Summary
Epidemiological studies indicate that overweight and obesity are associated with increased cancer risk. To study how obesity augments cancer risk and development, we focused on hepatocellular carcinoma (HCC), the common form of liver cancer whose occurrence and progression are the most strongly affected by obesity amongst all cancers. We now demonstrate that either dietary or genetic obesity is a potent bona fide liver tumor promoter in mice. Obesity-promoted HCC development was dependent on enhanced production of the tumor promoting cytokines IL-6 and TNF, which cause hepatic inflammation and activation of the oncogenic transcription factor STAT3. The chronic inflammatory response caused by obesity and enhanced production of IL-6 and TNF may also increase the risk of other cancers.
Introduction
Cancer development and progression are influenced by genetic, epigenetic and environmental factors. Epidemiological studies are particularly effective for identification of cancer risk factors but rarely elucidate the underlying mechanisms. For instance, a link between inflammation and cancer was first suggested by epidemiological studies but the underlying mechanisms were revealed through genetic and biochemical studies in appropriate mouse models (Johansson et al., 2008; Karin, 2006; Mantovani et al., 2008). Recently, a number of large scale epidemiological studies have pointed out that overweight and obesity, defined by body-mass-index (BMI) higher than 25, result in a substantial increase in cancer risk (Bianchini et al., 2002; Calle and Kaaks, 2004; Calle et al., 2003). It was pointed out that in men and women, respectively, excess body weight results in up to 1.52- or 1.62-fold increase in the relative risk of cancer-related death (Calle et al., 2003). The actual increase in risk is very much dependent on the type of cancer and the largest increase in cancer risk in individuals with high BMI was seen for hepatocellular carcinoma (HCC), the most common form of liver cancer. Men with BMI of 35-40 exhibited a staggering 4.52-fold increase in relative HCC risk (Calle et al., 2003). Even a modest elevation in BMI to the 25-30 range (grade 1 overweight) resulted in a significant increase in risk of death due to HCC, pancreatic cancer, cancers of the gastrointestinal (GI) tract and kidney cancer. Given the prevalence of overweight and obesity in the Western world [approximately two thirds of US adults are overweight or obese (Calle et al., 2003)] and their rapid increase in developing countries (there are about 300 million obese individuals worldwide), even a modest 1.5- to 1.6-fold increase in relative cancer risk represents a major public health problem. Indeed, more than 90,000 cancer-related deaths per year in the US alone are linked to excessive body weight (Calle et al., 2003).
Several mechanisms were proposed to explain how obesity increases cancer risk, including the prevalence of type II diabetes and insulin-resistance amongst obese individuals, which result in elevated circulating concentrations of insulin and insulin-like growth factor 1 (IGF-1), as well as increased production of sex steroids and cytokines by adipose tissue (Calle and Kaaks, 2004). However, these proposed mechanisms are based on correlative studies and have not been critically evaluated. Given the large impact of high BMI on HCC risk, we decided to investigate the mechanisms by which obesity promotes HCC development. Furthermore, an association between liver fat accumulation or hepatosteatosis and HCC development has been long known (Caldwell et al., 2004; El-Serag and Rudolph, 2007). Obesity-induced hepatosteatosis, together with its more severe complication nonalcoholic steatohepatitis (NASH), classified as nonalcoholic fatty liver disease (NAFLD) which affects up to 24% of the US population (Parekh and Anania, 2007), may match in their etiologic impact hepatitis C virus (HCV) infection, the major HCC risk factor in the US and Japan (Caldwell et al., 2004; El-Serag and Rudolph, 2007).
HCV infection and NAFLD, which can synergize to cause an even greater increase in HCC risk, can both lead to endoplasmic reticulum (ER) stress and accumulation of reactive oxygen species (ROS) within liver parenchymal cells resulting in chronic liver damage (Li et al., 2009; Parekh and Anania, 2007). It is thought that chronic liver injury that results in compensatory proliferation of differentiated hepatocytes, which are otherwise quiescent, is one of the major pathogenic mechanisms underlying HCC development (Bisgaard and Thorgeirsson, 1996; Fausto, 1999; Sakurai et al., 2006). The relevance of compensatory proliferation as a tumorigenic mechanism is supported by mouse genetic studies, using either the hepatic pro-carcinogen diethylnitrosamine (DEN) as an inducer of HCC (Hui et al., 2007; Maeda et al., 2005; Sakurai et al., 2008; Sakurai et al., 2006) or the conditional and complete inactivation of NF-κB signaling needed for maintenance of hepatocyte survival (Luedde et al., 2007).
We have used the DEN model to investigate whether dietary or genetic obesity is a direct promoter of HCC development and if so dissect its mechanism of action. We now show that obesity is a bona fide liver tumor promoter under conditions where DEN administration to lean mice does not result in HCC induction. We also demonstrate that one of the mechanisms that accounts for the tumor promoting effect of obesity is the low grade inflammatory response it induces (Hotamisligil, 2006; Shoelson et al., 2007), which results in elevated production of cytokines, such as TNF and IL-6, which was found to positively correlate with progression of chronic viral hepatitis to HCC in humans (Nakagawa et al., 2009; Wong et al., 2009). These cytokines are also required for propagation of steatohepatitis.
Results
Genetic and dietary obesity strongly promote DEN-induced hepatocarcinogenesis
Although obesity is a well established major HCC risk factor (El-Serag and Rudolph, 2007), critical mechanistic insights to its tumor promoting activity are currently lacking. To be able to employ genetic tools in our mechanistic analysis, we decided to conduct our work with mice rather than other rodents. Previous studies had revealed that hepatocyte-specific deletion of the IKK regulatory subunit NEMO/IKKγ results in spontaneous liver damage, hepatosteatosis, liver fibrosis and HCC development (Luedde et al., 2007). When placed on high fat diet (HFD), Nemo/IkkγΔhep mice exhibit the same protection from obesity-induced insulin resistance previously found in liver-specific IKKβ knock-out (IkkβΔhep) mice (Arkan et al., 2005), but display enhanced hepatosteatosis and elevated liver damage and eventually develop 4-times more HCCs than Nemo/IkkγΔhep mice kept on normal chow (Wunderlich et al., 2008). However, due to spontaneous liver damage and hepatosteatosis in Nemo/IkkγΔhep mice, it is difficult to determine whether obesity alone is a tumor promoter in this case. To address this issue more definitively, we decided to use wild type (WT) C57/BL6 mice which do not develop spontaneous liver disease or cancer but are susceptible to the hepatic procarcinogen DEN, which effectively induces HCC formation when given to 14-day old mice (Vesselinovitch and Mihailovich, 1983).
We administered DEN (25 mg/kg) to either male or female mice on postnatal day 14 and 4 wks later placed the mice on either normal chow (LFD) or high-fat diet (HFD), in which 60% of calories are fat-derived (protocol #1, Figure S1A). As expected, male mice maintained on HFD gained more weight than mice on LFD (Figure S1C) and their relative liver weight was increased (Figure S1D), as did liver triglycerides (TG) (Figure S1E). This was accompanied by increased serum TG (Figure S1F) and hepatosteatosis (Figure S1G). As expected, mice on HFD became glucose intolerant (Figure S1H). Importantly, when analyzed at 9 months of age, mice kept on HFD exhibited many more HCCs per liver than LFD-maintained mice (Figure 1A,B). Dietary obesity also increased tumor size and incidence. A similar effect was seen in female mice that were subjected to the same HCC induction protocol (Figure 1C). Due to their lower susceptibility to DEN-induced carcinogenesis (Naugler et al., 2007), female mice exhibited a greater enhancement of HCC incidence than male mice, although tumor multiplicity and its enhancement by obesity were lower in females. HFD increased body and liver weight as well as serum and liver TG also in female mice (Figure S1I-K), but the effects on liver weight and TG content were smaller than those seen in males.
Figure 1. Genetic and dietary obesity promote DEN-induced hepatocarcinogenesis.
