Abstract
In urodele amphibians, lens induction during development and regeneration occurs through different pathways. During development, the lens is induced from the mutual interaction of the ectoderm and the optic vesicle, whereas after lentectomy the lens is regenerated through the transdifferentiation of the iris-pigmented epithelial cells. Given the known role of fibroblast growth factors (FGFs) during lens development, we examined whether or not the expression and the effects of exogenous FGF during urodele lens regeneration were conserved. In this paper, we describe expression of FGF-1 and its receptors, FGFR-2 (KGFR and bek variants) and FGFR-3, in newts during lens regeneration. Expression of these genes was readily observed in the dedifferentiating pigmented epithelial cells, and the levels of expression were high in the lens epithelium and the differentiating fibers and lower in the retina. These patterns of expression implied involvement of FGFs in lens regeneration. To further elucidate this function, we examined the effects of exogenous FGF-1 and FGF-4 during lens regeneration. FGF-1 or FGF-4 treatment in lentectomized eyes resulted in the induction of abnormalities reminiscent to the ones induced during lens development in transgenic mice. Effects included transformation of epithelial cells to fiber cells, double lens regeneration, and lenses with abnormal polarity. These results establish that FGF molecules are key factors in fiber differentiation, polarity, and morphogenesis of the lens during regeneration even though the regenerating lens is induced by a different mechanism than in lens development. In this sense, FGF function in lens regeneration and development should be regarded as conserved. Such conservation should help elucidate the mechanisms of lens regeneration in urodeles and its absence in higher vertebrates.
Lens regeneration during adulthood is a remarkable phenomenon occurring only in some urodeles (1). After lentectomy, the pigment epithelial cells of the dorsal iris dedifferentiate, by shedding their pigments, proliferate, and eventually transdifferentiate to lens cells. These lens epithelial cells can subsequently differentiate to lens fibers (2–4). These morphogenetic events during lens regeneration differ from the corresponding events that take place during lens development. The developing lens is induced when the ectoderm interacts with the optic vesicle, which is the precursor of the retina. Once this induction takes place, the lens cup becomes independent and forms the lens vesicle. The posterior lens cells then differentiate to fibers. Comparative studies on these two types of lens induction at the molecular level are very limited. So far, Pax-6 has been found to be expressed during lens regeneration and development (5). On the other hand, regulation of crystallin synthesis appears to be different in regeneration and development (G. Eguchi, personal communication).
Several studies have demonstrated that molecules such as FGFs might play very important roles in determining the differentiation events during lens development. Most striking are the results indicating that FGF is present as a gradient in the eyeball with higher concentrations found in the posterior than in the anterior chamber (6). Such distribution renders FGF as a fiber differentiation factor, with a higher concentration needed to differentiate lens epithelial cells to fibers. Indeed, FGFs and their receptors have been found to be expressed in eye tissues. FGF-1 and FGF-2 have been found to be expressed in the mouse neural retina and in lens cells during development (7–9). Specifically, FGF-1 has been thought to be involved in lens-inductive interactions between ectoderm and optic vesicle (7). FGFR-1, FGFR-2, FGFR-3 and FGFR-4 are expressed in lens cells, while FGFR-1 and FGFR-2 have been implicated in retina development (10–12). More direct answers on the role of FGFs and their receptors in lens development have been obtained from studies involving transgenic mice. Mice made transgenic with FGF-1 develop abnormal lenses: abnormalities characterized by the transformation of lens epithelial cells to lens fibers, affected lens shape and polarity, and the development of cataracts (13). In similar studies, mice expressing a dominant-negative FGFR-1 showed that fibers were diminished by apoptosis (14). These studies clearly indicate that FGF is imperative for lens fiber differentiation.
