Abstract
γ-amino butyric acid type A (GABAA) receptors play an important role in mediating fast synaptic inhibition in the brain. They are ubiquitously expressed in the CNS and also represent a major site of action for clinically relevant drugs. Recent technological advances have greatly clarified the molecular and cellular roles played by distinct GABAA receptor subunit classes and isoforms in normal brain function. At the same time, postmortem and genetic studies have linked neuropsychiatric disorders including schizophrenia and bipolar disorder with GABAergic neurotransmission and various specific GABAA receptor subunits, while evidence implicating GABAAR-associated proteins is beginning to emerge. In this review we discuss the mounting genetic, molecular, and cellular evidence pointing toward a role for GABAA receptor heterogeneity in both schizophrenia etiology and therapeutic development. Finally, we speculate on the relationship between schizophrenia-related disorders and selected GABAA receptor associated proteins, key regulators of GABAA receptor trafficking, targeting, clustering, and anchoring that often carry out these functions in a subtype-specific manner.
Keywords: Schizophrenia, Psychiatric Disorders, Bipolar Disorder, GABA, GABAA-receptor
Introduction
Schizophrenia is a complex psychiatric disorder with a strong genetic component, affecting approximately 1% of the world population (Perala et al., 2007; Tsuang, 2000). To date, diagnosis relies solely on the presentation of clinical symptoms, which have been framed into a reliable set of diagnostic criteria that encompass the positive (delusions, hallucinations, thought disorder), negative (anhedonia, alogia, asociality), and cognitive (deficits in attention, executive function, and memory) features of schizophrenia (Lewis et al., 2008). Until recently, schizophrenia had limited prospective therapeutic targets, namely monoamine neurotransmitter receptors such as the dopamine D2 and serotonin 5HT2A receptors through the action of typical and atypical antipsychotics (Conn et al., 2008). While these compounds do not adequately address the negative and cognitive components of the syndrome, their efficacy in attenuating psychotic symptoms has led to the suggestion and subsequent demonstration that an excess in striatal dopamine release underlies the positive symptoms of schizophrenia (Morrison and Murray, 2005).
In addition to dopamine hyperfunction, a dysfunctional glutamate signaling hypothesis has also emerged, initially supported by findings that subanesthetic doses of noncompetitive N-methyl-D-aspartate receptor (NMDAR) antagonists such as ketamine recapitulate schizophrenia symptoms in healthy human subjects (Krystal et al., 1994; Malhotra et al., 1996) and exacerbate symptoms in schizophrenic patients (Lahti et al., 1995). Reinforcing this idea, it has become increasingly apparent, through advances in our understanding of the underlying biology, that a significant number of emerging candidate risk genes for schizophrenia are implicated in various aspects of glutamatergic neurotransmission, such as synaptic architecture (DISC1, Neuregulin-1, Dystrophin/Dysbindin), NMDAR function (DAAO, D-Serine Racemase), the interaction of glutamatergic and dopaminergic systems (RSG4, COMT), as well as the function of other glutamate receptors (mGluR3) (Camargo et al., 2007; Carter, 2006; Harrison and Weinberger, 2005). There is also strong evidence implicating impairments of γ-aminobutyric acid (GABA) signaling in the pathophysiology of schizophrenia. This notion, initially based on early findings that GABA has a profound influence on dopamine activity (Roberts, 1972; Stevens et al., 1974; Van Kammen, 1977), was ultimately demonstrated through postmortem studies finding reductions in cortical GABA in schizophrenic patients (Perry et al., 1979). This hypofunctional GABA hypothesis is now gaining wide acceptance as genetic, molecular, and circuit-based studies clarify the contribution of GABA signaling abnormalities to the disease, as well as shed light on the relationship between GABAergic dysfunction and other affected signaling systems. From a therapeutic standpoint, GABAA receptors (GABAARs) hold enormous potential for pharmacological modulation and specificity, owing to the high degree of receptor subtype heterogeneity combined with differential regional, cellular, and subcellular distributions of receptor subtypes within the brain. Along these lines, GABAAR functional expression and distribution are under a high degree of subtype-specific regulation, mediated in part by the interaction of these postsynaptic receptors with a number of accessory proteins (Fig. 2; Table 2). As will be discussed, cognitive deficits are considered to be core features of schizophrenia (Elvevag and Goldberg, 2000) and there is strong evidence that disturbances in GABA signaling may contribute to these deficits. Therefore, in this review, we explore the current clinical, genetic and molecular evidence implicating components of GABA signaling systems, including GABAAR subunits and some associated proteins, both as they relate to the etiology of schizophrenia as well as how they may serve as entry points for therapeutic intervention.
Figure 2. Summary of modified GABAergic signaling components in psychiatric disease.
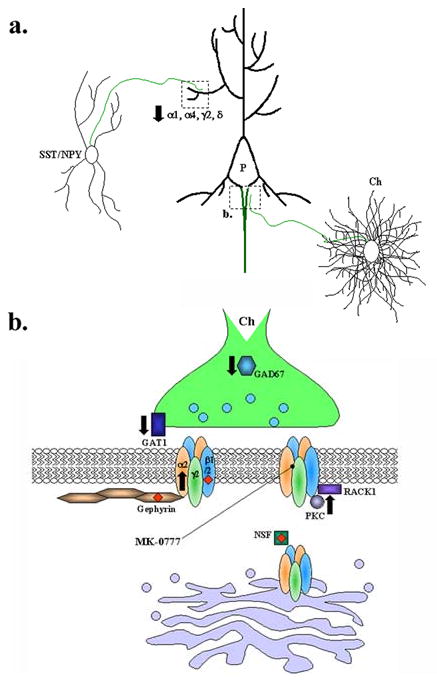
A. Schematic of relevant GABAergic interneuron synapses onto layer III pyramidal neurons (P) in the dorsolateral prefrontal cortex (DLPFC). Distal dendrites receive GABAergic input from Somatostatin (SST) and neuropeptide Y-expressing Interneurons (NPY) (upper left). The axon initial segment (AIS) is contacted by axons originating from chandelier neurons (Ch) located in layer IV of the DLPFC (lower right). Axons of SST/NPY, P, and Ch neurons are shown in green. B. Hypothetical AIS synapse highlighting some GABAergic synaptic components implicated in schizophrenia and/or bipolar affective disorder. Black arrows indicate reported reductions or elevations in mRNA or protein expression. Red diamonds indicate genetic association with schizophrenia or bipolar disorder.
Table II. Summary of Proteins that regulate GABAA trafficking and stability.
| Associated Protein; Gene Name | Interacting GABAAR Subunit(s) | Function | Chromosomal Location | Genetic Association | References |
|---|---|---|---|---|---|
| BIG2; ARFGEF2 | β1-3 | GABAAR trafficking from trans-Golgi network. | 20q13 | Linkage of 20q13 to psychotic bipolar disorder. | Becher et al., 2002; Charych et al., 2004; Park et al., 2004 |
| CAML | γ2 | GABAAR synaptic accumulation and endocytic recycling. | 5q23 | None. | Yuan et al., 2008 |
| Collybistin; ARHGEF9 | Indirect, gephyrin-binding | Synaptic clustering of GABAARs and gephyrin. | Xq11.1 | Associated with epilepsy, anxiety, aggression, and mental retardation. | Harvey et al., 2004; Kalscheuer et al., 2009; Kins et al., 2000; Marco et al., 2008; Papadopoulos et al., 2008; Papadopoulos et al., 2007 |
| Dystrophin; DMD | Indirect | Receptor stabilization. | Xp21.2 | DMD-deficient boys have cognitive impairment and a lower IQ. Associated proteins γ-syntrophin and dysbindin associated with schizophrenia. | Anderson et al., 2002; Kneussel et al., 1999; Lencz et al., 2007; Straub et al., 2002 |
| GABARAP | γ1, γ2S/L | Receptor trafficking and clustering | 17p13.1 | Locus associated with schizophrenia and bipolar disorder. | Kittler et al., 2000; Klei et al., 2005; Kneussel et al., 2000; Wang et al., 1999 |
| Gephyrin; GPHN | α2 | Receptor clustering and anchoring. | 14q23.3 | Run of homozygosity within GPHN gene associated with schizophrenia. | Craig et al., 1996; Essrich et al., 1998;Lencz et al., 2007; Levi et al., 2004; Toyota et al., 2003 |
| GRIF-1; TRAK2 | β2 | Receptor trafficking, mitochondrial transport. | 2q33 | 2q33 linkage with affective disorder in northern Swedish isolated population. | Beck et al., 2002; Brickley et al., 2005; Iyer et al., 2003; MacAskill et al., 2009; Venken et al., 2005 |
| GODZ; ZDHHC3 | γ1, γ2S/L | Palmitoyltransferase. | 3p21.31 | None. | Keller et al., 2004; Uemura et al., 2002 |
| HAP-1; HAP1 | β1 | Vesicular trafficking. | 17q21.2-q21.3 | None. | Kittler et al., 2004; Liao et al., 2005; McGuire et al., 2006 |
| Neuroligin-2; NLGN2 | Indirect | Cell adhesion, synapse formation. | 17p13 | Close to ALOX12 gene (17p13.1), linked to bipolar disorder. | Chih et al., 2004; Graf et al., 2004; Varoqueaux et al., 2004 |
| NSF | β1-3 | GABAAR exocytosis. | 17q2 | Run of homozygosity associated with schizophrenia. | Goto et al., 2005; Kittler et al., 2001; Lencz et al., 2007 |
| Plic-1; UBQLN1 | α1,α2, α3, α6 and β1–3 | GABAAR recycling. | 9q21.2-21.3 | UBQLN1 SNPs are associated with early-onset Alzheimer's disease. | Bedford et al., 2001; Hovatta et al., 1999; Kamboh et al., 2006 |
| PRIP-1; PLCL1 | β1-3 and γ2 | Regulation of subunit phosphorylation. | 2q33-34 | 2q33 linkage with affective disorder in northern Swedish isolated population. | Kanematsu and Hirata, 2003; Kanematsu et al., 2002; Terunuma et al., 2004; Uji et al., 2002; Venken et al., 2005; Yamaguchi et al., 2004 |
| RACK1; GNB2L1 | β1-3 | Modulation of GABAAR channel activity. | 5q35 | In risk locus for schizophrenia in Portuguese island families. | Brandon et al., 2000; Brandon et al., 2002; Brandon et al., 1999; Sklar et al., 2004 |
| Radixin; RDX | α5 | Membrane-cytoskeletal crosslinking; GABAAR clustering. | 11q23 | No genetic association. Associated protein EBP50 reduced in peripheral blood lymphocytes of schizophrenic patients. | Bowden et al., 2006; Loebrich et al., 2006 |
| Septin 11; SEPT11 | Indirect | Synaptic structure and dendritic morphology. | 4q21.1 | No genetic association. Septin 11 mRNA and protein levels are elevated in DLPFC of schizophrenic and bipolar subjects. | Li et al., 2009; Pennington et al., 2008 |
Abbreviations: BIG2, Brefeldin A-inhibited GDP/GTP exchange factor 2; ARFGEF2, ADP-ribosylation factor GDP/GTP exchange factor 2; CAML, calcium-modulating cycophilin ligand; GABARAP, GABAAR-associated protein; GRIF-1, GABAAR-interacting factor; GODZ, golgi-specific DHHC zinc-finger-domain protein; HAP-1, Huntingtin associated protein 1; NSF, N-ethylmaleimide-sensitive factor; Plic-1, protein linking IAP to the cytoskeleton-1; PRIP-1, phosopholipase C-related catalytically inactive protein-1; RACK1, receptor for activated C kinase-1.
Implications of GABAA receptor structural heterogeneity
The ionotropic GABA type A receptors (GABAARs) mediate the majority of fast synaptic inhibition in the mammalian brain. These postsynaptic receptors are heteropentamers that allow the inward flux of Cl- in response to binding of presynaptically released GABA, resulting in inward, anionic currents that transiently decrease local membrane excitability (Olsen and Sieghart, 2009). A remarkable feature of the brain GABAARs is the diversity of subunit isoforms available for assembly into the receptor heteropentamer. At present, 16 subunits, each encoded by separate genes, have been cloned (α1–6, β1–3, γ1, γ2 [short and long splice forms], γ3, δ, ε, π and θ) (Barnard et al., 1998; Bonnert et al., 1999; Jacob et al., 2008; McKernan and Whiting, 1996; Sieghart, 1995; Whiting et al., 1995). Sequence homology places the GABAAR in the superfamily of ligand-gated ion channels that include the nicotinic acetylcholine receptor (nAChR), the strychnine-sensitive glycine receptor (GlyR) and the serotonin type-3 receptor (5HT3R) (Grenningloh et al., 1987; Julius, 1991; Maricq et al., 1991; Schofield et al., 1987). Subunits of all superfamily members share the same predicted transmembrane topology (Fig. 1). These subunit polypeptides contain four transmembrane domains with a large extracellular N-terminal region, a large intracellular loop between transmembrane domains 3 and 4 (TM 3 and 4), and a small, extracellular C-terminal domain (Fig. 1) (MacDonald et al., 2005). When assembled, the native GABAAR subunits are arranged in a pentameric array such that the second transmembrane region (TM2) of each subunit contributes to the lining of the chloride channel pore (Fig. 1) (Imoto et al., 1986; Tierney et al., 1998).
Figure 1. The Structure of GABAA Receptor Subunit.
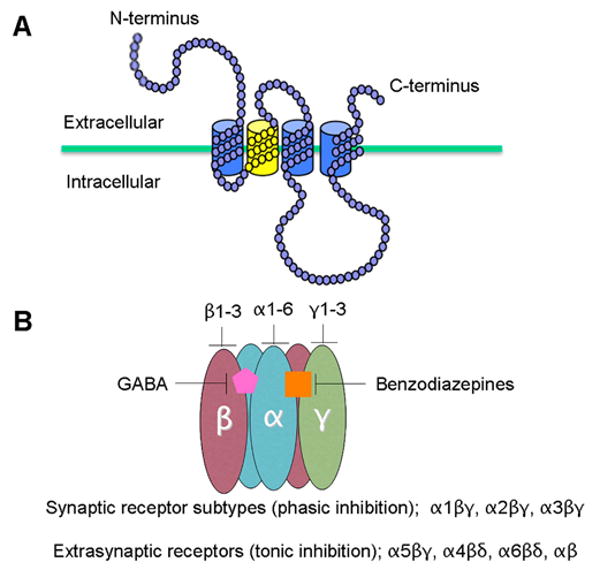
A. The membrane topology of an individual GABAA receptor subunit, TM1-3 are indicated in blue and TM2 in yellow. B. The tertiary structure of assembled GABAA receptors. Receptor α subunits are illustrated in blue, β subunits in pink and δ/γ in green. The benzodiazepine binding pocket is formed between α and γ subunits (orange square) and the GABA binding pocket is formed between α and β subunits (pink pentagon).