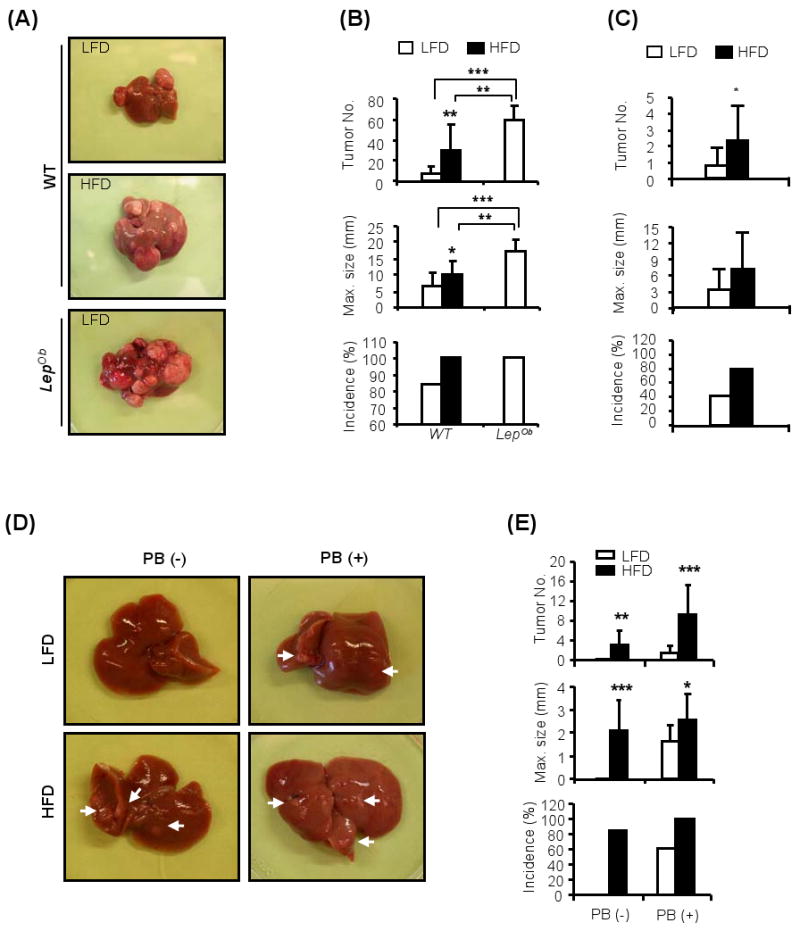
(A). Livers of male WT and LepOb mice kept on normal chow (LFD) or high fat diet (HFD) from week 6 to week 36 after the administration of DEN (25 mg/kg) at 2 weeks of age (protocol #1; Figure S1A).
(B). Tumor multiplicity, size and incidence in livers of DEN-injected WT and LepOb male mice kept on LFD □ or HFD ■ as above. Results are averages ± s.d. (n=10-12).
(C). Tumor multiplicity, size and incidence in livers of WT females that were given DEN at 2 weeks of age and fed as above. Results are averages ± s.d. (n=10-12).
(D). Livers of WT male mice given DEN (80 mg/kg) at 16 weeks of age and kept on LFD or HFD from week 6 to week 50 (protocol #2, Figure S1B). When indicated, phenobarbital (PB) was given as a tumor promoter in drinking water (0.05%) from 4 weeks after DEN administration until sacrifice. Arrows indicate HCCs.
(E). HCC multiplicity, size and incidence in WT male mice treated as in (D). Results are means ± s.d. (n=10-13). *P<0.05, **P<0.01, ***P<0.001 denote significant differences between the groups. See also Supplemental Figures S1 and S2.
To rule out the contribution of any potential carcinogens or tumor promoters that could be present in HFD but not in normal chow, we examined whether genetic obesity also enhances HCC induction and development. As leptin-deficient mice (LepOb) spontaneously develop obesity even when kept on normal chow (Friedman et al., 1991; Pelleymounter et al., 1995), we used them as a model for genetic obesity. We administrated 25 mg/kg DEN to 2 weeks-old LepOb male mice and found that they exhibited greatly enhanced HCC development relative to WT mice kept on LFD (Figure 1A,B). These results strongly suggest that it is either obesity per se or hepatosteatosis that enhances HCC induction and development rather than presence of unknown carcinogens or tumor promoters in the particular HFD we used.
We next examined whether dietary obesity functions as a tumor promoter using conditions under which lean WT mice do not develop any liver tumors when given DEN. We took advantage of the earlier observation that when DEN administration is delayed until 4-6 weeks of age, it fails to induce HCC, even in males, unless combined with a tumor promoter, such as phenobarbital (PB) (Ward et al., 1986). We kept WT male mice on either LFD or HFD for 10 weeks prior to DEN administration at 16 weeks of age (protocol #2, Figure S1B). As expected, HFD consumption initially resulted in weight gain and led to elevated serum TG (Figure S2A,B). However, immediately after DEN administration, HFD-kept mice lost weight but regained it a few weeks later. This sudden weight loss is likely to be due to enhanced DEN-induced liver toxicity in obese mice, first noted when HFD-kept mice were given the standard carcinogenic dose of 100 mg/kg DEN, which is not toxic in lean mice. Under these conditions, all of the obese mice died within 13 days after DEN administration (Figure S2C). Death was likely to have been caused by liver failure as indicated by histological analysis (Figure S2D), TUNEL staining (Figure S2E) and massive release of the liver enzyme, alanine aminotransferase (ALT) into the circulation (Figure S2F). Obese mice also exhibited enhanced DEN-induced ROS accumulation (Figure S2G). We therefore reduced the dose of DEN used in this protocol (protocol #2, Figure S1B) to 80 mg/kg. At this dose, despite initial weight loss (Figure S2A), no lethality was observed in the HFD group. Remarkably, close to 90% of mice kept on HFD developed HCC without PB administration (Figure 1D,E). By contrast, and as expected, mice kept on LFD did not develop any HCC unless treated with PB after DEN administration. Furthermore, the tumor promoting effect of dietary obesity was at least as strong as that of PB, a bona fide liver tumor promoter (Ward et al., 1986).
Obesity is associated with increased cancer cell proliferation
Enhanced DEN-induced hepatocyte death, as observed in IKKβ- and p38α- liver specific knockout mice (IkkβΔhep, p38αΔhep) or spontaneous liver damage in Nemo/IkkγΔhep mice is associated with enhanced compensatory proliferation and augmented HCC development (Hui et al., 2007; Luedde et al., 2007; Maeda et al., 2005; Sakurai et al., 2008). As mentioned above, DEN administration to obese mice induced more liver damage than in lean mice. Nonetheless, HCCs in obese mice exhibited reduced apoptotic cell death relative to HCCs in lean mice (Figure 2A,B). Concurrently, HCCs in obese mice exhibited more proliferating cells than HCCs in lean mice (Figure 2C,D), as well as elevated cyclin D1 mRNA expression (Figure 2E). Thus, despite the early increase in both DEN-induced apoptosis and compensatory proliferation in obese mice given DEN at 16 weeks of age (Figure 2F), the long term effect of obesity on HCC cell kinetics is to decrease cell death and enhance cell proliferation. These findings suggest that alterations in signal transduction pathways that modulate hepatoma cell proliferation independently of liver damage and compensatory proliferation may underlie the tumor promoting effect of obesity especially at later time points.
Figure 2. Obesity in HCC-bearing mice is associated with increased cancer cell proliferation and reduced apoptosis.
