye-antennal imaginal discs from third instar larvae were dissected in PBS, fixed in 50 mM EGTA/4% formaldehyde in PBS, rinsed in methanol, and stored in ethanol at −20°. Discs were treated with ethanol/xylene (1:1), rinsed with ethanol, post-fixed in 5% formaldehyde in PBS plus 0.1% Tween (PBT), washed with PBT, and digested with Proteinase K (5 μg/ml). Tissue was post-fixed again and pre-hybridized in hybridization buffer (50% deionized formamide, 5X SSC, 1 mg/ml glycogen, 100 μg/ml salmon sperm DNA, 0.1% Tween) at 48°C. Discs were hybridized overnight at 55°C with 2 μl digoxigenin-labeled antisense RNA probe in 100 μl hybridization buffer. The hybridized imaginal discs were washed extensively with hybridization buffer at 55°C followed by PBT washes at room temperature. Discs were incubated with alkaline phosphatase-conjugated anti-digoxigenin antibody (1:2,000, Roche Applied Science, Indianapolis, IN) overnight at 4°C. Discs were washed with PBT and gene expression was visualized with staining solution (100 mM NaCl, 50 mM MgCl2, 100 mM Tris pH 9.5, 0.1% Tween) containing NBT/BCIP (Roche Applied Science). Stained imaginal discs were mounted and photographed using an Axioskop plus/AxioCamHRc (Carl Zeiss Inc.,). For the double labeling experiment, digoxigenin-labeled and FITC-labeled antisense RNA probes were prepared and hybridized as described above. Following detection of the digoxigenin-labeled probe with NBT/BCIP, the FITC-labeled probe was detected with rabbit anti-FITC (1:200, Jackson Immunoresearch Laboratories, Inc.,) followed by signal amplification with biotinylated goat anti-rabbit (1:700) and streptavidin conjugated HRP (1:100) using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) and an AlexaFluor 488 tyramide using a tyramide signal amplification kit (Molecular Probes Inc., Eugene OR). The AlexaFluor 488 signal and NBT/BCIP reaction products were visualized by confocal microscopy.51
Acknowledgments
This work was supported by grants R01EY12423 and R01EY18376 from the NIH. We thank Lauren Chesnut at the University of Texas Health Science Center at San Antonio for the SEM work, Dorothy Dill for help with epon sectioning, Stephen Crews and Michael Levine for cDNAs, and Matthew Freeman, Terence Davis and John Roote for stocks and helpful discussion.
References
- 1.Heberlein U, Wolff T, Rubin GM. The TGFbeta homolog dpp and the segment polarity gene hedgehog are required for propagation of a morphogenetic wave in the Drosophila retina. Cell. 1993;75:913–26. doi: 10.1016/0092-8674(93)90535-x. [DOI] [PubMed] [Google Scholar]
- 2.Hardie RC. Functional organization of the fly retina. In: Autrum H, Ottoson D, Perl ER, Schmidt RF, Shimazu H, Willis WD, editors. Progress in sensory physiology. Berlin: Springer-Verlag; 1985. pp. 1–79. [Google Scholar]
- 3.Zuker CS, Cowman AF, Rubin GM. Isolation and structure of a rhodopsin gene from D. melanogaster. Cell. 1985;40:851–8. doi: 10.1016/0092-8674(85)90344-7. [DOI] [PubMed] [Google Scholar]
- 4.O’Tousa JE, Baehr W, Martin RL, Hirsh J, Pak WL, Applebury ML. The Drosophila ninaE gene encodes an opsin. Cell. 1985;40:839–50. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- 5.Feiler R, Harris WA, Kirschfeld K, Wehrhahn C, Zuker CS. Targeted misexpression of a Drosophila opsin gene leads to altered visual function. Nature. 1988;333:737–41. doi: 10.1038/333737a0. [DOI] [PubMed] [Google Scholar]
- 6.Kirschfeld K, Feiler R, Franceschini N. A photostable pigment within the rhabdomeres of fly photoreceptors no.7. J Comp Physiol. 1978;125:275–84. [Google Scholar]
- 7.Franceschini N, Kirschfeld K, Minke B. Fluorescence of photoreceptor cells observed in vivo. Science. 1981;213:1264–7. doi: 10.1126/science.7268434. [DOI] [PubMed] [Google Scholar]