These patterns of expression of FGFs and their receptors as well as the role that they play in lens morphogenesis has prompted us to study their expression and effects of exogenous FGF during lens regeneration. The reader should bear in mind that during lens regeneration, a lens is produced but the inductive mechanisms are different than those occurring in normal development. Does the FGF pathway operate differently in the case of lens regeneration? Has the mechanism of lens morphogenesis been conserved in the two different cases of induction? The answers to these questions could provide useful insights into the mechanism of lens regeneration and lens morphogenesis in general. The urodele system for lens regeneration offers a unique opportunity to study such expression patterns and effects of exogenous FGF. In this study, we examined the expression of FGF-1 and its receptors, FGFR-2 (both KGFR and bek variants) and FGFR-3. Once it was established that these molecules were indeed expressed during transdifferentiation, the effects of exogenous FGF-1 and FGF-4 on lens regeneration were ascertained. Our results demonstrate that FGFs act and control lens differentiation during regeneration the same way they do during lens development, indicating conservation of molecular mechanisms in two different inductive pathways.
MATERIALS AND METHODS
Operations and Tissue Collection.
Animals were lentectomized under anesthesia and their eyes were collected at different stages of regeneration. The collection from newts started at 1 hr postlentectomy and continued at 6 hr, 1 day, 5 days, 10 days, 15 days, 20 days, and 25 days. For comparison we used the axolotl, a urodele that is not capable of lens regeneration. Eyes were collected from unoperated or lentectomized animals 12 days after lens removal. This could help correlate expression with the process of lens regeneration. In addition, we used developing eyes from axolotl embryos of stage 36 and from hatched animals to compare with the regenerating eyes. The eyes were embedded in paraffin, sectioned, and processed for in situ hybridization.
Exogenous Administration of FGF.
We used newt recombinant FGF-1 made from our full-length cDNA (17) and human recombinant FGF-4 (R & D Systems). Each of these FGF isoforms was mixed with heparin beads (Sigma) at a concentration of 5 μg/μl. After incubation with the beads for a few hours or overnight at 4°C, one bead was implanted in the eye cavity immediately after lentectomy. These beads have been shown to slowly release FGF.
Probes.
FGFR-2 (both KGFR and bek variants) were cloned from newt limb blastema as previously described (15, 16). A partial FGF-1 clone was obtained via reverse transcriptase (RT)-PCR using degenerate primers designed from human sequences. With this fragment, the whole cDNA was cloned via 5′ and 3′ RACE (rapid amplification of cDNA ends) (17). The full-length newt FGF-1 cDNA was cloned into Nde/BglII sites of pET20b(+) and designated nrFGF1. A 340-bp PstI-XhoI fragment isolated from nrFGF1 was cloned into PstI/XhoI sites of Bluescript SK(+). Antisense and sense probes were generated for each of the probes using the DIG-RNA labeling kit from Boehringer Mannheim. The Xenopus FGFR-3 was kindly provided to us by I. Hongo (National Institute for Bioscience and Human Technology, Tsukuba, Japan).
In Situ Hybridization.
Slides containing paraffin sections were deparaffinized in xylene and subsequently hydrated through ethanol series. The slides were rinsed in 1× PBS and then fixed in 4% paraformaldehyde for 15 min. After a rinse with 1× PBS, the slides were incubated with 250 μg/ml of pepsin at 37°C for 15 min. Subsequently, the slides were rinsed again with 1× PBS and then treated with 0.1 M triethanolamine/0.25% acetic anhydride. The slides were then washed one last time with 1× PBS and dehydrated using ethanol series. After 1 hr of air drying, the sections were hybridized at 50°C for 16 hr with hybridization solution (50% formamide/1 mM EDTA/10 mM Tris⋅HCl, pH 7.5/600 mM NaCl/0.25% SDS/10% PEG 6000/1× Denhart’s/200 μg/ml tRNA/250 ng/ml of digoxigenin-labeled probes). The next day the slides were washed with 4× SSC followed by a treatment with 50 μg/ml of RNase at 37°C for 1 hr. Subsequently, the slides were incubated in 2× SSC at 50°C two times for 30 min each time, and then in 0.1× SSC at 50°C two times for 30 min each. For the immunological detection, the slides were rinsed in buffer 1 (0.1 M Tris⋅HCl, pH 7.5/0.15 M NaCl) and then incubated in buffer 2 (buffer 1 with 1% blocking reagent; Boehringer Mannheim) for 1 hr at room temperature. The sections were then incubated with anti-DIG antibody alkaline phosphatase conjugate in buffer 2 at 1:2,500 for 1 hr at room temperature. After 3 washes with buffer 1 for 30 min each, the slides were incubated in buffer 3 (0.1 M Tris⋅HCl, pH 9.5/0.1 M NaCl/50 mM MgCl2) for 10 min and later incubated in the same solution plus nitroblue tetrazolium/5′-bromo-4-chloro-3-indolylphosphate for 16–24 hr. The reaction was stopped with TE (10 mM Tris/1 mM EDTA), and finally the sections were mounted with crystal mount. Pictures were produced with a Sony video printer (Tokyo).