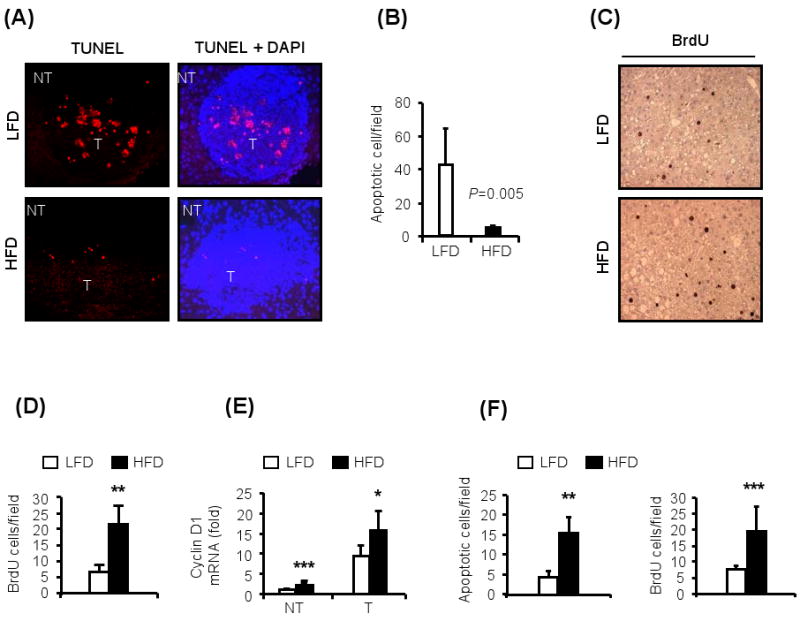
HCC-bearing (protocol #1) male mice were subjected to histological and biochemical analyses at 9 months of age.
(A). Obesity reduces cell death in HCCs. Paraffin-embedded liver sections were TUNEL labeled (left panels) and counter stained with DAPI (right panels). Note the presence of apoptotic cells in tumor (T) area in lean mice. Magnification: 20×.
(B). Numbers of apoptotic cells per field in (A) was determined by Image Tool software (n=5).
(C). Obesity enhanced cell proliferation in HCCs. Mice were BrdU pulsed 2 hrs prior to sacrifice. Paraffin embedded liver sections were stained with anti-BrdU antibody.
(D). Numbers of BrdU-positive proliferating cells in (C) was determined with Image Tool software (n=5).
(E). Expression of cyclin D1 mRNA in non-tumor liver (NT) and tumors (T) was determined by qRT-PCR (n=5-6). Values were normalized to cyclophilin mRNA.
(F). Mice kept on LFD or HFD for 10 wks were injected with DEN (100 mg/kg) and their livers were isolated 48 hrs after DEN administration and 2 hrs after BrdU pulse. Paraffin embedded liver sections were subjected to TUNEL and BrdU staining and positive cells were counted as above. (n=5).
All values represent means ± s.d. *P<0.05, **P<0.01, ***P<0.001 denote significant differences between the groups.
To further examine this notion and separate effects of obesity on tumor growth and progression from effects on tumor initiation, we transplanted established hepatoma cells (derived from a DEN-induced HCC) into 2 months-old lean mice that were kept after tumor cell inoculation on either LFD or HFD for 4 weeks. We also inoculated the same number of hepatoma cells into 8 months-old mice that were kept on HFD for the preceding 6 months or were genetically obese (LepOb). In all cases, tumor growth was monitored over the course of 4 weeks after inoculation. The greater the degree of host obesity, the faster the tumors grew, reaching the largest size in LepOb mice (Figure 3). We also treated some of the mice with the JAK inhibitor AG490 (Eriksen et al., 2001) to inhibit STAT3 activation (see below). AG490 exerted a stronger inhibitory effect on tumor growth in 2 months old mice kept on HFD than in mice kept on LFD (Figure 3B,C) and as expected inhibited STAT3 phosphorylation (Figure 3D).
Figure 3. Obesity promotes tumorigenic growth of hepatoma cells.
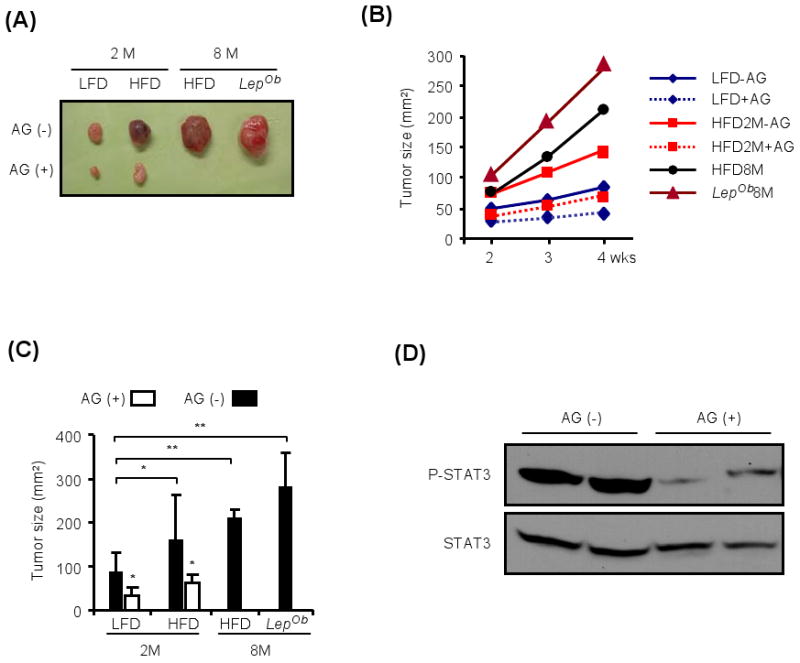
(A). Two months old lean mice (2 M) were subcutaneously injected with established hepatoma cells (2.5×106 cells/mouse) and kept on LFD or HFD for 4 weeks after inoculation with (AG+) or without (AG-) AG490 (0.5 mg/mouse/day), a JAK inhibitor that blocks STAT3 activation. The same number of hepatoma cells was also transplanted into 8 months-old mice that were kept on HFD for the preceding 6 months or were genetically obese (LepOb). Tumor growth was monitored by measurement with a caliper once a week, from week 2 to week 4. The images show representative solid tumors dissected at week 4.
(B). Tumor growth measured over the course of a 2 week period in mice described in (A). The results are means for 3-6 mice per group.
(C). Tumor sizes at week 4 post-inoculation are shown as a histogram. Results are means ± s.d. (n=3-6).
(D). Tumors grown in mice given HFD without (AG-) or with (AG+) AG490, administered as above, were collected at week 4 post-inoculation, homogenized and STAT3 phosphorylation and expression were analyzed by immunoblotting.
All values represent means ± s.d. *P<0.05, **P<0.01 denote significant differences between the groups.
Obese HCC-bearing mice exhibit elevated STAT3 and ERK activation and liver inflammation
To identify signaling pathways responsible for enhanced hepatoma cell survival and proliferation, we first examined the effect of obesity on several protein kinases known to be affected by metabolic state. As expected (Tremblay et al., 2007; Um et al., 2004), obesity was associated with decreased AKT phosphorylation and increased phosphorylation of the mTOR target S6 kinase and its substrate ribosomal protein S6 (Figure 4A). This was observed in both normal livers and HCCs, strongly suggesting that counter to a previous hypothesis (Calle and Kaaks, 2004), AKT activation, driven by insulin or IGF-1, is unlikely to be responsible for enhanced cell survival and proliferation in HCCs of obese individuals.
Figure 4. Obese HCC-bearing mice exhibit activated STAT3 and elevated expression of inflammatory cytokines.