Studies employing recombinant receptor expression as well as immunoprecipitation of native receptors have demonstrated that, despite an immense number of possible permutations of the 16 GABAAR subunit isoforms that could be combined to form the heteropentamer, native GABAAR stoichiometry is guided and limited by general rules of assembly. Heterologous expression studies using various subunit combinations have demonstrated the requirement for the coassembly of α, β and γ subunits in order to replicate the major electrophysiological and pharmacological properties of the native GABAAR (Pritchett et al., 1988; Pritchett et al., 1989). In the brain, the majority of GABAAR subtypes are assemblages of two α, two β and one γ subunit (Fig. 1) (Chang et al., 1996; Khan et al., 1996; Klausberger et al., 2000). The most abundant of these, comprising about 40% of the total GABAAR pool in the brain, consists of two α1, two β2 and one γ2 subunit (McKernan and Whiting, 1996).
GABAARs are sensitive to a considerable number of pharmacological agents (benzodiazepines, barbiturates, neurosteroids, and ethanol) and different GABAAR subtypes have been shown to exhibit unique pharmacological profiles (Costa, 1998; Vicini, 1991). The benzodiazepine-binding site is formed between α and γ subunits (Fig. 1) (Amin and Weiss, 1993; Smith and Olsen, 1995), and the α subunit influences the sensitivity of a given subtype to different benzodiazepine site ligands (Hadingham et al., 1993). In addition, different subunit compositions confer different affinities for GABA and determine the desensitization kinetics and channel properties (Gingrich et al., 1995; Lavoie and Twyman, 1996; Verdoorn, 1994; Wafford et al., 1993).
As described above, GABAAR subunit heterogeneity leads to pharmacological and functional diversity, which is paralleled by the differential regional distribution of GABAAR subtypes throughout the brain as well as at the subcellular level (Fritschy and Brunig, 2003). The combined efforts of many groups have characterized the differential mRNA and protein distributions of the GABAAR subunits throughout the brain (reviewed by Olsen and Sieghart, 2009). With respect to GABAAR subcellular distribution, it has been reported for example that in cerebellar granule cells, GABAARs containing the γ2 subunit are synaptically localized while those containing the δ subunit in place of the γ2 subunit are localized extrasynaptically (Fig. 1) (Nusser et al., 1998). In forebrain pyramidal neurons, GABAARs containing the α1 subunit are expressed throughout the somatodendritic region while those containing the α2 subunit are concentrated preferentially at the axon initial segment (Loup et al., 1998; Nusser et al., 1996). Taken together, subunit structural heterogeneity is the major determinant of pharmacological profile, channel kinetics, and subcellular localization of distinct GABAAR subtypes. That these subunits are expressed at varying levels in different cell types throughout the brain suggests that distinct GABAAR subtypes are regionally distributed in a manner that is specific for the neural circuits in which they participate. Since the pathological entity of schizophrenia and related disorders in the adult brain is ultimately characterized by deficits in neural circuitry (Carlsson, 2006; Lisman et al., 2008), GABAARs are ideal therapeutic targets because of their putative role in circuit dysfunction (as described below) combined with circuit-specific expression of subtypes exhibiting unique pharmacological properties.
Modified GABAAR expression in schizophrenia
The role of DLPFC GABAergic dysfunction in schizophrenia
Studies using functional magnetic resonance imaging (fMRI) during working memory tasks in schizophrenic subjects indicate that deficits in working memory, the cognitive processes involved in maintaining and manipulating information, is correlated with disturbances in dorsolateral prefrontal cortical (DLPFC) activity (Lewis et al., 2004). Furthermore, working memory deficits as well as disturbances in DLPFC activity are predictive of the severity of cognitive disorganization in patients with schizophrenia (Perlstein et al., 2001). These deficits appear to be specific for schizophrenia, as they are not present in individuals with major depression (Barch et al., 2003) or nonschizophrenia-related psychosis (MacDonald et al., 2005). At the same time, studies have also revealed that GABAergic interneuron activity is essential for spatial tuning in the DLPFC during working memory tasks (Rao et al., 1999) and that local injection of GABAAR antagonists to the DLPFC disrupts working memory performance in macaque monkeys (Sawaguchi et al., 1989). Thus, in the DLPFC, GABAergic inhibition controls the timing of principal neuron activities and, in doing so, controls the temporal flow of information during working memory tasks (Constantinidis et al., 2002).
Alterations in the expression of DLPFC GABAergic signaling components in schizophrenia
Numerous postmortem studies have indicated that mRNA expression of the GABA-synthesizing enzyme, glutamic acid decarboxylase of 67 kD (GAD67), is reduced in a subset of GABAergic interneurons in schizophrenic patients (Fig. 2) (Akbarian et al., 1995; Guidotti et al., 2000; Hashimoto et al., 2008a; Hashimoto et al., 2008b; Hashimoto et al., 2005; Mirnics et al., 2000; Straub et al., 2007; Vawter et al., 2002; Volk et al., 2002). Although corresponding decreases in GAD67 protein has only been demonstrated in one of these studies (Guidotti et al., 2000), these data are likely related to the observed deficits in cortical GABA reported in early postmortem studies of schizophrenic patients (Perry et al., 1979) as well as a more recent study in living patients using 2D proton magnetic resonance (Rosso et al., 2006). Interestingly, these losses in GAD67 were largely confined to parvalbumin (PV)-expressing chandelier and wide-arbor basket interneurons located in the middle layers of the cortex. PV is a Ca2+-binding protein that is thought to reduce residual Ca2+ levels in axon terminals during repetitive firing, but is also postulated to prolong neurotransmitter release by maintaining elevated Ca2+ levels (Collin et al., 2005; Lisman et al., 2008). These fast-spiking PV-expressing interneurons target the perisomatic regions (basket interneurons) and the axon initial segments (chandelier interneurons) of multiple pyramidal neurons simultaneously (Fig. 2) (Conde et al., 1994; Lewis and Lund, 1990; Peters et al., 1982; Somogyi, 1977) and can thus synchronize the activity of local pyramidal cell populations (McBain and Fisahn, 2001). Such synchronized networks give rise to oscillatory activity in the gamma band frequency range (30-80 Hz), which has been correlated with working memory load in healthy human subjects (Howard et al., 2003; Tamas et al., 2000) but is impaired in schizophrenic patients (Cho et al., 2006). Thus, disturbances in executive function of schizophrenic patients, such as working memory, might result from disruptions in the synchronized firing activity of cortical networks normally coordinated by PV-interneurons, the latter of which are deficient in GABA release due to a selective loss in GAD67 expression.
In addition to reductions in GAD67 mRNA expression in PV-interneurons, concurrent reductions in mRNA levels for GAT1 (the high-affinity GABA transporter; Fig. 2) and PV have also been observed in these interneurons (Lewis et al., 2005; Woo et al., 1998). Moreover, decreases in GAT1 immunoreactivity (IR) at chandelier cell axon terminals, as well as increases in the IR of GABAAR α2 subunit at the AIS of pyramidal neurons, which are contacted by chandelier terminals, have also been described (Fig. 2) (Lewis et al., 2005). As noted in the previous section, GABAARs containing the α2 subunit are selectively localized to the AIS of forebrain pyramidal neurons (Nusser et al., 1996). While the GABAAR α2 is not expressed only on the AIS of pyramidal neurons, it is worth noting that, when measured in hippocampal pyramidal neurons, the GABAAR α2 subunit was found to be present in greater than 80% of all pyramidal cell AIS synapses (Nusser et al., 1996; Nyiri et al., 2001) whereas it is thought that only 15% of all GABAARs contain the α2 subunit (Fritschy and Mohler, 1995). This is a significant point since this upregulation of α2 subunit IR is thought to indicate a compensatory attempt to increase GABAergic synaptic strength precisely at the chandelier-AIS synapse. In fact, the alterations in GAT1, GABAAR α2 subunit, and PV expression are thought to reflect compensatory changes that arise in response to the primary pathology of GAD67 loss in these interneurons (Lewis et al., 2005), which itself is thought to result from altered methylation of GAD1, the gene encoding GAD67 (Benes et al., 2007; Costa et al., 2003; Huang and Akbarian, 2007; Ruzicka et al., 2007; Tochigi et al., 2008; Veldic et al., 2007). Recently, it has been reported that in addition to those associated with GABAergic transmission at the chandelier-pyramidal cell synapse, mRNA expression of GABAAR subunits associated with other interneurons in the DLPFC is also altered (Hashimoto et al., 2008a). For example, significant reductions in GABAAR α1, α4, γ2, and δ subunit expression was detected in the distal dendrites of pyramidal cells, contacted by somatostatin/neuropeptide Y-expressing neurons, in the DLPFC of schizophrenic subjects (Fig. 2) (Hashimoto et al., 2008a). The reduction in γ2 subunit expression reinforces an earlier study which found a significant reduction in the short isoform of the GABAAR γ2 subunit (γ2S) over the long isoform (γ2L) in the DLPFC of schizophrenic patients compared to control subjects (Huntsman et al., 1998). The γ2S isoform is identical to the γ2L isoform except that the γ2S form lacks an 8 amino acid insert in the large intracellular loop containing a protein kinase C (PKC) phosphorylation site that, when phosphorylated, causes a reduction in GABA-mediated current amplitudes (Krishek et al., 1994). Whether these alterations in γ2 expression reflect cause or consequence of the disease is not known, however it stands to reason that a preferential reduction in the γ2S isoform might lead to significant reductions in GABAergic inhibition in the DLPFC since 1) this change would result in an overrepresentation of GABAAR subtypes containing the γ2L subunit isoform, 2) observations with recombinant GABAARs containing γ2L suggest the remaining γ2L-containing subtypes in the DLPFC will exhibit a diminished response to GABA, and 3) the γ2 subunit is the most ubiquitous synaptic GABAAR subunit in the brain (Olsen and Sieghart, 2009).
Alterations in GABA signaling components associated with schizophrenia are not restricted to the DLPFC. Within the granule cell layer of the cerebellum, reductions in the mRNA levels of GAD65/67, with elevations in the mRNA levels of GABAAR α6 and δ subunits have been reported (Bullock et al., 2008). In the hippocampus, mRNA levels of GAD65/67 were reduced in all layers of CA2/3 as well as in the stratum oriens of CA1, as determined by laser-capture microdissection in postmortem tissue (Benes et al., 2007). Moreover, cortical regions outside the DLPFC such as the primary visual cortex, primary motor cortex, and anterior cingulate cortex have also been shown to exhibit reductions in the mRNA levels of GAD67, GAT1, and GABAAR α1 and δ subunits (Hashimoto et al., 2008b).
GABAAR as a therapeutic target for schizophrenia
Given that cognitive deficits are considered to be core features of schizophrenia (Elvevag and Goldberg, 2000), and given the strong evidence implicating disturbances in GABA signaling as contributing to these deficits, it is not surprising that the effects of GABA-modulating drugs on clinical measures related to schizophrenia, including cognitive and positive symptoms, have been investigated. One early study investigated bretazenil (Ro 16-6028), a short-acting partial benzodiazepine agonist, on clinical outcome measures predictive of antipsychotic efficacy in schizophrenic patients (Delini-Stula and Berdah-Tordjman, 1996; Delini-Stula et al., 1992). Using semi-quantitative measures of psychosis, results of these studies indicated that approximately half of the subjects responded favorably to treatment compared to placebo control. Other studies have demonstrated the efficacy of diazepam, a classical benzodiazepine agonist, in preventing psychotic symptom progression in schizophrenic patients (Carpenter et al., 1999; Kirkpatrick et al., 1989).
Recently, a clinical study was carried out to test the effect of benzodiazepines on working memory performance in schizophrenic subjects. Curiously, this study concluded that lorazepam, a relatively non-selective GABAAR positive allosteric modulator, exacerbated working memory deficits in schizophrenic patients, while flumazenil, a GABAAR partial inverse agonist, ameliorated working memory deficits in these patients (Menzies et al., 2007). While the efficacy of a partial inverse agonist in improving working memory does not appear to be in line with the hypothesis that GABA deficiencies contribute to deficits in working memory, flumazenil has been reported to enhance learning and memory in rodents, presumably by increasing arousal and anxiety (Lal et al., 1988). This arousal- or anxiety-related effect may reflect the broad binding profile of flumazenil, however it has also been reported that α5 subunit-selective inverse agonists, such as alpha5IA, improve working memory in rodents (Chambers et al., 2004; Dawson et al., 2006; Sternfeld et al., 2004). This is consistent with studies showing that the GABAAR α5 subunit is highly enriched in the hippocampus over other brain regions (Fritschy and Mohler, 1995) and that α5 null mutant mice exhibit enhanced cognition (Collinson et al., 2002). While these studies point toward the α5-selective alpha5IA as a candidate for overcoming cognitive deficits associated with schizophrenia, prolonged clinical studies have been excluded since a metabolite of this compound was shown to be highly insoluble leading to renal toxicity in preclinical studies (Atack, 2008). Moreover, α5-selective compounds, such as alpha5IA, have not been tested in rodent models of schizophrenia. Since it is postulated that hippocampal hyperactivity may underlie the excessive dopamine release associated with psychosis (Lodge et al., 2009; Lodge and Grace, 2008), antagonizing a GABAAR subtype highly enriched in the hippocampus might be expected to exacerbate psychotic symptoms. Nevertheless, these studies underscore the potential utility of GABAAR subtype-selective compounds in addressing the cognitive deficiencies central to schizophrenia psychopathology.
A similar approach in targeting specific GABAAR subtypes for improving working memory in schizophrenia has exploited the restricted localization of α2 subunit-containing GABAARs to the pyramidal neuron AIS, combined with the putative role chandelier neuron inhibition plays in generating pyramidal cell network oscillations (Lewis et al., 2005; Lewis et al., 2004). Moreover, the compensatory upregulation of α2-containing receptors in schizophrenia suggests that further agonism at this receptor subtype might be beneficial. Thus, treatment with a α2-selective benzodiazepine site agonist would be predicted to selectively potentiate GABA responses predominately at chandelier interneuron synapses onto pyramidal cell AIS, raising the possibility of enhancing DLPFC pyramidal cell network oscillations at the gamma band frequency. The development of the α2/3-selective compound, TPA023, suggests that this is indeed a feasible and attractive pharmacological approach, since this compound exhibits minimal liabilities normally associated with benzodiazepines such as sedation, ethanol interaction, dependence, and withdrawal effects (Atack et al., 2006). Moreover, a small proof-of-concept clinical trial conducted by David Lewis et al. (University of Pittsburgh) has tested this hypothesis with the Merck compound MK-0777, a GABAAR α2/3-selective benzodiazepine-like compound, on 15 male subjects with chronic schizophrenia (Fig. 2). The authors found that, compared to placebo control, MK-0777, administered over four weeks, improved subject performance in three tasks for working memory and/or cognitive control, as well as increased gamma band power during one of these tasks (Lewis et al., 2008). This study provides preliminary evidence that selectively potentiating the postsynaptic response of α2 subunit-containing GABAARs results in cognitive improvement in schizophrenia, providing a potential new adjunctive therapy that could ameliorate the cognitive deficits of this disorder.
