Abstract
The over-expression of saccharides such as Globo-H, LewisY and Tn antigen is a common feature of oncogenic transformed cells. Endeavors to exploit this aberrant glycosylation for cancer vaccine development has been complicated by difficulties of eliciting high titers of IgG antibodies against classical conjugates of tumor-associated carbohydrates to carrier proteins. We have designed, chemical synthesized and immunologically evaluated a number of fully synthetic vaccine candidates to establish strategies to overcome the poor immunogenicity of tumor-associated carbohydrates and glycopeptides. We have found that a three-component vaccine composed of a TLR2 agonist, a promiscuous peptide T-helper epitope and a tumor-associated glycopeptide, can elicit in mice exceptionally high titers of IgG antibodies that can recognize cancer cells expressing the tumor-associated carbohydrate. The superior properties of the vaccine candidate are attributed to the local production of cytokines, upregulation of co-stimulatory proteins, enhanced uptake by macrophages and dendritic cells and avoidance of epitope suppression.
A broad and expanding body of preclinical and clinical studies1-4 demonstrates that naturally acquired, passively administered or actively induced antibodies against carbohydrate-associated tumor antigens are able to eliminate circulating tumor cells and micro-metastases in cancer patients. Tumor-associated saccharides are, however, of low antigenicity, because they are self-antigens and consequently tolerated by the immune system. In addition, foreign carrier proteins such as keyhole limpet hemocyanin (KLH) and bovine serum albumin (BSA) and the linker that attach the saccharides to the carrier protein can elicit strong B-cell responses, which may lead to the suppression of antibody responses against the carbohydrate epitope5,6. It is clear that the successful development of carbohydrate-based cancer vaccines requires novel strategies for the more efficient presentation of tumor-associated carbohydrate epitopes to the immune system, resulting in a more efficient class switch to IgG antibodies7-17.
We reasoned that a three-component vaccine composed of a tumor-associated carbohydrate B-epitope, a promiscuous peptide T-helper (Th) epitope and a Toll-like receptor (TLR) ligand will circumvent immune suppression caused by a carrier protein or the linker region of a classical conjugate vaccine. Such a vaccine candidate contains, however, all mediators required for eliciting a strong IgG immune response. In the first instance, vaccine candidates 1 and 2 were designed, which contain as a B-epitope a tumor-associated glycopeptide derived from MUC11,18 and the well-documented murine MHC class II restricted Th epitope KLFAVWKITYKDT derived from the polio virus19 (Fig. 1). Furthermore, compound 1 contains as an built-in adjuvant the lipopeptide Pam2CysSK4, which is a potent activator of TLR2/6, whereas compound 2 contains Pam3CysSK4, which induces cellular activation through TLR1/220.
Figure 1.
Structures of synthetic compounds.
Compound 1 was prepared by a solid-phase peptide synthesis (SPPS) protocol using a Rink amide AM resin, N-fluorenylmethoxycarbonyl (Nα-Fmoc) protected amino acids and Nα-Fmoc-Thr-(AcO3-α-D-GalNAc) (3)21. After assembly of the glycopeptide, the acetyl esters of the saccharide moiety were cleaved by treatment with 80% hydrazine in MeOH. Next, the resulting product was coupled manually with Nα-Fmoc-R-(2,3-bis(palmitoyloxy)-(2R-propyl)-(R)-cysteine (Nα-Fmoc-Pam2Cys-OH) (4)22 followed by removal of the Nα-Fmoc group using piperidine (20%) in N, N-dimethylformamide (DMF) and cleavage from resin using reagent B to give compound 1. Unfortunately, a similar linear synthesis of compound 2 gave a product that was difficult to purify to homogeneity. Therefore, 2 was prepared by liposome-mediated native chemical ligation (NCL) of building blocks 5a, 6 and 723. Thus, a film of dodecylphosphocholine, thiol 5a and thioester 6 was hydrated in a phosphate buffer (pH 7.5) in the presence of carboxyethyl phosphine and EDTA and then ultra-sonicated. The resulting vesicles were sized to 1 μm by passing through a polycarbonate membrane filter. The ligation was initiated by the addition of sodium 2-mercaptoethane sulfonate and LC-MS showed completion of the reaction after 2 hours. Homogenous glycopeptide 8a was obtained after removal of the acetamidomethyl (Acm) protecting group of the ligation product using mercury(II)acetate and purification by RP-HPLC over a C-4 column. A second liposome-mediated NCL of the free sulfhydryl moiety of 8a with thioester 7 gave glycolipopeptide 2 in an excellent overall yield. (For synthetic details see Supplementary Methods online).