Immunostaining.
A rabbit antibody against human FGFR-2 (bek) that crossreacts with newt tissues is commercially available from Santa Cruz Biotechnology. We took advantage of this to test for the presence of FGFR-2 protein as well. Paraffin sections were deparaffinized in xylene, and after hydrating them through ethanol series they were treated with 0.1% sodium borohydride in 1× PBS three times for 10 min each to prevent autofluoresence. The sections were then treated with 10% normal goat serum containing 0.1% Saponin in PBS (SNB) for 20 min. The primary antibody was used at a dilution of 1:30 for 2 hr in Saponin-NGS-PBS (SNB). After that, the sections were again incubated with SNB and then they were treated with anti-rabbit IgG conjugated with fluorescein isothiocyanate (1:300) in SNB for 1 hr. After three washes with 1× PBS the slides were mounted with glycerol and observed via fluorescent microscopy.
RESULTS AND DISCUSSION
Upon lentectomy certain events are initiated in the newt eye. The dorsal iris-pigmented epithelium undergoes dedifferentiation within a few days, and at about 10 days after lentectomy it forms the beginning of the lens vesicle. After this event, differentiation of the primary and secondary lens fibers ensues at about day 15. Lens regeneration is considered complete at about 25 days. To study the expression and the roles of FGF in this process, we selected FGF-1 and its receptor, FGFR-2. FGFR-2 can be produced in two different, alternatively spliced forms, the KGFR and bek, resulting in recognition of different FGF ligands (18). We examined the expression of both alternative transcripts, as well as FGFR-3 transcript, which also binds FGF-1.
Expression of FGF-1.
FGF-1 was found to be expressed in the retina (all layers, ganglion cells, amacrine cells, and photoreceptor cells) and the lens epithelium of the adult eye. Upon lentectomy, expression was persistent in the retina. Not much change was observed in the retina layers throughout the regeneration process. However, FGF-1 was expressed at high levels during dedifferentiation of the iris pigment epithelial cells at 10-day postlentectomy and in the initial lens vesicle. At day 15, there was strong expression in the lens epithelium and the lens fibers. This strong expression was observed at day 20 as well (Fig. 1). This expression was obviously higher in the lens when compared with retina (Fig. 1 E′ and F′). As expected FGF-1 was expressed very strongly in the developing and hatched axolotl eye as well (Fig. 2).
Figure 1.