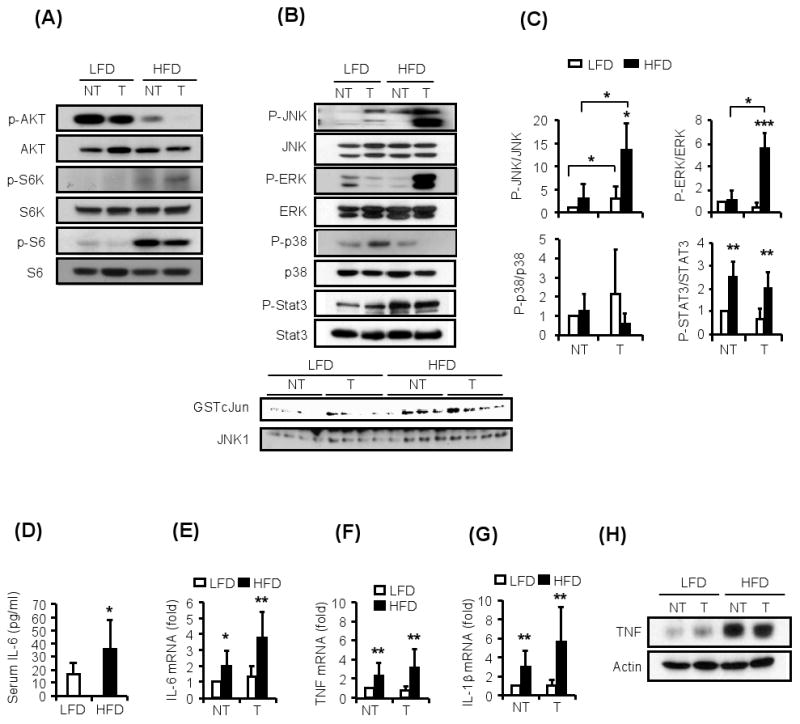
The HCC-bearing mice from Figure 2 were subjected to additional biochemical and gene expression analyses.
(A). Activation state of metabolically-regulated protein kinases. Non-tumor (NT) liver and HCC (T) were lysed, gel separated and the activation (phosphorylation) states of AKT and S6 kinase (S6K) were examined by immunoblotting.
(B). Non-tumor (NT) liver and HCC (T) were analyzed for phosphorylation of JNK, ERK, p38, and STAT3 by immunoblotting as above. The bottom panel shows JNK kinase assays in non-tumor liver and HCCs of 4 different mice per dietary group.
(C). The results of several immunoblots similar to the one shown in B, each representing a different mouse, were quantitated using Image J software and mean values were determined (n=3-4).
(D). Serum IL-6 in HCC-bearing mice was determined by ELISA (n=10-12).
(E-G). Relative amounts of IL-6 (E), TNF (F) and IL-1β (G) mRNAs in non-tumor (NT) liver and HCC (T) were determined by qRT-PCR and normalized to cyclophilin mRNA (n=6-8).
(H). TNF amounts in non-tumor liver (NT) and HCC (T) were examined by immunoblotting of tissue lysates.
All values represent means ± s.d. *P<0.05, **P<0.01, ***P<0.001 denote significant differences between the groups. See also Supplemental Figure S3.
We next examined the effect of obesity on several signaling molecules affected by inflammation and known to modulate HCC development (Hui et al., 2007; Maeda et al., 2005; Sakurai et al., 2008; Sakurai et al., 2006). Consistent with previous findings (Hirosumi et al., 2002; Solinas et al., 2007), obese mice exhibited elevated JNK activity in liver and even a larger increase in JNK phosphorylation in HCCs (Figure 4B,C). HCCs in obese mice also exhibited greatly elevated ERK phosphorylation relative to HCCs of lean mice, but p38 phosphorylation was not altered in obese liver and was even reduced in HCCs of obese mice (Figure 4B,C). Remarkably, both non-tumor liver tissue and HCCs from obese mice displayed a substantial increase in STAT3 phosphorylation, indicative of activation of this oncogenic transcription factor (Figure 4B,C).
Consistent with the increase in STAT3 activation, we found that obese mice exhibited elevated circulating IL-6 (Figure 4D), a potent STAT3 activating cytokine (Kamimura et al., 2003). Elevated IL-6 mRNA was observed in both non-tumor liver and HCCs of obese mice (Figure 4E). Obesity also enhanced expression of TNF and IL-1β mRNAs (Figure 4F,G). The amount of TNF protein was also greatly elevated in normal liver and HCC from obese mice (Figure 4H). Elevated IL-6, TNF and IL-1β expression was also observed in obese female tumor-bearing mice (Figure S3A-D). Consistent with elevated inflammatory cytokines, livers from obese mice contained higher amounts of macrophages and other leukocytes (Figure S3E-I).
Enhanced IL-6 production is required for obesity-induced tumor promotion
Given the marked increase in STAT3 phosphorylation and circulating IL-6 in obese mice and their tumors, and knowing that IL-6 functions as a downstream mediator for both IL-1 and TNF (Kamimura et al., 2003), we examined whether IL-6 is an important component of the tumor promoting mechanism activated by obesity. To this end, we subjected WT and IL6-/- mice to protocol #1 (Figure S1A), in which DEN is given to two weeks-old mice followed by either LFD or HFD. Remarkably, IL6-/- male mice, which developed much fewer HCCs than WT mice when kept on LFD, an observation consistent with previous findings (Naugler et al., 2007), hardly exhibited any augmentation of tumor multiplicity, size and incidence when placed on HFD (Figure 5A,B). Importantly, the HCC load in IL6-/- male mice is identical to that of WT females (Naugler et al., 2007), but unlike WT females which produce more IL-6 when rendered obese (Figure S3A, B) and develop more HCC, no significant increase in tumor load was seen in obese IL6-/- males (Figure 5C). Although IL6-/- males did not gain weight as rapidly as WT males, they reached almost the same weight as WT mice after 8 months on HFD (Figure 5D) and exhibited elevated serum TG (Figure 5E). IL6-/- males also showed increased liver TG content after placement on HFD, although the extent of liver TG accumulation was lower than in WT males. IL6-/- male mice also presented with elevated serum insulin after placement on HFD, but the increase was not as robust as in obese WT males (Figure 5E).
Figure 5. Enhanced IL-6 production is required for obesity-induced tumor promotion.
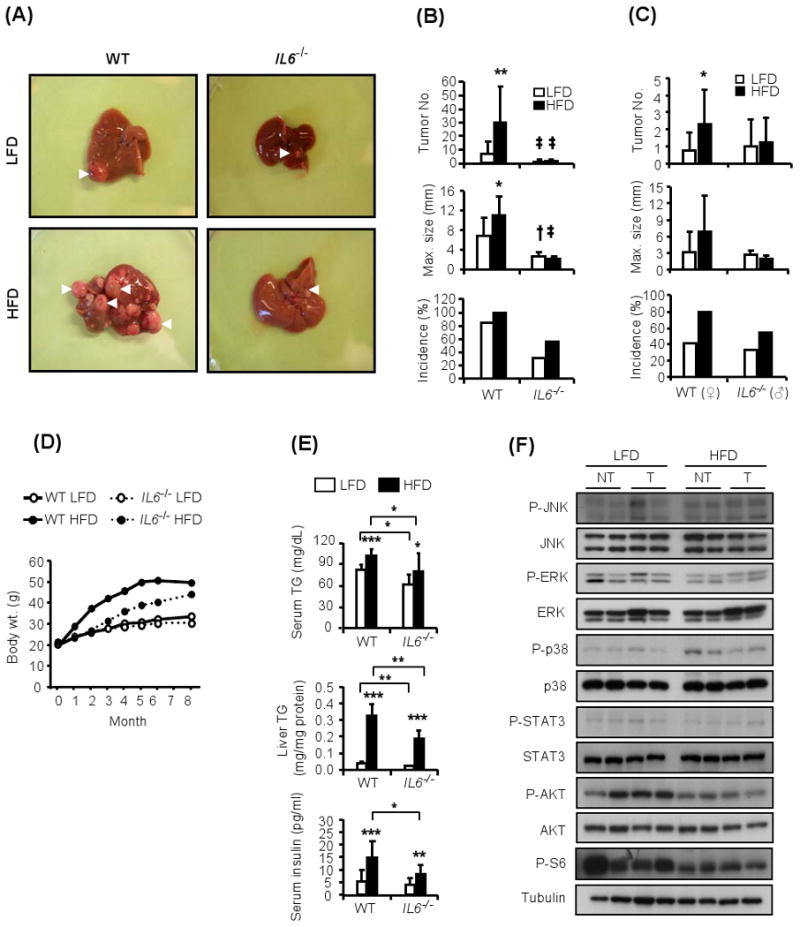
(A). Livers of WT and IL-6 deficient (IL6-/-) male mice that were kept on LFD or HFD after the administration of DEN (25 mg/kg) at 14 days of age.
