Abstract
Human peptidoglycan recognition proteins (PGLYRPs) is a novel family of pattern recognition receptors, and also act as antibacterial proteins. This study was to explore the toll-like receptor (TLR)-mediated regulation of PGLYRPs in human corneal epithelial cells (HCECs). Fresh human donor corneoscleral tissues were used to prepare cryosections. Primary HCECs, established from limbal explants, were treated with microbial ligands to TLRs 1–9 for 4–48 hours, with or without pretreatment of TLR antibodies, NFκB inhibitor, or siRNA transfection. The mRNA of PGLYRPs was evaluated by RT and real time PCR, and their proteins and NF-κB activation were determined by immunostaining and Western blot. The nuclear IRF3 activity was quantified using an ELISA-based TransAM kit. PGLYRP-2, -3 and -4 were found to be expressed by human corneal epithelium while PGLYRP-1 was not detected. In primary HCEC cultures, PGLYRP-3 and -4 were constitutively expressed while PGLYRP-2 was largely inducible. PGLYRP-2 was induced by bacterial components, Pam3CSK4, PGN, flagellin and FSL-1, ligands for TLR2/1, 2, 5 and 2/6, respectively. Interestingly, PGLYRP-2 was strongest stimulated by polyI:C representing viral dsRNA. TLR3 antibody or NF-κB inhibitor blocked IRF3 and NF-κB p65 activation as well as polyI:C-stimulated PGLYRP-2. RNA interference indicates that the polyI:C-induced PGLYRP-2 was dramatically blocked in the cells transfected with siRNA-TRIF but neither siRNA-MyD88 nor the negative control siRNA-F. These findings suggest that human corneal epithelium may response to viral or bacterial infection by producing PGLYRPs through TLRs, and the induction of PGLYRP-2 by dsRNA was through TLR3-TRIF-IRF3-NFκB signaling pathways.
Keywords: Cornea, Epithelium, Toll-like receptors, PGLYRP, NF-κB
INTRODUCTION
In addition to physical barrier function, epithelial cells have been recently recognized to play a vital role in regulation of immune responses. Innate immunity, the first line of defense against microorganisms, can detect the pathogen and start a rapid defensive response through pattern recognition receptors (PRRs), which detect pathogens via the recognition of pathogen-associated molecular patterns (PAMPs) (Kumar et al., 2004; Unterholzner and Bowie, 2008). The recognition of pathogens has been largely ascribed toll-like receptors (TLRs), which can transduce signals in response to microbes, such as bacteria, fungi and viruses (Takeda and Akira, 2005). Activation of TLRs leads to the activation and recruitment of neutrophils and macrophages to sites of infection and enhances production of antimicrobial factors.
Peptidoglycan (PGN), a major constituent of the cell wall of Gram positive bacteria, represents an excellent target for innate immune recognition. In addition to TLR2, peptidoglycan recognition proteins (PGRPs), a novel family of PRR conserved from insects (Werner et al., 2000; Yoshida et al., 1996) to mammals (Dziarski, 2004; Kang et al., 1998), recognize bacterial PGN as an innate immunity molecule. The PGRP family currently includes nearly 100 members, all of which have a homologous PGN-binding domain of ~165 amino acids in length (Dziarski and Gupta, 2006b). Insects have up to 19 PGRPs, while mammals have only four PGRPs: PGRP-S (small PGRP, 24 kDa), PGRP-L (Long PGRP, 64 kDa), PGRP-I α (Intermediate PGRP-α, 38 kDa) and PGRP-I β (Intermediate PGRP-β, 46 kDa) (Liu et al., 2001). The Human Genome Organization Gene Nomenclature Committee has renamed these as peptidoglycan recognition proteins (PGLYRPs) 1–4, respectively. Distinct from insects, mammalian PGLYRPs not only recognize bacterial PGN as an innate immune response, but also act as anti-bacterial factors with direct bactericidal function (Dziarski and Gupta, 2006a). All four mammalian PGLYRPs are soluble intracellular or secreted molecules, which kill bacteria in the absence of enzymatic activity. PGLYRP-1 participates in killing phagocytized bacteria (Dziarski et al., 2003; Liu et al., 2000); PGLYRP-2 is an N-acetylmuramoyl-L-alanine amidase (Gelius et al., 2003; Wang et al., 2003; Zhang et al., 2005) that hydrolyzes bacterial peptidoglycan; PGLYRP-3 and PGLYRP-4 are co-expressed in the same cells and are exclusively secreted as homo- or heterodimers with antibacterial function (Lu et al., 2006).
Interestingly, we have recently found that the PGLYRPs were stimulated in human corneal epithelial cells (HCECs) not only in response to PGN extracted from Staphylococcus aureus, but also to polyinosine-polycytidylic acid (polyI:C), a synthetic analog of double-stranded RNA (dsRNA) that is a molecular pattern associated with viral infection. These findings prompted us to explore the potential interactions between TLRs and PGLYRPs, an important regulation of innate immune response that has not been elucidated. Since human corneal epithelial cells have been shown to express functional TLRs 1–9, except for TLR8 (Kumar and Yu, 2006), we hypothesize that these cells can be utilized as an in vitro model to investigate the innate epithelial response. The purpose of this study was to explore the expression, regulation and signaling pathways utilized by human corneal epithelium to produce PGLYRPs in response to a variety of TLR ligands.