In addition to benzodiazepines, GABAARs are also sites for endogenous neuroactive steroids such as allopregnanolone, which potentiate the response to GABA with greater potency than benzodiazepine binding (Majewska et al., 1986; Morrow et al., 1990; Morrow et al., 1987). Interestingly, it has been shown that the atypical antipsychotics olanzapine (Marx et al., 2000; Marx et al., 2003) and clozapine (Barbaccia et al., 2001; Marx et al., 2003) elevate endogenous allopregnanolone to levels that are sufficient to modulate GABAAR-mediated neuronal activity. Furthermore, it was shown that administration of allopregnanolone significantly potentiated olanzapine-induced, but not risperidone- or haloperidol-induced, inhibition of the conditioned avoidance response and apomorphine-induced climbing, two rodent models used to predict antispsychotic efficacy (Ugale et al., 2004). These studies support a hypothesis in which atypical antipsychotics such as olanzapine may ameliorate psychotic symptoms, in part, through the action of elevating allopregnanolone levels. The role it may play in the pathophysiology of schizophrenia and its therapeutic potential in humans is not yet clear, however it has recently been shown in postmortem studies that allopregnanolone levels are reduced in the parietal cortex of schizophrenic subjects compared to controls (Marx et al., 2006). Moreover, a recent proof-of-concept pilot trial of another neuroactive steroid, pregnenolone, demonstrated clinical efficacy in improving cognitive and negative symptoms in schizophrenic subjects (Marx et al., 2009). Pregnenolone and its derivative pregnenolone sulfate have been shown to enhance learning and memory in rodents (Akwa et al., 2001; Darnaudery et al., 2002; Flood et al., 1992, 1995; Ladurelle et al., 2000; Mayo et al., 1993; Meziane et al., 1996; Pallares et al., 1998; Vallee et al., 1997; Vallee et al., 2001), likely due in part to the ability of pregnenolone sulfate to act as a positive allosteric modulator at the NMDA receptor (Bowlby, 1993; Irwin et al., 1994; Wu et al., 1991). Interestingly, pregnenolone is the biosynthetic precursor to allopregnanolone and administration of pregnenolone resulted in serum increases in allopregnanolone in this trial (Marx et al., 2009). Furthermore, the levels of serum allopreganaolone were strongly correlated with cognitive improvement as measured by composite Brief Assessment of Cognition in Schizophrenia (BACS) score (Marx et al., 2009). Thus, it is conceivable that metabolism of pregnenolone to allopregnanolone contributed to its efficacy in this study. To date, however, it is unclear why neurosteroids such as allopregnanolone might enhance cognition or atypical antipsychotic efficacy when these molecules are thought to be more potent than benzodiazepines and barbiturates in potentiating GABAAR activity. This is especially puzzling in light of the above studies showing that lorazepam, a relatively non-selective GABAAR positive allosteric modulator, exacerbated working memory deficits in schizophrenic patients, while flumazenil, a GABAAR partial inverse agonist, ameliorated working memory deficits in these patients (Menzies et al., 2007). The answer might be related to the increased sensitivity to allopregnanolone of GABAARs containing a δ subunit in place of a γ subunit (Belelli et al., 2002; Bianchi et al., 2002; Wohlfarth et al., 2002) combined with the notion that these δ-containing receptors are localized extrasynaptically (Nusser et al., 1998) and mediate tonic rather than phasic inhibition, the former of which can be enhanced by neuroactive steroids (Stell et al., 2003). Thus, treatment with allopregnanolone might represent yet another approach to selectively targeting specific GABAAR subtypes, though the consequences of targeting these extrasynaptic receptors are not fully understood in the context of schizophrenia.
Experimental genetics studies linking GABA hypofunction and schizophrenia
At present, few experimental genetics studies have specifically addressed the role of GABA hypofunction in the etiology of schizophrenia and related psychiatric disorders. One notable study that has begun to address this issue, conducted by Heldt et al.(Heldt et al., 2004), generated mutant mice in which the 65 kD isoform of GAD was deleted. These mice exhibited robust deficits in prepulse inhibition (PPI) of the acoustic startle response, a behavioral phenomenon in which the response to a startling acoustic stimulus is suppressed when the startling stimulus is preceded by a weaker one. PPI is used as a measure of intact sensorimotor gating, a mechanism that 1) results in the attribution of salience to behaviorally relevant sensory stimuli at the expense of irrelevant stimuli, 2) is largely influenced by mesolimbic dopaminergic neuron activity, and 3) is deficient in untreated schizophrenic patients as well as in a variety of other psychiatric disorders, including bipolar disorder. Interestingly, the PPI deficits exhibited by these GAD65-/- mice were reversed by the atypical antipsychotic clozapine.
Another key study, conducted by Yee et al. (Yee et al., 2005), generated mutant mice lacking the GABAAR α3 subunit (α3KO). The authors also demonstrated that these α3KO mice exhibit deficits in PPI and, further, the PPI deficits were reversed by administration of the dopamine D2 receptor antagonist haloperidol. Since the GABAAR α3 subunit is a major isoform expressed in dopaminergic neurons of the ventral tegmental area (Okada et al., 2004), a likely scenario is one in which the loss of function of a major GABAAR subtype leads to disinhibition of these dopaminergic neurons, resulting in an excess in striatal dopamine, which is thought to underlie deficits in PPI (Lisman and Grace, 2005). Thus, distinct GABAAR subtypes may play a prominent role not only in regions involved in cognitive disturbances such as the DLPFC, but also within the dopaminergic mesolimbic system, which is heavily implicated in the positive symptoms of schizophrenia. While this last point has not been directly tested, it would be interesting to determine whether the α2/3-selective compound MK-0777 (described above) might also influence dopamine release as well as dopamine-related behavioral phenotypes in animal models of schizophrenia. One such model is based on methylazoxymethanol (MAM)-administration to pregnant rats during a narrow gestational window (gestational day 17), resulting in preferentially reduced GABAergic interneurons and elevated VTA activity, resulting in excessive dopamine release in response to amphetamine challenge in adult offspring (Lisman et al., 2008). These studies raise the interesting possibility that attenuation of GABAergic inhibition may be related to the hyperdopaminergic state, a major neurochemical hallmark of schizophrenia pathology. This concept has been reviewed extensively (Grace et al., 2007).
Human genetics studies linking GABAAR subunits to schizophrenia and related disorders
The chromosome 5q GABAAR gene cluster and schizophrenia
The genes encoding the GABAAR α1 (GABRA1), α6 (GABRA6), β2 (GABRB2), γ2 (GABRG2), and π (GABRP) subunits form a cluster in human chromosome 5q34-q35, a region that in a meta-analysis had been shown to be the second most compelling schizophrenia susceptibility locus in the genome (Lewis et al., 2003). A genome-wide linkage scan in Portuguese Island families identified 5q31-5q35 as a susceptibility locus for both schizophrenia and psychosis (Sklar et al., 2004). A further association study of this GABAAR gene cluster identified SNPs and haplotypes in GABRA1, GABRA6 and GABRP associated with schizophrenia in a Portuguese sample (Petryshen et al., 2005). In the same study, Petryshen and colleagues also looked for effects of disease-related haplotypes on microarray mRNA expression of GABAAR subunits and found that a haplotype within GABRA1 was associated with reduced expression of GABAAR α6 subunit mRNA in schizophrenia patients. An additional haplotype in GABRA1 was associated with increased expression of a set of genes functionally related to GABAAR function (a group of pre-synaptic proteins and a group of neurotransmitter receptors). Together, this not only implicates the α1 subunit risk alleles in GABAAR-specific alterations in expression but also suggests that this subunit can influence the expression of other relevant proteins, further highlighting the crucial role the GABAARs may play in the etiology of this disease.
The GABAAR β2 subunit and schizophrenia
In an initial Chinese population-based study, positive associations were identified between intronic SNPs and haplotypes in the GABAAR β2 subunit gene (GABRB2) and schizophrenia (Lo et al., 2004). This initial finding was later replicated with other samples in multiple independent linkage and association studies (Liu et al., 2005; Lo et al., 2007a; Lo et al., 2007b; Petryshen et al., 2005; Yu et al., 2006). In fact, in a recent meta analysis of 12 candidate genes associated with schizophrenia, only the GABRB2 association survived corrections for multiple testing for all the meta analyses performed in the study (Shi et al., 2008). Interestingly, a postmortem study exploring the validity of these genomic associations using real-time PCR found that mRNA expression for the long isoform of the β2 GABAAR subunit (β2L) was decreased to a greater extent than that for short isoform (β2S) in the DLPFC of schizophrenic patients (Zhao et al., 2006). Thus, the expression of alternative splice forms of the GABAAR β2 subunit might be differentially affected in schizophrenia. Although the functional consequences of this is not fully understood, a putative calcium-calmodulin dependent kinase II (CaMKII) phosphorylation site is present within the large intracellular loop of the β2L isoform but not in the β2S isoform (McKinley et al., 1995). Moreover, it was demonstrated that recombinant β2L-containing GABAARs exhibit greater GABA-mediated current rundown compared to β2S-containing receptors, and it has been suggested that differential phosphorylation may account for these distinct electrophysiological properties (Zhao et al., 2006). Taken together, GABRB2 is the strongest candidate GABAAR subunit gene associated with schizophrenia, implicated by several independent reports including a follow-up validation study and two independent meta-analyses (Allen et al., 2008; Liu et al., 2005; Lo et al., 2007a; Lo et al., 2004; Lo et al., 2007b; Petryshen et al., 2005; Shi et al., 2008; Yu et al., 2006; Zhao et al., 2006; Zhao et al., 2007). While β2 subunit-containing GABAARs are the most abundant in the brain (McKernan and Whiting, 1996), it is worth noting that β2-containing GABAARs are highly enriched over β1- and β3-containing GABAARs in the globus pallidus of the rat (Schwarzer et al., 2001), the major target for GABAergic medium spiny output neurons of the striatum. These medium spiny neurons receive dopaminergic inputs from both the substantia nigra and ventral tegmental area and are thought to be critical components of the circuitry underlying psychosis associated with excess dopamine release. This, combined with the importance of the β subunits in GABAAR trafficking (Jacob et al., 2008) may prove critical in understanding the genetic basis for hypofunctional GABA systems in schizophrenia.
The GABAAR β1 subunit and bipolar disorder
A growing number of genetic variants that confer risk for psychiatric disorders such as schizophrenia and bipolar disorder are beginning to emerge by whole genome association scans (GWAS), an unbiased approach to detect correlations between genetic variation and disease susceptibility (Hirschhorn and Daly, 2005). This approach employs microarray platform technologies to examine hundreds of thousands of individual single-nucleotide polymorphisms (SNPs) across genomes of large cohorts of cases and healthy controls (Hirschhorn and Daly, 2005). Recently, the Wellcome Trust Case Control Consortium (WTCCC) undertook a GWAS study of ∼3000 shared controls and ∼2000 cases for each of seven human diseases including bipolar disorder (WTCCC, 2007). While this study did not include cases formally diagnosed with schizophrenia, there is increasing evidence pointing to an overlap in genetic susceptibility for schizophrenia and bipolar disorder (Craddock and Owen, 2005). Among the highest ranked GWAS signals in the bipolar disorder dataset of the Wellcome Trust study, which was derived from 1868 cases, was the GABRB1 gene encoding the GABAAR β1 subunit (WTCCC, 2007). In a follow-up study, the GABRB1 risk allele identified in the Wellcome Trust study was found to be strongly associated with a subset of cases (279 of the 1868 bipolar cases) that met the criteria for schizoaffective bipolar type, a phenotype characterized by psychotic symptoms (delusions and/or hallucinations) (Craddock et al., 2008). Moreover, when only these 279 schizoaffective bipolar cases were tested for association at other GABAAR genes, additional significant associations for GABAAR α4, α5, and β3 were revealed (Craddock et al., 2008). Interestingly, no association to GABAAR genes were found when those 1589 cases that did not meet the criteria for schizoaffective bipolar type were compared to control subjects (Craddock et al., 2008).
Specificity of GABAAR modification to schizophrenia etiology
While the above studies relate schizophrenia and similar disorders to altered GABAAR subunit expression and genetic variation, it remains to be firmly established whether these alterations 1) are in fact etiological rather than compensatory or in some other way a response to the disease process, particularly with respect to GABAAR subunit expression, and 2) are specific to schizophrenia and related disorders rather than common features of psychiatric disorders. Indeed, the increase in GABAARAR α2 subunit immunoreactivity in the axon initial segment of cortical pyramidal neurons of subjects with schizophrenia is thought to play a compensatory role in response to reduced GABA release by PV-containing interneurons (Volk et al., 2002). This, however, does not preclude the value of α2-containing GABAARs as therapeutic targets for schizophrenia, as has recently been demonstrated (Lewis et al., 2008). Moreover, this increase appeared to be specific for schizophrenia since it was shown that the increase in α2 immunoreactivity was not detected in matched subjects with major depressive disorder, even when this disorder was accompanied by psychotic symptoms (Volk et al., 2002). Thus, the increase in α2 subunit immunoreactivity at cortical pyramidal neuron axon initial segment does not appear to be characteristic of psychosis in general.
It might also be argued that these changes in α2 subunit expression are related to comorbid substance abuse since 1) numerous reports have demonstrated that genetic variation in the gene encoding the α2 subunit is strongly associated with risk for alcoholism (Covault et al., 2004; Covault et al., 2008; Dick et al., 2006; Edenberg et al., 2004; Fehr et al., 2006; Lappalainen et al., 2005; Soyka et al., 2008) and drug abuse (Agrawal et al., 2006; Drgon et al., 2006), and 2) substance abuse, including that of alcohol and cannabis, is the most common psychiatric comorbidity among patients with schizophrenia (Cantor-Graae et al., 2001; Mueser et al., 1990). However, the increase in α2 subunit expression does not appear to result from mutation within the α2 subunit gene, since alleles associated with increased risk for schizophrenia have not been identified within the GABAAR α2 subunit gene. In addition, studies have demonstrated that GABAAR α2 subunit mRNA is decreased rather than increased when rats were subjected to prolonged ethanol exposure (Mhatre et al., 1993; Montpied et al., 1991), while human postmortem studies found that GABAAR α2 subunit mRNA levels were unchanged in the cerebral cortex of alcoholic cases compared to that of control subjects (Thomas et al., 1998).
Reductions in GABAAR α1 and δ subunit mRNA in the DLPFC, as well as other cortical regions, of schizophrenic subjects were recently reported (Hashimoto et al., 2008a; Hashimoto et al., 2008b). At the same time, reductions in α1 and δ subunit mRNA have been detected in the frontopolar region of postmortem samples obtained from suicide victims (Merali et al., 2004), which may confound interpretation of results from schizophrenic subjects where the cause of death was suicide. However, in the studies cited, the reduced levels of α1 and δ subunit mRNA in schizophrenic subjects compared to controls could not be attributed solely to samples obtained from subjects whose cause of death was suicide (Hashimoto et al., 2008a).
