Abstract
Objective
An inversion polymorphism of approximately 900kb on chromosome 17q21, which includes the microtubule-associated protein tau (MAPT) gene defines two haplotype clades, H1 and H2. Several small case–control studies have observed a marginally significant excess of the H1/H1 diplotype among patients with Parkinson’s disease (PD), and one reported refining the association to a region spanning exons 1 to 4 of MAPT. We sought to replicate these findings.
Methods
We genotyped 1,762 PD patients and 2,010 control subjects for a single nucleotide polymorphism (SNP) that differentiates the H1 and H2 clades. We also analyzed four SNPs that define subhaplotypes within H1 previously reported to associate with PD or other neurodegenerative disorders.
Results
After adjusting for age, sex, and site, we observed a robust association between the H1/H1 diplotype and PD risk (odds ratio for H1/H1 vs H1/H2 and H2/H2, 1.46; 95% confidence interval, 1.25–1.69; p = 8 × 10−7). The effect was evident in both familial and sporadic subgroups, men and women, and early- and late-onset disease. Within H1/H1 individuals, there was no significant difference between cases and control subjects in the overall frequency distribution of H1 subhaplotypes.
Interpretation
Our data provide strong evidence that the H1 clade, which contains MAPT and several other genes, is a risk factor for PD. However, attributing this finding to variants within a specific region of MAPT is premature. Thorough fine-mapping of the H1 clade in large numbers of individuals is now needed to identify the underlying functional variant(s) that alter susceptibility for PD.
The microtubule-associated protein tau, encoded by the MAPT gene, is primarily expressed in neurons and plays a key role in the organization and integrity of the cytoskeleton.1,2 Filamentous neuronal tau inclusions define a set of neurodegenerative diseases collectively known as the tauopathies, which include Alzheimer’s disease (AD), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and frontotemporal dementia with parkinsonism linked to chromosome 17.1,2 MAPT sequence variation was first linked to the etiopathogenesis of tauopathies by the discovery of mutations resulting in frontotemporal dementia with parkinsonism linked to chromosome 17, and subsequently an extended common haplotype (H1) across the gene was shown to associate with disease risk in PSP and CBD.3–7
Though Parkinson’s disease (PD) shares some clinical features with the tauopathies, it has been assigned to a distinct subset of neurodegenerative diseases (the α-synucleinopathies) by histopathology because it is characterized by intraneuronal accumulation of α-synuclein, rather than tau.8 Thus, initial reports that the MAPT H1 haplotype was also associated with PD were intriguing but met with some skepticism. Since then the association between MAPT variants and PD has remained tenuous with a number of small case–control studies reporting either a marginal or no significant overrepresentation of the H1/H1 diplotype among cases (Table 1).9–23However, a recent meta-analysis of 10 such studies in white subjects supported the hypothesis that the H1 haplotype might confer susceptibility for PD (pooled odds ratio [OR] for H1/H1 vs H1/H2 and H2/H2, 1.49; confidence interval [CI], 1.28 –1.74).24
Table 1.
Summary of Studies on MAPT H1 Diplotype as Risk Factor for Parkinson‘s Disease
| Study | Populationb | Cases | Control Subjects | H1/H1 vs All Othersa |
|||
|---|---|---|---|---|---|---|---|
| N | H1/H1 (%) |
N | H1/H1 (%) |
OR | 95% CI | ||
| Hoenicka and colleagues, 19999 |
White (Spain) | 18 | 83.3 | 79 | 51.9 | 4.63 | 1.24–17.28 |
| Morris and colleagues, 199910 |
European (UK) |
50 | 68.0 | 75 | 66.7 | 1.06 | 0.49–2.28 |
| Pastor and colleagues, 200011 |
Europeanc (Spain) |
152 | 68.4 | 150 | 58.0 | 1.57 | 0.98–2.51 |
| Maraganore and colleagues, 200112 |
European (US) |
252 | 73.4 | 152 | 65.1 | 1.54 | 0.98–2.44 |
| de Silva and colleagues, 200213 |
European (UK) |
157 | 63.1 | 157 | 56.1 | 1.34 | 0.85–2.10 |
| Farrer and colleagues, 200214 |
European (Norway) |
96 | 85.4 | 68 | 51.5 | 5.52 | 2.64–11.57 |
| Clark and colleagues, 200315 |
Non-Hispanic white (US) |
72 | 66.7 | 129 | 56.6 | 1.53 | 0.84–2.80 |
| Peplonska and colleagues, 200316 |
European (Poland) |
100 | 76.0 | 100 | 74.0 | 1.11 | 0.59–2.11 |
| Zappia and colleagues, 200317 |
European (Italy) |
300 | 46.3 | 197 | 68.5 | 0.40 | 0.27–0.58 |
| Levecque and colleagues, 200418 |
European (France) |
208 | 64.4 | 483 | 52.4 | 1.71 | 1.20–2.43 |
| Kwok and colleagues, 200419 |
European (Australia) |
206 | 66.5 | 169 | 58.0 | 1.44 | 0.94–2.19 |
| Healy and colleagues, 200420 |
White (UK) | 580 | 65.0 | 513 | 56.5 | 1.43 | 1.12–1.82 |
| Johansson and colleagues, 200521 |
Europeanc (Sweden) |
105 | 78.1 | 160 | 70.0 | 1.53 | 0.86–2.71 |
| Fidani and colleagues, 200622 |
European (Greece) |
133 | 66.2 | 113 | 53.1 | 1.73 | 1.03–2.90 |
| Fung and colleagues, 200623d |
European (Greece) |
224 | 57.1 | 215 | 62.3 | 0.80 | 0.55–1.18 |
| Fung and colleagues, 200623d |
European (Finland) |
134 | 85.1 | 140 | 83.6 | 1.12 | 0.58–2.15 |
The odds ratios (ORs) and 95% confidence intervals (CIs) indicated were taken from the original publication (if available) or were calculated from the diplotype counts provided using a standard Pearson’s χ2 test.