METHODS
Materials and reagents
The TLR ligands Pam3csk4, PGN from staphylococcus aureus (PGN-SA), flagellin from S. typhimurium, synthetic diacylated lipoprotein (FSL-1), imiquimod (R837), single-stranded GU-rich oligonuleotide complexed with LyoVec (ssRNA40/LyoVec), and Type C CpG oligonucleotide (ODN) were purchased from InvivoGen (San Diego, CA). Polyinosine-polycytidylic acid (polyI:C), LPS from Escherichia coli were from Sigma-Aldrich (St. Louis, MO). Affinity-purified goat polyclonal antibody (Ab) against PGLYRP-2, rabbit polyclonal Abs against TLR3 (M-300) and p65 were from Santa Cruz Biotechnology (Santa Cruz, CA); mouse antibodies against PGLYRP-3 and -4 from Imgenex (San Diego, CA). RNeasy® Mini kit and HiperFect transfection reagent from Qiagen (Valencia, CA). TaqMan® Gene Expression Assay, real-time PCR Master Mix, and Silencer Select® pre-designed siRNAs were from Applied Biosystems (Foster City, CA).
Human corneal tissue and primary HCEC cultures for PGLYRPs induction
Fresh human corneoscleral tissues (<72 hours after death) not suitable for clinical use, from donors aged 21 to 68 years, were obtained from the Lions Eye Bank of Texas (Houston, TX). The tissues were cut, frozen and sectioned for immunostaining. The corneal epithelium was scraped and lysed for mRNA analysis. HCECs were established from limbal explants in a supplemented hormonal epidermal medium containing 5%FBS (SHEM) using our previous methods (Kim et al., 2004; Li et al., 2001; Solomon et al., 2000). Corneal epithelial cell growth was carefully monitored, and only the epithelial cultures without visible fibroblast contamination were used for this study. Confluent corneal epithelial cultures were switched to serum-free SHEM and treated for different time periods (4, 8, 16, 24, or 48 hours) with a series of microbial components with multiple concentrations. Each experiment was repeated at least three times.
TLRs and NFκB signaling pathway assay
HCECs were pre-incubated with specific TLR3 antibody (10µg/ml) or NFκB activation inhibitor quinazoline (NFκB-I, 10µM) for 1 hour before adding polyI:C for 4–48 hours respectively. The cells in 6-well plates treated with 4 hours were lysed for extraction of cytoplasmic and nuclear proteins, and stored at −80 °C for Western blot analysis or IRF3 activation assay. The cells treated with 16 hours in 12-well plates were lysed for real time PCR analysis. The cells treated with 4 or 48 hours in 8-chamber slides were fixed for p65 or PGLYRPs immunofluorescent staining, respectively.
RNA interference
RNA interference experiments were performed as our previous method (Chen et al., 2006) with modification. In brief, primary HCECs were transfected with annealed double-stranded siRNA (20 nM) specific for TRIF (TIR-domain-containing adapter-inducing interferon-β), or MyD88 (myeloid differentiation factor 88) with a non-coding sequence siRNA-fluorescein (siRNA-F) as a negative control (also serve as visible monitor for transfection efficiency) using HiperFect reagent for 16 hours. After incubation for additional 48 hours, the cells were treated with polyI:C 25 µg/ml for 4–48 hours. The sequences of target TRIF or MyD88 mRNAs used in this study are 5'- GAAUCAUCAUCGGAACAGA-3' or 5'-GAUGAUUACCUGCAGAGCA-3' respectively. The cell viability after transfection for 4days was more than 90%, as assessed by a 0.2% trypan blue exclusion test and morphological observation.
Total RNA extraction, reverse transcription (RT) and quantitative real-time PCR
Total RNA was extracted from corneal tissues or HCECs using a Qiagen RNeasy® Mini kit according to manufacturer’s protocol, quantified by NanoDrop® ND-1000 Spectrophotometer, and stored at −80 °C. The first strand cDNA was synthesized by RT from 1 µg of total RNA using Ready-To-Go You-Prime First-Strand Beads as previously described (Luo et al., 2004; Yoon et al., 2007). The real-time PCR was performed in Mx3005P™ system (Stratagene) with 20µl reaction volume containing 5µl of cDNA that was generated from 50ng/ml of total RNA, 1µl of TaqMan® Gene Expression Assay primers and probe for PGLYRP-1 (Hs00175475_m1), PGLYRP-2 (Hs00277228_m1), PGLYRP-3 (Hs00364657_m1), PGLYRP-4 (Hs00220648_m1) or GAPDH (Hs99999905_m1), and 10µl TaqMan® Gene Expression Master Mix. The thermocycler parameters were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. A non-template control was included to evaluate DNA contamination. The results were analyzed by the comparative Ct method and normalized by GAPDH, and presented as relative fold change in the expression levels by the treated groups versus untreated controls (de Paiva et al., 2006; Yoon et al., 2007).
Immunoflluoresent staining
The human corneal frozen sections or corneal epithelial cells on 8-chamber slides were fixed in acetone at −30°C for 5 minutes. Cell cultures were permeabilized with 0.2% Triton X-100 in PBS at room temperature (RT), for 10 min. Indirect immunofluorescent staining was performed with our previous methods (Chen et al., 2004; Li and Tseng, 1995). using primary rabbit antibodies against human PGLYRP-2(1:50, 4µg/ml), -3 or -4 (1:100, 5µg/ml). Alexa-Fluor 488 conjugated secondary antibodies was applied, and propidium iodide (PI) was used for nuclear counterstaining. Secondary antibody alone without primary antibodies applied, or isotype IgG were used as negative controls. The staining will be photographed with Zeiss laser scanning confocal microscope (LSCM510META, Thornwood, NY) (Yu et al., 2007).