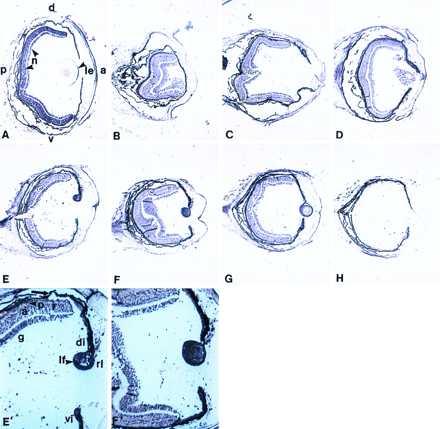
Expression of FGF-1 in newt lens regeneration. (A) Expression in the intact eye. FGF-1 transcripts are present in neural retina (n) and lens epithelium (le). a, anterior; p, posterior; d, dorsal; v, ventral. (B–G) Expression during the process of lens regeneration, 1, 5, 10, 15, 20, and 25 days postlentectomy. E′ and F′ are higher magnification of E and F to better show the differences in expression between the lens and the neural retina. Expression in lens is higher when compared with the retina. g, ganglion; a, amacrine; p, photoreceptors; rl, regenerating lens; lf, lens fibers; di, dorsal iris; vi, ventral iris. The arrow indicates the pigment epithelium. (H) Hybridization with the sense probe to show the background.
Figure 2.
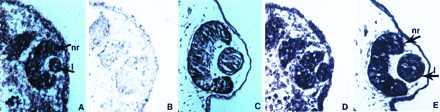
Expression of FGF-1 and KGFR in axolotl developing eyes. (A) Expression of FGF-1 at stage 36 embryo showing strong reaction in the neural retina (nr) and the lens (l) (arrows). (B) Hybridization with the sense probe to show the background. (C) Expression of FGF-1 in the eye of a hatched animal. (D and E) Expression of KGFR in the eye of a stage 36 and hatched animal, respectively (arrows).
Expression of FGFR-2.
Similar to FGF-1, FGFR-2, its receptor, in the form of both KGFR and bek variants, was expressed in the retina and lens epithelium of the intact newt eye. The expression patterns of both variants were very similar. After lentectomy, expression in the dedifferentiated cells and in the regenerating lens was similar to FGF-1. Retina as well as both ventral and dorsal ciliary body and iris were positive. There was a strong expression in the lens epithelium and the forming fibers (Fig. 3). One difference from the FGF-1 pattern was that KGFR seemed to be down-regulated in the retina (especially in the amacrine and photoreceptor cells) pretty much throughout the regeneration process. In this sense, expression in the regenerating lens was highly up-regulated. The expression patterns of FGFR-2 variants in the developing axolotl eye were similar to those of FGF-1 (Fig. 2). In Fig. 3, the expression patterns of KGFR during the regeneration series are shown. The availability of an antibody against FGFR-2 that crossreacts with newt tissues provided us with the opportunity to determine the presence of the protein as well. The product for FGFR-2 seemed to follow the patterns observed for the transcript. The protein was present in the retina, ciliary body, and the lens epithelium of the intact eye. During regeneration, the protein appeared in the dorsal dedifferentiated iris and subsequently in the regenerating lens. However, it was also present in the retina, ciliary body, and ventral iris with no apparent regulation (Fig. 4).
Figure 3.
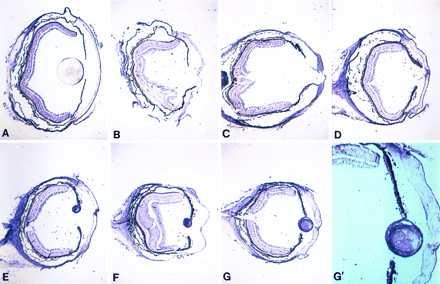
Expression of FGFR-2 (KGFR) in newt lens regeneration. (A–G) Same series as in Fig. 1. (G′) Higher magnification of G to show the higher expression in the lens when compared with the retina.
Figure 4.