Next, 1 and 2 were incorporated into phospholipid-based small uni-lamellar vesicles (SUVs) by hydration of a thin film of the synthetic compounds, egg phosphatidylcholine (PC), phosphatidylglycerol (PG) and cholesterol in a HEPES buffer followed by extrusion through a 100 nm Nuclepore® polycarbonate membrane. Groups of five female BALB/c mice were immunized intra-peritoneal four times at weekly intervals with liposomes containing 3 μg of saccharide. To explore the adjuvant properties of the vaccine candidates, liposomes were administered with or without the potent saponin immuno-adjuvant QS-2124.
Anti-MUC1 antibody titers were determined by coating microtiter plates with CTSAPDT(α-D-GalNAc)RPAP conjugated to bromoacetyl-modified BSA and detection was accomplished with anti-mouse IgM and IgG antibodies labeled with alkaline phosphatase. Mice immunized with 2 elicited exceptionally high titers of anti-MUC1 IgG antibodies (Table 1). Sub-typing of the IgG antibodies indicated a bias towards a Th2 response25. Furthermore, the observed high IgG3 titer is typical of an anti-carbohydrate response. Co-administering of adjuvant QS-21 did not lead to a significant increase of IgG antibodies; however, in this case a mixed Th1/Th2 response was observed. Surprisingly, the use of glycolipopeptide 1, which contains Pam2CysSK4 instead of Pam3CysSK4, gave lower titers of IgG antibodies. Compounds 1 and 2 elicited low titers of antibodies against the T-epitope indicating that the vaccine does not suffer from immune suppression.
Table 1.
ELISA anti-MUC1 antibody titers* after 4 immunizations with various preparations.
| Immunization** | IgG total*** | IgG1 | IgG2a | IgG2b | IgG3 | IgM |
|---|---|---|---|---|---|---|
| 1 | 20,900 | 66,900 | 700 | 900 | 7,300 | 1,400 |
| 1 and QS-21 | 30,200 | 113,100 | 23,000 | 6,600 | 17,800 | 1,100 |
| 2 | 169,600 | 389,300 | 56,500 | 42,700 | 116,800 | 7,200 |
| 2 and QS-21 | 322,800 | 371,300 | 378,900 | 56,800 | 263,500 | 5,000 |
| 8b and 9 | 16,600 | 26,800 | 3,300 | 3,100 | 7,800 | 1,000 |
| 8b and 9 (saline) | 600 | 400 | 400 | 800 | 400 | 400 |
| 5b, 9 and 10 | 2,300 | 8,400 | 0 | 0 | 0 | 300 |
Anti-MUC1 antibody titers are presented as the median for groups of five mice. ELISA plates were coated with BSA-BrAc-MUC1 conjugate and titers were determined by linear regression analysis, plotting dilution vs. absorbance. Titers are defined as the highest dilution yielding an optical density of 0.1 or greater over that of normal control mouse sera.
Liposomal preparations were employed, except for 8b and 9 (saline).
A statistical significant difference (P < 0.05) was observed between 1vs. 2, 2vs. 8b/9, 2vs. 8b/9 (saline), 2vs. 5b/9/10, and 8b/9vs. 8b/9 (saline).