Western Blot Analysis
Western blot analysis was performed using a previous method (Luo et al., 2004). In brief, the cytoplasm and nuclear protein samples (30 µg per lane), prepared with Nuclear Extract Kit (Active Motif, Carlsbad CA) and measured by a BCA protein assay kit (Pierce, Rockford, IL), were mixed with 6× SDS reducing sample buffer and boiled for 5 min before loading. Proteins were separated by SDS PAGE gel and transferred electronically to PVDF membranes. The membranes were blocked with 5% non-fat milk in TTBS (50 mM Tris [pH 7.5], 0.9% NaCl, and 0.1% Tween-20) for 1 hour at RT, incubated with primary antibodies against NFκB p65 or PGLYRP-2 overnight at 4°C, and then with horseradish peroxidase-conjugated secondary antibody for I hour at RT. The signals were detected with an ECL chemiluminescence reagent (GE Healthcare) and the images were acquired by a Kodak image station 2000R (Eastman Kodak).
Nuclear IRF3 activation assay
The nuclear interferon regulatory factor 3 (IRF3) activity was quantified using an ELISA-based TransAM IRF3 kit (Active Motif) according to manufacturer’s protocol. In brief, the nuclear protein extracts (10 µg per well) were added to a 96-well plate that was immobilized with oligonucleotide containing IRF3 element. IRF3 contained in nuclear extracts binds specifically to this oligonucleotide during ncubation for two hours at room temperature. IRF-3 antibody (100 µl, at 1:1000 dilution) was then added to each well for 1.5 hours followed by 100 µl of horseradish peroxidase (HRP)-conjugated antibody (1:1000 dilution) for 1 hour. After adding 100 µl of Developing Solution for up to 15 minutes and colorimetric reaction was stopped, the IRF3 activity was determined by reading absorbance on a spectrophotometer at 450 nm with a reference wavelength of 655 nm.
Statistical analysis
Student’s t-test was used to compare difference between two groups. One-way ANOVA test was used to make comparisons among three groups, and the Dunnett’s test was further used to compare each treated group with the control group. Statistical significance was retained for P values <0.05.
RESULTS
Three members of PGLYRPs were detected in human corneal epithelial tissues and primary cultured HCECs
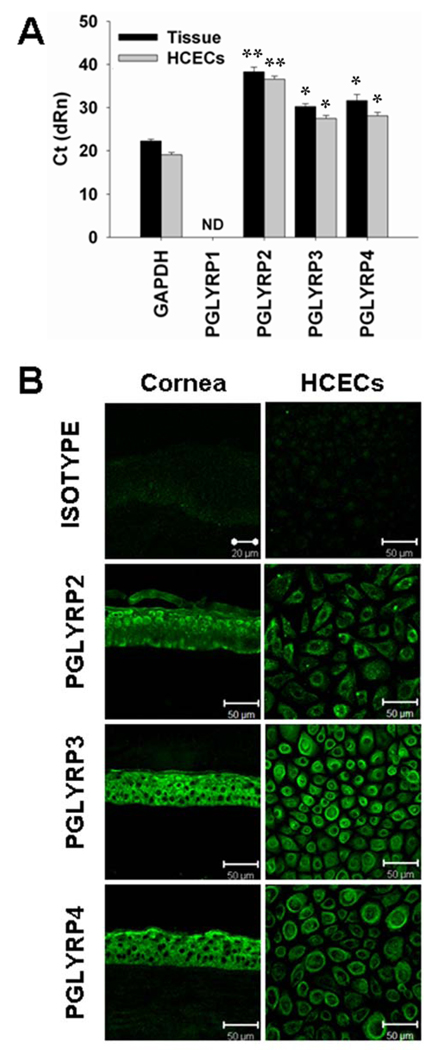
The mRNA expression of PGLYRPs in human corneal epithelial tissues and primary cultured HCECs was evaluated by real-time PCR. As shown in Figure 1A, GAPDH, an internal control, was detected at threshold cycles (Ct) 22.03±0.45 in human corneal epithelial tissues and at Ct 19.02±0.55 in HCECs, while PGLYRP-1 mRNA was not detected in 45 cycles. PGLYRP-2 expression was detected at low level at Ct 38.28±1.11 or 36.58±0.7 in corneal tissue or cultures, respectively. More abundantly expressed than PGLYRP-2, PGLYRP-3 and PGLYRP-4 have similar Ct levels of 30.23±0.66 or 31.64±0.1.42, respectively in corneal tissues and Ct 27.47±0.71 or Ct 28.07±0.85, respectively in HCECs.
Figure 1.
Presence of PGLYRPs in human corneal tissue sections and primary cultured human corneal epithelial cells (HCECs). A. The levels of cycle threshold (Ct), evaluated by RT and real-time PCR, represent the mRNA levels of PGLYRPs expressed in total RNAs extracted from fresh human corneal epithelial tissues and HCECs, respectively; ND: not detectable. The Ct data shown are mean ± SD of three independent experiments, *, p < 0.01; **, p < 0.005 compared with GAPDH. B. Representative images showing immunofluorescent staining of PGLYRPs 2–4 (green color) with isotype IgG as negative control on frozen sections of human corneal tissues and HCECs. Propidium iodide (PI) was used as nuclear counterstaining (red color, data not shown). Magnification: ×400 (bar = 50 µm).
Immunofluorescent staining was performed to localize three members of PGLYRPs in human corneal tissues and primary cultured HCECs. As shown in the Figure 1B, PGLYRP-2 was immuno-localized mainly in suprabasal epithelial layers while PGLYRP-3 and -4 were localized throughout all epithelial layers of the human cornea. No PGLYRPs were detected in corneal stroma. In primary cultured HCECs, these three PGLYRPs were found in most cells and mainly localized to the cell membrane and cytoplasm. The immunoreactivity to PGLYRP-2 was lower than PGLYRP-3 and -4, which was consistent with their mRNA levels.