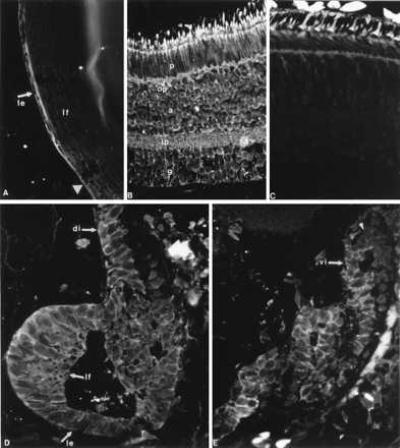
Immunohistochemical detection of the FGFR-2 protein in the intact eye and the regenerating lens. Positive reaction is obvious as a fluorescent ring around the cells. (A) Presence in the lens epithelium (le) of an intact lens. lf, lens fibers. The arrowhead points to the equator. (B) Presence in all layers of intact retina. p, photoreceptors; a, amacrine cells; g, ganglion cells; op, outer plexiform layer; ip, inner plexiform layer. (C) Negative control with a section through adult retina, to show the background. (D) FGFR-2 protein is detected in the regenerating lens (le, lens epithelium; lf, differentiating lens fibers) and the dorsal iris (di, arrow) 15 days after lentectomy. (E) FGFR-2 presence in the ventral iris (vi, arrow) 15 days after lentectomy. Reaction is particularly visible in iris cells that are depigmented. At the tip of the iris (arrowhead) expression is obscured by the pigments.
Expression of FGFR-3.
FGFR-3 showed identical patterns of expression as those of FGFR-2. It was expressed in the retina, the ciliary body, and iris (both dorsal and ventral) and very strongly in the dedifferentiated pigment epithelial cells and the lens fibers (Fig. 5).
Figure 5.
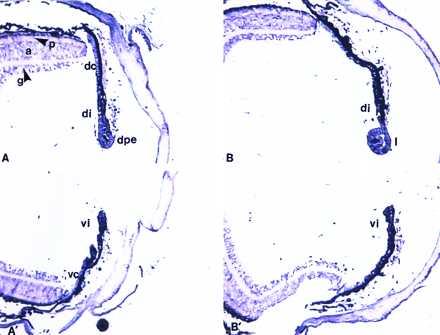
Expression of FGFR-3. (A and A′) Expression 10 days postlentectomy in the dorsal and ventral part of the eye, respectively. dpe, dedifferentiated pigment epithelium; di, dorsal iris; dc, dorsal ciliary body; vi, ventral iris; vc, ventral ciliary body. g, a, and p designate the three layers of neural retina, ganglion, amacrine, and photoreceptors. (B and B′) Expression 15 days postlentectomy. l, lens. Note no differences in expression between dorsal and ventral iris, but higher expression in the lens when compared with retina.
Our expression experiments provided clear evaluation of the presence of FGF-1, FGFR-2, and FGFR-3 in the newt intact adult eye and in comparison with developing and regenerating lens. This is a comprehensive study on the expression of these molecules during urodele lens development and regeneration. The expression patterns of FGF-1 and its receptors in the developing, intact, and regenerating urodele eye seem to be consistent with the developmental role of these molecules. Expression in the retina might be important for the ability of these animals to regenerate their retina as adults as well. When part of the newt retina is removed, regeneration occurs from the retinal pigment epithelium (19). In the embryonic chicken eye, FGF has been shown to be able to account for retina regeneration from the pigment epithelium (20). In the newt, expression in the dedifferentiating cells of the dorsal iris after lentectomy strongly implies that FGF-1 and its receptors play a significant role in this process as well. Consistent with these expression patterns is that FGF can enhance transdifferentiation of pigment epithelial cells to lens cells in vitro (21). Consistent with a role of FGF in lens regeneration in vivo is that proteoglycans, proteins known to bind FGF, disappear sequentially from the dorsal iris after lentectomy (22, 23). It could be that loss of proteoglycans renders FGF accessible for its function as mitogen after dedifferentiation. Strong expression in the lens obviously is correlated with the role of FGF in lens fiber differentiation.