Restricted to populations of European, “white,“ or “Caucasian“ ancestry. The primary geographic region from which the subjects were recruited is indicated in parentheses.
The population group of the sample was not explicitly stated but was assumed to be European.
The data from Fung and colleagues23 on two geographically distinct case-control samples are displayed as two separate studies.
The H1 and H2 haplotypes actually represent two distinct clades or families of subhaplotypes that arose from an inversion of 900kb on chromosome 17q21 approximately 3 million years ago.25 Since the event, the two inverted regions, which contain MAPT and several other genes, have been recombinationally suppressed and have accumulated sequence variation independently. As a result, any one of a large number of single nucleotide polymorphisms (SNPs) can be genotyped to differentiate the two haplotype clades (referred to hereafter as H1–H2 SNPs). However, because these SNPs are in perfect linkage disequilibrium (LD) with one another (r2 =1) across the entire region, the association of a given H1–H2 SNP with disease risk could be due to functional variation within any segment of MAPT or a neighboring gene.26
To more precisely map the disease-associated region in PD, Skipper and colleagues27 analyzed 14 SNPs specific to the H1 clade (ie, SNPs that were polymorphic on H1 chromosomes but monomorphic on H2 chromosomes) in subjects of the H1/H1 diplotype. A subhaplotype composed of two such “H1-SNPs” (rs242562 and rs2435207) spanning MAPT exons 1 to 4 was significantly overrepresented in cases versus control subjects refining the PD-associated interval from approximately 900 to 90kb. More comprehensive analyses of H1-SNPs have subsequently identified disease-associated H1 subhaplotypes overlapping the same MAPT region in PSP, CBD, and AD.28–30 In this study, we sought to confirm whether the H1 clade is associated with PD, and if so, whether the effect can be attributed to specific H1-SNPs or subhaplotypes previously implicated in PD and the tauopathies.
Subjects and Methods
Subjects
We recruited 1,762 PD patients (mean age at onset [AAO], 58.7 ± 11.6 years; range, 24–93 years; mean age at enrollment, 68.0 ± 10.6 years; male sex, 67.7%) and 2,010 control subjects (mean age at enrollment, 67.4 ± 18.3 years; male sex, 37.3%) through the NeuroGenetics Research Consortium, which includes movement disorder clinics in Albany, New York, Atlanta, Georgia, Portland, Oregon, and Seattle, Washington. All patients met UK PD Society Brain Bank clinical diagnostic criteria31 for PD as determined by a movement disorder specialist and were consecutively recruited except that patients who had an AAO less than 20 years, whose race was not solely classified as “white” (by self-report), or who carried pathogenic mutations in LRRK2 or PARK2 (homozygotes/compound heterozygotes) were excluded from the study population. Patients with an AAO ≤ 50 years were defined as early-onset PD and comprised 25.4% of all cases. Among patients, 22.9% reported a family history of PD in at least one first- or second-degree relative and were classified as “familial” PD for this analysis. In such instances, only one affected individual from each family was included in the study.
Control subjects had no history of parkinsonism and were either spouses of PD patients (31.2%) or healthy volunteers from the local community (68.8%). The institutional review boards at each participating site approved the study, and all subjects gave informed consent.
Marker Selection and Genotyping
Our primary objective was to select MAPT variants previously reported to modify PD susceptibility. To do this, we chose a single H1–H2 SNP (rs1800547) to differentiate the H1 and H2 haplotype clades,3,14,25 and three H1-SNPs (rs242562, rs3785883, and rs2435207) that defined the two and three locus H1 subhaplotypes that Skipper and colleagues27 observed to associate with PD.