The extent to which SNPs and haplotypes within the 5q34 GABAAR gene cluster are specific for schizophrenia risk is confounded by recent reports showing that this region is also associated with mood disorders in female patients (Yamada et al., 2003). Approximately half of the mood disorder cases in this study were formally diagnosed with bipolar disorder, with which 5q34 SNPs were significantly associated, consistent with a previous study (Horiuchi et al., 2004). This might be consistent with recent studies highlighting the increasingly recognized overlap in genetic vulnerability between schizophrenia and bipolar disorder (Badner and Gershon, 2002; Berrettini, 2003; Cardno et al., 2002; Fallin et al., 2005; Lichtenstein et al., 2009). However, these SNPs were also significantly associated with unipolar mood disorder (Yamada et al., 2003), a finding less easily reconciled. It should be noted, however, that the functional consequences of these SNPs in mood disorder patients have yet to be elucidated. This may be important, since different risk alleles, even within the same gene, may be associated with distinct functional consequences. For example, Petryshen and colleagues (2005) have shown that the haplotype within the GABAAR α1 gene that was overrepresented in schizophrenic patients was correlated with reductions in GABAAR α6 mRNA expression (Petryshen et al., 2005). Similar explorations into the specific effects of genetic variation are necessary in order to reconcile the apparent overlap in susceptibility loci between schizophrenia and other neuropsychiatric disorders.
GABAA receptor associated proteins and psychiatric disease
Much of the sequence diversity among the GABAAR subunits is attributed to the intracellular loop between TM3 and TM4, which represents the largest intracellular domain with the highest amino acid sequence variability among the GABAAR subunit isoforms (Olsen and Tobin, 1990). The specific interaction of defined amino acids in the GABAAR intracellular loops with intracellular interacting proteins is thought to mediate key regulatory processes such as intracellular vesicular trafficking, plasma membrane insertion, synaptic clustering, turnover, and functional modulation by protein phosphorylation, palmitoylation and ubiquitination. These GABAAR-interacting proteins have been identified over the last 10 years by a combination of biochemical, cell biological, and physiological assays (for further detail, see reviews by (Arancibia-Carcamo and Moss, 2006; Chen and Olsen, 2006). Given the emerging roles for these proteins in regulating the functional expression of GABAARs, we have evaluated their possible roles in the etiology of psychiatric disorders.
GABAAR associated proteins implicated by whole genome homozygosity association
While the GWAS approach has revolutionized the search for rare disease-related genetic variants, it has been argued that GWAS requires especially conservative statistical thresholds, which might lead to false negative results (Lencz et al., 2007). An extension of this approach, termed whole genome homozygosity association (WGHA), addresses this potential limitation, by first identifying regions of SNPs that exhibit extended homozygosity, called runs of homozygosity (ROH), followed by association analysis of these regions to identify susceptibility loci. This approach is based on the notion that large regions of homozygous SNPs can be found in common between groups of individuals without direct common lineage, reflecting loci with low recombination rates (Lencz et al., 2007). In the first study to employ this technique, Lencz et al. (2007) found that genes encoding two GABAAR-associated proteins, gephyrin (GPHN) and N-ethylmaleimide sensitive factor (NSF) were located within ROHs identified as susceptibility loci for schizophrenia.
Gephyrin (Fig. 2) was originally identified as a 93 kD protein that co-purified with the glycine receptor (GlyR) (Schmitt et al., 1987) and was found to interact with an 18 amino acid region of the large intracellular loop of the GlyR β subunit (Meyer et al., 1995). In addition to its association with the GlyR, colocalization of gephyrin and GABAAR clusters has been demonstrated both in the rat brain and in cultured neurons (Christie and de Blas, 2002; Christie et al., 2002; Craig et al., 1996; Danglot et al., 2003; Essrich et al., 1998; Giustetto et al., 1998; Sassoe-Pognetto et al., 2000). Although convincing evidence for direct binding of gephyrin to any GABAAR subunit has eluded investigators for more than 10 years, gephyrin was recently reported to interact with a 10 amino acid hydrophobic motif within the large intracellular loop of the GABAAR α2 subunit and, further, this domain was shown to regulate GABAAR synaptic accumulation in a gephyrin-dependent manner (Tretter et al., 2008). Earlier studies using gephyrin and GABAAR γ2 subunit knockout mutant mice revealed the mutual dependence of gephyrin and the γ2 subunit in the clustering and maintenance of α2/γ2-containing GABAARs at GABAergic synapses (Essrich et al., 1998; Kneussel et al., 1999; Schweizer et al., 2003). For example, the loss of GABAAR clustering in γ2 knockout mice was paralleled by a loss of synaptic gephyrin clusters (Essrich et al., 1998). Studies using hippocampal pyramidal cells derived from gephyrin-deficient mice suggested that gephyrin is essential for postsynaptic localization of α2/γ2-containing GABAARs (Kneussel et al., 1999). Furthermore, gephyrin might be important for GABAAR insertion or stabilization at the synaptic membrane, since the loss of synaptic α2 and γ2 immunoreactive puncta was paralleled by an increase in intracellular α2 and γ2 microclusters in gephyrin-deficient neurons, but not accompanied by a change in overall levels of α2 or γ2 subunits (Kneussel et al., 1999). However, studies with spinal chord sections of gephyrin-deficient mutant mice provided evidence for the existence of gephyrin-independent clustering mechanisms for the α1 and α5 subunit-containing GABAARs, the synaptic clusters of which were not abolished in the spinal cord sections of gephyrin-deficient mutants (Kneussel et al., 2001). Moreover, contrary to earlier findings, another study using cultured hippocampal neurons derived from gephyrin-deficient mice showed only a partial decrease in the number of synaptic GABAAR α2/γ2-containing GABAAR clusters (Levi et al., 2004). Thus, gephyrin alone might not be sufficient for synaptic clustering of α2/γ2-containing GABAARs, but may instead participate in a complex mechanism whereby it acts to reduce the lateral mobility of GABAARs, facilitating the accumulation of α2/γ2-containing GABAARs at sites apposed to GABAergic terminals (Jacob et al., 2005). Although the functional implications of the occurrence of the GEPHN gene within a schizophrenia-associated ROH have not been investigated, it is tempting to speculate on the relationship between gephyrin-mediated synaptic accumulation of α2/γ2-containing GABAARs, the importance of α2/γ2-containing GABAARs concentrated in the AIS of DLPFC pyramidal neurons, and the role of chandelier-AIS synapses in the generation of γ-oscillatory network activity, that latter of which may underlie cognitive deficits in schizophrenia (Lewis et al., 2005).
NSF (N-ethyl maleimide-sensitive factor) is known for its role in transport vesicle fusion to acceptor membranes and was previously demonstrated to be involved in the trafficking of AMPA receptors (Nishimune et al., 1998; Noel et al., 1999). It was reported that another GABAAR-associated protein, GABARAP, interacts with NSF and that these two proteins colocalize in cultured hippocampal neurons (Kittler et al., 2001). GABARAP has been postulated to play a role in the intracellular trafficking of GABAARs, because of its association with NSF combined with its localization predominantly at cisternae of endoplasmic reticulum and Golgi apparatus (Fig. 2), consistent with a role in protein transport (Moss and Smart, 2001). More recently, NSF itself was shown to interact directly with the β subunits of the GABAARs, and that overexpression of NSF with recombinant GABAARs decreased receptor expression at the cell surface of transfected COS7 cells (Goto et al., 2005), consistent with an important role in the exocytosis of assembled GABAARs. While the functional significance of the NSF gene within a schizophrenia-associated ROH has not been investigated, its interaction with the GABAAR β subunits (see previous discussion of GABRB2) combined with a role in GABAAR trafficking implies a critical role in the functional expression of GABAARs, a process which mounting evidence suggests is impaired in schizophrenia.
PKC and RACK1
GABAARs are regulated by direct phosphorylation by protein kinase C (PKC) on conserved serine residues within the large intracellular loop of all β subunits (Moss and Smart, 2001) via direct interaction with the βII isoform of PKC (Brandon et al., 1999). Moreover, the receptor for activated C kinase (RACK1), a PKC interacting protein involved in the subcellular targeting of PKC, also interacts independently with the intracellular loops of the GABAAR β subunits (Brandon et al., 1999). Functional analysis of these interactions suggest that enhancing PKC activity results in a reduction in GABAAR channel activity (Brandon et al., 2000) and that the direct, independent binding of RACK1 to GABAAR β subunits serves to potentiate the catalytic activity of GABAAR-bound PKC (Brandon et al., 2002). Thus, regulation of the stoichiometry of GABAAR β subunit phosphorylation plays a key regulatory role in GABAAR function.
It has been shown that PKC activity is increased in frontal cortex from postmortem brains of subjects with bipolar affective disorder (Wang and Friedman, 1996), raising the possibility that GABAAR channel activity may be reduced under these conditions by virtue of the role played by PKC phosphorylation on GABAAR channel activity (Brandon et al., 2000). Consistent with this hypothesis, it has also been shown that the association of RACK1 and PKC is increased in the frontal cortex of postmortem brains of subjects with bipolar affective disorder (Wang and Friedman, 2001), which may explain the increase in PKC activity in these subjects and lends further support to the notion that PKC phosphorylation of GABAAR β subunits may be enhanced in bipolar disorder, leading to reductions in GABAAR channel activity in this region, an emerging feature of psychiatric disease.
Septin 11
Septins are a family of GTPases that form polymeric filaments and ring-like structures, are expressed in various tissues, including brain, and are thought to act as diffusion barriers and scaffolds in a range of cellular processes (Barral and Kinoshita, 2008). It was recently reported that one septin family member, Septin 11, was identified by mass-spectrometry analysis in a brain fraction enriched in the GABAergic postsynaptic complex (Li et al., 2009). Furthermore, it was demonstrated in cultured hippocampal neurons that RNAi-mediated knockdown of septin 11 resulted in a decrease in the density of γ2 subunit-containing GABAARs as well as a reduction in the number of GABAergic synaptic contacts to those neurons where septin 11 expression was attenuated (Li et al., 2009). Interestingly, a recent postmortem study concluded that protein and mRNA expression of septin 11, in addition to other septin family members, is significantly elevated in the DLPFC of both schizophrenic and bipolar cases compared to controls (Pennington et al., 2008). Given the findings that septin 11 appears to positively regulate GABAAR localization and GABAergic synapse formation, the upregulation of septin 11 might reflect a compensatory response to the loss of GABAergic signaling in schizophrenia and bipolar disorder.
GABAAR-associated proteins indirectly associated with schizophrenia
Segments of a large run of homozygosity (ROH) associated with schizophrenia was found to occur directly in the coding region of SNTG1 (Lencz et al., 2007), a gene encoding γ-syntrophin, a brain-enriched PDZ domain-containing scaffolding protein that binds to dystrophin and is part of the dystrophin protein complex (Alessi et al., 2006). In addition to γ-syntrophin, a number of reports have linked dysbindin (DTNBP1), another member of the dystrophin complex thought to play a role in trafficking and tethering postsynaptic receptors including GABAARs, to schizophrenia (Straub et al., 2002). Dystrophin is found colocalized with α2 and γ2 GABAAR subunit clusters in pyramidal cells as well as in α1 and γ2 clusters in Purkinje cells of the cerebellum (Knuesel et al., 1999). The dystrophin gene plays an important role in Duchenne muscular dystrophy (DMD), the second most commonly occurring genetically inherited disease in humans. Studies of mdx mice (dystrophin mutant), a model of Duchenne muscular dystrophy, have shown neural shrinkage as well as a 50% decrease in neuron number in regions of the cerebral cortex and brainstem. Histological evidence shows a reduction in the density of GABAA channel clusters in mdx Purkinje cells and hippocampal CA1 neurons, and in particular a marked reduction in the number of clusters immunoreactive for the GABAARs α1 and α2, indicating that dystrophin may play an important role in the clustering or stabilization of GABAARs. Interestingly, dystrophin has also been identified as a component of the so-called DISC1-interactome, a network of protein-protein interactions around the key schizophrenia risk gene DISC1 (Camargo et al., 2007). To date, DISC1 has not been associated with inhibitory synapses but it may be worth examining such a link in the future. DISC1 has been associated genetically not only to schizophrenia but also to bipolar disorder, Asperger syndrome, and Autism (Hennah et al., 2008; Kilpinen et al., 2008).
mRNA expression of the PDZ domain-containing Ezrin/Radixin/Moesin (ERM)-binding phosphoprotein 50 (EBP50), a protein required for the maintenance of active, phosphorylated ERM proteins at the cell surface (Morales et al., 2004), is significantly reduced in peripheral blood lymphocytes derived from schizophrenic patients (Bowden et al., 2006). The actin-binding protein radixin, a member of the ERM family, directly binds to the large intracellular loop of the GABAAR α5 subunit (Loebrich et al., 2006). This interaction requires the activation of radixin by phosphorylation at a C-terminal threonine residue, resulting in a shift from an inactive, closed conformation to an active open conformation. The binding of activated radixin with the GABAAR α5 subunit was demonstrated to be essential for clustering and localization of extrasynaptic α5-containing GABAARs (Loebrich et al., 2006). Conceivably, a reduction in EBP50 protein could result in a loss of active radixin at the cell surface and, consequently, compromised extrasynaptic clustering and localization of α5-containing GABAARs. Recently, an α5-specific benzodiazepine site radioligand ([11C]Ro15-4513), was used in a positron emission tomography (PET) study, which found that [11C]Ro15-4513 binding in the prefrontal cortex and hippocampus was negatively correlated with PANSS negative symptoms scores in patients with schizophrenia (Asai et al., 2008). These data are consistent with a loss of extrasynaptic α5-containing GABAAR localization, without a concurrent loss in α5 subunit mRNA or protein levels, which might be expected if α5-specific clustering mechanisms, such as that mediated by radixin binding, were compromised.
General Conclusions
Modern genetics has allowed us to make great progress in cataloging the genetic links between GABAAR function in inhibitory neurotransmission and psychiatric disorders. However, until now most of the genetic polymorphisms have been found in non-coding regions, such as introns and other untranslated regions. One can only speculate that these non-coding regions may affect gene transcription which, in turn, may affect subunit protein levels, observations which, in some cases, have been substantiated in post-mortem tissue. By combining the ever-increasing power of genetic association with invaluable insights gained from sound biological validation, a deeper understanding of the underlying mechanisms that give rise to these disorders are beginning to take shape. A growing body of evidence suggests that a malfunction in cortical GABAergic transmission resulting in a disturbance in cortical network activity is a critical factor underlying such psychiatric disorders as schizophrenia and bipolar disorder. Therefore the development of novel and innovative pharmacological agents that target individual GABAAR subtypes hold enormous potential for a novel, highly specific therapeutic approach to schizophrenia and other psychiatric conditions.