That we did not observe any differences in expression across the dorsal–ventral iris is quite telling. FGFR-3 protein has been found to be in higher amounts in the intact dorsal than the ventral iris and during the dedifferentiation process (24). FGFR-3 protein was not specific to dorsal iris, but it was found at higher levels in the dorsal iris. The authors of that paper, however, failed to provide an explanation for the opposite gradient (more in ventral, less in dorsal iris) that they observed at day 36 after lentectomy, a stage that is considered equivalent to normal intact eye (24). In our study, we did not observe this gradient for FGFR-3 transcripts. The most likely explanation, if the protein data were correct (24), is that during the regeneration process there is translational or posttranslational control. Interestingly, during newt limb regeneration, the muscle cells can reenter the cell cycle and dedifferentiate by phosphorylation of the retinoblastoma protein (25). The retinoblastoma gene normally is transcribed in unamputated limb, blastema, muscle cells, or blastema cells, but what is unique during dedifferentiation is a posttranslational control (phosphorylation) and not a transcriptional one (25). Similar events might happen with other factors as well, whether they are transcriptional regulators or they are downstream targets in limb and lens regeneration, where in both cases cells must reenter the cell cycle. We believe that in light of our results, that FGFs or their receptors could be involved in lens regeneration by translational or posttranslational modification events. If this is true it could revolutionize research in the regeneration field.
Effects of Exogenous FGF-1 and FGF-4 on Lens Morphogenesis.
The expression patterns observed for FGF-1 and its receptors, FGFR-2 and FGFR-3, are consistent with the role of these molecules in lens fiber differentiation. To further elucidate this role, we applied exogenously newt recombinant FGF-1 (which binds both KGFR and bek). Human recombinant FGF-4 (which binds bek) was also used. This experiment was undertaken to see whether or not increase in exogenously added FGF would perturb its normal distribution and affect fiber differentiation. The compounds were absorbed in heparin beads and were administered immediately after lentectomy. Twenty-five days later, the eyes were examined histologically to find out whether any effects on lens morphogenesis had resulted. Several interesting abnormalities were found in the regenerated lens (Fig. 6). The normal lens epithelium is composed of cuboidal cells, found in the anterior part, that extend up to the equator of the lens where they elongate and become fiber cells (Fig. 6A). Fig. 6B shows an FGF-1-treated eye where the epithelial cells in the anterior of the lens have lost their typical cuboidal shape and have become elongated (arrowheads), more reminiscent of the cells that are found in the equator, which eventually become fibers. In other cases, more severe abnormalities were observed. Fig. 6 C and D shows what seem to be cases of double lens formation due to FGF-4 treatment. In Fig. 6C we observed a primary lens that does not have the typical round shape but is elongated and protrudes to a more anterior part of the eye. This lens is not covered by cells of cuboidal shape but rather of elongated ones (arrowhead). On the top of the big (primary) lens there is a small lens (arrow) also generated from the dorsal iris. In Fig. 6D we observe a lens with abnormal polarity (the primary fibers are found at the dorsal tip, arrow). These are probably two lenses fused together if we judge from the dynamics of the secondary fibers and the two-lobed morphology. An explanation for this could be that the mitogenic effects of FGF induces the uncontrolled proliferation of the depigmented iris cells. Alternatively, FGF might induce double-fused lens (as in the case in Fig. 6D) through the differentiation of lens epithelial cells to fibers in different areas of the lens. An interesting feature of the small lens in Fig. 6C is that it is vacuolated. These vacuoles are signs of cataractous lens and have been observed mostly when lens regenerates concomitantly with retina in newt eyes where both the neural retina as well as the lens have been removed (26).
Figure 6.