We sought additional MAPT variants for analysis based on published data from case–control studies on tauopathies. Three H1-SNPs (rs242557, rs3785883, and rs2471738), either individually or together as a three-locus subhaplotype, have been shown to associate with disease risk in PSP, CBD, and AD.28–30 One of these (rs3785883) was already selected for analysis. To avoid unnecessary genotyping, we assessed LD between the remaining two SNPs (rs242557 and rs2471738) and the three PD-associated H1-SNPs described earlier using data on 60 unrelated individuals of European ancestry from the International HapMap Project CEU population (http://www.hapmap.org).32 We used the LD-select algorithm33 as implemented on the SeattleSNPs Genome Variation Server (http://gvs.gs.washington.edu/GVS/) to estimate LD (measured as r2) among these markers. Of the two nonoverlapping tauopathy-linked SNPs, one (rs242557) was discarded because it was highly correlated with rs242562 (r2 = 0.96). The other (rs2471738) was selected for analysis because it was imperfectly correlated to all SNPs in the group (r2 = 0.03– 0.71).
Genotyping was performed by TaqMan assay using an ABI 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). To assess accuracy, we also genotyped each SNP by resequencing in 94 DNA samples.
Data Analysis
We assessed each SNP for Hardy–Weinberg equilibrium in cases and control subjects using an exact test. For all markers, we first tested for association between allele frequencies and PD using a Pearson’s χ2 test. We examined the H1–H2 SNP using a recessive model (H1/H1 vs H1/H2 and H2/H2). For H1-SNPs, we examined the association between genotype and PD using logistic regression, with homozygotes for the more common allele as the reference. Tests of H1-SNPs were restricted to H1/H1 individuals. To account for differences in important variables between cases and control subjects, we then used logistic regression to test for genotypic associations, adjusting for sex, site, and age at enrollment (by three age strata: <50, 50–80, and >80 years). The Breslow–Day test was used to test the homogeneity of the ORs for the H1–H2 SNP across sites. We assessed the effect of the H1–H2 SNP on AAO by comparing the mean AAO in the H1/H1 versus the combined H1/H2 and H2/H2 groups by Student’s t test. These analyses were performed using STATA version 8.
We used HAPSTAT34 to reconstruct H1 subhaplotypes from unphased genotype data and to test subhaplotype–disease associations without adjustment and after adjustment for sex, site, and age. We excluded subhaplotypes with an estimated frequency of <0.01 in cases and control subjects. For a given set of H1-SNPs, we first performed a global likelihood ratio test to assess whether the overall subhaplotype frequency distribution differed between cases and control subjects. In instances in which the overall distribution significantly differed or where the effects of a specific subhaplotype had been reported in the literature, we then examined the effects of each individual subhaplotype compared with all others. We used coefficient estimates and standard errors given by the program to construct ORs and 95% CIs. We calculated pairwise LD (measured as D′) between H1-SNPs in cases and control subjects, and created graphic representations of the data using Haploview.35
Results
There were no discordant calls between genotypes determined by TaqMan assay or resequencing. No significant deviation from Hardy–Weinberg equilibrium was observed for any of the SNPs except rs2471738, for which a marginally significant deviation was seen in cases ( p = 0.02) but not control subjects ( p = 0.27).
We observed a significantly greater frequency of the H1 haplotype in cases compared with control subjects (81.8 vs 77.4%; χ2 = 22.6; OR, 1.31; 95% CI, 1.17– 1.47; p = 2 × 10−6). The H1/H1 diplotype was strongly associated with PD and remained so after adjustment for age, sex, and site (Table 2; OR, 1.46; 95% CI, 1.25–1.69; p = 8 × 10−7). The effect was seen in both familial and sporadic subgroups, in early-and late-onset disease, and in both sexes (Table 2 and Table 3). The ORs for the H1/H1 diplotype were not significantly different across sites (χ2 = 3.95; p = 0.27), and the direction of the effect was the same at each site (Georgia: OR, 1.39; 95% CI, 0.79 –2.46; New York: OR, 1.76; 95% CI, 1.27–2.44; Oregon: OR, 1.24; 95% CI, 1.01–1.53; Washington: OR, 1.58; 95% CI, 1.25–2.01). We did not observe a significant effect of the H1/H1 diplotype on AAO (mean AAO H1/H1, 58.4 ± 11.7 years; H1/H2 and H2/H2, 59.3 ± 11.5 years; p = 0.10).
Table 2.