Table I. Genetic association and modified expression patterns of GABAA receptor subunits in schizophrenia and related disorders.
| GABAAR Subunit; Gene Name | Chromosomal Location | Evidence for Genetic Association | Evidence for Modified Expression | References |
|---|---|---|---|---|
| α1; GABRA1 | 5q34-q35 | Identified as within susceptibility locus for schizophrenia in Portuguese Island families. | Reduced mRNA expression in pyramidal neurons of DLPFC of schizophrenic patients. | Hashimoto et al., 2008a; Sklar et al., 2004 |
| α2; GABRA2 | 4p12 | None. | Elevated expression of GABAAR α2 subunit at chandelier-pyramidal neuron AIS synapses in schizophrenic patients. | Volk et al., 2002 |
| α4; GABRA4 | 4p12 | Association with schizoaffective disorder, bipolar type. | Reduced mRNA expression in DLPFC of schizophrenic patients. | Craddock et al., 2008; Hashimoto et al., 2008a |
| α5; GABRA5 | 15q11.2-q12 | Association with schizoaffective disorder, bipolar type and bipolar disorder. | α5-specific PET ligand binding negatively correlated with PANSS negative symptoms scores. | Asai et al., 2008; Craddock et al., 2008; Papadimitriou et al., 2001; Papadimitriou et al., 1998 |
| α6; GABRA6 | 5q34 | Identified as within susceptibility locus for schizophrenia in Portuguese Island families. | Reduced mRNA expression in patients with an associated haplotype for the GABRA1 gene. | Sklar et al., 2004 |
| β1; GABRB1 | 4p12 | GWAS association with schizoaffective disorder, bipolar type. | None. | 2007; Craddock et al., 2008 |
| β2; GABRB2 | 5q34 | Multiple linkage, association, and meta-analyses. | Differential mRNA expression of long versus short isoforms n the DLPFC of schizophrenic patients. | 2007; Allen et al., 2008; Craddock et al., 2008; Liu et al., 2005; Lo et al.,2007a; Lo et al., 2004; Lo et al., 2007b; Petryshen et al., 2005; Shi et al., 2008; Yu et al., 2006; Zhao et al., 2006; Zhao et al., 2007 |
| γ1; GABRG | 4p12 | None. | Reduced mRNA expression in DLPFC of schizophrenic patients. | Hashimoto et al., 2008a |
| γ2; GABRG2 | 5q31.1-q33.2 | Identified as within susceptibility locus for schizophrenia in Portuguese Island families. | Differential mRNA expression of long versus short isoforms in schizophrenic patients. Reduced mRNA expression in pyramidal neurons of DLPFC of schizophrenic patients. | Hashimoto et al., 2008a; Huntsman et al., 1998; Sklar et al., 2004 |
| δ; GABRD | 1p36.3 | None. | Reduced mRNA expression in pyramidal neurons of DLPFC of schizophrenic patients. | Hashimoto et al., 2008a |
| π;GABRP | 5q33-q34 | Identified as within susceptibility locus for schizophrenia in Portuguese Island families. | None. | Sklar et al., 2004 |
Abbreviations
- AIS
axon initial segment
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid
- DISC1
disrupted in schizophrenia-1
- DLPFC
dorsolateral prefrontal cortex
- ERM
ezrin-radixin-moesin family
- GABA
γ-aminobutyric acid
- GABAAR
γ-aminobutyric acid type A receptor
- GAD
glutamic acid decarboxylase
- GAT1
GABA transporter-1
- GlyR
glycine receptor
- GWAS
genome-wide association scan
- IR
immunoreactivity
- NMDAR
N-methyl-D-aspartic acid-sensitive receptor
- NSF
N-ethylmaleimide-sensitive factor
- PET
positron emission tomography
- PKC
protein kinase C
- PPI
prepulse inhibition
- PV
parvalbumin
- RACK1
receptor for activated C kinase-1
- ROH
run of homozygosity
- SNP
single-nucleotide polymorphism
- TM
transmembrane domain
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal A, Edenberg HJ, Foroud T, Bierut LJ, Dunne G, Hinrichs AL, Nurnberger JI, Crowe R, Kuperman S, Schuckit MA, Begleiter H, Porjesz B, Dick DM. Association of GABRA2 with drug dependence in the collaborative study of the genetics of alcoholism sample. Behav Genet. 2006;36:640–650. doi: 10.1007/s10519-006-9069-4. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Akwa Y, Ladurelle N, Covey DF, Baulieu EE. The synthetic enantiomer of pregnenolone sulfate is very active on memory in rats and mice, even more so than its physiological neurosteroid counterpart: distinct mechanisms? Proc Natl Acad Sci U S A. 2001;98:14033–14037. doi: 10.1073/pnas.241503698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi A, Bragg AD, Percival JM, Yoo J, Albrecht DE, Froehner SC, Adams ME. gamma-Syntrophin scaffolding is spatially and functionally distinct from that of the alpha/beta syntrophins. Exp Cell Res. 2006;312:3084–3095. doi: 10.1016/j.yexcr.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, Tanzi RE, Bertram L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- Amin J, Weiss DS. GABAA receptor needs two homologous domains of the beta-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Head SI, Rae C, Morley JW. Brain function in Duchenne muscular dystrophy. Brain. 2002;125:4–13. doi: 10.1093/brain/awf012. [DOI] [PubMed] [Google Scholar]
- Arancibia-Carcamo IL, Moss SJ. Molecular organization and assembly of the central inhibitory postsynapse. Results Probl Cell Differ. 2006;43:25–47. doi: 10.1007/400_017. [DOI] [PubMed] [Google Scholar]
- Asai Y, Takano A, Ito H, Okubo Y, Matsuura M, Otsuka A, Takahashi H, Ando T, Ito S, Arakawa R, Asai K, Suhara T. GABAA/Benzodiazepine receptor binding in patients with schizophrenia using [11C]Ro15-4513, a radioligand with relatively high affinity for alpha5 subunit. Schizophr Res. 2008;99:333–340. doi: 10.1016/j.schres.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Atack JR. GABA(A) receptor subtype-selective efficacy: TPA023, an alpha2/alpha3 selective non-sedating anxiolytic and alpha5IA, an alpha5 selective cognition enhancer. CNS Neurosci Ther. 2008;14:25–35. doi: 10.1111/j.1527-3458.2007.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atack JR, Wafford KA, Tye SJ, Cook SM, Sohal B, Pike A, Sur C, Melillo D, Bristow L, Bromidge F, Ragan I, Kerby J, Street L, Carling R, Castro JL, Whiting P, Dawson GR, McKernan RM. TPA023 [7-(1,1-dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluor ophenyl)-1,2,4-triazolo[4,3-b]pyridazine], an agonist selective for alpha2- and alpha3-containing GABAA receptors, is a nonsedating anxiolytic in rodents and primates. J Pharmacol Exp Ther. 2006;316:410–422. doi: 10.1124/jpet.105.089920. [DOI] [PubMed] [Google Scholar]
- Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry. 2002;7:405–411. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Affricano D, Purdy RH, Maciocco E, Spiga F, Biggio G. Clozapine, but not haloperidol, increases brain concentrations of neuroactive steroids in the rat. Neuropsychopharmacology. 2001;25:489–497. doi: 10.1016/S0893-133X(01)00254-8. [DOI] [PubMed] [Google Scholar]
- Barch DM, Sheline YI, Csernansky JG, Snyder AZ. Working memory and prefrontal cortex dysfunction: specificity to schizophrenia compared with major depression. Biol Psychiatry. 2003;53:376–384. doi: 10.1016/s0006-3223(02)01674-8. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Barral Y, Kinoshita M. Structural insights shed light onto septin assemblies and function. Curr Opin Cell Biol. 2008;20:12–18. doi: 10.1016/j.ceb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Becher P, Thiel HJ, Collins M, Brownlie J, Orlich M. Cellular sequences in pestivirus genomes encoding gamma-aminobutyric acid (A) receptor-associated protein and Golgi-associated ATPase enhancer of 16 kilodaltons. J Virol. 2002;76:13069–13076. doi: 10.1128/JVI.76.24.13069-13076.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M, Brickley K, Wilkinson HL, Sharma S, Smith M, Chazot PL, Pollard S, Stephenson FA. Identification, molecular cloning, and characterization of a novel GABAA receptor-associated protein, GRIF-1. J Biol Chem. 2002;277:30079–30090. doi: 10.1074/jbc.M200438200. [DOI] [PubMed] [Google Scholar]
- Bedford FK, Kittler JT, Muller E, Thomas P, Uren JM, Merlo D, Wisden W, Triller A, Smart TG, Moss SJ. GABA(A) receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nat Neurosci. 2001;4:908–916. doi: 10.1038/nn0901-908. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci U S A. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini W. Evidence for shared susceptibility in bipolar disorder and schizophrenia. Am J Med Genet C Semin Med Genet. 2003;123C:59–64. doi: 10.1002/ajmg.c.20014. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. Alpha1 and alpha6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABA(A) receptors containing the delta subunit. Neuropharmacology. 2002;43:492–502. doi: 10.1016/s0028-3908(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Bonnert TP, McKernan RM, Farrar S, le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Brown N, Wafford KA, Whiting PJ. theta, a novel gamma-aminobutyric acid type A receptor subunit. Proc Natl Acad Sci U S A. 1999;96:9891–9896. doi: 10.1073/pnas.96.17.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden NA, Weidenhofer J, Scott RJ, Schall U, Todd J, Michie PT, Tooney PA. Preliminary investigation of gene expression profiles in peripheral blood lymphocytes in schizophrenia. Schizophr Res. 2006;82:175–183. doi: 10.1016/j.schres.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Bowlby MR. Pregnenolone sulfate potentiation of N-methyl-D-aspartate receptor channels in hippocampal neurons. Mol Pharmacol. 1993;43:813–819. [PubMed] [Google Scholar]
- Brandon NJ, Delmas P, Kittler JT, McDonald BJ, Sieghart W, Brown DA, Smart TG, Moss SJ. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J Biol Chem. 2000;275:38856–38862. doi: 10.1074/jbc.M004910200. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Jovanovic JN, Smart TG, Moss SJ. Receptor for activated C kinase-1 facilitates protein kinase C-dependent phosphorylation and functional modulation of GABA(A) receptors with the activation of G-protein-coupled receptors. J Neurosci. 2002;22:6353–6361. doi: 10.1523/JNEUROSCI.22-15-06353.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Uren JM, Kittler JT, Wang H, Olsen R, Parker PJ, Moss SJ. Subunit-specific association of protein kinase C and the receptor for activated C kinase with GABA type A receptors. J Neurosci. 1999;19:9228–9234. doi: 10.1523/JNEUROSCI.19-21-09228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley K, Smith MJ, Beck M, Stephenson FA. GRIF-1 and OIP106, members of a novel gene family of coiled-coil domain proteins: association in vivo and in vitro with kinesin. J Biol Chem. 2005;280:14723–14732. doi: 10.1074/jbc.M409095200. [DOI] [PubMed] [Google Scholar]
- Bullock WM, Cardon K, Bustillo J, Roberts RC, Perrone-Bizzozero NI. Altered expression of genes involved in GABAergic transmission and neuromodulation of granule cell activity in the cerebellum of schizophrenia patients. Am J Psychiatry. 2008;165:1594–1603. doi: 10.1176/appi.ajp.2008.07121845. [DOI] [PubMed] [Google Scholar]
- Camargo LM, Collura V, Rain JC, Mizuguchi K, Hermjakob H, Kerrien S, Bonnert TP, Whiting PJ, Brandon NJ. Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- Cantor-Graae E, Nordstrom LG, McNeil TF. Substance abuse in schizophrenia: a review of the literature and a study of correlates in Sweden. Schizophr Res. 2001;48:69–82. doi: 10.1016/s0920-9964(00)00114-6. [DOI] [PubMed] [Google Scholar]
- Cardno AG, Rijsdijk FV, Sham PC, Murray RM, McGuffin P. A twin study of genetic relationships between psychotic symptoms. Am J Psychiatry. 2002;159:539–545. doi: 10.1176/appi.ajp.159.4.539. [DOI] [PubMed] [Google Scholar]
- Carlsson A. The neurochemical circuitry of schizophrenia. Pharmacopsychiatry 39 Suppl. 2006;1:S10–14. doi: 10.1055/s-2006-931483. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Jr, Buchanan RW, Kirkpatrick B, Breier AF. Diazepam treatment of early signs of exacerbation in schizophrenia. Am J Psychiatry. 1999;156:299–303. doi: 10.1176/ajp.156.2.299. [DOI] [PubMed] [Google Scholar]
- Carter CJ. Schizophrenia susceptibility genes converge on interlinked pathways related to glutamatergic transmission and long-term potentiation, oxidative stress and oligodendrocyte viability. Schizophr Res. 2006;86:1–14. doi: 10.1016/j.schres.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Chambers MS, Atack JR, Carling RW, Collinson N, Cook SM, Dawson GR, Ferris P, Hobbs SC, O'Connor D, Marshall G, Rycroft W, Macleod AM. An orally bioavailable, functionally selective inverse agonist at the benzodiazepine site of GABAA alpha5 receptors with cognition enhancing properties. J Med Chem. 2004;47:5829–5832. doi: 10.1021/jm040863t. [DOI] [PubMed] [Google Scholar]
- Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABAA receptor. J Neurosci. 1996;16:5415–5424. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charych EI, Yu W, Miralles CP, Serwanski DR, Li X, Rubio M, De Blas AL. The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J Neurochem. 2004;90:173–189. doi: 10.1111/j.1471-4159.2004.02481.x. [DOI] [PubMed] [Google Scholar]
- Chen ZW, Olsen RW. GABA(A) receptor associated proteins: a key factor regulating GABA(A) receptor function. J Neurochem. 2006 doi: 10.1111/j.1471-4159.2006.04206.x. [DOI] [PubMed] [Google Scholar]
- Chih B, Afridi SK, Clark L, Scheiffele P. Disorder-associated mutations lead to functional inactivation of neuroligins. Hum Mol Genet. 2004;13:1471–1477. doi: 10.1093/hmg/ddh158. [DOI] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, de Blas AL. alpha5 Subunit-containing GABA(A) receptors form clusters at GABAergic synapses in hippocampal cultures. Neuroreport. 2002;13:2355–2358. doi: 10.1097/00001756-200212030-00037. [DOI] [PubMed] [Google Scholar]
- Christie SB, Li RW, Miralles CP, Riquelme R, Yang BY, Charych E, Wendou Y, Daniels SB, Cantino ME, De Blas AL. Synaptic and extrasynaptic GABAA receptor and gephyrin clusters. Prog Brain Res. 2002;136:157–180. doi: 10.1016/s0079-6123(02)36015-1. [DOI] [PubMed] [Google Scholar]
- Collin T, Chat M, Lucas MG, Moreno H, Racay P, Schwaller B, Marty A, Llano I. Developmental changes in parvalbumin regulate presynaptic Ca2+ signaling. J Neurosci. 2005;25:96–107. doi: 10.1523/JNEUROSCI.3748-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Tamminga C, Schoepp DD, Lindsley C. Schizophrenia: moving beyond monoamine antagonists. Mol Interv. 2008;8:99–107. doi: 10.1124/mi.8.2.7. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Williams GV, Goldman-Rakic PS. A role for inhibition in shaping the temporal flow of information in prefrontal cortex. Nat Neurosci. 2002;5:175–180. doi: 10.1038/nn799. [DOI] [PubMed] [Google Scholar]