Differential induction of PGLYRPs was observed in HCECs exposed to microbial ligands
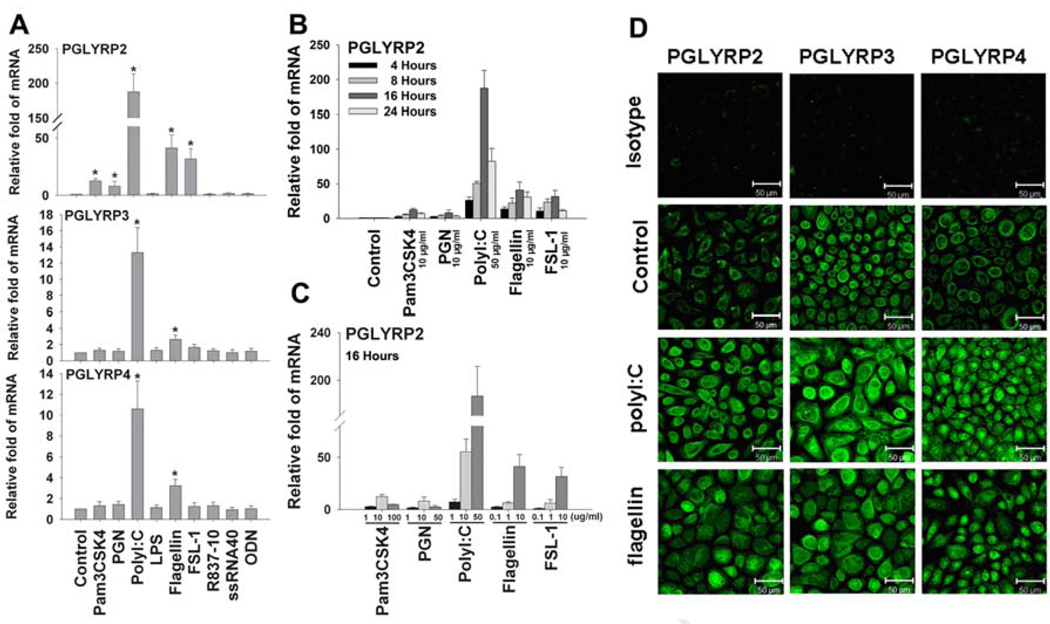
The primary cultured HCECs were challenged by nine extracted or synthetic microbial components, representing the TLR ligands respective to TLRs 1–9, in different concentrations for different time periods (4–48h). Induction of three PGLYRPs was evaluated by real time PCR and immunofluorescent staining. Interestingly, PGLYRP-2 was largely induced by several microbial components, although it was weakly expressed by normal HCECs. As shown in Figure 2A, PGLYRP-2 mRNA level was markedly stimulated in the HCECs challenged by certain bacterial or viral components including Pam3CSK4, PGN, polyI:C, flagellin and FSL-1, the ligands respective to TLRs 1, 2, 3, 5 and 6. PGLYRP-2 induction was not observed in HCECs exposed to LPS (1–10 µg/ml), Imiquimod (R837) (1–50 µg/ml), ssRNA40 (0.1–10 µg/ml) or unmethylated DNA CpG motifs (ODN, 1–50 µg/ml), the ligands respective to TLRs 4, 7, 8 and 9. The induction of PGLYRP-2 mRNA reached to the peak levels at 16 hours (Figure 2B) and was stimulated in a concentration-dependent fashion in HCECs challenged by these major microbial ligands (Figure 2C). The PGLYRP-2 mRNA was stimulated to the highest level (up to 187 fold) by TLR3 ligand polyI:C representing viral double stranded RNA (dsRNA). It was also dramatically stimulated by several bacterial components, including TLR2/1 ligand Pam3CSK4 (up to 12 fold), TLR2 ligand PGN (up to 8 fold), TLR5 ligand flagellin (up to 41 fold) and TLR2/6 ligand FSL-1 (up to 32 fold). In contrast, PGLYRP-3 and -4 appeared to be much less inducible in HCECs in response to microbial components, although they were constitutively expressed by normal HCECs at more abundant levels than PGLYRP-2. Their mRNA expression was only induced by polyI:C and flagellin, and the stimulated levels of PGLYRP-3 and -4 (up to 12 or 3 fold, respectively by polyI:C, and up to 8 or 3 fold, respectively by flagellin) were much less than PGLYRP-2 in HCECs (Figure 2A). The induction of these three PGLYRPs by polyI:C and flagellin was confirmed at protein level by the immunofluorescent staining (Figure 2D).
Figure 2.
Differential induction of PGLYRPs by microbial ligands in HCECs. A. The confluent primary HCECs were incubated with 50µg/ml polyI:C or 10µg/ml of Pam3CSK4, PGN, LPS, flagellin, FSL-1, R-837, ssRNA40 or C-CpG-ODN for 16 hours for evaluating PGLYRP-2, -3 and -4 mRNA expression by RT and real-time PCR; B. The time course of PGLYRP-2 mRNA induction by RT and real-time PCR in HCECs exposed to 50 µg/ml of polyI:C, or 10 µg/ml of Pam3CSK4, PGN, flagellin or FSL-1 for 4–24 hours; C. Concentration-dependent induction of PGLYRP-2 in HCECs by microbial ligands, 1–100 µg/ml Pam3CSK4, 1–50 µg/ml PGN, 5–50 µg/ml polyI:C, or 0.1–10 µg/ml of flagellin or FSL-1 for 16 hours; D. The induced protein production of PGLYRPs 2–4 by polyI:C and flagellin in HCECs was shown by immuoflurecent staining. The mRNA data shown are mean ± SD of three independent experiments, *, p < 0.05; representative images from three independent experiments are shown.