Logistic Regression Analysis of MAPT H1 Diplotype in Parkinson‘s Disease
| Subjects | Diplotype, n (%) | N | Model 1a | Model 2b | |||||
|---|---|---|---|---|---|---|---|---|---|
| H1/H2 or H2/ H2 |
H1/H1 | OR | 95% CI | p | OR | 95% CI | p | ||
| All PD | 575 (33) | 1,187 (67) | 1,762 | 1.42 | 1.24–1.62 | 2.7 × 10−7 | 1.46 | 1.25–1.69 | 8.0 × 10−7 |
| Familial PDc | 119 (30) | 284 (70) | 403 | 1.64 | 1.30–2.07 | 2.8 × 10−5 | 1.70 | 1.33–2.17 | 2.2 × 10−5 |
| Sporadic PDd |
456 (34) | 903 (66) | 1,359 | 1.36 | 1.18–1.57 | 2.5 × 10−5 | 1.38 | 1.18–1.62 | 6.8 × 10−5 |
| Control subjects |
819 (41) | 1,191 (59) | 2,010 | — | — | — | — | — | — |
Unadjusted.
Adjusted for age, sex, and site.
Patients with at least one first- or second-degree relative with Parkinson’s disease (PD).
Patients with no first- or second-degree relatives with PD.
OR = odds ratio; CI = confidence interval.
Table 3.
Logistic Regression Analysis of MAPT H1 Diplotype in Parkinson‘s Disease Stratified by Sex and Age
| Subjects | Diplotype, n (%) | Model 1a | Model 2b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| H1/H2 or H2/H2 |
H1/H1 | N | OR | 95% CI | p | OR | 95% CI | p | |
| Male PD | 379 (32) | 814 (68) | 1,193 | 1.54 | 1.28–1.86 | 7.6 × 10−6 | 1.61 | 1.31–1.98 | 6.6 × 10−6 |
| Male control subjectsc |
313 (42) | 436 (58) | 749 | — | — | — | — | — | — |
| Female PD | 196 (34) | 373 (66) | 569 | 1.27 | 1.04–1.56 | 0.022 | 1.31 | 1.05–1.62 | 0.015 |
| Female control subjectsc |
505 (40) | 755 (60) | 1,260 | — | — | — | — | — | — |
| Early-onset PDd,e |
143 (32) | 304 (68) | 447 | 1.33 | 0.99–1.78 | 0.056 | 1.37 | 0.98–1.92 | 0.066 |
| Young control subjectsf |
138 (38) | 221 (62) | 359 | — | — | — | — | — | — |
| Late-onset PDe,g |
430 (33) | 882 (67) | 1,312 | 1.44 | 1.24–1.68 | 2.3 × 10−6 | 1.46 | 1.24–1.72 | 4.6 × 10−6 |
| Older control subjectsh |
681 (41) | 970 (59) | 1,651 | — | — | — | — | — | — |
Unadjusted.
For analyses stratified by sex, adjustment was made for age and site. For analyses stratified by age, adjustment was made for sex and site.
The sex of one control subject was unknown.
Age at onset ≤ 50 years.
The age at onset of three cases was unknown.
Age at enrollment ≤ 50 years.
Age at onset > 50 years.
Age at enrollment > 50 years.
OR = odds ratio; CI = confidence interval; PD = Parkinson‘s disease.
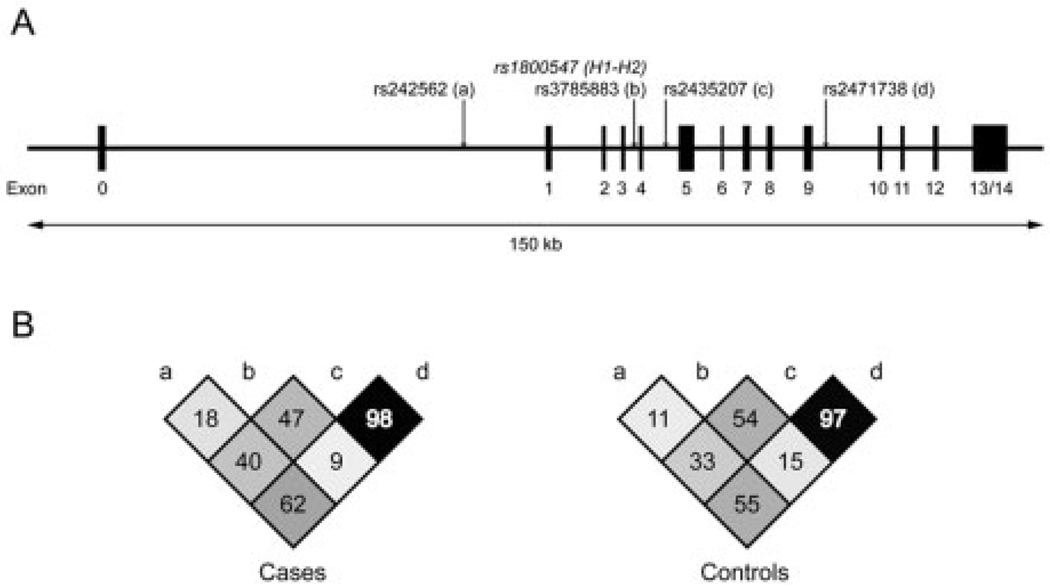
To determine whether the observed effects on PD risk were attributable to variation within the H1 clade, we then examined four H1-SNPs in all subjects of the H1/H1 diplotype (n = 1,187 cases and 1,191 control subjects). These four markers spanned a distance of approximately 49kb across MAPT (Fig), and only two (rs2435207 and rs2471738) of the four were in strong LD with one another (cases: D′ = 0.98; control subjects: D′ = 0.97; see Fig). The pattern of LD among H1-SNPs was similar in cases versus control subjects (see Fig).