(B). HCC multiplicity, size, and incidence (n=10-12) in DEN-administered male mice of the indicated genotype kept on either LFD □ or HFD ■.
(C). HCC multiplicity, size, and incidence (n=10-12) in DEN-administered WT females or IL-6-/- males of the indicated genotype kept on either LFD □ or HFD ■.
(D). Body weight gain during tumor development in WT and IL-6-/- male mice kept on LFD or HFD after DEN administration.
(E). Serum and liver triglycerides (TG) in mice from (D) were determined by a colorimetric assay at 9 months of age. Serum insulin was determined by ELISA at same age (serum, n=8-12; liver, n=5).
(F). HCCs (T) and non-tumor (NT) liver tissue from IL6-/- male mice kept on either LFD or HFD were isolated, homogenized and subject to immunoblot analysis to determine the phosphorylation state of the indicated proteins. Shown are the results from two separate tumors for each condition.
All values are means ± s.d. *P<0.05, **P<0.01, ***P<0.001 denote significant differences between the groups. †P<0.05, ‡P<0.01 presents significant differences from WT mice. See also Supplemental Figure S4.
These data suggest that obesity-associated chronic elevation in IL-6, a tumor promoting cytokine whose expression can be induced by both TNF and IL-1, is an important contributing factor to liver tumorigenesis.
To better understand how the absence of IL-6 prevents obesity-induced tumor promotion, we examined its effect on stress/inflammation- and metabolism-responsive signaling molecules. The absence of IL-6 prevented the obesity-induced increase in JNK and ERK phosphorylation in non-tumor liver and HCCs and completely reversed the decrease in p38 phosphorylation previously seen in HCCs of obese mice (Figures 5F and S4A; compare to Figures 4B and C). As expected, the absence of IL-6 prevented STAT3 phosphorylation in liver and HCC of obese mice (Figures 5F and S4A). Interestingly, the IL-6 deficiency also prevented much of the obesity induced increase in S6 phosphorylation in both liver and HCCs and partially attenuated the decrease in AKT phosphorylation (Figures 5F and S4A). These finding suggest that IL-6 is not only required for STAT3, ERK and JNK activation in the obese liver but that it also contributes (indirectly, see below) to the alterations in AKT and mTOR-S6K signaling.
TNF receptor signaling is required for obesity-induced tumor promotion
In addition to IL-6, HFD increases expression of TNF, a pro-inflammatory cytokine that stimulates IL-6 production. We previously found that TNF signaling via its type 1 receptor, TNFR1, is not required for DEN-induced hepatocarcinogenesis in lean mice (Naugler et al., 2007). Nonetheless, given the dramatic increase in liver TNF production in obese mice (Figure 4H), we examined whether TNFR1 signaling contributes to obesity-induced tumor promotion. Remarkably, ablation of TNFR1 almost completely abolished obesity-enhanced HCC development without affecting HCC induction in lean mice (Figure 6A,B). TNFR1-/- mice, however, gained almost as much weight as WT mice (Figure 6C). The rate of weight gain was also very similar in the two strains.
Figure 6. TNFR1 signaling is required for obesity-induced tumor promotion.
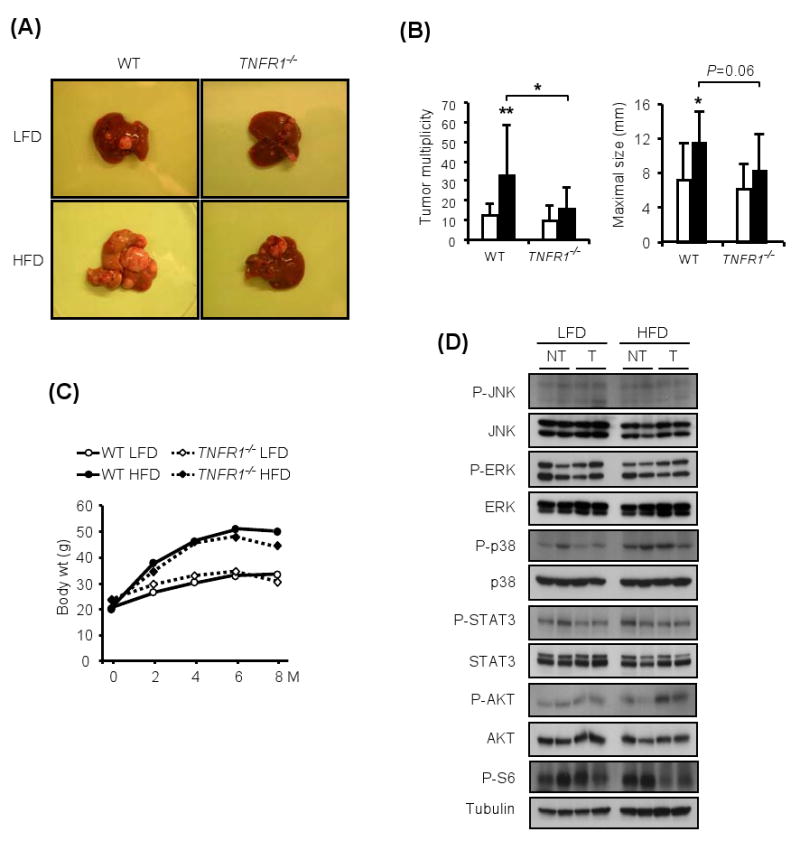
(A). Livers of WT or TNFR1 deficient male mice kept on LFD or HFD after DEN administration (25 mg/kg) at 14 days of age.
(B). Tumor multiplicity and maximal sizes in DEN-administered male mice of the indicated genotypes kept on either LFD □ or HFD ■ (n=10-13).
(C). Body weight gain during tumor development in WT or TNFR1-/- male mice kept on LFD or HFD after DEN administration.
(D). Non-tumor (NT) liver and HCC (T) from the mice described in (A) were isolated, lysed, gel separated and the phosphorylation states of the indicated proteins were examined by immunoblotting. Shown are the results for two separate HCCs per condition.
Ablation of TNFR1 reduced JNK and p38 phosphorylation in both lean and obese mouse livers and prevented the increase in JNK activity associated with HCC development (Figures 6D and S4B). In addition, TNFR1-/- mice did not show the obesity-induced increase in STAT3 and ERK phosphorylation. TNFR1 ablation also prevented the decrease in AKT phosphorylation seen in HFD-fed WT mice and attenuated the changes in S6 phosphorylation (Figures 6D and S4B).
TNFR1 signaling and IL-6 promote steatohepatitis
We further examined IL6-/- and TNFR1-/- tumor-bearing mice to identify a common mechanism that could explain their resistance to obesity-induced tumor promotion. As expected, the absence of TNFR1 reduced the obesity-induced increase in IL-6 production, resulting in lower circulating IL-6 and less IL-6 mRNA in liver and in HCCs of obese mice (Figure 7A,B). Conversely, the absence of IL-6 reduced the obesity-induced increase in TNF production in tumor-bearing mice (Figure 7C). The absence of either IL-6 or TNFR1 in tumor-bearing mice reduced HFD-induced liver lipid accumulation (Figure 7D,E). The absence of either IL-6 or TNFR1 also reduced macrophage and neutrophil accumulation in livers of HFD-fed mice (Figure 7F,G). We therefore conclude that both IL-6 and TNF signaling via TNFR1 are important for liver lipid accumulation (hepatosteatosis) and fat-induced liver inflammation (steatohepatitis), which together define NAFLD, a condition that greatly increases the risk of HCC development (El-Serag and Rudolph, 2007; Parekh and Anania, 2007).
Figure 7. TNFR1 signaling and IL-6 support steatohepatitis.