TLR and NFκB signal pathways mediated the induction of PGLYRP-2 by dsRNA in HCECs
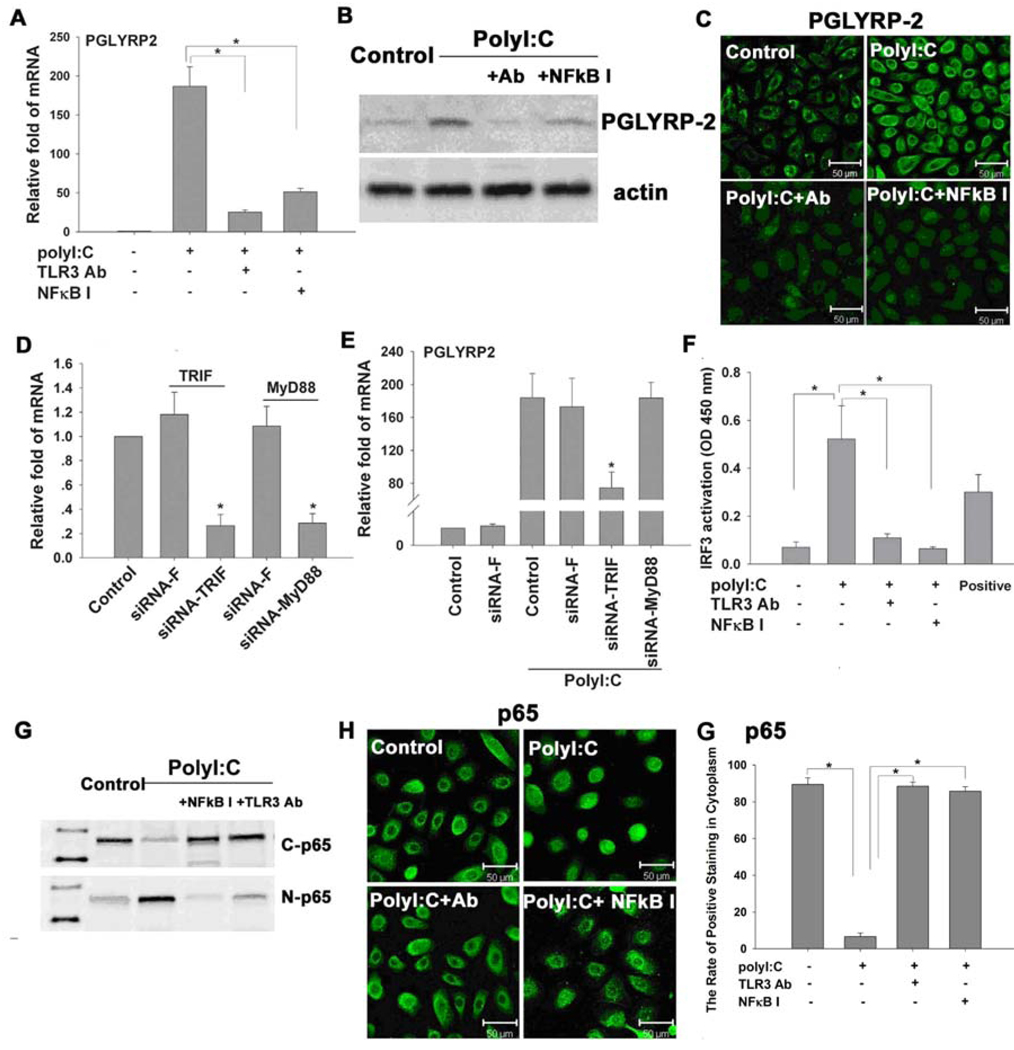
Since PGLYRP-2 was the most inducible in response to different microbial components, and synthetic dsRNA polyI:C was the strongest stimulator, we used this model to further investigate the signaling pathways that mediated the polyI:C-induced production of PGLYRP-2. When HCECs were pre-incubated with a rabbit TLR3 antibody (10µg/ml) or NFκB activation inhibitor quinazoline (NFκB-I, 10µM) one hour before challenged by TLR3 ligand polyI:C (50µg/ml) for 16 or 48 hours, the polyI:C stimulated induction of PGLYRP-2 transcript (Figure 3A) and protein (Figure 3 B and C) was dramatically blocked, as evaluated by real-time PCR, immunostaining and Western blot, respectively. When the cells were pre-transfected with siRNA-TRIF or siRNA-MyD88, using non-coding sequence siRNA-F as a negative control for 48 hours before challenged by polyI:C for 16 hours, the transcripts of TRIF or MyD88 were significantly decreased (up to 70% or more knocked down, Figure 3D) in the cells successfully transfected with the respective siRNA at a transfection rate reaching more than 80% for each siRNA (data not shown). In addition, the polyI:C stimulated levels of PGLYRP-2 transcripts were dramatically blocked in the cells transfected with siRNA-TRIF but neither siRNA-MyD88 nor the negative control siRNA-F (Figure 3E). Evaluated by an ELISA-based nuclear transcription factor activity assay using the Cos-7 cell nuclear extract (supplied in kit) as a positive control, the nuclear IRF3 activity, specific for TRIF pathway activation, was significantly stimulated in the HCECs exposed to polyI:C for 4 hours; and this stimulated nuclear IRF3 activity was markedly blocked by pre-incubation with TLR3 antibody or NFκB-I, respectively (Figure 3F). As evaluated by immunostaining and Western blot analysis (Figure 3 G and H), the NF-κB pathway activation was confirmed by p65 protein translocation from cytoplasm to nucleus in the cells treated with poly I:C for 4 hours. This p65 nuclear translocation was largely blocked by pre-incubation with TLR3 antibody or NFκB-I.
Figure 3.