Fig. 1.
Schematic of the MAPT gene and patterns of linkage disequilibrium (LD). (A) The positions of the single nucleotide polymorphism (SNP; rs1800547) used to define the H1 and H2 haplotype clades and of the four H1-SNPs (a–d) used to construct subhaplotypes are indicated by arrows. (B) Comparison of LD among H1-SNPs (a–d) in cases and control subjects. The numbers in each plot represent pairwise D′ values × 100.
To test the relation between H1-SNPs and PD risk, we first assessed each marker individually and found no significant difference in genotype frequencies between cases and control subjects after Bonferroni correction for multiple testing ( p corrected > 0.07, data not shown). We then reconstructed H1 subhaplotypes and tested for association with PD using the full marker set or subsets of SNPs.
For the full marker set, 11 of the 16 possible subhaplotypes were observed at a frequency ≥ 1% (data not shown). The frequency distribution of these subhaplotypes was not significantly different between cases and control subjects (χ2 = 10.57; degrees of freedom, 10; global p = 0.39). Next, we considered a subset of two H1-SNPs (rs242562 and rs2435207) that defined the “AA” and “AG” subhaplotypes previously reported to associate with PD.27 There was no significant difference in the overall distribution of subhaplotypes (χ2 = 4.78; degrees of freedom, 3; global p = 0.19) or in the frequencies of the AA and AG subhaplotypes in cases versus control subjects (see Supplementary Table S1). Of the remaining subhaplotypes, one (GA) was underrepresented among cases at a marginally significant level (OR, 0.83; 95% CI, 0.69–0.99).
Finally, we examined a subset of three H1-SNPs (rs242562, rs3785883, and rs2471738) that best captured the variation within the “H1c” subhaplotype28 reported to associate with the tauopathies.28–30 There was no significant difference in the overall subhaplotype distribution (χ2 = 5.85; degrees of freedom, 7; global p = 0.56) or in the frequencies of any of the eight individual subhaplotypes observed between cases and control subjects (see Supplementary Table S2). Subhaplotype frequencies from a previous study on PSP and CBD29 are provided for reference. The frequencies of the “AGT” and “GGC” subhaplotypes, which are equivalent to the two subhaplotypes reported to associate with PSP,29 were nearly identical in PD patients and control subjects (AGT: OR, 1.04; 95% CI, 0.89 –1.22; GGC: OR, 1.04; 95% CI, 0.92–1.18).
Discussion
Nearly all of the genes nominated as risk factors for typical late-onset PD have either not yet undergone or failed appropriate replication,36–39 with a few notable exceptions (eg, SNCA REP1 polymorphism).40 Two previous meta-analyses concluded that the MAPT H1 haplotype might confer susceptibility for PD,20,24 but these conclusions must be interpreted with caution for two reasons: (1) individual-level data were not available to account for important covariates, and (2) substantial differences in study design likely existed among the publications selected for analysis (eg, method of PD diagnosis). We believe that our study, which replicates this finding in a large case–control sample in which standardized diagnostic criteria and data collection procedures were used, constitutes a major step toward establishing the MAPT H1 clade as a risk factor in PD. Although the association we observed between the H1/H1 diplotype and PD was highly significant ( p = 8 × 10−7), the effect size was rather modest (OR, 1.46; 95% CI, 1.25–1.69) but fell within the range thought typical for genetic determinants in complex diseases.41 This underscores the need for investigators to conduct future PD genetic association studies in large samples and might explain, in part, the lack of reproducibility among previous case–control studies on MAPT variants in PD (see Table 1).
Given that the H1 clade represents a risk factor for PD, the true (functional) risk allele(s) could, in theory, reside at any position within an approximately 900kb region that includes genes other than MAPT such as CRHR1 and IMP5. Thus, Skipper and colleagues’ report27 that a subhaplotype within the H1 clade was overrepresented in PD was potentially important because, if validated, it would narrow the location of the risk variant(s) to the 5′ half of MAPT itself. Among H1/H1 subjects, the authors observed a twofold greater frequency of the “AA” subhaplotype, defined by rs242562 and rs2435207, in a subset of 81 “probable” PD patients in comparison with 81 matched control subjects from an isolated population in Norway (see Supplementary Table S1). They also noted substantially greater LD among these and nearby H1-SNPs in cases versus control subjects. In contrast, we found nearly identical frequencies of the AA subhaplotype and similar patterns of LD in cases (n = 1,187) and control subjects (n = 1,191) of the H1/H1 diplotype (see Supplementary Table S1 and the Fig). Assuming that our data are representative of the outbred “white” population of North America, there are two plausible explanations for this discrepancy. First, population isolates often have greater overall levels of LD than do outbred populations.42 Thus, it is possible that an untyped risk variant exists that is present in both populations but is in LD with rs242562 and rs2435207 only in the Norwegian isolate. Second, the association between the AA subhaplotype and PD in the Norwegian sample might simply represent a false-positive finding. Notably, when the authors of that study included all available H1/H1 cases (possible and probable PD, n = 201) and control subjects (n = 278) in the analysis, the association of the AA subhaplotype with PD was of only marginal significance ( p < 0.02), particularly because multiple testing was not accounted for.