Individual titers for IgG total, IgG1, IgG2a, IgG2b, IgG3 and IgM are reported in Supplementary Figs. 4 and 5 online.
Titers after 3 and 5 immunizations for 2 and 2/QS-21 are listed in Supplementary Table 1 online.
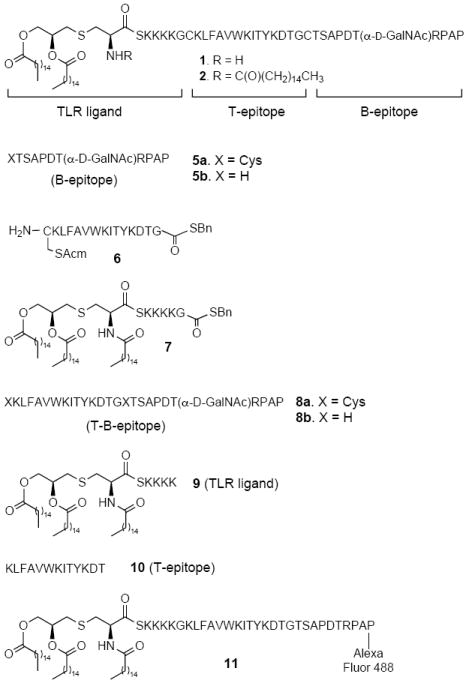
To ensure that the mouse sera were able to recognize the native MUC1 antigen present on cancer cells, binding of the sera to the MUC1 expressing MCF7 human breast cancer cell line was examined by flow cytometry. The anti-sera elicited against 1 and 2 reacted strongly with the MUC1 positive tumor cells whereas no binding was observed when SK-MEL 28 cells, which do not express the MUC1 antigen, were employed (Fig. 2). Further studies showed that both compounds induced the secretion of cytokines such as tumor necrosis factor alpha (TNF-α) in a TLR2-dependent manner leading to the upregulation of co-stimulatory proteins such as CD80, CD83 and CD86 (Supplementary Figs. 1-3 online).
Figure 2.
Flow cytometry analysis for specific anti-MUC1 antibodies. Reactivity was tested on MCF7 (a) and SK-MEL-28 (b) cells. Fluorescence intensity of serum (1:50 diluted) was assessed before (serum control; pink) and after 4 immunizations with 2 (orange). Also shown are medium (red) and conjugate (blue) controls. This is a representative example of the group of mice immunized with 2 (n=5). Similar results were obtained for mice immunized with 2 in the presence of QS-21 and 1 in the absence and presence of QS-21.
Uptake and proteolytic processing of antigen and subsequent presentation of the peptide T-epitope as a complex with MHC class II on the cell surface of antigen presenting cells (APCs), is critical for eliciting IgG antibodies. Thus, immunizations with a liposomal preparation of the individual components of 2 will not require such processing and hence may lead to even more robust antigenic responses. The influence of covalent attachment of the various components of the vaccine candidate on antigenic responses was investigated by immunizing mice with a liposomal preparation of compounds 8b, which is composed of the T-epitope linked to the B-epitope and the adjuvant Pam3CysSK4 (9). Significantly lower titers of IgG antibodies were determined compared to the use of 2 (Table 1). No or very low IgG antibody responses were observed when the two compounds were administered as a saline solution indicating that a liposomal preparation contributes to antigenicity. The importance of covalent attachment was further highlighted in an experiment in which the B-epitope (5b), T-epitope (10) and adjuvant Pam3CysSK4 (9) were administered as a liposomal preparation resulting in low titers of IgG antibodies.