TLR3 and NFκB signaling pathways involved in PGLYRP-2 induction by polyI:C in HCECs. A. PGLYRP-2 mRNA levels in cells treated with polyI:C for 16 hours and pre-incubated in the absence or presence of 10 µg/ml TLR3 mAb or 10µM NFκB activation inhibitor quinazoline (NFκB-I) for 1 hour; B. Whole protein of cells treated for 48 hours were extracted with RIPA buffer for western blot of PGLYRP-2 production; C. PGLYRP-2 immunofluorescent staining of cells on 8-chamber microscope slides treated with polyI:C for 48 hours and pre-incubated in the presence or absence of 10 µg/ml TLR3 mAb or 10µM NFκB-I for 1 hour; D. RNA interference results showing up to 70% of TRIF or MyD88 mRNA knocked down by respective siRNA for TRIF or MyD88; E. PolyI:C stimulated PGLYRP-2 mRNA levels were blocked by siRNA-TRIF, but not by siRNA-MyD88 nor siRNA-F; F. The nuclear transcription factor IRF3 activity in the cells treated with polyI:C for 4 hours and pre-incubated in the absence or presence of 10 µg/ml TLR3 mAb or 10µM NFκB-I for 1 hour; G. Western blot analysis showing NFκB p65 translocation from cytoplasm to nuclei in cells treated with polyI:C for 4 hours pre-incubated in the absence or presence of 10 µg/ml TLR3 mAb or 10µM NFκB-I for 1 hour. C-p65: cytoplasmic p65; N-p65: nuclear p65; H. NFκB p65 immunofluorescent staining in cells of 8-chamber slides treated with polyI:C for 4 hours and pre-incubated in the presence or absence of 10 µg/ml TLR3 mAb or 10µM quinazoline (NFκB-I) for 1 hour; I. The rate of NFκB p65 positive staining in cytoplasm of human corneal epithelial cells in experiments H was quantified. The mRNA data shown are mean ± SD of three independent experiments, *, p < 0.05; representative images from three independent experiments are shown.
DISCUSSION
Epithelial cells have been recently recognized to detect and respond to various pathogens through the innate immune pathway with PRRs that recognize PAMPs (Ishihara et al., 2006; Kumar et al., 2004; Uehara et al., 2005). PGN is known to be recognized by PGRPs, TLR2, and nucleotide-binding oligomerization domain-containing proteins (NODs) that detect some soluble PGN motifs termed muropeptides (Franchi et al., 2006; Uehara et al., 2005). All three PRR families recognize PGN, but the interactions between TLRs and PGRPs have not been elucidated.
The discovery of the TLRs, the most important family among the PRRs, is a breakthrough in the understanding of the ability of the innate immune system to rapidly recognize pathogens. At least 10 human TLRs have been identified to date. Each TLR has unique ligand specificity. TLRs are expressed on the immune cells that are most likely to first encounter microbes, such as neutrophils, monocytes, macrophages, and dendritic cells (Takeda et al., 2003). In addition to innate immune cells, an array of TLRs is expressed by epithelial cells. HCECs have been shown to express functional TLRs 1–9, except for TLR8 (Kumar and Yu, 2006), and to be capable of recognizing a variety of microbial ligands from bacteria, fungi and viruses.
Although TLR2 has been shown to be able to detect PGN, the PGRPs, especially the human PGLYRPs, are more novel, which not only recognize bacterial PGN as an innate immune response, but also have directly bactericidal function (Dziarski and Gupta, 2006a). Using fresh human corneoscleral tissue and primary cultured corneal epithelial cells, we have revealed that PGLYRP-2, -3 and -4 were constitutively expressed and differentially regulated in HCECs in response to a variety of microbial ligands through TLR and NFκB signaling pathways.
Human PGLYRPs are selectively expressed in different organs. PGLYRP-1 is found in high level in granulocyte granules (Dziarski et al., 2003; Liu et al., 2000; Tydell et al., 2006). PGLYRP-2 is constitutively produced in the liver (Liu et al., 2001; Wang et al., 2003) and inducible in epithelial cells (Uehara et al., 2005). PGLYRP-3 and -4 are expressed in the skin, eyes, salivary glands, throat, tongue, esophagus, stomach and intestine. In the present study, we revealed that three PGLYRPs 2–4 were expressed in human corneal epithelium at mRNA and protein levels, while PGLYRP-1 was not detected. PGLYRP-2 was expressed at very low levels while PGLYRP-3 and -4 were abundantly produced by human corneal epithelium. This diversity suggests that different PGLYRPs may induce distinct biological effects.
A previous study reported that chemically synthesized PAMPs increase the expression of PGLYRPs via TLRs 2 and 4 in human oral epithelial cells (Uehara et al., 2005). In the present study, HCECs were challenged by nine extracted or synthetic microbial components, ligands to TLRs 1–9, respectively. We observed that PGLYRP-3 and -4 were much less inducible, although they were expressed at abundantly constitutive levels in normal conditions. Among the 9 microbial ligands challenged, only TLR5 ligand flagellin, a major component from bacterial flagellar filament, and TLR3 ligand polyI:C, a synthetic viral double stranded RNA (dsRNA) could stimulated the mRNA of PGLYRP-3 and PGLYRP-4 up to 2–3 fold or 11–13 fold, respectively. Consistent with and expanded from the previous results (Uehara et al., 2005), we explored that the expression of PGLYRP-2 was largely induced by a variety of bacteria components, including Pam3CSK4, PGN, flagellin and FSL-1, the ligands for TLR2/1, 2, 5 and 2/6 respectively. Interestingly, we further revealed that PGLYRP-2 was stimulated to the greatest degree (up to 187 fold) by TLR3 ligand polyI:C, representing viral dsRNA. PolyI:C has been shown to induce the expression of key proinflammatory cytokines, chemokines and antiviral genes that help in the defense of the cornea against viral infection (Kumar et al., 2006). Very recently, we reported that polyI:C induced production of the pro-allergic cytokine, thymic stromal lymphopoietin (TSLP) in HCECs (Ma et al., 2009). There are various mechanisms in epithelial cells to sense viral components and initiate intracellular signal transduction for responding rapidly to viral infections. Our findings suggested that corneal epithelial cells are important sentinels of the innate immune system against viral infection. Further investigations, including in vivo cornea infection studies, are necessary to clarify whether PGLYRPs may have a potential anti-viral role in addition to known anti-bacterial efficacy.