There is increasing evidence that risk variants within the H1 clade overlap among the tauopathies28–30; thus, it is possible that these same variants alter susceptibility to PD. We addressed this by genotyping three H1-SNPs that have been reported to either directly associate with the tauopathies (rs3785883 and rs2471738) or are highly correlated with a SNP that does (rs242562). However, we found no significant association between these SNPs and PD individually or together as three-locus subhaplotypes (see Supplementary Table S2). Though a slight deviation from Hardy–Weinberg equilibrium was observed for one of these SNPs (rs2471738) in cases ( p = 0.02), we believe that this probably resulted from random chance and was unlikely to mask a true disease association.
Taken together, these data suggest that although the H1 clade conveys susceptibility for PD, there is currently insufficient evidence to refine the disease association to a specific region within MAPT or neighboring genes. The as yet unidentified PD risk variant(s) might therefore exist within the H1 clade or be among the many H1–H2 SNPs that define the two clades. If the former is true, then more thorough fine mapping of the region with dense sets of H1-SNPs is likely to narrow the search to a limited number of candidate variants that can then be assessed in functional assays. If the latter is correct, then association mapping will be unable to differentiate a risk variant from among a large number of highly correlated SNPs. For example, within the MAPT region alone, which spans approximately 135kb, there are at least 109 H1–H2 SNPs in the HapMap CEU population (http://www.hapmap.org) that are in perfect LD with one another.
Assuming that MAPT is, in fact, the PD susceptibility gene within the H1–H2 region, how then does variation within the gene convey risk for a number of seemingly distinct neurodegenerative diseases? The explanation can be viewed along a continuum. At one end, a variant unique to each disease (eg, PD, PSP, CBD, AD) determines risk by a distinct functional effect on MAPT. Such effects include varying gene expression or altering ratios of the six major isoforms of tau present in adult brain that result from alternative splicing of exons 2, 3, and 10.2 At the other end of the spectrum, risk is conveyed by a single functional variant and disease outcome is determined by its interaction with other genetic and environmental factors. Our data indicate that the answer might lie between the two extremes in that the tauopathies share disease-associated H1-SNPs28–30 that do not appear to alter risk in PD. The identity of these functional variants remains to be determined, but evidence from in vitro experiments and assays of postmortem human brain indicate that variation within the H1 and H2 clades might influence both overall levels of MAPT expression19,30 and the ratio of four- to three-repeat isoforms. 43 The mechanism by which such effects on MAPT lead to PD is unknown, but some have speculated that a direct interaction between tau and α-synuclein might be involved.44
Further mapping of the H1 clade in PD patients and control subjects from both isolated and outbred populations is now necessary to locate the underlying risk allele(s). Such studies should use maximally informative “tagging” SNPs, be undertaken in samples large enough to detect effects of modest size (OR, 1.1–1.5),41 and will require independent validation. These rigorous requirements will ensure that an optimum set of candidate risk variants is then selected for assessment in both in vitro and in vivo model systems. These endeavors promise to provide important insights into the pathophysiology not only of PD, but of a number of other neurodegenerative diseases as well.
Supplementary Material
Acknowledgments
This work was supported by the Michael J. Fox Foundation (Edmond J. Safra Global Genetics Consortia Initiative, H.P.), NIH (NINDS, K08 NS044138, C.P.Z.; N1A P30 AG008017, NINDS, R01 NS036960 H.P.), Department of Veterans Affairs Merit Review Award (C.P.Z.), the Veterans Integrated Service Network 20 Geriatric, Mental Illness, and Parkinson’s Disease Research Education and Clinical Centers; and the Wadsworth Center, New York State Department of Health.
We thank the individuals who participated in the study, and S. Ayres, S. Evans, E. Martinez, and G. Richards for technical support and assistance with subject recruitment.