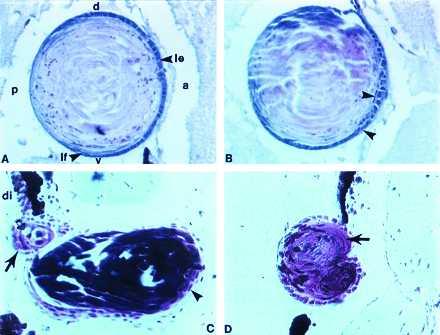
Effects of exogenous FGF on lens regeneration. (A) A normal lens regenerated 25 days postlentectomy without any treatment (control bead). Note the characteristic morphology of the lens epithelium. The cuboidal lens epithelial cells (le) in the anterior become elongated at the equator as they differentiate to lens fibers (lf). a, anterior; p, posterior; d, dorsal; v, ventral. (B) Abnormal epithelial cell morphology in a lens regenerated under the influence of FGF-1. Many of the epithelial cells do not show cuboidal morphology, but they are elongated and present in the anterior part of the lens (arrowheads). (C) Abnormal double lens regeneration due to FGF-4 treatment. A large abnormal lens is protruded to the anterior chamber. Epithelial cells are elongated (arrowhead). A second small lens (arrow) can be seen regenerating at the dorsal iris (di). This lens has vacuoles (open arrow). (D) An abnormal lens, induced by FGF-4 treatment, which also seems to be the product of two fused lenses. The polarity is also abnormal with the fibers showing at the dorsal margin (arrow) instead the posterior. All sections are through regenerates 25 days postlentectomy.
In mice it has been clearly shown that FGF isoforms are the major players for the determination and differentiation of lens fibers and lens polarity. Indeed, FGF-1 transgenic mice show vacuolated lens with abnormal shape and transformation of the anterior lens epithelial cells into more posterior fiber cells. Vacuoles in lens result in cataracts. This effect is conferred by overexpression of FGF-1 in lens cells (13). Similar effects have been noted in all (FGF-2 to FGF-9) transgenic mice (27). In newt, transgenic technologies have not been developed for such studies, but the regenerative ability of the lens from the dorsal iris provides an excellent system to overcome the transgenesis and study the overexpression effects by adding FGF in a releasing devise (bead) that can be placed easily in the eye after lentectomy. Such an experiment showed strikingly similar effects of FGF on lens morphology during regeneration as in developing lens of transgenic mice. Consistent with the mouse model, we observed abnormalities in the anterior lens epithelium cell shape and in lens morphogenesis. These similarities argue that despite the different mechanisms of developmental lens induction and lens regeneration, the function of FGF is conserved even when compared in different species. Likewise, such conservation might provide the molecular tools to investigate lens regeneration and its restriction in some urodeles only.
Acknowledgments
This work was supported by National Institutes of Health Grant EY10540 to P.A.T.
ABBREVIATION
- FGF
fibroblast growth factor
References
- 1.Stone L S. J Exp Zool. 1967;164:87–104. doi: 10.1002/jez.1401640109. [DOI] [PubMed] [Google Scholar]
- 2.Eguchi G. Embryologia. 1963;8:45–62. [Google Scholar]
- 3.Eguchi G, Kodama R. Curr Opin Cell Biol. 1993;5:1023–1028. doi: 10.1016/0955-0674(93)90087-7. [DOI] [PubMed] [Google Scholar]
- 4.Reyer R W. In: Handbook of Sensory Physiology. Crescitelli F, editor. VII/5. Berlin: Springer; 1977. pp. 309–390. [Google Scholar]