TLR3 is constitutively present on endosomal membranes inside cells (Kumar and Yu, 2006) or on cell surface (Ueta et al., 2005) to recognize virus nucleic acids. Stimulation of TLR3 by viral dsRNA has been reported to be able to transduce signals to activate transcription factors via MyD88-independent signalling pathways (Ueta et al., 2005). In the present study, we showed that synthetic dsRNA polyI:C induced PGLYRP-2 through a TLR3 signaling cascade that activated NF-κB and IRF3. TLR3 antibody or NF-κB inhibitor markedly blocked the activation of NF-κB and IRF3, and also inhibited the polyI:C-stimulated induction of PGLYRP-2 in HCECs. The gene silencing experiments using siRNA further confirmed the TLR3-TRIF-IRF3 signaling pathways activated by dsRNA in HCECs. When siRNA-TRIF knocked down the expression of TRIF mRNA, the polyI:C induced stimulation of PGLYRP-2 expression and production was also significantly blocked in HCECs. In contrast, polyI:C-induced PGLYRP-2 expression was not inhibited in MyD88-silenced cells. These results indicated that PGLYRP-2 induction by polyI:C was via TLR3 and NFκB signaling pathways in HCECs. TLR3 may function as a sensor for detection of viral infection and for initiation of the antiviral response in the cornea.
In summary, we have demonstrated that human corneal epithelium expresses three PGLYRPs, among which PGLYRP-3 and -4 were constitutively expressed, while PGLYRP-2 was most inducible by a variety of bacterial and viral ligands. Interestingly, dsRNA was the strongest stimulator for PGLYRPs in HCECs, and the induction of PGLYRP-2 by dsRNA was through TLRs-TRIF-IRF3-NFκB signaling pathways. These findings suggest that PGLYRPs may participate in antimicrobial defense through innate immune response in human corneal epithelium.
ACKNOWLEDGEMENT
The authors thank the Lions Eye Bank of Texas for their kindly providing human corneoscleral tissues. This work was supported in part by Department of Defense CDMRP PRMRP grant FY06 PR064719 (DQL), National Institutes of Health grant EY11915 (SCP), an unrestricted grant from Research to Prevent Blindness, the Oshman Foundation and the William Stamps Farish Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li D-Q. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Li D-Q, Tong L, Stewart P, Chu C, Pflugfelder SC. Targeted inhibition of p57 and p15 blocks transforming growth factor beta-inhibited proliferation of primary cultured human limbal epithelial cells. Mol. Vis. 2006;12:983–994. [PMC free article] [PubMed] [Google Scholar]
- de Paiva CS, Corrales RM, Villarreal AL, Farley WJ, Li D-Q, Stern ME, Pflugfelder SC. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp. Eye Res. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Dziarski R. Peptidoglycan recognition proteins (PGRPs) Mol. Immunol. 2004;40:877–886. doi: 10.1016/j.molimm.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Dziarski R, Gupta D. Mammalian PGRPs: novel antibacterial proteins. Cell Microbiol. 2006a;8:1059–1069. doi: 10.1111/j.1462-5822.2006.00726.x. [DOI] [PubMed] [Google Scholar]
- Dziarski R, Gupta D. The peptidoglycan recognition proteins (PGRPs) Genome Biol. 2006b;7:232. doi: 10.1186/gb-2006-7-8-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziarski R, Platt KA, Gelius E, Steiner H, Gupta D. Defect in neutrophil killing and increased susceptibility to infection with nonpathogenic gram-positive bacteria in peptidoglycan recognition protein-S (PGRP-S)-deficient mice. Blood. 2003;102:689–697. doi: 10.1182/blood-2002-12-3853. [DOI] [PubMed] [Google Scholar]
- Franchi L, McDonald C, Kanneganti TD, Amer A, Nunez G. Nucleotide-binding oligomerization domain-like receptors: intracellular pattern recognition molecules for pathogen detection and host defense. J. Immunol. 2006;177:3507–3513. doi: 10.4049/jimmunol.177.6.3507. [DOI] [PubMed] [Google Scholar]
- Gelius E, Persson C, Karlsson J, Steiner H. A mammalian peptidoglycan recognition protein with N-acetylmuramoyl-L-alanine amidase activity. Biochem. Biophys. Res. Commun. 2003;306:988–994. doi: 10.1016/s0006-291x(03)01096-9. [DOI] [PubMed] [Google Scholar]
- Ishihara S, Rumi MA, Ortega-Cava CF, Kazumori H, Kadowaki Y, Ishimura N, Kinoshita Y. Therapeutic targeting of toll-like receptors in gastrointestinal inflammation. Curr. Pharm. Des. 2006;12:4215–4228. doi: 10.2174/138161206778743448. [DOI] [PubMed] [Google Scholar]