- 8.Feiler R, Bjornson R, Kirschfeld K, Mismer D, Rubin GM, Smith DP, et al. Ectopic expression of ultraviolet-rhodopsins in the blue photoreceptor cells of Drosophila: visual physiology and photochemistry of transgenic animals. J Neurosci. 1992;12:3862–8. doi: 10.1523/JNEUROSCI.12-10-03862.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montell C, Jones K, Zuker C, Rubin G. A second opsin gene expressed in the ultraviolet sensitive R7 photoreceptor cells of Drosophila melanogaster. J Neurosci. 1987;7:1558–66. doi: 10.1523/JNEUROSCI.07-05-01558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou WH, Huber A, Bentrop J, Schulz S, Schwab K, Chadwell LV, et al. Patterning of the R7 and R8 photoreceptor cells of Drosophila: evidence for induced and default cell-fate specification. Development. 1999;126:607–16. doi: 10.1242/dev.126.4.607. [DOI] [PubMed] [Google Scholar]
- 11.Salcedo E, Huber A, Henrich S, Chadwell LV, Chou WH, Paulsen R, et al. Blue- and green-absorbing visual pigments of Drosophila: ectopic expression and physiological characterization of the R8 photoreceptor cell-specific Rh5 and Rh6 rhodopsins. J Neurosci. 1999;19:10716–26. doi: 10.1523/JNEUROSCI.19-24-10716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuker C, Montell C, Jones K, Laverty T, Rubin G. A rhodopsin gene expressed in photoreceptor cell R7 of the Drosophila eye: homologies with other signal-transducing molecules. J Neurosci. 1987;7:1550–7. doi: 10.1523/JNEUROSCI.07-05-01550.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou WH, Hall KJ, Wilson DB, Wideman CL, Townson SM, Chadwell LV, et al. Identification of a novel Drosophila opsin reveals specific patterning of the R7 and R8 photoreceptor cells. Neuron. 1996;17:1101–15. doi: 10.1016/s0896-6273(00)80243-3. [DOI] [PubMed] [Google Scholar]
- 14.Bell ML, Earl JB, Britt SG. Two types of Drosophila R7 photoreceptor cells are arranged randomly: a model for stochastic cell-fate determination. J Comp Neurol. 2007;502:75–85. doi: 10.1002/cne.21298. [DOI] [PubMed] [Google Scholar]
- 15.Wernet MF, Mazzoni EO, Celik A, Duncan DM, Duncan I, Desplan C. Stochastic spineless expression creates the retinal mosaic for colour vision. Nature. 2006;440:174–80. doi: 10.1038/nature04615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortini ME, Rubin GM. The optic lobe projection pattern of polarization-sensitive photoreceptor cells in Drosophila melanogaster. Cell Tissue Res. 1991;265:185–91. doi: 10.1007/BF00318153. [DOI] [PubMed] [Google Scholar]
- 17.Wernet MF, Labhart T, Baumann F, Mazzoni EO, Pichaud F, Desplan C. Homothorax switches function of Drosophila photoreceptors from color to polarized light sensors. Cell. 2003;115:267–79. doi: 10.1016/s0092-8674(03)00848-1. [DOI] [PubMed] [Google Scholar]
- 18.Tomlinson A. Patterning the peripheral retina of the fly: decoding a gradient. Dev Cell. 2003;5:799–809. doi: 10.1016/s1534-5807(03)00326-5. [DOI] [PubMed] [Google Scholar]
- 19.Earl JB, Britt SG. Expression of Drosophila rhodopsins during photoreceptor cell differentiation: Insights into R7 and R8 cell subtype commitment. Gene Expr Patterns. 2006;6:687–94. doi: 10.1016/j.modgep.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Papatsenko D, Sheng G, Desplan C. A new rhodopsin in R8 photoreceptors of Drosophila: evidence for coordinate expression with Rh3 in R7 cells. Development. 1997;124:1665–73. doi: 10.1242/dev.124.9.1665. [DOI] [PubMed] [Google Scholar]