Footnotes
This article includes supplementary materials available via the Internet at http://www.interscience.wiley.com/jpages/0364-5134/suppmat
References
- 1.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 2.Pittman AM, Fung HC, de Silva R. Untangling the tau gene association with neurodegenerative disorders. Hum Mol Genet. 2006;15 suppl 2:R188–R195. doi: 10.1093/hmg/ddl190. [DOI] [PubMed] [Google Scholar]
- 3.Baker M, Litvan I, Houlden H, et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet. 1999;8:711–715. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- 4.Houlden H, Baker M, Morris HR, et al. Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology. 2001;56:1702–1706. doi: 10.1212/wnl.56.12.1702. [DOI] [PubMed] [Google Scholar]
- 5.Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 6.Poorkaj P, Bird TD, Wijsman E, et al. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- 7.Spillantini MG, Murrell JR, Goedert M, et al. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci U S A. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galpern WR, Lang AE. Interface between tauopathies and synucleinopathies: a tale of two proteins. Ann Neurol. 2006;59:449–458. doi: 10.1002/ana.20819. [DOI] [PubMed] [Google Scholar]
- 9.Hoenicka J, Perez M, Perez-Tur J, et al. The tau gene A0 allele and progressive supranuclear palsy. Neurology. 1999;53:1219–1225. doi: 10.1212/wnl.53.6.1219. [DOI] [PubMed] [Google Scholar]
- 10.Morris HR, Janssen JC, Bandmann O, et al. The tau gene A0 polymorphism in progressive supranuclear palsy and related neurodegenerative diseases. J Neurol Neurosurg Psychiatry. 1999;66:665–667. doi: 10.1136/jnnp.66.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pastor P, Ezquerra M, Munoz E, et al. Significant association between the tau gene A0/A0 genotype and Parkinson’s disease. Ann Neurol. 2000;47:242–245. [PubMed] [Google Scholar]
- 12.Maraganore DM, Hernandez DG, Singleton AB, et al. Case-control study of the extended tau gene haplotype in Parkinson’s disease. Ann Neurol. 2001;50:658–661. doi: 10.1002/ana.1228. [DOI] [PubMed] [Google Scholar]
- 13.de Silva R, Hardy J, Crook J, et al. The tau locus is not significantly associated with pathologically confirmed sporadic Parkinson’s disease. Neurosci Lett. 2002;330:201–203. doi: 10.1016/s0304-3940(02)00742-5. [DOI] [PubMed] [Google Scholar]
- 14.Farrer M, Skipper L, Berg M, et al. The tau H1 haplotype is associated with Parkinson’s disease in the Norwegian population. Neurosci Lett. 2002;322:83–86. doi: 10.1016/s0304-3940(02)00106-4. [DOI] [PubMed] [Google Scholar]
- 15.Clark LN, Levy G, Tang MX, et al. The Saitohin ‘Q7R’ polymorphism and tau haplotype in multi-ethnic Alzheimer disease and Parkinson’s disease cohorts. Neurosci Lett. 2003;347:17–20. doi: 10.1016/s0304-3940(03)00635-9. [DOI] [PubMed] [Google Scholar]
- 16.Peplonska B, Zekanowski C, Religa D, et al. Strong association between Saitohin gene polymorphism and tau haplotype in the Polish population. Neurosci Lett. 2003;348:163–166. doi: 10.1016/s0304-3940(03)00788-2. [DOI] [PubMed] [Google Scholar]
- 17.Zappia M, Annesi G, Nicoletti G, et al. Association of tau gene polymorphism with Parkinson’s disease. Neurol Sci. 2003;24:223–224. doi: 10.1007/s10072-003-0141-z. [DOI] [PubMed] [Google Scholar]
- 18.Levecque C, Elbaz A, Clavel J, et al. Association of polymorphisms in the Tau and Saitohin genes with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:478–480. doi: 10.1136/jnnp.2003.015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwok JB, Teber ET, Loy C, et al. Tau haplotypes regulate transcription and are associated with Parkinson’s disease. Ann Neurol. 2004;55:329–334. doi: 10.1002/ana.10826. [DOI] [PubMed] [Google Scholar]
- 20.Healy DG, Abou-Sleiman PM, Lees AJ, et al. Tau gene and Parkinson’s disease: a case-control study and meta-analysis. J Neurol Neurosurg Psychiatry. 2004;75:962–965. doi: 10.1136/jnnp.2003.026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson A, Zetterberg H, Hakansson A, et al. TAU haplotype and the Saitohin Q7R gene polymorphism do not influence CSF Tau in Alzheimer’s disease and are not associated with frontotemporal dementia or Parkinson’s disease. Neurodegener Dis. 2005;2:28–35. doi: 10.1159/000086428. [DOI] [PubMed] [Google Scholar]
- 22.Fidani L, Kalinderi K, Bostantjopoulou S, et al. Association of the Tau haplotype with Parkinson’s disease in the Greek population. Mov Disord. 2006;21:1036–1039. doi: 10.1002/mds.20864. [DOI] [PubMed] [Google Scholar]