- Costa E. From GABAA receptor diversity emerges a unified vision of GABAergic inhibition. Annu Rev Pharmacol Toxicol. 1998;38:321–350. doi: 10.1146/annurev.pharmtox.38.1.321. [DOI] [PubMed] [Google Scholar]
- Costa E, Grayson DR, Guidotti A. Epigenetic downregulation of GABAergic function in schizophrenia: potential for pharmacological intervention? Mol Interv. 2003;3:220–229. doi: 10.1124/mi.3.4.220. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129B:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Jensen K, Anton R, Kranzler HR. Markers in the 5′-region of GABRG1 associate to alcohol dependence and are in linkage disequilibrium with markers in the adjacent GABRA2 gene. Neuropsychopharmacology. 2008;33:837–848. doi: 10.1038/sj.npp.1301456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, Jones L, Jones IR, Kirov G, Green EK, Grozeva D, Moskvina V, Nikolov I, Hamshere ML, Vukcevic D, Caesar S, Gordon-Smith K, Fraser C, Russell E, Norton N, Breen G, St Clair D, Collier DA, Young AH, Ferrier IN, Farmer A, McGuffin P, Holmans PA, Donnelly P, Owen MJ, O'Donovan MC. Strong genetic evidence for a selective influence of GABA(A) receptors on a component of the bipolar disorder phenotype. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, Owen MJ. The beginning of the end for the Kraepelinian dichotomy. Br J Psychiatry. 2005;186:364–366. doi: 10.1192/bjp.186.5.364. [DOI] [PubMed] [Google Scholar]
- Craig AM, Banker G, Chang W, McGrath ME, Serpinskaya AS. Clustering of gephyrin at GABAergic but not glutamatergic synapses in cultured rat hippocampal neurons. J Neurosci. 1996;16:3166–3177. doi: 10.1523/JNEUROSCI.16-10-03166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danglot L, Triller A, Bessis A. Association of gephyrin with synaptic and extrasynaptic GABAA receptors varies during development in cultured hippocampal neurons. Mol Cell Neurosci. 2003;23:264–278. doi: 10.1016/s1044-7431(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Darnaudery M, Pallares M, Piazza PV, Le Moal M, Mayo W. The neurosteroid pregnenolone sulfate infused into the medial septum nucleus increases hippocampal acetylcholine and spatial memory in rats. Brain Res. 2002;951:237–242. doi: 10.1016/s0006-8993(02)03166-9. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, Choudhury HI, McDonald LM, Pillai G, Rycroft W, Smith AJ, Sternfeld F, Tattersall FD, Wafford KA, Reynolds DS, Seabrook GR, Atack JR. An inverse agonist selective for alpha5 subunit-containing GABAA receptors enhances cognition. J Pharmacol Exp Ther. 2006;316:1335–1345. doi: 10.1124/jpet.105.092320. [DOI] [PubMed] [Google Scholar]
- Delini-Stula A, Berdah-Tordjman D. Antipsychotic effects of bretazenil, a partial benzodiazepine agonist in acute schizophrenia--a study group report. J Psychiatr Res. 1996;30:239–250. doi: 10.1016/0022-3956(96)00003-9. [DOI] [PubMed] [Google Scholar]
- Delini-Stula A, Berdah-Tordjman D, Neumann N. Partial benzodiazepine agonists in schizophrenia: expectations and present clinical findings. Clin Neuropharmacol. 1992;1540(Pt A) 1:5A–406A. doi: 10.1097/00002826-199201001-00211. [DOI] [PubMed] [Google Scholar]
- Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, Kuperman S, Hesselbrock V, Schuckit M, Almasy L, Tischfield J, Porjesz B, Begleiter H, Nurnberger J, Jr, Xuei X, Edenberg HJ, Foroud T. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Drgon T, D'Addario C, Uhl GR. Linkage disequilibrium, haplotype and association studies of a chromosome 4 GABA receptor gene cluster: candidate gene variants for addictions. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:854–860. doi: 10.1002/ajmg.b.30349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr, O'Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Fallin MD, Lasseter VK, Avramopoulos D, Nicodemus KK, Wolyniec PS, McGrath JA, Steel G, Nestadt G, Liang KY, Huganir RL, Valle D, Pulver AE. Bipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am J Hum Genet. 2005;77:918–936. doi: 10.1086/497703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Sander T, Tadic A, Lenzen KP, Anghelescu I, Klawe C, Dahmen N, Schmidt LG, Szegedi A. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatr Genet. 2006;16:9–17. doi: 10.1097/01.ypg.0000185027.89816.d9. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE, Roberts E. Memory-enhancing effects in male mice of pregnenolone and steroids metabolically derived from it. Proc Natl Acad Sci U S A. 1992;89:1567–1571. doi: 10.1073/pnas.89.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Morley JE, Roberts E. Pregnenolone sulfate enhances post-training memory processes when injected in very low doses into limbic system structures: the amygdala is by far the most sensitive. Proc Natl Acad Sci U S A. 1995;92:10806–10810. doi: 10.1073/pnas.92.23.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Brunig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the alpha-subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol. 1995;489(Pt 2):529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustetto M, Kirsch J, Fritschy JM, Cantino D, Sassoe-Pognetto M. Localization of the clustering protein gephyrin at GABAergic synapses in the main olfactory bulb of the rat. J Comp Neurol. 1998;395:231–244. doi: 10.1002/(sici)1096-9861(19980601)395:2<231::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Goto H, Terunuma M, Kanematsu T, Misumi Y, Moss SJ, Hirata M. Direct interaction of N-ethylmaleimide-sensitive factor with GABA(A) receptor beta subunits. Mol Cell Neurosci. 2005;30:197–206. doi: 10.1016/j.mcn.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenningloh G, Gundelfinger E, Schmitt B, Betz H, Darlison MG, Barnard EA, Schofield PR, Seeburg PH. Glycine vs GABA receptors. Nature. 1987;330:25–26. doi: 10.1038/330025b0. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Hadingham KL, Wingrove P, Le Bourdelles B, Palmer KJ, Ragan CI, Whiting PJ. Cloning of cDNA sequences encoding human alpha 2 and alpha 3 gamma-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant alpha 1-, alpha 2-, alpha 3-, and alpha 5-containing human gamma-aminobutyric acidA receptors. Mol Pharmacol. 1993;43:970–975. [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- Harvey K, Duguid IC, Alldred MJ, Beatty SE, Ward H, Keep NH, Lingenfelter SE, Pearce BR, Lundgren J, Owen MJ, Smart TG, Luscher B, Rees MI, Harvey RJ. The GDP-GTP exchange factor collybistin: an essential determinant of neuronal gephyrin clustering. J Neurosci. 2004;24:5816–5826. doi: 10.1523/JNEUROSCI.1184-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008a;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008b;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, Sun Z, Sampson AR, Lewis DA. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Green A, Ressler KJ. Prepulse inhibition deficits in GAD65 knockout mice and the effect of antipsychotic treatment. Neuropsychopharmacology. 2004;29:1610–1619. doi: 10.1038/sj.npp.1300468. [DOI] [PubMed] [Google Scholar]
- Hennah W, Thomson P, McQuillin A, Bass N, Loukola A, Anjorin A, Blackwood D, Curtis D, Deary IJ, Harris SE, Isometsa ET, Lawrence J, Lonnqvist J, Muir W, Palotie A, Partonen T, Paunio T, Pylkko E, Robinson M, Soronen P, Suominen K, Suvisaari J, Thirumalai S, Clair DS, Gurling H, Peltonen L, Porteous D. DISC1 association, heterogeneity and interplay in schizophrenia and bipolar disorder. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.22. [DOI] [PubMed] [Google Scholar]
- Hirschhorn JN, Daly MJ. Genome-wide association studies for common diseases and complex traits. Nat Rev Genet. 2005;6:95–108. doi: 10.1038/nrg1521. [DOI] [PubMed] [Google Scholar]
- Horiuchi Y, Nakayama J, Ishiguro H, Ohtsuki T, Detera-Wadleigh SD, Toyota T, Yamada K, Nankai M, Shibuya H, Yoshikawa T, Arinami T. Possible association between a haplotype of the GABA-A receptor alpha 1 subunit gene (GABRA1) and mood disorders. Biol Psychiatry. 2004;55:40–45. doi: 10.1016/s0006-3223(03)00689-9. [DOI] [PubMed] [Google Scholar]
- Hovatta I, Varilo T, Suvisaari J, Terwilliger JD, Ollikainen V, Arajarvi R, Juvonen H, Kokko-Sahin ML, Vaisanen L, Mannila H, Lonnqvist J, Peltonen L. A genomewide screen for schizophrenia genes in an isolated Finnish subpopulation, suggesting multiple susceptibility loci. Am J Hum Genet. 1999;65:1114–1124. doi: 10.1086/302567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Huang HS, Akbarian S. GAD1 mRNA expression and DNA methylation in prefrontal cortex of subjects with schizophrenia. PLoS ONE. 2007;2:e809. doi: 10.1371/journal.pone.0000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntsman MM, Tran BV, Potkin SG, Bunney WE, Jr, Jones EG. Altered ratios of alternatively spliced long and short gamma2 subunit mRNAs of the gamma-amino butyrate type A receptor in prefrontal cortex of schizophrenics. Proc Natl Acad Sci U S A. 1998;95:15066–15071. doi: 10.1073/pnas.95.25.15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto K, Methfessel C, Sakmann B, Mishina M, Mori Y, Konno T, Fukuda K, Kurasaki M, Bujo H, Fujita Y, et al. Location of a delta-subunit region determining ion transport through the acetylcholine receptor channel. Nature. 1986;324:670–674. doi: 10.1038/324670a0. [DOI] [PubMed] [Google Scholar]
- Irwin RP, Lin SZ, Rogawski MA, Purdy RH, Paul SM. Steroid potentiation and inhibition of N-methyl-D-aspartate receptor-mediated intracellular Ca++ responses: structure-activity studies. J Pharmacol Exp Ther. 1994;271:677–682. [PubMed] [Google Scholar]
- Iyer SP, Akimoto Y, Hart GW. Identification and cloning of a novel family of coiled-coil domain proteins that interact with O-GlcNAc transferase. J Biol Chem. 2003;278:5399–5409. doi: 10.1074/jbc.M209384200. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D. Molecular biology of serotonin receptors. Annu Rev Neurosci. 1991;14:335–360. doi: 10.1146/annurev.ne.14.030191.002003. [DOI] [PubMed] [Google Scholar]
- Kalscheuer VM, Musante L, Fang C, Hoffmann K, Fuchs C, Carta E, Deas E, Venkateswarlu K, Menzel C, Ullmann R, Tommerup N, Dalpra L, Tzschach A, Selicorni A, Luscher B, Ropers HH, Harvey K, Harvey RJ. A balanced chromosomal translocation disrupting ARHGEF9 is associated with epilepsy, anxiety, aggression, and mental retardation. Hum Mutat. 2009;30:61–68. doi: 10.1002/humu.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboh MI, Minster RL, Feingold E, DeKosky ST. Genetic association of ubiquilin with Alzheimer's disease and related quantitative measures. Mol Psychiatry. 2006;11:273–279. doi: 10.1038/sj.mp.4001775. [DOI] [PubMed] [Google Scholar]
- Kanematsu T, Hirata M. PRIP-1 involved in GABAA receptor trafficking. Seikagaku. 2003;75:378–382. [PubMed] [Google Scholar]
- Kanematsu T, Jang IS, Yamaguchi T, Nagahama H, Yoshimura K, Hidaka K, Matsuda M, Takeuchi H, Misumi Y, Nakayama K, Yamamoto T, Akaike N, Hirata M. Role of the PLC-related, catalytically inactive protein p130 in GABA(A) receptor function. Embo J. 2002;21:1004–1011. doi: 10.1093/emboj/21.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred M, Sassoe-Pognetto M, Luscher B. The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J Neurosci. 2004;24:5881–5891. doi: 10.1523/JNEUROSCI.1037-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZU, Gutierrez A, De Blas AL. The alpha 1 and alpha 6 subunits can coexist in the same cerebellar GABAA receptor maintaining their individual benzodiazepine-binding specificities. J Neurochem. 1996;66:685–691. doi: 10.1046/j.1471-4159.1996.66020685.x. [DOI] [PubMed] [Google Scholar]
- Kilpinen H, Ylisaukko-Oja T, Hennah W, Palo OM, Varilo T, Vanhala R, Nieminen-von Wendt T, von Wendt L, Paunio T, Peltonen L. Association of DISC1 with autism and Asperger syndrome. Mol Psychiatry. 2008;13:187–196. doi: 10.1038/sj.mp.4002031. [DOI] [PubMed] [Google Scholar]
- Kins S, Betz H, Kirsch J. Collybistin, a newly identified brain-specific GEF, induces submembrane clustering of gephyrin. Nat Neurosci. 2000;3:22–29. doi: 10.1038/71096. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, Waltrip RW, 2nd, Jauch D, Carpenter WT., Jr Diazepam treatment of early symptoms of schizophrenic relapse. J Nerv Ment Dis. 1989;177:52–53. doi: 10.1097/00005053-198901000-00009. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Rostaing P, Schiavo G, Fritschy JM, Olsen R, Triller A, Moss SJ. The subcellular distribution of GABARAP and its ability to interact with NSF suggest a role for this protein in the intracellular transport of GABA(A) receptors. Mol Cell Neurosci. 2001;18:13–25. doi: 10.1006/mcne.2001.1005. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Thomas P, Tretter V, Bogdanov YD, Haucke V, Smart TG, Moss SJ. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating gamma-aminobutyric acid type A receptor membrane trafficking. Proc Natl Acad Sci U S A. 2004;101:12736–12741. doi: 10.1073/pnas.0401860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Fuchs K, Mayer B, Ehya N, Sieghart W. GABA(A) receptor assembly. Identification and structure of gamma(2) sequences forming the intersubunit contacts with alpha(1) and beta(3) subunits. J Biol Chem. 2000;275:8921–8928. doi: 10.1074/jbc.275.12.8921. [DOI] [PubMed] [Google Scholar]
- Klei L, Bacanu SA, Myles-Worsley M, Galke B, Xie W, Tiobech J, Otto C, Roeder K, Devlin B, Byerley W. Linkage analysis of a completely ascertained sample of familial schizophrenics and bipolars from Palau, Micronesia. Hum Genet. 2005;117:349–356. doi: 10.1007/s00439-005-1320-1. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Gasnier B, Feng G, Sanes JR, Betz H. Gephyrin-independent clustering of postsynaptic GABA(A) receptor subtypes. Mol Cell Neurosci. 2001;17:973–982. doi: 10.1006/mcne.2001.0983. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Laube B, Stahl S, Muller U, Betz H. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Haverkamp S, Fuhrmann JC, Wang H, Wassle H, Olsen RW, Betz H. The gamma-aminobutyric acid type A receptor (GABAAR)-associated protein GABARAP interacts with gephyrin but is not involved in receptor anchoring at the synapse. Proc Natl Acad Sci U S A. 2000;97:8594–8599. doi: 10.1073/pnas.97.15.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel I, Mastrocola M, Zuellig RA, Bornhauser B, Schaub MC, Fritschy JM. Short communication: altered synaptic clustering of GABAA receptors in mice lacking dystrophin (mdx mice) Eur J Neurosci. 1999;11:4457–4462. doi: 10.1046/j.1460-9568.1999.00887.x. [DOI] [PubMed] [Google Scholar]