- 21.Mikeladze-Dvali T, Wernet MF, Pistillo D, Mazzoni EO, Teleman AA, Chen YW, et al. The Growth Regulators warts/lats and melted Interact in a Bistable Loop to Specify Opposite Fates in Drosophila R8 Photoreceptors. Cell. 2005;122:775–87. doi: 10.1016/j.cell.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 22.Krivshenko J. New mutants report. Drosophila Information Service. 1959;33:95–6. [Google Scholar]
- 23.Krivshenko E, Krivshenko J. Linkage Data. Drosophila Information Service. 1960;34:55. [Google Scholar]
- 24.Ashburner M, Detwiler C, Tsubota S, Woodruff RC. The genetics of a small autosomal region of Drosophila melanogaster containing the structural gene for alcohol dehydrogenase VI. Induced revertants of scutoid. Genetics. 1983;104:405–31. doi: 10.1093/genetics/104.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashburner M, Tsubota S, Woodruff RC. The genetics of a small chromosome region of Drosophila melanogaster containing the structural gene for alcohol dehydrogenase IV: scutoid, an antimorphic mutation. Genetics. 1982;102:401–20. doi: 10.1093/genetics/102.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGill S, Chia W, Karp R, Ashburner M. The molecular analyses of an antimorphic mutation of Drosophila melanogaster, Scutoid. Genetics. 1988;119:647–61. doi: 10.1093/genetics/119.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuse N, Matakatsu H, Taniguchi M, Hayashi S. Snail-type zinc finger proteins prevent neurogenesis in Scutoid and transgenic animals of Drosophila. Dev Genes Evol. 1999;209:573–80. doi: 10.1007/s004270050291. [DOI] [PubMed] [Google Scholar]
- 28.Hemavathy K, Ashraf SI, Ip YT. Snail/slug family of repressors: slowly going into the fast lane of development and cancer. Gene. 2000;257:1–12. doi: 10.1016/s0378-1119(00)00371-1. [DOI] [PubMed] [Google Scholar]
- 29.Mauhin V, Lutz Y, Dennefeld C, Alberga A. Definition of the DNA-binding site repertoire for the Drosophila transcription factor SNAIL. Nucleic Acids Res. 1993;21:3951–7. doi: 10.1093/nar/21.17.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boulay JL, Dennefeld C, Alberga A. The Drosophila developmental gene snail encodes a protein with nucleic acid binding fingers. Nature. 1987;330:395–8. doi: 10.1038/330395a0. [DOI] [PubMed] [Google Scholar]
- 31.Ip YT, Park RE, Kosman D, Bier E, Levine M. The dorsal gradient morphogen regulates stripes of rhomboid expression in the presumptive neuroectoderm of the Drosophila embryo. Genes Dev. 1992;6:1728–39. doi: 10.1101/gad.6.9.1728. [DOI] [PubMed] [Google Scholar]
- 32.Nibu Y, Zhang H, Bajor E, Barolo S, Small S, Levine M. dCtBP mediates transcriptional repression by Knirps, Kruppel and Snail in the Drosophila embryo. EMBO J. 1998;17:7009–20. doi: 10.1093/emboj/17.23.7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stathopoulos A, Levine M. Linear signaling in the Toll-Dorsal pathway of Drosophila: activated Pelle kinase specifies all threshold outputs of gene expression while the bHLH protein Twist specifies a subset. Development. 2002;129:3411–9. doi: 10.1242/dev.129.14.3411. [DOI] [PubMed] [Google Scholar]
- 34.Stathopoulos A, Levine M. Dorsal gradient networks in the Drosophila embryo. Dev Biol. 2002;246:57–67. doi: 10.1006/dbio.2002.0652. [DOI] [PubMed] [Google Scholar]
- 35.Steward R, Govind S. Dorsal-ventral polarity in the Drosophila embryo. Curr Opin Genet Dev. 1993;3:556–61. doi: 10.1016/0959-437x(93)90090-c. [DOI] [PubMed] [Google Scholar]