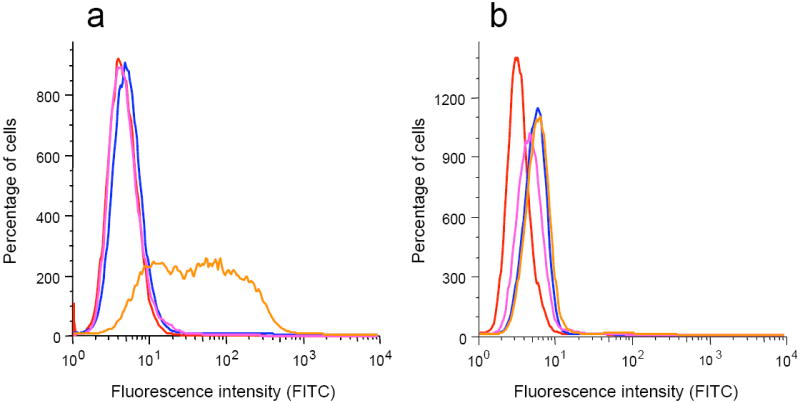
It was anticipated that in addition to initiating the production of cytokines and stimulating the upregulation of co-stimulatory proteins, the lipopeptide Pam3CysSK4 may facilitate selective uptake by antigen presenting cells in a TLR2-dependent manner. To test this hypothesis, compound 11, which contains an Alexa Fluor 488 fluorescence label, was prepared (Supplementary Methods) and administered to mouse macrophages. After 30 min, the cells were harvested, lysed and the fluorescence measured. To account for possible cell surface binding without internalization, the cells were also treated with trypsin before lyses and then examined for fluorescence. A significant quantity of 11 was internalized, whereas a small amount was attached to the cell surface. To determine whether the uptake was mediated by TLR2, uptake studies were also performed using wild type HEK 293T cells and HEK 293T cells stable transfected with murine TLR2, TLR2/TLR6 or TLR4/MD2 (Fig. 3). Importantly, significant uptake was only observed when the cells were transfected with TLR2, indicating that uptake is mediated by this receptor. It is important to note that macrophage galactose type C-lectin may also facilitate uptake of the vaccine candidates by binding to the Tn-antigen, which is not probed by compound 1126.
Figure 3.
Cellular uptake of compound 11. Cells (RAW 264.7 γNO(-), HEK 293T wild type and HEK 293T stable transfected with murine TLR2, TLR2/TLR6 or TLR4/MD2) were exposed to Alexa fluor 488-labeled compound 11 (1 μg/ml) for 30 min. After cells were washed and lysed (total cell interaction; grey) or washes, treated with trypsin and then lysed (internalization only; black) fluorescence (absorbance 485 nm, emission 538 nm) was measured. Fluorescence values were normalized for maximum possible fluorescence (100%). Data represent mean values ± s.d. (n=3).
Most efforts aimed at developing carbohydrate-based cancer vaccines have been focused on the use of chemically synthesized tumor-associated carbohydrates linked through an artificial linker to a carrier protein1-4. It has been established that the use of KLH as a carrier protein in combination with the powerful adjuvant QS-21 gives the best results. However, a drawback of this approach is that KLH is a very large and cumbersome protein that can elicit high titers of anti-KLH-antibodies, leading to immune suppression of the tumor-associated carbohydrate epitope27. Furthermore, the conjugation chemistry is often difficult to control resulting in conjugates with ambiguities in composition. Also, the linker moiety can elicit strong B-cell responses5,6. Not surprisingly, preclinical and clinical studies with carbohydrate-protein conjugates have led to results of mixed merit. For example, mice immunizations with a trimeric cluster of Tn-antigens conjugated to KLH (Tn(c)-KLH) in the presence of the adjuvant QS-21 elicited modest titers of IgG antibodies28. In a clinical trial of relapsed prostate cancer patients, the vaccine gave low median IgG and IgM antibody titers29. In another study, it was found that only KLH conjugates of glycopeptides composed of multiple repeat units that are highly glycosylated with Tn antigens elicited IgG antibodies30.