- Krishek BJ, Xie X, Blackstone C, Huganir RL, Moss SJ, Smart TG. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron. 1994;12:1081–1095. doi: 10.1016/0896-6273(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Ladurelle N, Eychenne B, Denton D, Blair-West J, Schumacher M, Robel P, Baulieu E. Prolonged intracerebroventricular infusion of neurosteroids affects cognitive performances in the mouse. Brain Res. 2000;858:371–379. doi: 10.1016/s0006-8993(00)01953-3. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- Lal H, Kumar B, Forster MJ. Enhancement of learning and memory in mice by a benzodiazepine antagonist. Faseb J. 1988;2:2707–2711. doi: 10.1096/fasebj.2.11.3135223. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Krupitsky E, Remizov M, Pchelina S, Taraskina A, Zvartau E, Somberg LK, Covault J, Kranzler HR, Krystal JH, Gelernter J. Association between alcoholism and gamma-amino butyric acid alpha2 receptor subtype in a Russian population. Alcohol Clin Exp Res. 2005;29:493–498. doi: 10.1097/01.alc.0000158938.97464.90. [DOI] [PubMed] [Google Scholar]
- Lavoie AM, Twyman RE. Direct evidence for diazepam modulation of GABAA receptor microscopic affinity. Neuropharmacology. 1996;35:1383–1392. doi: 10.1016/s0028-3908(96)00077-9. [DOI] [PubMed] [Google Scholar]
- Lencz T, Lambert C, DeRosse P, Burdick KE, Morgan TV, Kane JM, Kucherlapati R, Malhotra AK. Runs of homozygosity reveal highly penetrant recessive loci in schizophrenia. Proc Natl Acad Sci U S A. 2007;104:19942–19947. doi: 10.1073/pnas.0710021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Logan SM, Tovar KR, Craig AM. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J Neurosci. 2004;24:207–217. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, Williams NM, Schwab SG, Pulver AE, Faraone SV, Brzustowicz LM, Kaufmann CA, Garver DL, Gurling HM, Lindholm E, Coon H, Moises HW, Byerley W, Shaw SH, Mesen A, Sherrington R, O'Neill FA, Walsh D, Kendler KS, Ekelund J, Paunio T, Lonnqvist J, Peltonen L, O'Donovan MC, Owen MJ, Wildenauer DB, Maier W, Nestadt G, Blouin JL, Antonarakis SE, Mowry BJ, Silverman JM, Crowe RR, Cloninger CR, Tsuang MT, Malaspina D, Harkavy-Friedman JM, Svrakic DM, Bassett AS, Holcomb J, Kalsi G, McQuillin A, Brynjolfson J, Sigmundsson T, Petursson H, Jazin E, Zoega T, Helgason T. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, Montrose D. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Lund JS. Heterogeneity of chandelier neurons in monkey neocortex: corticotropin-releasing factor- and parvalbumin-immunoreactive populations. J Comp Neurol. 1990;293:599–615. doi: 10.1002/cne.902930406. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Volk DW, Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology (Berl) 2004;174:143–150. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- Li X, Serwanski DR, Miralles CP, Nagata K, De Blas AL. Septin 11 is present in GABAergic synapses and plays a functional role in the cytoarchitecture of neurons and GABAergic synaptic connectivity. J Biol Chem. 2009;284:17253–17265. doi: 10.1074/jbc.M109.008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M, Shen J, Zhang Y, Li SH, Li XJ, Li H. Immunohistochemical localization of huntingtin-associated protein 1 in endocrine system of the rat. J Histochem Cytochem. 2005;53:1517–1524. doi: 10.1369/jhc.5A6662.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Liu J, Shi Y, Tang W, Guo T, Li D, Yang Y, Zhao X, Wang H, Li X, Feng G, Gu N, Zhu S, Liu H, Guo Y, Shi J, Sang H, Yan L, He L. Positive association of the human GABA-A-receptor beta 2 subunit gene haplotype with schizophrenia in the Chinese Han population. Biochem Biophys Res Commun. 2005;334:817–823. doi: 10.1016/j.bbrc.2005.06.167. [DOI] [PubMed] [Google Scholar]
- Lo WS, Harano M, Gawlik M, Yu Z, Chen J, Pun FW, Tong KL, Zhao C, Ng SK, Tsang SY, Uchimura N, Stober G, Xue H. GABRB2 association with schizophrenia: commonalities and differences between ethnic groups and clinical subtypes. Biol Psychiatry. 2007a;61:653–660. doi: 10.1016/j.biopsych.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Lo WS, Lau CF, Xuan Z, Chan CF, Feng GY, He L, Cao ZC, Liu H, Luan QM, Xue H. Association of SNPs and haplotypes in GABAA receptor beta2 gene with schizophrenia. Mol Psychiatry. 2004;9:603–608. doi: 10.1038/sj.mp.4001461. [DOI] [PubMed] [Google Scholar]
- Lo WS, Xu Z, Yu Z, Pun FW, Ng SK, Chen J, Tong KL, Zhao C, Xu X, Tsang SY, Harano M, Stober G, Nimgaonkar VL, Xue H. Positive selection within the Schizophrenia-associated GABA(A) receptor beta2 gene. PLoS ONE. 2007b;2:e462. doi: 10.1371/journal.pone.0000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Hippocampal dysfunction and disruption of dopamine system regulation in an animal model of schizophrenia. Neurotox Res. 2008;14:97–104. doi: 10.1007/BF03033801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebrich S, Bahring R, Katsuno T, Tsukita S, Kneussel M. Activated radixin is essential for GABAA receptor alpha5 subunit anchoring at the actin cytoskeleton. Embo J. 2006;25:987–999. doi: 10.1038/sj.emboj.7600995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loup F, Weinmann O, Yonekawa Y, Aguzzi A, Wieser HG, Fritschy JM. A highly sensitive immunofluorescence procedure for analyzing the subcellular distribution of GABAA receptor subunits in the human brain. J Histochem Cytochem. 1998;46:1129–1139. doi: 10.1177/002215549804601005. [DOI] [PubMed] [Google Scholar]
- MacAskill AF, Brickley K, Stephenson FA, Kittler JT. GTPase dependent recruitment of Grif-1 by Miro1 regulates mitochondrial trafficking in hippocampal neurons. Mol Cell Neurosci. 2009;40:301–312. doi: 10.1016/j.mcn.2008.10.016. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, Stenger VA, Cohen JD. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. 2005;162:475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14:301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- Marco EJ, Abidi FE, Bristow J, Dean WB, Cotter P, Jeremy RJ, Schwartz CE, Sherr EH. ARHGEF9 disruption in a female patient is associated with X linked mental retardation and sensory hyperarousal. J Med Genet. 2008;45:100–105. doi: 10.1136/jmg.2007.052324. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- Marx CE, Duncan GE, Gilmore JH, Lieberman JA, Morrow AL. Olanzapine increases allopregnanolone in the rat cerebral cortex. Biol Psychiatry. 2000;47:1000–1004. doi: 10.1016/s0006-3223(99)00305-4. [DOI] [PubMed] [Google Scholar]
- Marx CE, Keefe RS, Buchanan RW, Hamer RM, Kilts JD, Bradford DW, Strauss JL, Naylor JC, Payne VM, Lieberman JA, Savitz AJ, Leimone LA, Dunn L, Porcu P, Morrow AL, Shampine LJ. Proof-of-concept trial with the neurosteroid pregnenolone targeting cognitive and negative symptoms in schizophrenia. Neuropsychopharmacology. 2009;34:1885–1903. doi: 10.1038/npp.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CE, Stevens RD, Shampine LJ, Uzunova V, Trost WT, Butterfield MI, Massing MW, Hamer RM, Morrow AL, Lieberman JA. Neuroactive steroids are altered in schizophrenia and bipolar disorder: relevance to pathophysiology and therapeutics. Neuropsychopharmacology. 2006;31:1249–1263. doi: 10.1038/sj.npp.1300952. [DOI] [PubMed] [Google Scholar]
- Marx CE, VanDoren MJ, Duncan GE, Lieberman JA, Morrow AL. Olanzapine and clozapine increase the GABAergic neuroactive steroid allopregnanolone in rodents. Neuropsychopharmacology. 2003;28:1–13. doi: 10.1038/sj.npp.1300015. [DOI] [PubMed] [Google Scholar]
- Mayo W, Dellu F, Robel P, Cherkaoui J, Le Moal M, Baulieu EE, Simon H. Infusion of neurosteroids into the nucleus basalis magnocellularis affects cognitive processes in the rat. Brain Res. 1993;607:324–328. doi: 10.1016/0006-8993(93)91524-v. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- McGuire JR, Rong J, Li SH, Li XJ. Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J Biol Chem. 2006;281:3552–3559. doi: 10.1074/jbc.M509806200. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- McKinley DD, Lennon DJ, Carter DB. Cloning, sequence analysis and expression of two forms of mRNA coding for the human beta 2 subunit of the GABAA receptor. Brain Res Mol Brain Res. 1995;28:175–179. doi: 10.1016/0169-328x(94)00228-7. [DOI] [PubMed] [Google Scholar]
- Menzies L, Ooi C, Kamath S, Suckling J, McKenna P, Fletcher P, Bullmore E, Stephenson C. Effects of gamma-aminobutyric acid-modulating drugs on working memory and brain function in patients with schizophrenia. Arch Gen Psychiatry. 2007;64:156–167. doi: 10.1001/archpsyc.64.2.156. [DOI] [PubMed] [Google Scholar]
- Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Anisman H. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci. 2004;24:1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Kirsch J, Betz H, Langosch D. Identification of a gephyrin binding motif on the glycine receptor beta subunit. Neuron. 1995;15:563–572. doi: 10.1016/0896-6273(95)90145-0. [DOI] [PubMed] [Google Scholar]
- Meziane H, Mathis C, Paul SM, Ungerer A. The neurosteroid pregnenolone sulfate reduces learning deficits induced by scopolamine and has promnestic effects in mice performing an appetitive learning task. Psychopharmacology (Berl) 1996;126:323–330. doi: 10.1007/BF02247383. [DOI] [PubMed] [Google Scholar]
- Mhatre MC, Pena G, Sieghart W, Ticku MK. Antibodies specific for GABAA receptor alpha subunits reveal that chronic alcohol treatment down-regulates alpha-subunit expression in rat brain regions. J Neurochem. 1993;61:1620–1625. doi: 10.1111/j.1471-4159.1993.tb09795.x. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Montpied P, Morrow AL, Karanian JW, Ginns EI, Martin BM, Paul SM. Prolonged ethanol inhalation decreases gamma-aminobutyric acidA receptor alpha subunit mRNAs in the rat cerebral cortex. Mol Pharmacol. 1991;39:157–163. [PubMed] [Google Scholar]
- Morales FC, Takahashi Y, Kreimann EL, Georgescu MM. Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc Natl Acad Sci U S A. 2004;101:17705–17710. doi: 10.1073/pnas.0407974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison PD, Murray RM. Schizophrenia. Curr Biol. 2005;15:R980–984. doi: 10.1016/j.cub.2005.11.059. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Pace JR, Purdy RH, Paul SM. Characterization of steroid interactions with gamma-aminobutyric acid receptor-gated chloride ion channels: evidence for multiple steroid recognition sites. Mol Pharmacol. 1990;37:263–270. [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–485. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2:240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Yarnold PR, Levinson DF, Singh H, Bellack AS, Kee K, Morrison RL, Yadalam KG. Prevalence of substance abuse in schizophrenia: demographic and clinical correlates. Schizophr Bull. 1990;16:31–56. doi: 10.1093/schbul/16.1.31. [DOI] [PubMed] [Google Scholar]
- Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- Noel J, Ralph GS, Pickard L, Williams J, Molnar E, Uney JB, Collingridge GL, Henley JM. Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron. 1999;23:365–376. doi: 10.1016/s0896-6273(00)80786-2. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of alpha(2)-subunit-containing GABA(A) receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- Okada H, Matsushita N, Kobayashi K, Kobayashi K. Identification of GABAA receptor subunit variants in midbrain dopaminergic neurons. J Neurochem. 2004;89:7–14. doi: 10.1111/j.1471-4159.2004.02271.x. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA(A) receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Tobin AJ. Molecular biology of GABAA receptors. Faseb J. 1990;4:1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- Pallares M, Darnaudery M, Day J, Le Moal M, Mayo W. The neurosteroid pregnenolone sulfate infused into the nucleus basalis increases both acetylcholine release in the frontal cortex or amygdala and spatial memory. Neuroscience. 1998;87:551–558. doi: 10.1016/s0306-4522(98)00174-2. [DOI] [PubMed] [Google Scholar]
- Papadimitriou G, Dikeos D, Daskalopoulou E, Karadima G, Avramopoulos D, Contis C, Stefanis C. Association between GABA-A receptor alpha 5 subunit gene locus and schizophrenia of a later age of onset. Neuropsychobiology. 2001;43:141–144. doi: 10.1159/000054882. [DOI] [PubMed] [Google Scholar]
- Papadimitriou GN, Dikeos DG, Karadima G, Avramopoulos D, Daskalopoulou EG, Vassilopoulos D, Stefanis CN. Association between the GABA(A) receptor alpha5 subunit gene locus (GABRA5) and bipolar affective disorder. Am J Med Genet. 1998;81:73–80. [PubMed] [Google Scholar]
- Papadopoulos T, Eulenburg V, Reddy-Alla S, Mansuy IM, Li Y, Betz H. Collybistin is required for both the formation and maintenance of GABAergic postsynapses in the hippocampus. Mol Cell Neurosci. 2008;39:161–169. doi: 10.1016/j.mcn.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Papadopoulos T, Korte M, Eulenburg V, Kubota H, Retiounskaia M, Harvey RJ, Harvey K, O'Sullivan GA, Laube B, Hulsmann S, Geiger JR, Betz H. Impaired GABAergic transmission and altered hippocampal synaptic plasticity in collybistin-deficient mice. Embo J. 2007;26:3888–3899. doi: 10.1038/sj.emboj.7601819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park N, Juo SH, Cheng R, Liu J, Loth JE, Lilliston B, Nee J, Grunn A, Kanyas K, Lerer B, Endicott J, Gilliam TC, Baron M. Linkage analysis of psychosis in bipolar pedigrees suggests novel putative loci for bipolar disorder and shared susceptibility with schizophrenia. Mol Psychiatry. 2004;9:1091–1099. doi: 10.1038/sj.mp.4001541. [DOI] [PubMed] [Google Scholar]