- 23.Fung HC, Xiromerisiou G, Gibbs JR, et al. Association of tau haplotype-tagging polymorphisms with Parkinson’s disease in diverse ethnic Parkinson’s disease cohorts. Neurodegener Dis. 2006;3:327–333. doi: 10.1159/000097301. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Song Y, Chen H, Fan D. The tau gene haplotype h1 confers a susceptibility to Parkinson’s disease. Eur Neurol. 2005;53:15–21. doi: 10.1159/000082956. [DOI] [PubMed] [Google Scholar]
- 25.Stefansson H, Helgason A, Thorleifsson G, et al. A common inversion under selection in Europeans. Nat Genet. 2005;37:129–137. doi: 10.1038/ng1508. [DOI] [PubMed] [Google Scholar]
- 26.Pittman AM, Myers AJ, Duckworth J, et al. The structure of the tau haplotype in controls and in progressive supranuclear palsy. Hum Mol Genet. 2004;13:1267–1274. doi: 10.1093/hmg/ddh138. [DOI] [PubMed] [Google Scholar]
- 27.Skipper L, Wilkes K, Toft M, et al. Linkage disequilibrium and association of MAPT H1 in Parkinson disease. Am J Hum Genet. 2004;75:669–677. doi: 10.1086/424492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myers AJ, Kaleem M, Marlowe L, et al. The H1c haplotype at the MAPT locus is associated with Alzheimer’s disease. Hum Mol Genet. 2005;14:2399–2404. doi: 10.1093/hmg/ddi241. [DOI] [PubMed] [Google Scholar]
- 29.Pittman AM, Myers AJ, Abou-Sleiman P, et al. Linkage disequilibrium fine mapping and haplotype association analysis of the tau gene in progressive supranuclear palsy and corticobasal degeneration. J Med Genet. 2005;42:837–846. doi: 10.1136/jmg.2005.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rademakers R, Melquist S, Cruts M, et al. High-density SNP haplotyping suggests altered regulation of tau gene expression in progressive supranuclear palsy. Hum Mol Genet. 2005;14:3281–3292. doi: 10.1093/hmg/ddi361. [DOI] [PubMed] [Google Scholar]
- 31.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altshuler D, Brooks LD, Chakravarti A, et al. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlson CS, Eberle MA, Rieder MJ, et al. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet. 2004;74:106–120. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin DY, Zeng D, Millikan R. Maximum likelihood estimation of haplotype effects and haplotype-environment interactions in association studies. Genet Epidemiol. 2005;29:299–312. doi: 10.1002/gepi.20098. [DOI] [PubMed] [Google Scholar]
- 35.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 36.Elbaz A, Nelson LM, Payami H, et al. Lack of replication of thirteen single-nucleotide polymorphisms implicated in Parkinson’s disease: a large-scale international study. Lancet Neurol. 2006;5:917–923. doi: 10.1016/S1474-4422(06)70579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Healy DG, Abou-Sleiman PM, Casas JP, et al. UCHL-1 is not a Parkinson’s disease susceptibility gene. Ann Neurol. 2006;59:627–633. doi: 10.1002/ana.20757. [DOI] [PubMed] [Google Scholar]
- 38.Borlak J, Reamon-Buettner SM. N-acetyltransferase 2 (NAT2) gene polymorphisms in Parkinson’s disease. BMC Med Genet. 2006;7:30. doi: 10.1186/1471-2350-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zintzaras E, Hadjigeorgiou GM. The role of G196A polymorphism in the brain-derived neurotrophic factor gene in the cause of Parkinson’s disease: a meta-analysis. J Hum Genet. 2005;50:560–566. doi: 10.1007/s10038-005-0295-z. [DOI] [PubMed] [Google Scholar]
- 40.Maraganore DM, de Andrade M, Elbaz A, et al. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA. 2006;296:661–670. doi: 10.1001/jama.296.6.661. [DOI] [PubMed] [Google Scholar]
- 41.Ioannidis JP, Trikalinos TA, Khoury MJ. Implications of small effect sizes of individual genetic variants on the design and interpretation of genetic association studies of complex diseases. Am J Epidemiol. 2006;164:609–614. doi: 10.1093/aje/kwj259. [DOI] [PubMed] [Google Scholar]
- 42.Service S, DeYoung J, Karayiorgou M, et al. Magnitude and distribution of linkage disequilibrium in population isolates and implications for genome-wide association studies. Nat Genet. 2006;38:556–560. doi: 10.1038/ng1770. [DOI] [PubMed] [Google Scholar]
- 43.Caffrey TM, Joachim C, Paracchini S, et al. Haplotype-specific expression of exon 10 at the human MAPT locus. Hum Mol Genet. 2006;15:3529–3537. doi: 10.1093/hmg/ddl429. [DOI] [PubMed] [Google Scholar]
- 44.Giasson BI, Forman MS, Higuchi M, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300:636–640. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.