The excellent antigenicity of the here reported three-component vaccine is attributed to a number of features. Firstly, it does not have any unnecessary components that are antigenic and may induce immune suppression. The chemical attachment of the TLR2 agonist Pam3CysSK4 to the B- and T-epitopes ensures that cytokines are produced at the site where the vaccine interacts with immune cells, leading to a high local concentration of cytokines facilitating maturation of relevant immune cells. It also facilitates uptake by TLR2-expressing cells such as APCs, which will assist antigen processing and full activation. Finally, a fully synthetic approach makes it possible to optimize the various components of the candidate vaccine by structure-activity relationship studies. In this respect, proper design of the three-component vaccine is essential, because previously we showed that a compound composed of the inferior adjuvant Pam3Cys which lacks the important tetra-lysine moiety and a human Th epitope elicits low titers of IgG antibodies7,14. Furthermore, it has been found that covalent attachment of the three components is important for optimal antigenic responses. Probably, the lipid moiety of the vaccine candidate facilitates proper presentation and retention into liposomes. In this respect, liposomes can present the B-epitopes multivalently facilitating B-cell receptor clustering resulting in B-cell activation.
METHODS
General methods for solid-phase peptide synthesis (SPPS)
Peptides were synthesized by established protocols on a ABI 433A peptide synthesizer (Applied Biosystems) equipped with UV-detector using Nα-Fmoc-protected amino acids and 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyl hexafluorophosphate (HBTU)/N-hydroxybenzotriazole (HOBt) as the activating reagents. Single coupling steps were performed with conditional capping. The following protected amino acids were employed: Nα-Fmoc-Arg(Pbf)-OH, Nα-Fmoc-Asp(OtBu)-OH, Nα-Fmoc-Cys(Acm)-OH, Nα-Fmoc-Cys(Trt)-OH, Nα-Fmoc-Lys(Boc)-OH, Nα-Fmoc-Ser(tBu)-OH, Nα-Fmoc-Thr(tBu)-OH, Nα-Fmoc-Tyr(tBu)-OH. Nα-Fmoc groups were cleaved by piperidine (20%) in DMF for 1 h. The couplings of glycosylated amino acid Nα-Fmoc-Thr-(AcO3-α-d-GalNAc) (3)21 and Nα-Fmoc-R-(2,3-bis(palmitoyloxy)-(2R-propyl)-(R)-cysteine (4), which was prepared from R-glycidol,22 were carried out manually using O-(7-azabenzotriazol-1-yl)-N, N, N’, N’-tetramethyl-uronium hexafluorophosphate (HATU)/1-hydroxy-7-azabenzotriazole (HOAt) and benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium (PyBOP)/HOBt as coupling reagents, respectively. Progress of the manual couplings was monitored by standard Kaiser test. Compounds 1, 5a, 5b, 8b, 9 and 10 were prepared on Rink amide AM resin and thioesters 6 and 7 were synthesized on sulfamylbutyryl Novasyn TG resin. The preparation of 11 was carried out on universal NovaTag resin. Liposome mediated native chemical ligation of 7 with 8a to give 2 was performed as described previously23. A similar procedure was used for the synthesis of compound 8b using 5a and 6. (Glyco)(lipo)peptides were purified by HPLC using semi-preparative a C4 and C8 columns and linear gradients of 0 to 95% solvent B (0.1% trifluoroacetic acid (TFA) in acetonitrile) in solvent A (0.1% TFA in water). Details of the chemical synthesis and analytical data of the compounds are provided in the Supplementary Methods.
General procedure for the preparation of liposomes
Egg PC, PG, cholesterol and synthetic compound (15 μmol, molar ratios, 65/25/50/10) were dissolved in a mixture of trifluoroethanol and MeOH (1/1, v/v, 5 ml). The solvents were removed in vacuo to give a thin lipid film, which was hydrated by shaking in HEPES buffer (10 mM, pH 6.5) containing NaCl (145 mM) (1 ml) under Ar atmosphere at 41 °C for 3 h. The vesicle suspension was sonicated for 1 min and then extruded successively through 1.0, 0.4, 0.2 and 0.1 μm polycarbonate membranes (Whatman, Nucleopore Track-Etch Membrane) at 50 °C to obtain SUVs. The GalNAc content was determined by heating a mixture of SUVs (50 μl) and aqueous TFA (2M, 200 μl) in a sealed tube for 4 h at 100 °C. The solution was then concentrated in vacuo and analyzed by high pH anion exchange chromatography using a pulsed amperometric detector (HPAEC-PAD) and a CarboPac PA-1 column.