- Pennington K, Beasley CL, Dicker P, Fagan A, English J, Pariante CM, Wait R, Dunn MJ, Cotter DR. Prominent synaptic and metabolic abnormalities revealed by proteomic analysis of the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder. Mol Psychiatry. 2008;13:1102–1117. doi: 10.1038/sj.mp.4002098. [DOI] [PubMed] [Google Scholar]
- Perala J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsa E, Pirkola S, Partonen T, Tuulio-Henriksson A, Hintikka J, Kieseppa T, Harkanen T, Koskinen S, Lonnqvist J. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64:19–28. doi: 10.1001/archpsyc.64.1.19. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Perry TL, Kish SJ, Buchanan J, Hansen S. Gamma-aminobutyric-acid deficiency in brain of schizophrenic patients. Lancet. 1979;1:237–239. doi: 10.1016/s0140-6736(79)90767-0. [DOI] [PubMed] [Google Scholar]
- Peters A, Proskauer CC, Ribak CE. Chandelier cells in rat visual cortex. J Comp Neurol. 1982;206:397–416. doi: 10.1002/cne.902060408. [DOI] [PubMed] [Google Scholar]
- Petryshen TL, Middleton FA, Tahl AR, Rockwell GN, Purcell S, Aldinger KA, Kirby A, Morley CP, McGann L, Gentile KL, Waggoner SG, Medeiros HM, Carvalho C, Macedo A, Albus M, Maier W, Trixler M, Eichhammer P, Schwab SG, Wildenauer DB, Azevedo MH, Pato MT, Pato CN, Daly MJ, Sklar P. Genetic investigation of chromosome 5q GABAA receptor subunit genes in schizophrenia. Mol Psychiatry. 2005;10:1074–1088. 1057. doi: 10.1038/sj.mp.4001739. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Gorman CM, Kettenmann H, Seeburg PH, Schofield PR. Transient expression shows ligand gating and allosteric potentiation of GABAA receptor subunits. Science. 1988;242:1306–1308. doi: 10.1126/science.2848320. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol. 1999;81:1903–1916. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- Roberts E. Prospects for research on schizophrenia. An hypotheses suggesting that there is a defect in the GABA system in schizophrenia. Neurosci Res Program Bull. 1972;10:468–482. [PubMed] [Google Scholar]
- Ruzicka WB, Zhubi A, Veldic M, Grayson DR, Costa E, Guidotti A. Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol Psychiatry. 2007;12:385–397. doi: 10.1038/sj.mp.4001954. [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Panzanelli P, Sieghart W, Fritschy JM. Colocalization of multiple GABA(A) receptor subtypes with gephyrin at postsynaptic sites. J Comp Neurol. 2000;420:481–498. doi: 10.1002/(sici)1096-9861(20000515)420:4<481::aid-cne6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Matsumura M, Kubota K. Delayed response deficits produced by local injection of bicuculline into the dorsolateral prefrontal cortex in Japanese macaque monkeys. Exp Brain Res. 1989;75:457–469. doi: 10.1007/BF00249897. [DOI] [PubMed] [Google Scholar]
- Schmitt B, Knaus P, Becker CM, Betz H. The Mr 93,000 polypeptide of the postsynaptic glycine receptor complex is a peripheral membrane protein. Biochemistry. 1987;26:805–811. doi: 10.1021/bi00377a022. [DOI] [PubMed] [Google Scholar]
- Schofield PR, Darlison MG, Fujita N, Burt DR, Stephenson FA, Rodriguez H, Rhee LM, Ramachandran J, Reale V, Glencorse TA, et al. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor superfamily. Nature. 1987;328:221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Berresheim U, Pirker S, Wieselthaler A, Fuchs K, Sieghart W, Sperk G. Distribution of the major gamma-aminobutyric acid(A) receptor subunits in the basal ganglia and associated limbic brain areas of the adult rat. J Comp Neurol. 2001;433:526–549. doi: 10.1002/cne.1158. [DOI] [PubMed] [Google Scholar]
- Schweizer C, Balsiger S, Bluethmann H, Mansuy IM, Fritschy JM, Mohler H, Luscher B. The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci. 2003;24:442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- Shi J, Gershon ES, Liu C. Genetic associations with schizophrenia: Meta-analyses of 12 candidate genes. Schizophr Res. 2008;104:96–107. doi: 10.1016/j.schres.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Sklar P, Pato MT, Kirby A, Petryshen TL, Medeiros H, Carvalho C, Macedo A, Dourado A, Coelho I, Valente J, Soares MJ, Ferreira CP, Lei M, Verner A, Hudson TJ, Morley CP, Kennedy JL, Azevedo MH, Lander E, Daly MJ, Pato CN. Genome-wide scan in Portuguese Island families identifies 5q31-5q35 as a susceptibility locus for schizophrenia and psychosis. Mol Psychiatry. 2004;9:213–218. doi: 10.1038/sj.mp.4001418. [DOI] [PubMed] [Google Scholar]
- Smith GB, Olsen RW. Functional domains of GABAA receptors. Trends Pharmacol Sci. 1995;16:162–168. doi: 10.1016/s0165-6147(00)89009-4. [DOI] [PubMed] [Google Scholar]
- Somogyi P. A specific ‘axo-axonal’ interneuron in the visual cortex of the rat. Brain Res. 1977;136:345–350. doi: 10.1016/0006-8993(77)90808-3. [DOI] [PubMed] [Google Scholar]
- Soyka M, Preuss UW, Hesselbrock V, Zill P, Koller G, Bondy B. GABA-A2 receptor subunit gene (GABRA2) polymorphisms and risk for alcohol dependence. J Psychiatr Res. 2008;42:184–191. doi: 10.1016/j.jpsychires.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternfeld F, Carling RW, Jelley RA, Ladduwahetty T, Merchant KJ, Moore KW, Reeve AJ, Street LJ, O'Connor D, Sohal B, Atack JR, Cook S, Seabrook G, Wafford K, Tattersall FD, Collinson N, Dawson GR, Castro JL, MacLeod AM. Selective, orally active gamma-aminobutyric acidA alpha5 receptor inverse agonists as cognition enhancers. J Med Chem. 2004;47:2176–2179. doi: 10.1021/jm031076j. [DOI] [PubMed] [Google Scholar]
- Stevens J, Wilson K, Foote W. GABA blockade, dopamine and schizophrenia: experimental studies in the cat. Psychopharmacologia. 1974;39:105–119. doi: 10.1007/BF00440842. [DOI] [PubMed] [Google Scholar]
- Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV, Harris-Kerr C, Wormley B, Sadek H, Kadambi B, Cesare AJ, Gibberman A, Wang X, O'Neill FA, Walsh D, Kendler KS. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002;71:337–348. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, Vakkalanka RK, Kolachana BS, Kleinman JE, Weinberger DR. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Lorincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- Terunuma M, Jang IS, Ha SH, Kittler JT, Kanematsu T, Jovanovic JN, Nakayama KI, Akaike N, Ryu SH, Moss SJ, Hirata M. GABAA receptor phospho-dependent modulation is regulated by phospholipase C-related inactive protein type 1, a novel protein phosphatase 1 anchoring protein. J Neurosci. 2004;24:7074–7084. doi: 10.1523/JNEUROSCI.1323-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GJ, Harper CG, Dodd PR. Expression of GABA(A) receptor isoform genes in the cerebral cortex of cirrhotic and alcoholic cases assessed by S1 nuclease protection assays. Neurochem Int. 1998;32:375–385. doi: 10.1016/s0197-0186(97)00102-2. [DOI] [PubMed] [Google Scholar]
- Tierney ML, Birnir B, Cromer B, Howitt SM, Gage PW, Cox GB. Two threonine residues in the M2 segment of the alpha 1 beta 1 GABAA receptor are critical for ion channel function. Receptors Channels. 1998;5:113–124. [PubMed] [Google Scholar]
- Tochigi M, Iwamoto K, Bundo M, Komori A, Sasaki T, Kato N, Kato T. Methylation status of the reelin promoter region in the brain of schizophrenic patients. Biol Psychiatry. 2008;63:530–533. doi: 10.1016/j.biopsych.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Toyota T, Yamada K, Detera-Wadleigh SD, Yoshikawa T. Analysis of a cluster of polymorphisms in AKT1 gene in bipolar pedigrees: a family-based association study. Neurosci Lett. 2003;339:5–8. doi: 10.1016/s0304-3940(02)01428-3. [DOI] [PubMed] [Google Scholar]
- Tretter V, Jacob TC, Mukherjee J, Fritschy JM, Pangalos MN, Moss SJ. The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunits to gephyrin. J Neurosci. 2008;28:1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang M. Schizophrenia: genes and environment. Biol Psychiatry. 2000;47:210–220. doi: 10.1016/s0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- Uemura T, Mori H, Mishina M. Isolation and characterization of Golgi apparatus-specific GODZ with the DHHC zinc finger domain. Biochem Biophys Res Commun. 2002;296:492–496. doi: 10.1016/s0006-291x(02)00900-2. [DOI] [PubMed] [Google Scholar]
- Ugale RR, Hirani K, Morelli M, Chopde CT. Role of neuroactive steroid allopregnanolone in antipsychotic-like action of olanzapine in rodents. Neuropsychopharmacology. 2004;29:1597–1609. doi: 10.1038/sj.npp.1300460. [DOI] [PubMed] [Google Scholar]
- Uji A, Matsuda M, Kukita T, Maeda K, Kanematsu T, Hirata M. Molecules interacting with PRIP-2, a novel Ins(1,4,5)P3 binding protein type 2: Comparison with PRIP-1. Life Sci. 2002;72:443–453. doi: 10.1016/s0024-3205(02)02275-0. [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Darnaudery M, Corpechot C, Young J, Koehl M, Le Moal M, Baulieu EE, Robel P, Simon H. Neurosteroids: deficient cognitive performance in aged rats depends on low pregnenolone sulfate levels in the hippocampus. Proc Natl Acad Sci U S A. 1997;94:14865–14870. doi: 10.1073/pnas.94.26.14865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Le Moal M. Role of pregnenolone, dehydroepiandrosterone and their sulfate esters on learning and memory in cognitive aging. Brain Res Brain Res Rev. 2001;37:301–312. doi: 10.1016/s0165-0173(01)00135-7. [DOI] [PubMed] [Google Scholar]
- Van Kammen DP. gamma-Aminobutyric acid (Gaba) and the dopamine hypothesis of schizophrenia. Am J Psychiatry. 1977;134:138–143. doi: 10.1176/ajp.134.2.138. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Crook JM, Hyde TM, Kleinman JE, Weinberger DR, Becker KG, Freed WJ. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: a preliminary study. Schizophr Res. 2002;58:11–20. doi: 10.1016/s0920-9964(01)00377-2. [DOI] [PubMed] [Google Scholar]
- Veldic M, Kadriu B, Maloku E, Agis-Balboa RC, Guidotti A, Davis JM, Costa E. Epigenetic mechanisms expressed in basal ganglia GABAergic neurons differentiate schizophrenia from bipolar disorder. Schizophr Res. 2007;91:51–61. doi: 10.1016/j.schres.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken T, Claes S, Sluijs S, Paterson AD, van Duijn C, Adolfsson R, Del-Favero J, Van Broeckhoven C. Genomewide scan for affective disorder susceptibility Loci in families of a northern Swedish isolated population. Am J Hum Genet. 2005;76:237–248. doi: 10.1086/427836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoorn TA. Formation of heteromeric gamma-aminobutyric acid type A receptors containing two different alpha subunits. Mol Pharmacol. 1994;45:475–480. [PubMed] [Google Scholar]
- Vicini S. Pharmacologic significance of the structural heterogeneity of the GABAA receptor-chloride ion channel complex. Neuropsychopharmacology. 1991;4:9–15. [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Whiting PJ, Kemp JA. Differences in affinity and efficacy of benzodiazepine receptor ligands at recombinant gamma-aminobutyric acidA receptor subtypes. Mol Pharmacol. 1993;43:240–244. [PubMed] [Google Scholar]
- Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW. GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature. 1999;397:69–72. doi: 10.1038/16264. [DOI] [PubMed] [Google Scholar]
- Wang H, Friedman E. Increased association of brain protein kinase C with the receptor for activated C kinase-1 (RACK1) in bipolar affective disorder. Biol Psychiatry. 2001;50:364–370. doi: 10.1016/s0006-3223(01)01147-7. [DOI] [PubMed] [Google Scholar]
- Wang HY, Friedman E. Enhanced protein kinase C activity and translocation in bipolar affective disorder brains. Biol Psychiatry. 1996;40:568–575. doi: 10.1016/0006-3223(95)00611-7. [DOI] [PubMed] [Google Scholar]
- Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ, McKernan RM, Wafford KA. Structure and pharmacology of vertebrate GABAA receptor subtypes. Int Rev Neurobiol. 1995;38:95–138. doi: 10.1016/s0074-7742(08)60525-5. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FS, Gibbs TT, Farb DH. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol Pharmacol. 1991;40:333–336. [PubMed] [Google Scholar]
- Yamada K, Watanabe A, Iwayama-Shigeno Y, Yoshikawa T. Evidence of association between gamma-aminobutyric acid type A receptor genes located on 5q34 and female patients with mood disorders. Neurosci Lett. 2003;349:9–12. doi: 10.1016/s0304-3940(03)00611-6. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Kubota T, Kanematsu T, Nakayama K, Hirata M, Yamamoto T. Hypersensitivity to pentylenetetrazol-induced convulsion in mice lacking the PLC-related inactive protein-1. Brain Res. 2004;1025:237–240. doi: 10.1016/j.brainres.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Yee BK, Keist R, von Boehmer L, Studer R, Benke D, Hagenbuch N, Dong Y, Malenka RC, Fritschy JM, Bluethmann H, Feldon J, Mohler H, Rudolph U. A schizophrenia-related sensorimotor deficit links alpha 3-containing GABAA receptors to a dopamine hyperfunction. Proc Natl Acad Sci U S A. 2005;102:17154–17159. doi: 10.1073/pnas.0508752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Chen J, Shi H, Stoeber G, Tsang SY, Xue H. Analysis of GABRB2 association with schizophrenia in German population with DNA sequencing and one-label extension method for SNP genotyping. Clin Biochem. 2006;39:210–218. doi: 10.1016/j.clinbiochem.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Yuan X, Yao J, Norris D, Tran DD, Bram RJ, Chen G, Luscher B. Calcium-modulating cyclophilin ligand regulates membrane trafficking of postsynaptic GABA(A) receptors. Mol Cell Neurosci. 2008;38:277–289. doi: 10.1016/j.mcn.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Xu Z, Chen J, Yu Z, Tong KL, Lo WS, Pun FW, Ng SK, Tsang SY, Xue H. Two isoforms of GABA(A) receptor beta2 subunit with different electrophysiological properties: Differential expression and genotypical correlations in schizophrenia. Mol Psychiatry. 2006;11:1092–1105. doi: 10.1038/sj.mp.4001899. [DOI] [PubMed] [Google Scholar]
- Zhao X, Qin S, Shi Y, Zhang A, Zhang J, Bian L, Wan C, Feng G, Gu N, Zhang G, He G, He L. Systematic study of association of four GABAergic genes: glutamic acid decarboxylase 1 gene, glutamic acid decarboxylase 2 gene, GABA(B) receptor 1 gene and GABA(A) receptor subunit beta2 gene, with schizophrenia using a universal DNA microarray. Schizophr Res. 2007;93:374–384. doi: 10.1016/j.schres.2007.02.023. [DOI] [PubMed] [Google Scholar]