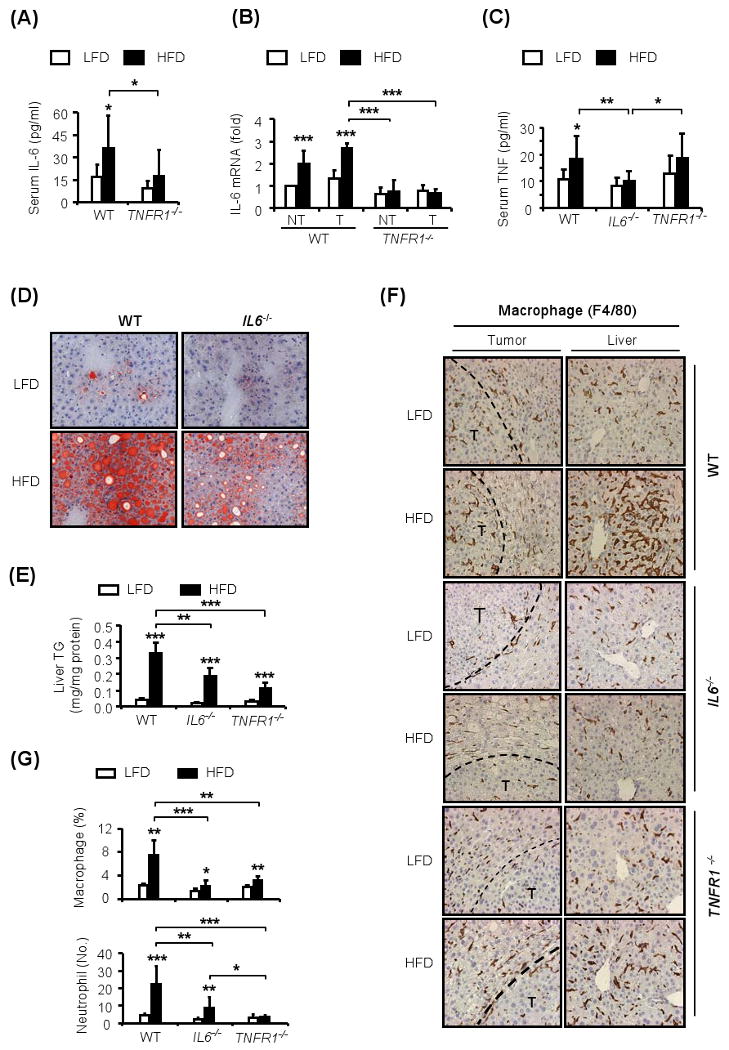
(A and C). Serum IL-6 (A) and TNF (C) were measured by ELISA in HCC bearing WT, IL6-/- or TNFR1-/- mice kept on LFD □ or HFD ■. The analyses were done at 9 months of age (n=8-10).
(B). IL-6 mRNA amounts in HCC-bearing livers and HCCs from WT or TNFR1-/- mice kept on HFD or LFD were measured by qRT-PCR at 9 months of age. The bars depict the relative increase in normalized mRNA amount, with the amount in non-tumor (NT) liver of lean mice given an arbitrary value of 1.0 (n=5-6).
(D). Liver fat accumulation in HCC-bearing WT and IL6-/- mice kept on LFD or HFD was examined by oil red O (ORO) staining of liver sections collected at 9 months of age (magnification: 40×).
(E). Liver TG levels in HCC-bearing WT, IL6-/- or TNFR1-/- mice were determined at 9 months of age.
(F-G). Macrophage (F and G) and neutrophil (G) infiltration in non-tumor liver and HCCs of lean and obese 9 months old WT, IL6-/- and TNFR1-/- male mice. Paraffin embedded sections were stained with F4/80 and Gr-1 specific antibodies to visualize macrophages and neutrophils, respectively. Results from F4/80 staining are shown in (F). Data from several experiments (n=4-5) were quantitated with Image J software and were averaged (G).
All values represent means ± s.d. *P<0.05, **P<0.01, **P<0.001 denote significant differences between the groups.
Discussion
Overweight and obesity greatly increase HCC risk, especially in men (Calle and Kaaks, 2004; Calle et al., 2003). Despite the magnitude of the effect and the very large number (900,000) of individuals on which the epidemiological study that provided this insight was based, the mechanisms by which obesity increases risk of death from HCC and other cancers remained unknown. We now describe that genetic or dietary obesity greatly enhances the development of chemically induced HCC in laboratory mice. By administering a chemical carcinogen (DEN) to adult mice in which it fails to induce HCC on its own, we demonstrated that obesity is a bona fide tumor promoter, whose effect is at least as strong as that of the validated liver tumor promoter phenobarbital. Furthermore, we found that the tumor promoting effect of obesity in HCC depends to a large extent on the low grade inflammatory response it induces, which involves elevated production of TNF and IL-6, both of which are tumor promoting cytokines (Lin and Karin, 2007).
While our work was being completed, it was described that consumption of HFD resulted in increased formation of pre-neoplastic lesions in response to DEN administration in rats (Wang et al., 2009). However, these rats were not evaluated for HCC and the mechanism underlying increased formation of pre-neoplastic lesions was not determined. Another recent study using Nemo/IkkγΔhep mice, that spontaneously develop liver damage, hepatosteatosis and HCC on a normal diet (Luedde et al., 2007), found that consumption of HFD enhanced the development of hepatosteatosis and ensuing liver damage and accelerated the appearance of liver tumors (Wunderlich et al., 2008). However, the pre-existing hepatosteatosis and spontaneous liver damage in Nemo/IkkγΔhep mice that are kept on LFD prevented the conclusive demonstration that obesity per se rather than chronic liver damage caused by the absence of NF-κB is responsible for tumor promotion. Furthermore, the mechanism by which obesity accelerates HCC development in these mice was not determined. Enhanced liver tumorigenesis is not unique to Nemo/IkkγΔhep mice, and was also seen upon DEN administration to IkkβΔhep and p38αΔhep mice, which do not manifest spontaneous liver damage or hepatosteatosis (Hui et al., 2007; Maeda et al., 2005; Sakurai et al., 2008). It was proposed that enhanced liver damage driven by ROS accumulation, which gives rise to compensatory proliferation of initiated hepatocytes, may explain augmented HCC development in all of these mouse strains, which are more susceptible to DEN-induced hepatotoxicity (Sakurai et al., 2006; 2008). Enhanced liver damage is also seen in mice that have been rendered obese by consumption of HFD for 10 weeks and then challenged with DEN. This elevated hepatotoxic response may contribute to the tumor promoting effect of obesity. We also found that obese mice accumulate more ROS both in non-tumor liver tissue and in HCCs (Figure S2G). However, this persistent ROS accumulation, which is also seen during chronic HCV infection (Wang and Weinman, 2006), does not lead to increased cell death in HCCs of obese mice. By contrast, HCCs in obese mice display substantially lower apoptotic rates than HCCs in lean mice (Figure 2A). Despite the reduction in apoptosis, HCCs in obese mice contain more proliferating cells (Figure 2C,D), suggesting that enhanced compensatory proliferation driven by hepatocyte death does not account for mid to late tumor promotion in obese mice. Instead, mid to late tumor promotion in obese mice correlates with enhanced proliferation and reduced death of malignant hepatoma cells. Indeed, fully initiated hepatoma cells exhibit higher tumor growth rates when transplanted into obese mice and this accelerated tumor growth is slowed down by administration of a JAK inhibitor that prevents STAT3 activation. Notably, IL-6 signaling through ERK and STAT3 can reduce epithelial cell apoptosis while stimulating cell proliferation, as recently found in colitis associated cancer (Grivennikov et al., 2009).