Dose and immunization schedule
Groups of five mice (female BALB/c, age 8-10 weeks) were immunized four or five times at 1-week intervals. Each boost included 3 μg of saccharide in the liposome formulation. In some immunizations, the external immuno-adjuvant QS-21 (10 μg; Antigenics Inc.) was included. Serum samples were obtained before immunization (pre-bleed) and one week after the final immunization. The final bleeding was done by cardiac bleed.
Serologic assays
Anti-MUC1 IgG, IgG1, IgG2a, IgG2b and IgG3 antibody titers were determined by enzyme-linked immunosorbent assay (ELISA), as described previously5. Briefly, ELISA plates (Thermo Electron Corp.) were coated with a conjugate of the MUC1 glycopeptide conjugated to BSA through a bromoacetyl linker (BSA-BrAc-MUC1). Serial dilutions of the sera were allowed to bind to immobilized MUC1. Detection was accomplished by the addition of phosphate-conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc.), IgG1 (Zymed), IgG2a (Zymed), IgG2b (Zymed) or IgG3 (BD Biosciences Pharmingen) antibodies. After addition of p-nitrophenyl phosphate (Sigma), the absorbance was measured at 405 nm with wavelength correction set at 490 nm using a microplate reader (BMG Labtech). The antibody titer was defined as the highest dilution yielding an optical density of 0.1 or greater over that of normal control mouse sera.
Flow cytometry analysis
Pre- and post-immunization sera were diluted 50-fold and incubated with MCF7 and SK-MEL-28 single-cell suspensions for 30 min on ice. Next, the cells were washed and incubated with goat anti-mouse IgG γ-chain specific antibody conjugated to fluorescein isothiocyanate (FITC; Sigma) for 20 min. Cells were analyzed by flow cytometry using the FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems) and data analysis was performed with FlowJo software (Tree Star, Inc.).
Binding and uptake assay
RAW 264.7 γNO (-) cells, HEK293T cells and HEK293T cells stable transfected with murine TLR2, TLR2/TLR6 or TLR4/MD2 (2.6 × 106 cells ml-1) were exposed to Alexa Fluor 488-labeled compound 11 (1 μg ml-1) for 30 min at 37 °C. Cells were harvested and washed in HNE buffer (HEPES, 20 mM; NaCl, 150 mM; EDTA, 1 mM). Samples that were assessed for internalization only were treated with trypsin (500 μg ml-1) for 1 min and washed in HNE buffer. Next, cells were lysed in Passive Lysis Buffer (Promega) and fluorescence (absorbance 485 nm, emission 538 nm) of the cell lysates was measured using the POLARstar OPTIMA combination luminometer/fluorometer (BMG Labtech). Fluorescence values were normalized for maximum possible fluorescence (100%), using untreated cell lysates spiked with the fluorescent compound. The data are presented as the means ± s.d. of triplicate treatments, with each experiment being repeated three times.
Other methods
See Supplementary Methods for sources of reagents, cell maintenance, TNF-α assay, evaluation of materials for contamination by LPS, dendritic cell maturation, transfection and NF-κB activation assay.
Supplementary Material
Acknowledgments
We thank Dr. L. Jaso-Friedmann for helpful discussions and Dr. G. K. Lewis for the dendritic cell maturation measurements. This research was supported by the National Cancer Institute of the National Institutes of Health (Grant No. RO1 CA88986).
Footnotes
COMPETING INTERESTS STATEMENT The authors declare that they have no competing financial interests.