- 5.Del Rio-Tsonis K, Washabaugh C H, Tsonis P A. Proc Natl Acad Sci USA. 1995;92:5092–5096. doi: 10.1073/pnas.92.11.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caruelle D, Groux-Muscatelli B, Gaudric A, Sestier C, Coscas G, Caruelle J P, Darritault D. J Cell Biochem. 1989;39:156–159. doi: 10.1002/jcb.240390204. [DOI] [PubMed] [Google Scholar]
- 7.de Iongh R, McAvoy J W. Dev Dyn. 1993;198:190–202. doi: 10.1002/aja.1001980305. [DOI] [PubMed] [Google Scholar]
- 8.Lovicu F J, McAvoy J W. Invest Ophthalmol Visual Sci. 1993;34:3355–3365. [PubMed] [Google Scholar]
- 9.McAvoy J W, Chamberlain C G. Development (Cambridge, UK) 1989;107:221–228. doi: 10.1242/dev.107.2.221. [DOI] [PubMed] [Google Scholar]
- 10.Orr-Urtreger A, Givol D, Yayon A, Yarden Y, Lonai P. Development (Cambridge, UK) 1991;113:1419–1434. doi: 10.1242/dev.113.4.1419. [DOI] [PubMed] [Google Scholar]
- 11.Launay C, Fromentoux V, Thery C, Shi D-L, Boucaut J-C. Differentiation. 1994;58:101–111. doi: 10.1046/j.1432-0436.1995.5820101.x. [DOI] [PubMed] [Google Scholar]
- 12.Tcheng M, Fuhrmann G, Hartmann M-P, Courtois Y, Jeanny J-C. Exp Eye Res. 1994;58:351–358. doi: 10.1006/exer.1994.1025. [DOI] [PubMed] [Google Scholar]
- 13.Robinson M L, Overbeek P A, Verran D J, Grizzle W E, Stockard C R, Friesel R, Maciag T, Thompson J A. Development (Cambridge, UK) 1995;121:505–514. doi: 10.1242/dev.121.2.505. [DOI] [PubMed] [Google Scholar]
- 14.Chow R L, Roux G D, Roghani M, Palmer M A, Rifkin D B, Moscatelli D A, Lang R A. Development (Cambridge, UK) 1995;121:4383–4393. doi: 10.1242/dev.121.12.4383. [DOI] [PubMed] [Google Scholar]
- 15.Poulin M L, Chiu I-M. Dev Dyn. 1995;202:378–387. doi: 10.1002/aja.1002020407. [DOI] [PubMed] [Google Scholar]
- 16.Poulin M L, Patrie K M, Botelho M J, Tassava R A, Chiu I-M. Development (Cambridge, UK) 1993;119:353–361. doi: 10.1242/dev.119.2.353. [DOI] [PubMed] [Google Scholar]
- 17.Patrie K M, Botelho M J, Ray S K, Mehta V B, Chiu I-M. Growth Factors. 1996;14:39–57. doi: 10.3109/08977199709021509. [DOI] [PubMed] [Google Scholar]
- 18.Patrie K M, Kudla A J, Olwin B B, Chiu I-M. J Biol Chem. 1995;270:29018–29024. doi: 10.1074/jbc.270.48.29018. [DOI] [PubMed] [Google Scholar]
- 19.Mitashov V I. Int J Dev Biol. 1996;40:833–844. [PubMed] [Google Scholar]
- 20.Park C M, Hollenberg M J. Dev Biol. 1989;134:201–205. doi: 10.1016/0012-1606(89)90089-4. [DOI] [PubMed] [Google Scholar]
- 21.Hyuga M, Kodama R, Eguchi G. Int J Dev Biol. 1993;37:319–326. [PubMed] [Google Scholar]
- 22.Zalik S E, Scott V. Nature (London) 1973;244:212–214. doi: 10.1038/newbio244212a0. [DOI] [PubMed] [Google Scholar]
- 23.Tsonis P A, Del Rio-Tsonis K. J Chromatogr A. 1995;698:361–367. doi: 10.1016/0021-9673(94)00989-m. [DOI] [PubMed] [Google Scholar]
- 24.McDevitt D S, Brahma S K, Courtois Y, Jeanny J-C. Dev Dyn. 1997;208:220–226. doi: 10.1002/(SICI)1097-0177(199702)208:2<220::AID-AJA9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka E M, Gann A A F, Gates P B, Brockes J P. J Cell Biol. 1997;136:155–165. doi: 10.1083/jcb.136.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone L S, Steinitz H. J Exp Zool. 1953;124:435–467. [Google Scholar]
- 27.Lovicu E J, Shrinivasan Y, Overbeek P A. Exp Eye Res. 1996;63:S16. (abstr.). [Google Scholar]