A survey of different signaling molecules in human HCC revealed frequent activation of ERK and STAT3, especially in HCCs with poor prognosis, as well as reduced p38 activation, relative to the surrounding liver tissue (Calvisi et al., 2006). Similar changes were observed in HCCs of WT obese mice (Figure 4A-C). We have also confirmed frequent STAT3 activation in human HCC and using liver-specific STAT3 knockout mice (Stat3Δhep), found that this major transcriptional target for IL-6 is required for HCC development (He et al., submitted). Furthermore, expression of IL-6, a major STAT3 activator in the liver, is elevated in cirrhosis and HCC (Tilg et al., 1992; Trikha et al., 2003). Elevated serum IL-6 also correlates with a higher risk of progression from chronic viral hepatitis to HCC (Nakagawa et al., 2009; Wong et al., 2009). A role for IL-6 in HCC development was first suggested by our findings that differential IL-6 production following DEN-induced liver damage accounts for the gender bias in HCC induction; female mice produce two-fold less IL-6 after DEN challenge than males and therefore develop less HCC (Naugler et al., 2007). Here we show that IL6-/- male mice no longer respond to the tumor promoting effect of obesity but female mice which can produce more IL-6 when placed HFD, are susceptible to obesity-induced tumor promotion, even though their basal HCC induction rate is very low and similar to that of IL-6-deficient males. Furthermore, inactivation of TNFR1, a receptor whose engagement by TNF contributes to obesity-induced IL-6 production (Figure 7A,B), prevents obesity-promoted hepatocarcinogenesis without affecting DEN-induced hepatocarcinogenesis in lean mice.
Although TNF and IL-6 may not be the only mediators responsible for obesity-induced tumor promotion, it is well-established that their production is elevated in obese mice due to the low grade inflammatory response caused by lipid accumulation (Arkan et al., 2005; Hotamisligil, 2006; Shoelson et al., 2007; Solinas et al., 2007). In humans, IL-6 is thought to be produced by adipose tissue during non-inflammatory conditions and is therefore considered to be an adipokine (Fried et al., 1998; Mohamed-Ali et al., 1997). Not surprisingly, IL-6 in serum shows positive correlation with BMI (Bastard et al., 2000). IL-6 is also produced by Kupffer cells where it is induced in response to hepatocyte death, which results in IL-1 release (Sakurai et al., 2008). Not only IL-1, but TNF, whose production is elevated in the obese liver, also leads to IL-6 production and our results reveal a substantial reduction in obesity-induced IL-6 in TNFR1-/- mice. Regardless of its source, it is established that IL-6 can propagate inflammation (Kamimura et al., 2003) and stimulate triglyceride secretion (Nonogaki et al., 1995). However, the complete absence of IL-6 in mice was reported to have controversial effects on adiposity. Whereas one study found that IL6-/- mice became spontaneously obese as they got older (Wallenius et al., 2002), another study failed to find any basal alteration in body weight and fat deposition in IL6-/- mice kept on LFD (Di Gregorio et al., 2004). When placed on HFD, IL6-/- mice were found to gain weight slower than WT mice, but eventually did become obese (Di Gregorio et al., 2004) as seen in our study (Figure 5D). Thus the absence of obesity-induced tumor promotion in IL6-/- mice is not due to their failure to gain weight. Importantly, however, while total body weight and serum triglycerides are still elevated, liver lipid accumulation was substantially reduced in both IL6-/- and TNFR1-/- mice (Figure 7D,E). Thus, both TNF and IL-6 contribute to the maintenance of hepatosteatosis. Furthermore, the absence of TNFR1 or IL-6 prevented steatohepatitis, resulting in much lower amounts of HFD-induced macrophage and neutrophil accumulation in the liver relative to WT mice (Figure 7F,G). In humans, steatohepatitis is the more severe manifestation of NAFLD and it, rather than insulin-resistance, is likely to be the major contributor to obesity-induced tumor promotion in the liver.
In addition to propagation of steatohepatitis, IL-6 leads to STAT3 activation in hepatocytes (Naugler et al., 2007) and STAT3 can stimulate the proliferation and progression of initiated hepatocytes (He et al., submitted) and thus could be a molecular mediator of tumor promotion. Given the involvement of STAT3 in several other cancers (Bromberg and Wang, 2009) and the elevation in circulating IL-6 in obese and overweight individuals (Bastard et al., 2000), it is likely that IL-6 and STAT3 may contribute to the general enhancement of cancer risk by high BMI. However, obesity, elevated IL-6 and activated STAT3 are unlikely to cause cancer on their own. Even genetic alterations that result in constitutive STAT3 activation in hepatocytes only cause benign hepatic adenomas, unless combined with oncogenic mutations (Rebouissou et al., 2009). Most likely, chronic activation of the IL-6-STAT3 axis increases the likelihood of expansion and further progression of transformed hepatocytes that have acquired oncogenic mutations upon exposure to environmental and dietary carcinogens. Notably, most xenobiotics are metabolized within the liver, but sometimes this metabolism results in the activation, rather than elimination, of pro-carcinogens (Sheweita and Tilmisany, 2003).
IL-6 induced STAT3 activation, however, is unlikely to be the only tumor promoting mechanism stimulated by obesity. It was suggested that another tumor promoting mechanism triggered by obesity is enhanced AKT activation caused by the high concentrations of circulating insulin and IGF-1 in overweight and obese individuals (Calle and Kaaks, 2004). However, obesity results in insulin resistance and actually leads to inhibition of AKT activation as shown above. Thus AKT activation is unlikely to contribute to enhanced HCC development in obese mice. A more relevant player whose contribution merits further and dedicated studies is mTOR, whose activity is elevated in the obese liver (Figure 4A), and is known to be an important regulator of cell and tumor growth (Guertin and Sabatini, 2007). The mouse models described above should be suitable for such studies.
Experimental Procedures
Mice, diet, tumor induction and analysis
IL-6 deficient (IL6-/-), TNFR1 deficient (TNFR1-/-) and leptin deficient (LepOb) mice on a C57BL/6 background, were used along with corresponding age-matched WT mice. All mice were maintained in filter topped cages on autoclaved regular chow diet (low-fat diet; LFD, composed of 12%-fat, 23%-protein, 65%-carbohydrates based on caloric content) or high-fat diet (HFD, composed of 59%-fat, 15%-protein, 26%-carbohydrates based on caloric content; BioServ) and water at UCSD according to NIH Guidelines.
In the first DEN-induced HCC model (Figure S1A, protocol #1), DEN (25 mg/kg) was injected intraperitoneally into 14 days old mice. After 4 weeks, mice were separated into two dietary groups and fed either LFD or HFD until sacrificed. In the second HCC model (Figure S1B, protocol #2), mice were fed either LFD or HFD for 10 weeks prior to DEN administration (i.p., 80 mg/kg) at 16 weeks of age, until sacrificed. Four weeks after DEN administration, mice received water with or without PB (0.05%, Sigma) until sacrificed. Serum insulin, IL-6 and TNF were measured by ELISA (eBioscience) and ALT was detected using Biotron kit. Tumors in each liver lobe were counted and measured with a caliper. Tumor and non-tumor tissue was collected and rapidly frozen for biochemical, histological and immunochemical analysis. In the HCC induction studies, mice were not treated with any hormone or drug except when indicated (PB administration). In the transplant study (Figure 3), mice did not receive any drug or hormone other than AG490 (LC Labs) when indicated.
Histological, immunohistochemical and immunofluorescent assay
Liver tissue was fixed in 10% formalin or embedded in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA) for paraffin and frozen block preparation, respectively. Paraffin embedded liver tissue was used for H&E, TUNEL (Roche), and immunohistochemical staining as described (Sakurai et al., 2008). TUNEL and BrdU positive cells were quantified using Image tool. Frozen tissue sections were stained with oil-red O (ORO) for lipid detection and dihydroethidine (DHE) to detect ROS accumulation (Maeda et al., 2005).
Triglyceride (TG), insulin and glucose measurement
Mice were fasted for 6 hrs after which their blood was analyzed for insulin (ELISA: Crystal Chem Inc) and TG content (calorimetric assay: Diagnostic Chemicals Limited). Liver TG was extracted in chloroform/methanol (2:1, v/v) (Folch et al., 1957) and measured as above. For glucose tolerance test, mice were injected with glucose (2 g/kg, i.p.) after 6 hrs of fasting. Blood glucose was measured using glucose meter (LifeScan).