Note: Supplementary information and chemical compound information is available on the Nature Chemical Biology website.
References
- 1.Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 2.Dube DH, Bertozzi CR. Glycans in cancer and inflammation. Potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 3.Ouerfelli O, Warren JD, Wilson RM, Danishefsky SJ. Synthetic carbohydrate-based antitumor vaccines: challenges and opportunities. Expert Rev Vaccines. 2005;4:677–685. doi: 10.1586/14760584.4.5.677. [DOI] [PubMed] [Google Scholar]
- 4.Slovin SF, Keding SJ, Ragupathi G. Carbohydrate vaccines as immunotherapy for cancer. Immunol Cell Biol. 2005;83:418–428. doi: 10.1111/j.1440-1711.2005.01350.x. [DOI] [PubMed] [Google Scholar]
- 5.Buskas T, Li YH, Boons GJ. The immunogenicity of the tumor-associated antigen Lewis(y) may be suppressed by a bifunctional cross-linker required for coupling to a carrier protein. Chem Eur J. 2004;10:3517–3524. doi: 10.1002/chem.200400074. [DOI] [PubMed] [Google Scholar]
- 6.Ni J, Song H, Wang Y, Stamatos NM, Wang LX. Toward a carbohydrate-based HIV-1 vaccine: synthesis and immunological studies of oligomannose-containing glycoconjugates. Bioconjug Chem. 2006;17:493–500. doi: 10.1021/bc0502816. [DOI] [PubMed] [Google Scholar]
- 7.Reichel F, Ashton PR, Boons GJ. Synthetic carbohydrate-based vaccines: synthesis of an L-glycero-D-manno-heptose antigen-T-epitope-lipopetide conjugate. Chem Commun. 1997;21:2087–2088. [Google Scholar]
- 8.Alexander J, et al. Linear PADRE T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. J Immunol Methods. 2000;164:1625–1633. doi: 10.4049/jimmunol.164.3.1625. [DOI] [PubMed] [Google Scholar]
- 9.Kudryashov V, et al. Toward optimized carbohydrate-based anticancer vaccines: epitope clustering, carrier structure, and adjuvant all influence antibody responses to Lewis(y) conjugates in mice. Proc Natl Acad Sci U S A. 2001;98:3264–3269. doi: 10.1073/pnas.051623598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo-Man R, et al. Anti-tumor immunity provided by a synthetic multiple antigenic glycopeptide displaying a tri-Tn glycotope. J Immunol Methods. 2001;166:2849–2854. doi: 10.4049/jimmunol.166.4.2849. [DOI] [PubMed] [Google Scholar]
- 11.Jiang ZH, Koganty RR. Synthetic vaccines: the role of adjuvants in immune targeting. Curr Med Chem. 2003;10:1423–1439. doi: 10.2174/0929867033457340. [DOI] [PubMed] [Google Scholar]
- 12.Jackson DC, et al. A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc Natl Acad Sci U S A. 2004;101:15440–5. doi: 10.1073/pnas.0406740101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo-Man R, et al. A fully synthetic therapeutic vaccine candidate targeting carcinoma-associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates. Cancer Res. 2004;64:4987–4994. doi: 10.1158/0008-5472.CAN-04-0252. [DOI] [PubMed] [Google Scholar]
- 14.Buskas T, Ingale S, Boons GJ. Towards a fully synthetic carbohydrate-based anticancer vaccine: synthesis and immunological evaluation of a lipidated glycopeptide containing the tumor-associated Tn antigen. Angew Chem Int Ed. 2005;44:5985–5988. doi: 10.1002/anie.200501818. [DOI] [PubMed] [Google Scholar]
- 15.Dziadek S, Kowalczyk D, Kunz H. Synthetic vaccines consisting of tumor-associated MUC1 glycopeptide antigens and bovine serum albumin. Angew Chem Int Ed. 2005;44:7624–7630. doi: 10.1002/anie.200501593. [DOI] [PubMed] [Google Scholar]