- 36.Birkholz DA, Chou WH, Phistry MM, Britt SG. Rhomboid mediates specification of blue- and green-sensitive R8 photoreceptor cells in Drosophila. J Neurosci. 2009;29:2666–75. doi: 10.1523/JNEUROSCI.5988-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golic KG. Site-specific recombination between homologous chromosomes in Drosophila. Science. 1991;252:958–61. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- 38.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–37. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 39.Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–60. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- 40.Hardie RC. Electrophysiological analysis of fly retina I: Comparative properties of R 1–6 and R 7 and 8. J Comp Physiol. 1979;129:19–33. [Google Scholar]
- 41.Hiromi Y, Mlodzik M, West SR, Rubin GM, Goodman CS. Ectopic expression of seven-up causes cell fate changes during ommatidial assembly. Development. 1993;118:1123–35. doi: 10.1242/dev.118.4.1123. [DOI] [PubMed] [Google Scholar]
- 42.Kramer S, West SR, Hiromi Y. Cell fate control in the Drosophila retina by the orphan receptor seven-up: its role in the decisions mediated by the ras signaling pathway. Development. 1995;121:1361–72. doi: 10.1242/dev.121.5.1361. [DOI] [PubMed] [Google Scholar]
- 43.Kanai MI, Okabe M, Hiromi Y. Seven-up Controls switching of transcription factors that specify temporal identities of Drosophila neuroblasts. Dev Cell. 2005;8:203–13. doi: 10.1016/j.devcel.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Gates J, Lam G, Ortiz JA, Losson R, Thummel CS. Rigor mortis encodes a novel nuclear receptor interacting protein required for ecdysone signaling during Drosophila larval development. Development. 2004;131:25–36. doi: 10.1242/dev.00920. [DOI] [PubMed] [Google Scholar]
- 45.Zelhof AC, Yao TP, Chen JD, Evans RM, McKeown M. Seven-up inhibits ultraspiracle-based signaling pathways in vitro and in vivo. Mol Cell Biol. 1995;15:6736–45. doi: 10.1128/mcb.15.12.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubin AE, Heald NL, Cleveland B, Carlson JR, Harris GL. Scutoid mutation of Drosophila melanogaster specifically decreases olfactory responses to short-chain acetate esters and ketones. J Neurobiol. 1995;28:214–33. doi: 10.1002/neu.480280208. [DOI] [PubMed] [Google Scholar]
- 47.Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117:965–79. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Couto A, Alenius M, Dickson BJ. Molecular, anatomical and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–47. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 49.Endo K, Aoki T, Yoda Y, Kimura K, Hama C. Notch signal organizes the Drosophila olfactory circuitry by diversifying the sensory neuronal lineages. Nat Neurosci. 2007;10:153–60. doi: 10.1038/nn1832. [DOI] [PubMed] [Google Scholar]
- 50.Fleiss JL, Levin BA, Paik MC. Statistical methods for rates and proportions. Hoboken NJ: Wiley-Interscience; 2003. [Google Scholar]
- 51.Trinh le A, McCutchen MD, Bonner-Fraser M, Fraser SE, Bumm LA, McCauley DW. Fluorescent in situ hybridization employing the conventional NBT/BCIP chromogenic stain. Biotechniques. 2007;42:756–9. doi: 10.2144/000112476. [DOI] [PubMed] [Google Scholar]
- 52.Ashburner M, Aaron CS, Tsubota S. The genetics of a small autosomal region of Drosophila melanogaster, including the structural gene for alcohol dehydrogenase V. Characterization of X-ray-induced Adh null mutations. Genetics. 1982;102:421–35. doi: 10.1093/genetics/102.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dubnau J, Chiang AS, Grady L, Barditch J, Gossweiler S, McNeil J, et al. The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr Biol. 2003;13:286–96. doi: 10.1016/s0960-9822(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 54.Ashburner M, Misra S, Roote J, Lewis SE, Blazej R, Davis T, et al. An exploration of the sequence of a 2.9-Mb region of the genome of Drosophila melanogaster: the Adh region. Genetics. 1999;153:179–219. doi: 10.1093/genetics/153.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]