Real-time quantitative PCR, immunoblotting and kinase assays
Total liver and HCC RNA was extracted with RNase Plus kit (Qiagen) and cDNA was synthesized using SuperScript® II kit (Invitrogen). Real-time PCR was performed using SYBR green (Bio-Rad) on a Bio-Rad iQ5 machine. Values were normalized to cyclophilin mRNA amount. Liver lysates prepared in standard RIPA buffer were gel separated and proteins were transferred to PVDF membranes (Whatman). Membranes were probed with primary antibodies and secondary HRP-conjugated antibodies (GE Healthcare UK), and developed using ECL detection reagent (Pierce). Protein band intensity was quantified using Image J software. JNK immunocomplex kinase assay was performed using GST-cJun(1-79) as a substrate and a JNK1/2 antibody (Pharmingen) as described (Solinas et al., 2007).
Statistical analysis
Data are presented as means ± standard deviation. Statistical significance was calculated with a Student's t-test. Significance was accepted at the level of P < 0.05 (*), P < 0.01 (**) or P < 0.001 (***).
Supplementary Material
Acknowledgments
We thank David Brenner and the anonymous reviewers for their critical comments and Giovanni Solinas for his advice on metabolic measurements. We also thank Soo Young Lee, Helen Tran, and Katsumi Miyai for technical assistance and advice on liver pathology. E.J.P. and J.H.L. were supported in part by postdoctoral fellowships from the Natural Sciences and Engineering Research Council of Canada and Korean Research Foundation, respectively. S.R.A. received a postdoctoral fellowship from the Philip Morris Foundation and G.Y.Y. was supported by an American Diabetes Association postdoctoral research grant to M.K. Work was supported by grants from the NIH (ES006376 and CA118165), the Superfund Research Program (P42-ES010337) and the Wellcome Trust (WT086755) to M.K., who is an American Cancer Society Research Professor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, Vidal H, Hainque B. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85:3338–3342. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- Bisgaard HC, Thorgeirsson SS. Hepatic regeneration. The role of regeneration in pathogenesis of chronic liver diseases. Clin Lab Med. 1996;16:325–339. [PubMed] [Google Scholar]
- Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AM. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, Factor VM, Thorgeirsson SS. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117–1128. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Di Gregorio GB, Westergren R, Enerback S, Lu T, Kern PA. Expression of FOXC2 in adipose and muscle and its association with whole body insulin sensitivity. Am J Physiol Endocrinol Metab. 2004;287:E799–803. doi: 10.1152/ajpendo.00155.2004. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- Eriksen KW, Kaltoft K, Mikkelsen G, Nielsen M, Zhang Q, Geisler C, Nissen MH, Ropke C, Wasik MA, Odum N. Constitutive STAT3-activation in Sezary syndrome: tyrphostin AG490 inhibits STAT3-activation, interleukin-2 receptor expression and growth of leukemic Sezary cells. Leukemia. 2001;15:787–793. doi: 10.1038/sj.leu.2402093. [DOI] [PubMed] [Google Scholar]
- Fausto N. Mouse liver tumorigenesis: models, mechanisms, and relevance to human disease. Semin Liver Dis. 1999;19:243–252. doi: 10.1055/s-2007-1007114. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Leibel RL, Siegel DS, Walsh J, Bahary N. Molecular mapping of the mouse ob mutation. Genomics. 1991;11:1054–1062. doi: 10.1016/0888-7543(91)90032-a. [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hui L, Bakiri L, Mairhorfer A, Schweifer N, Haslinger C, Kenner L, Komnenovic V, Scheuch H, Beug H, Wagner EF. p38alpha suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat Genet. 2007;39:741–749. doi: 10.1038/ng2033. [DOI] [PubMed] [Google Scholar]
- Johansson M, Denardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunol Rev. 2008;222:145–154. doi: 10.1111/j.1600-065X.2008.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Li S, Ye L, Yu X, Xu B, Li K, Zhu X, Liu H, Wu X, Kong L. Hepatitis C virus NS4B induces unfolded protein response and endoplasmic reticulum overload response-dependent NF-kappaB activation. Virology. 2009;391:257–264. doi: 10.1016/j.virol.2009.06.039. [DOI] [PubMed] [Google Scholar]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, Roskams T, Trautwein C, Pasparakis M. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Yoshida H, Tateishi R, Masuzaki R, Ohki T, Hayakawa Y, Kinoshita H, Yamakado M, Kato N, et al. Serum IL-6 levels and the risk for hepatocarcinogenesis in chronic hepatitis C patients: An analysis based on gender differences. Int J Cancer. 2009 doi: 10.1002/ijc.24720. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Fuller GM, Fuentes NL, Moser AH, Staprans I, Grunfeld C, Feingold KR. Interleukin-6 stimulates hepatic triglyceride secretion in rats. Endocrinology. 1995;136:2143–2149. doi: 10.1210/endo.136.5.7720663. [DOI] [PubMed] [Google Scholar]
- Parekh S, Anania FA. Abnormal lipid and glucose metabolism in obesity: implications for nonalcoholic fatty liver disease. Gastroenterology. 2007;132:2191–2207. doi: 10.1053/j.gastro.2007.03.055. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Rebouissou S, Amessou M, Couchy G, Poussin K, Imbeaud S, Pilati C, Izard T, Balabaud C, Bioulac-Sage P, Zucman-Rossi J. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature. 2009;457:200–204. doi: 10.1038/nature07475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Maeda S, Chang L, Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci U S A. 2006;103:10544–10551. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheweita SA, Tilmisany AK. Cancer and phase II drug-metabolizing enzymes. Curr Drug Metab. 2003;4:45–58. doi: 10.2174/1389200033336919. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Tilg H, Wilmer A, Vogel W, Herold M, Nolchen B, Judmaier G, Huber C. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992;103:264–274. doi: 10.1016/0016-5085(92)91122-k. [DOI] [PubMed] [Google Scholar]
- Tremblay F, Brule S, Hee Um S, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G, et al. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2007;104:14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikha M, Corringham R, Klein B, Rossi JF. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res. 2003;9:4653–4665. [PMC free article] [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- Vesselinovitch SD, Mihailovich N. Kinetics of diethylnitrosamine hepatocarcinogenesis in the infant mouse. Cancer Res. 1983;43:4253–4259. [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- Wang T, Weinman SA. Causes and consequences of mitochondrial reactive oxygen species generation in hepatitis C. J Gastroenterol Hepatol. 2006;21 3:S34–37. doi: 10.1111/j.1440-1746.2006.04591.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang XD. Nonalcoholic steatohepatitis induced by a high-fat diet promotes diethylnitrosamine-initiated early hepatocarcinogenesis in rats. Int J Cancer. 2009;124:540–546. doi: 10.1002/ijc.23995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Diwan BA, Ohshima M, Hu H, Schuller HM, Rice JM. Tumor-initiating and promoting activities of di(2-ethylhexyl) phthalate in vivo and in vitro. Environ Health Perspect. 1986;65:279–291. doi: 10.1289/ehp.8665279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VW, Yu J, Cheng AS, Wong GL, Chan HY, Chu ES, Ng EK, Chan FK, Sung JJ, Chan HL. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124:2766–2770. doi: 10.1002/ijc.24281. [DOI] [PubMed] [Google Scholar]
- Wunderlich FT, Luedde T, Singer S, Schmidt-Supprian M, Baumgartl J, Schirmacher P, Pasparakis M, Bruning JC. Hepatic NF-kappa B essential modulator deficiency prevents obesity-induced insulin resistance but synergizes with high-fat feeding in tumorigenesis. Proc Natl Acad Sci U S A. 2008;105:1297–1302. doi: 10.1073/pnas.0707849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