- 16.Krikorian D, Panou-Pomonis E, Voitharou C, Sakarellos C, Sakarellos-Daitsiotis M. A peptide carrier with a built-in vaccine adjuvant: construction of immunogenic conjugates. Bioconjug Chem. 2005;16:812–819. doi: 10.1021/bc049703m. [DOI] [PubMed] [Google Scholar]
- 17.Pan Y, Chefalo P, Nagy N, Harding C, Guo Z. Synthesis and immunological properties of N-modified GM3 antigens as therapeutic cancer vaccines. J Med Chem. 2005;48:875–883. doi: 10.1021/jm0494422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldus SE, Engelmann K, Hanisch FG. MUC1 and the MUCs: a family of human mucins with impact in cancer biology. Crit Rev Clin Lab Sci. 2004;41:189–231. doi: 10.1080/10408360490452040. [DOI] [PubMed] [Google Scholar]
- 19.Leclerc C, Deriaud E, Mimic V, van der Werf S. Identification of a T-cell epitope adjacent to neutralization antigenic site 1 of poliovirus type 1. J Virol. 1991;65:711–718. doi: 10.1128/jvi.65.2.711-718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spohn R, et al. Synthetic lipopeptide adjuvants and Toll-like receptor 2 - structure-activity relationships. Vaccine. 2004;22:2494–2499. doi: 10.1016/j.vaccine.2003.11.074. [DOI] [PubMed] [Google Scholar]
- 21.Cato D, Buskas T, Boons GJ. Highly efficient stereospecific preparation of Tn and TF building blocks using thioglycosyl donors and the Ph2SO/Tf2O promotor system. J Carbohydr Chem. 2005;24:503–516. [Google Scholar]
- 22.Metzger JW, Wiesmuller KH, Jung G. Synthesis of Nα-Fmoc protected derivatives of S-(2,3-dihydroxypropyl)-cysteine and their application in peptide synthesis. Int J Pept Protein Res. 1991;38:545–554. doi: 10.1111/j.1399-3011.1991.tb01538.x. [DOI] [PubMed] [Google Scholar]
- 23.Ingale S, Buskas T, Boons GJ. Synthesis of glyco(lipo) peptides by liposome-mediated native chemical ligation. Org Lett. 2006;8:5785–5788. doi: 10.1021/ol062423x. [DOI] [PubMed] [Google Scholar]
- 24.Kensil CR. Saponins as vaccine adjuvants. Crit Rev Ther Drug Carrier Syst. 1996;13:1–55. [PubMed] [Google Scholar]
- 25.Dabbagh K, Lewis DB. Toll-like receptors and T-helper-1/T-helper-2 responses. Curr Opin Infect Dis. 2003;16:199–204. doi: 10.1097/00001432-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Saeland E, et al. The C-type lectin MGL expressed by dendritic cells detects glycan changes on MUC1 in colon carcinoma. Cancer Immunol Immunother. 2006 doi: 10.1007/s00262-006-0274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musselli C, Livingston PO, Ragupathi G. Keyhole limpet hemocyanin conjugate vaccines against cancer: the Memorial Sloan Kettering experience. J Cancer Res Clin Oncol. 2001;127(Suppl 2):R20–R26. doi: 10.1007/BF01470995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuduk SD, et al. Synthetic and immunological studies on clustered modes of mucin-related Tn and TF O-linked antigens: the preparation of a glycopeptide-based vaccine for clinical trials against prostate cancer. J Am Chem Soc. 1998;120:12474–12485. [Google Scholar]
- 29.Slovin SF, et al. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with alpha-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J Clin Oncol. 2003;21:4292–4298. doi: 10.1200/JCO.2003.04.112. [DOI] [PubMed] [Google Scholar]
- 30.Sorensen AL, et al. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology. 2006;16:96–107. doi: 10.1093/glycob/cwj044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