- Kang D, Liu G, Lundstrom A, Gelius E, Steiner H. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc. Natl. Acad. Sci. U. S. A. 1998;95:10078–10082. doi: 10.1073/pnas.95.17.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Jun SX, de Paiva CS, Chen Z, Pflugfelder SC, Li D-Q. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp. Eye Res. 2004;79:41–49. doi: 10.1016/j.exer.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Yu FS. Toll-like receptors and corneal innate immunity. Curr. Mol. Med. 2006;6:327–337. doi: 10.2174/156652406776894572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Zhang J, Yu FS. Innate immune response of corneal epithelial cells to Staphylococcus aureus infection: role of peptidoglycan in stimulating proinflammatory cytokine secretion. Invest Ophthalmol. Vis. Sci. 2004;45:3513–3522. doi: 10.1167/iovs.04-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Zhang J, Yu FS. Toll-like receptor 3 agonist poly(I:C)-induced antiviral response in human corneal epithelial cells. Immunology. 2006;117:11–21. doi: 10.1111/j.1365-2567.2005.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D-Q, Lokeshwar BL, Solomon A, Monroy D, Ji Z, Pflugfelder SC. Regulation of MMP-9 production by human corneal epithelial cells. Exp. Eye Res. 2001;73:449–459. doi: 10.1006/exer.2001.1054. [DOI] [PubMed] [Google Scholar]
- Li D-Q, Tseng SC. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J. Cell Physiol. 1995;163:61–79. doi: 10.1002/jcp.1041630108. [DOI] [PubMed] [Google Scholar]
- Liu C, Gelius E, Liu G, Steiner H, Dziarski R. Mammalian peptidoglycan recognition protein binds peptidoglycan with high affinity, is expressed in neutrophils, and inhibits bacterial growth. J. Biol. Chem. 2000;275:24490–24499. doi: 10.1074/jbc.M001239200. [DOI] [PubMed] [Google Scholar]
- Liu C, Xu Z, Gupta D, Dziarski R. Peptidoglycan recognition proteins: a novel family of four human innate immunity pattern recognition molecules. J. Biol. Chem. 2001;276:34686–34694. doi: 10.1074/jbc.M105566200. [DOI] [PubMed] [Google Scholar]
- Lu X, Wang M, Qi J, Wang H, Li X, Gupta D, Dziarski R. Peptidoglycan recognition proteins are a new class of human bactericidal proteins. J. Biol. Chem. 2006;281:5895–5907. doi: 10.1074/jbc.M511631200. [DOI] [PubMed] [Google Scholar]
- Luo L, Li D-Q, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis. Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- Ma P, Bian F, Wang Z, Zheng X, Chotikavanich S, Pflugfelder SC, Li DQ. Human corneal epithelium-derived thymic stromal lymphopoietin links innate and adaptive immune responses via TLRs and Th2 cytokines. Invest. Ophthalmol. Vis. Sci. 2009;50:2702–2709. doi: 10.1167/iovs.08-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A, Rosenblatt M, Li D-Q, Monroy D, Ji Z, Lokeshwar BL, Pflugfelder SC. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Am. J. Ophthalmol. 2000;130:688. doi: 10.1016/s0002-9394(00)00755-8. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int. Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Tydell CC, Yuan J, Tran P, Selsted ME. Bovine peptidoglycan recognition protein-S: antimicrobial activity, localization, secretion, and binding properties. J. Immunol. 2006;176:1154–1162. doi: 10.4049/jimmunol.176.2.1154. [DOI] [PubMed] [Google Scholar]
- Uehara A, Sugawara Y, Kurata S, Fujimoto Y, Fukase K, Kusumoto S, Satta Y, Sasano T, Sugawara S, Takada H. Chemically synthesized pathogen-associated molecular patterns increase the expression of peptidoglycan recognition proteins via toll-like receptors, NOD1 and NOD2 in human oral epithelial cells. Cell Microbiol. 2005;7:675–686. doi: 10.1111/j.1462-5822.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- Ueta M, Hamuro J, Kiyono H, Kinoshita S. Triggering of TLR3 by polyI:C in human corneal epithelial cells to induce inflammatory cytokines. Biochem. Biophys. Res. Commun. 2005;331:285–294. doi: 10.1016/j.bbrc.2005.02.196. [DOI] [PubMed] [Google Scholar]
- Unterholzner L, Bowie AG. The interplay between viruses and innate immune signaling: recent insights and therapeutic opportunities. Biochem. Pharmacol. 2008;75:589–602. doi: 10.1016/j.bcp.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Wang ZM, Li X, Cocklin RR, Wang M, Wang M, Fukase K, Inamura S, Kusumoto S, Gupta D, Dziarski R. Human peptidoglycan recognition protein-L is an N-acetylmuramoyl-L-alanine amidase. J. Biol. Chem. 2003;278:49044–49052. doi: 10.1074/jbc.M307758200. [DOI] [PubMed] [Google Scholar]
- Werner T, Liu G, Kang D, Ekengren S, Steiner H, Hultmark D. A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13772–13777. doi: 10.1073/pnas.97.25.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KC, de Paiva CS, Qi H, Chen Z, Farley WJ, Li DQ, Pflugfelder SC. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol. Vis. Sci. 2007;48:2561–2569. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Kinoshita K, Ashida M. Purification of a peptidoglycan recognition protein from hemolymph of the silkworm, Bombyx mori. J. Biol. Chem. 1996;271:13854–13860. doi: 10.1074/jbc.271.23.13854. [DOI] [PubMed] [Google Scholar]
- Yu K, Ma P, Ge J, Willey CD, Yang P, Wang Z, Gao Q. Expression of protein kinase C isoforms in cultured human retinal pigment epithelial cells. Graefes Arch. Clin. Exp. Ophthalmol. 2007;245:993–999. doi: 10.1007/s00417-006-0467-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, van der FL, Voerman JS, Melief MJ, Laman JD, Wang M, Wang H, Wang M, Li X, Walls CD, Gupta D, Dziarski R. Identification of serum N-acetylmuramoyl-l-alanine amidase as liver peptidoglycan recognition protein 2. Biochim. Biophys. Acta. 2005;1752:34–46. doi: 10.1016/j.bbapap.2005.07.001. [DOI] [PubMed] [Google Scholar]